Abstract
A 76-year-old woman suddenly developed anasarca and a fever, and an examination revealed thrombocytopenia, reticulin fibrosis, and acute kidney injury, yielding the diagnosis of thrombocytopenia, anasarca, fever, reticulin fibrosis, organomegaly (TAFRO) syndrome. Renal replacement therapy and steroid treatment were soon started. Her proteinuria was minor at first; however, once the kidney function improved, nephrotic syndrome occurred. A kidney biopsy showed membranoproliferative glomerulonephritis-like glomerulopathy with massive macrophage infiltration. Although kidney dysfunction is often observed in TAFRO syndrome patients, its detailed mechanism is unclear. This case suggests that TAFRO syndrome involves both acute kidney injury with minor proteinuria and nephrotic syndrome, and these disorders can develop serially in the same patient.
Keywords: acute kidney injury, membranoproliferative glomerulonephritis-like glomerulopathy, TAFRO syndrome, nephrotic syndrome
Introduction
Thrombocytopenia, anasarca, fever, reticulin fibrosis, organomegaly (TAFRO) syndrome is a rare disorder characterized by thrombocytopenia, anasarca including pleural effusion and ascites, a fever, kidney dysfunction, myelofibrosis, and organomegaly (e.g. hepatosplenomegaly and lymphadenopathy) (1,2). The syndrome was originally reported in 2010 by Takai et al. and is regarded as a novel systemic inflammatory disease (1).
TAFRO syndrome shares several clinical and pathological features with multicentric Castleman's disease (MCD), and cases of human herpesvirus 8 (HHV-8)-negative MCD have been described as TAFRO syndrome (3). Elevated serum levels of interleukin (IL)-6 and vascular endothelial growth factor (VEGF) are hypothesized to induce the immunological disorder in TAFRO syndrome; however, the precise pathogenesis of this condition remains to be determined.
The detailed mechanisms of kidney dysfunction in TAFRO syndrome have also been unclear, and there are only a few case reports detailing the associated kidney pathology. We herein report the case of a patient with TAFRO syndrome who first presented with acute kidney injury (AKI) with minor proteinuria. Interestingly, the patient subsequently developed nephrotic syndrome, and a kidney biopsy showed membranoproliferative glomerulonephritis (MPGN)-like glomerulopathy.
Case Report
A 76-year-old woman presented with a fever, abdominal pain, and facial edema and was admitted to a nearby hospital. Antibiotics and diuretics were administered, but her general condition worsened. Two weeks after the admission, she was transferred to our hospital for further care.
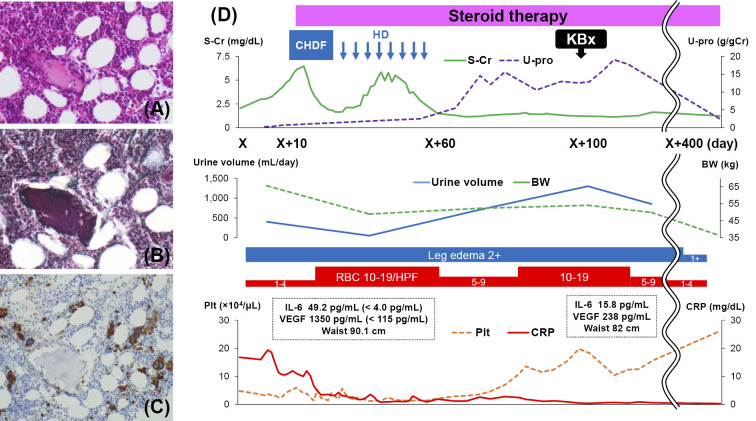
On admission to our hospital, the patient had a low-grade fever and anasarca with a 10-kg increase in body weight. The results of laboratory tests were as follows: white blood cell count, 14.1×103/μL (neutrophil count, 12.3×103/μL); hemoglobin level, 10.4 g/dL; platelet count, 3.6×104/μL; serum creatinine, 3.02 mg/dL; blood urea nitrogen, 64 mg/dL; lactate dehydrogenase, 206 U/L; alkaline phosphatase, 378 U/L; total protein without M-protein, 4.3 g/dL; albumin, 1.2 g/dL; C-reactive protein, 16.0 mg/dL; immunoglobulin G (IgG), 769 mg/dL; haptoglobin, 241 mg/dL; soluble IL-2 receptor, 3,110 U/mL; IL-6, 49.2 pg/mL (<4.0 pg/mL); and VEGF, 1,350 pg/mL (<115 pg/mL). Antinuclear antibody and rheumatoid factor studies were negative, and cryoglobulin and autoantibodies, including anti-double-stranded DNA antibody, anti-neutrophil cytoplasmic antibody, and platelet-associated IgG, were undetectable. There were no signs of a viral infection, including Epstein-Barr virus, cytomegalovirus, or HHV-8. Her urinalysis showed isomorphic hematuria [1-4 red blood cells (RBCs)/high-power field (HPF)] and a protein level of 0.30 g/day when the daily urine volume was 400 mL/day. Her urinary N-acetyl-beta-D-glucosaminidase (NAG) excretion was 16.4 U/L (<4.2 U/L), and the beta 2-microglobulin (β2-MG) excretion was 40 μg/L (<200 μg/L). Her fractional excretion of sodium (FENa) was 0.34%. Computed tomography revealed massive pleural effusion, ascites, and periaortic lymphadenopathy but did not show any evidence of malignant solid tumors or signs of infection. Bone marrow aspiration showed bone marrow hyperplasia, reticulin fibrosis (Fig. 1A and B), and an increased number of CD42b-positive megakaryocytes (Fig. 1C).
