Abstract
Toxoplasma gondii is an obligate intracellular parasite with global incidence. The acute infection, toxoplasmosis, is treatable but current regimens have poor host tolerance and no cure has been found for latent infections. This work builds upon a previous high throughput screen which identified benzoquinone acyl hydrazone (KG8) as the most promising compound; KG8 displayed potent in vitro activity against T. gondii but only marginal in vivo efficacy in a T. gondii animal model. To define the potential of this new lead compound, we now describe a baseline structure-activity relationship for this chemotype. Several derivatives displayed IC50's comparable to that of the control treatment pyrimethamine with little to no cytotoxicity. The best of these, KGW44 and KGW59, had higher metabolic stability than KG8. In an in vivo T. gondii murine model, KGW59 significantly increased survivorship. This work provides new insights for optimization of this novel chemotype.
Keywords: Toxoplasma gondii, Drug discovery, Lead compounds, Anti-parasitics
Graphical abstract
Highlights
-
•
Derivatives from a previously described anti T. gondii compound were characterized.
-
•
Top derivatives showed increased activity against T. gondii in vitro.
-
•
Derivatives demonstrated low cytotoxicity in host cell lines.
-
•
Derivative KGW59 showed efficacy in vivo with a strong therapeutic window.
-
•
These early-leads are suitable for further development as toxoplasmosis therapies.
1. Introduction
Toxoplasma gondii is a protozoan parasite capable of infecting virtually all classes of warm-blooded vertebrates. Initial infection often occurs from contaminated food and water or exposure to oocysts shed by members of the Felidae family. Upon host infection, the disease toxoplasmosis can occur. Acute toxoplasmosis is frequently described as mild flu-like symptoms, but healthy individuals will often be asymptomatic, while transition to a life-long latent infection is common (Tenter et al., 2000).
The immunocompromised populations who carry the parasitic infection require lifelong prophylactic treatment to prevent parasite induced encephalitis. Primary infections that occur in mothers during pregnancy can cause congenital toxoplasmosis in the fetus due to the ability of the parasite to cross the placenta. Such fetal infections can result in disastrous birth defects such as hydrocephaly and other malformations, causing T. gondii to be leading cause of birth defects worldwide (Tenter et al., 2000).
Current treatments exist to control acute toxoplasmosis, but the issues remain with poor host tolerance and frequent severe complications (Fung and Kirschenbaum, 1996). Front-line treatment most commonly includes pyrimethamine in combination with sulfadiazine but is associated with bone marrow toxicity and allergic reactions (Fung and Kirschenbaum, 1996). Some symptoms have been mitigated with the co-administration of folinic acid. Because of this, there is an imperative need for new therapeutics to be developed to combat acute infection. No FDA approved treatment has yet to be established for congenital toxoplasmosis, but it has been treated with clindamycin with varying degrees of success (McFarland et al., 2016).
While acute stage toxoplasmosis has been mitigated with current treatments, the latent infection has remained resistant to clearance and is presently life-long (Neville et al., 2015). Research efforts have produced few promising lead candidates against the chronic stage (Neville et al., 2015; McFarland et al., 2016).
A high-throughput phenotypic screen (HTS) of a chemical library produced a small collection of drug-like compounds with demonstrated efficacy against both Plasmodium falciparum and T. gondii in vitro (Guiguemde et al., 2010). The three compounds with the highest potency against T. gondii were further characterized as potential leads for further optimization and development (Sanford et al., 2018). The most promising of these was KG8 (Fig. 1). Given that kinases are thought to be promising T. gondii drug targets (Kamau et al., 2012), it is interesting to note that the closely related compound poloxin (Fig. 1), a derivative of thymoquinone, is an inhibitor of host polo-like kinase 1 (Liao et al., 2010). KG8 and closely related analogues have been previously shown to inhibit cyclooxgenase-2 and 5-lipoxygenase (Misra et al., 2013) in the host, and potentiate nerve growth factor (Eguchi et al., 2000). KG8 is also an acyl hydrazone derivative of the antibiotic isoniazid; however, the latter was inactive against T. gondii in vitro (Sanford et al., 2018). These data may suggest a host target of KG8, or potentially a parasite homolog target.
Fig. 1.
KG8 and poloxin structures.
We now describe our initial work to assess the potential of this chemotype by the generation of a small library of chemical analogues to increase potency and selectivity against T. gondii. We identified two promising derivatives (KGW44 and KGW59) and assessed their physicochemical and ADME profiles and their in vivo efficacy in a T. gondii animal model.
2. Methods
2.1. Experimental compounds
The synthesis of the KG8 derivatives is described in the supplemental material section.