Figure 1.
Hyperplastic bone marrow in a patient with TAFRO syndrome (A, Hematoxylin and Eosin staining) accompanied by reticulin fibrosis (B, silver stain). (C) Immunoperoxidase staining for CD42b shows an increased number of megakaryocytes (original magnification, A, B, C, 100×). (D) The patient’s clinical course shows that steroid treatment resulted in an improved kidney function, increased platelet count (Plt), and decreased body weight (BW), waist size, and levels of inflammatory markers including C-reactive protein (CRP), interleukin (IL) -6, and vascular endothelial growth factor (VEGF). In contrast, urine protein (U-pro) increased after steroid therapy was started. Day X is the admission day. CHDF: Continuous hemodiafiltration, HD: hemodialysis, KBx: kidney biopsy, S-Cr: serum creatinine
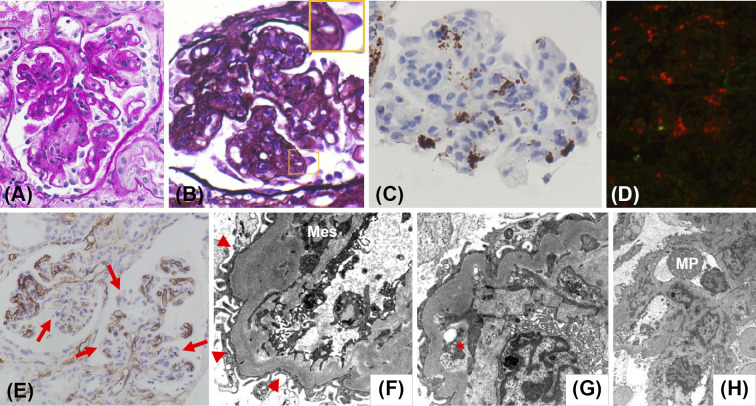
Although the patient presented with severe thrombocytopenia, hemolytic anemia was absent because the level of lactate dehydrogenase was not elevated and that of haptoglobin was not decreased, making thrombotic microangiopathy unlikely. In addition, an episode of preceding diarrhea suggesting hemolytic uremic syndrome and decreased a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13 (ADAMTS13) activity suggesting thrombotic thrombocytopenic purpura were absent. We diagnosed our patient with TAFRO syndrome and initiated steroid treatment, which improved her platelet count and decreased the inflammatory marker levels. However, her kidney function worsened, and we began treatment with continuous hemodiafiltration (CHDF) followed by hemodialysis (HD). Her clinical course is shown in Fig. 1D. Although her kidney function improved with steroid treatment and HD, massive proteinuria and dysmorphic hematuria (10-19 RBCs/HPF) were observed after we discontinued hemodialysis. At that point, the selectivity index of proteinuria was 0.44. Nephrotic syndrome was diagnosed, and a kidney biopsy was performed. An examination of the kidney tissue showed 29 glomeruli, of which 4 were globally sclerotic. Although crescent formation was absent, 60% of the glomeruli were lobulated and accompanied by mesangial cell proliferation, increased mesangial matrix, and focal double contouring of the glomerular capillary walls (Fig. 2A and B). Although mild neutrophil infiltration was noted, massive macrophage infiltration was noted within the glomeruli (brown-stained cells, Fig. 2C) and tubulointerstitial area (red-stained cells, Fig. 2D). In addition, decreased CD34 immunoreactivity was segmentally identified, suggesting glomerular endothelial cell injury (Fig. 2E). Focal tubular injury was also present.
Figure 2.
Histological features of a kidney biopsy in a patient with TAFRO syndrome. (A) Lobulation of a glomerulus and mesangial proliferation can be seen in light microscopy sections (periodic acid-Schiff stain). (B) The glomerular capillary walls show focal double contouring (periodic acid-methenamine-silver stain). (C) Immunoperoxidase staining for CD68 (Nichirei Biosciences, Tokyo, Japan) shows many intraglomerular macrophages. (D) Double immunofluorescence staining for neutrophils (neutrophil elastase; Calbiochem, San Diego, USA) labeled with Alexa Fluor 488 (Thermo Fisher Scientific, Waltham, USA; green) and macrophages (CD68; Agilent, Santa Clara, USA) labeled with Alexa Fluor 594 (Thermo Fisher Scientific; red) shows massive macrophage infiltration within the tubulointerstitial area. The neutrophil infiltration is unremarkable. (E) Immunoperoxidase staining for CD34 (Agilent) shows prominently decreased CD34 immunoreactivity in some glomerular endothelial cells (arrows). Effacement of the podocyte foot processes (arrowheads) and mesangial interposition (Mes, F), expansion of the subendothelial space (asterisk, G), and infiltration of macrophages (MP, H) are shown in electron microscopy sections. There were no electron-dense deposits present (original magnification, A, B, C, 100×, D, 66×, E, 25×).
Immunofluorescent staining showed no immunoglobulin or complement deposition in the glomeruli. Electron microscopy did not reveal any electron-dense deposits but did show effacement of the podocyte foot processes, mesangial interposition, expansion of subendothelial space, and infiltration of macrophages (Fig. 2F-H). Based on these findings, we diagnosed her with MPGN-like glomerulopathy with massive macrophage infiltration and glomerular endothelial cell injury accompanied by focal tubular injury. The patient's general condition improved, and her massive proteinuria decreased after long-term steroid treatment. She was then discharged home.
Discussion
We herein report the case of a patient with TAFRO syndrome and a kidney biopsy showing MPGN-like glomerulopathy with massive macrophage infiltration and glomerular endothelial cell injury accompanied by focal tubular injury. Although kidney dysfunction is commonly observed in patients with TAFRO syndrome, little is known about the kidney pathology associated with this diagnosis. There have been only a few reports in the English literature indexed within the PubMed database that report the pathologic kidney findings in patients with TAFRO syndrome (4-8). Notably, as in the present case, an MPGN lesion was also the main light microscopy finding in previous reports.
Massive macrophage infiltration was a prominent finding in the kidney biopsy of our patient with TAFRO syndrome, and it is possible that infiltrating macrophages play an important role in the pathogenesis of kidney injury in TAFRO syndrome. Indeed, activated macrophages may contribute significantly to the pathogenesis of MPGN lesion (9). Furthermore, if the kidney biopsy had been performed before the initiation of steroid treatment, more macrophages would have been observed in the biopsy performed following therapy.
CD34 immunostaining reportedly identifies the morphological changes associated with glomerular endothelial cell injury, and decreased CD34 staining is found in both acute and chronic glomerular lesions and is associated with glomerular sclerosis (10). We therefore considered that, in the present case, endothelial cell injury occurred in the glomerular endothelial cells with segmentally decreased CD34 immunoreactivity.
Tubulointerstitial nephritis was reported as a pathologic finding in TAFRO syndrome (11). However, in the present case, the levels of urinary NAG and β2-MG were not elevated before steroid treatment was started, and tubulointerstitial nephritis was not observed in the kidney biopsy. However, the patient's FENa was consistently low before we started renal replacement therapy, suggesting severe kidney ischemia, and the kidney biopsy revealed tubular injury. Thus, prerenal AKI and tubular injury may be other characteristic features of the kidney injury that occurs in TAFRO syndrome. These features may also be associated with decreased urination, which was observed in the early clinical course of the present case.
Increased levels of IL-6 and VEGF are reportedly involved in the pathogenesis of TAFRO syndrome, and these molecules may also contribute to TAFRO-associated kidney injury (5). IL-6 overproduction induces VEGF production, resulting in mesangial proliferation (5), glomerular endothelial cell injury (12), and MPGN lesions (13), all of which were observed in the kidney biopsy of the present case. Regarding this point, because activated macrophages can produce both IL-6 and VEGF (14), the kidney macrophage infiltration may also play a role in the formation of the lesions. In addition, because elevated VEGF enhances the vascular permeability (12), we speculate that increased systemic vascular permeability induced an intravascular dehydrated state and resultant severe kidney ischemia accompanied by prerenal AKI in our patient, and that overproduction of IL-6 and/or VEGF might be involved in the pathogenesis.
However, why these two forms of kidney injury developed serially in the present case is unclear. One possibility is that the MPGN-like glomerulopathy already existed at the onset of TAFRO syndrome but was masked due to decreased urination. Indeed, a kidney biopsy of the present case revealed some sclerotic MPGN lesions, such as increased mesangial matrix and double contouring of the glomerular capillary walls (Fig. 2A and B). Similarly, the proteinuria in the present case was minor at first but later increased, possibly due to increased urination following steroid therapy, which improved the kidney function. The further accumulation of cases in which a kidney biopsy is performed in the early clinical course is required to investigate this matter.
The optimum treatment for patients with TAFRO syndrome is still unknown, but the usual treatment at present is glucocorticoids alone or together with immunosuppressive medications (5). In our patient, long-term glucocorticoid treatment improved the kidney function, platelet count, and inflammatory marker levels as well as the clinical symptoms. We did not use immunosuppressive drugs because the patient's kidney function was preserved, but it should be kept in mind that additional immunosuppressive therapy may be needed in the future.
In conclusion, we herein report the case of a patient with TAFRO syndrome involving both AKI with minor proteinuria and nephrotic syndrome, in which MPGN-like glomerulopathy with massive macrophage infiltration and tubular injury were detected. The present case suggests that TAFRO syndrome is complicated by several forms of kidney injury and that these injuries can develop serially in the same patient.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Prof. Yasuharu Sato (Division of Pathophysiology, Okayama University Graduate School of Health Sciences) and Dr. Noriko Iwaki (Division of Hematology, Toyama Red Cross Hospital) for their valuable advice.
References
- 1.Kawabata H, Takai K, Kojima M, et al. . Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J Clin Exp Hematop 53: 57-61, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Masaki Y, Kawabata H, Takai K, et al. . Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol 103: 686-692, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Iwaki N, Fajgenbaum DC, Nabel CS, et al. . Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol 91: 220-226, 2016. [DOI] [PubMed] [Google Scholar]
- 4.José FF, Kerbauy LN, Perini GF, et al. . A life-threatening case of TAFRO syndrome with dramatic response to tocilizumab, rituximab, and pulse steroids: The first case report in Latin America. Medicine (Baltimore) 96: e6271, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawashima M, Usui T, Okada H, et al. . TAFRO syndrome: 2 cases and review of the literature. Mod Rheumatol 27: 1093-1097, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Tsujimoto H, Yamamoto K, Shimoda S, Oka K, Takeoka H. Clinicopathological features of progressive renal involvement in TAFRO syndrome: a case report and literature review. Medicine (Baltimore) 96: e8216, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamori A, Akagaki F, Yamaguchi Y, Arima R, Sugiura T. Nephrotic syndrome with thrombocytopenia, lymphadenopathy, systemic inflammation, and splenomegaly. Intern Med 57: 1123-1129, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis C, Vijgen S, Samii K, et al. . TAFRO Syndrome in caucasians: a case report and review of the literature. Front Med (Lausanne) 4: 149, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowalewska J, Okoń K, Szynaka B, Naumnik B. Expression of macrophage markers in cryoglobulinemic glomerulonephritis - a possible role of CXCL9. Adv Med Sci 58: 394-400, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Kusano T, Takano H, Kang D, et al. . Endothelial cell injury in acute and chronic glomerular lesions in patients with IgA nephropathy. Hum Pathol 49: 135-144, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Kubokawa I, Yachie A, Hayakawa A, et al. . The first report of adolescent TAFRO syndrome, a unique clinicopathologic variant of multicentric Castleman's disease. BMC Pediatr 14: 139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seida A, Wada J, Morita Y, et al. . Multicentric Castleman's disease associated with glomerular microangiopathy and MPGN-like lesion: does vascular endothelial cell-derived growth factor play causative or protective roles in renal injury? Am J Kidney Dis 43: E3-E9, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Soubrier M, Sauron C, Souweine B, et al. . Growth factors and proinflammatory cytokines in the renal involvement of POEMS syndrome. Am J Kidney Dis 34: 633-638, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Murakami M, Iwai S, Hiratsuka S, et al. . Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocyte/macrophages. Blood 108: 1849-1856, 2006. [DOI] [PubMed] [Google Scholar]