2.2. Cell line maintenance
Human foreskin fibroblasts (HFF), human osteosarcoma cells (U-20S), human embryonic kidney cells (HEK-293), human liver cells (HC-04), and murine macrophages (NR-9456) were all obtained from ATCC. HFF, U-20S, and all T. gondii strains (RH-dTom, ME49 and PRU) used were maintained in DMEM media (Lonza) supplemented with 10% heat inactivated Hyclone bovine serum (GE Healthcare Life Sciences), HyClone 2 mM L-glutamine (GE Healthcare Life Sciences), 100 μg/mL penicillin and streptomycin (Corning), 10% Medium 199 (Corning) and gentamicin sulfate (Corning) at 36.5 °C with 5% CO2. HEK-293 and HC-04 were maintained in the same medium without the addition of Medium 199. Macrophage NR-9456 was grown with the addition of 1 mM sodium pyruvate as recommended.
2.3. Parasite strains
RH (Type I) and PRU (Type II) strain T. gondii with inserted fluorescent transgene dimerized Tomato (dTom) were used for in vitro growth inhibition experiments. ME49 (Type II) strain T. gondii was used for the in vivo survivorship challenge in a murine model.
2.4. T. gondii growth inhibition in vitro screens (IC50)
HFF cells were plated in 96 well plates at approximately 20,000 cells per well and allowed to grow until confluent. Once confluency was reached, 2000 RH-dTom (or 8000 PRU-dTom) tachyzoites were added to each well and the plates were incubated at 37 °C for 24 h (48 h for PRU) to allow for infection. Media was then replaced, and compounds dissolved in DMSO (Fischer Scientific) were added at increasing concentration from 0 to 100 μM. The concentration of DMSO did not exceed 1% in all assays to prevent cytotoxicity. All concentrations were performed in duplicate and pyrimethamine was used as a positive control. A fluorescent reading was then taken with a BioTek Snergy HT plate reader at 530/25 excitation and 590/25 emission for 5 days. IC50s were determined after day 5 where half of the relative fluorescent units (RFU) were seen.
2.5. Mammalian cell line cytotoxicity screens (IC50)
Mammalian cell viability was determined using human foreskin fibroblasts (HFF), osteosarcoma (U-20S), embryonic kidney (HEK-283), hepatic carcinoma (HC-04), and a murine derived macrophage (NR-9456) cell line that were plated with approximately 20,000 cells per well and let grown until confluent. Once confluent, cells were treated with increasing compound concentrations from 0 to 100 μM for 24 h. This was followed by an Alamar Blue assay with 0.5 mM resazurin at 37 °C with 5% CO2 for 4 h. All concentrations were performed in duplicate. A fluorescent reading was then taken with a BioTek Snergy HT plate reader at 530/25 excitation and 590/25 emission. Host cell viability was determined by comparing the treatments with no compound treatment as 100% viability.
2.6. Bacterial reverse mutation assay
A modified form of the Ames assay (Environmental Bio-Detection Products Inc.) with Salmonella typhimurium (TA100 strain) was used to detect DNA single point mutagenicity. Compounds were tested at concentrations of 3x the averaged T. gondii IC50 values (Table 1) in sets of 48 replicates. A count of revertant colonies was performed and compared to the natural revertant control with the unpaired Student's t-test to assess statistical significance.
Table 1.
IC50 values of KG8 and derivatives against T. gondii strains and HFF.
| Derivative ID | RH T. gondii IC50 (μM) | PRU T. gondii IC50 (μM) | HFF IC50 (μM) |
|---|---|---|---|
| KG81 | 2.3 | 2.5 | 28.0 |
| KGW15 | 2.0 | – | >100 |
| KGW42 | 2.0 | – | >100 |
| KGW43 | 3.8 | – | >100 |
| KGW64b | 0.91 | 78 | >100 |
| KGW44a | 0.20 | 0.99 | >100 |
| KGW54 | 1.2 | 2.2 | >100 |
| KGW65b | 0.66 | >100 | >100 |
| KGW45 | 40 | – | >100 |
| KGW66b | >100 | – | >100 |
| KGW68b | 3.8 | – | >100 |
| KGW59a | 0.13 | 1.4 | >100 |
| KGW72 | 13 | – | >100 |
| KGW73 | >100 | – | >100 |
Derivatives of KG8 (Sanford et al., 20181) were screened against both RH-dTom and Pru-dTom T. gondii.
Compounds that were effective in inhibiting T. gondii viability were selected for further cell viability screening. All compounds were also screened in a cell viability screen against human foreskin fibroblasts (HFF). No compound was highly toxic against HFF.
Compounds KGW64-68 were found to be auto fluorescent and were excluded from further analysis.
2.7. Host cell and extracellular parasite pre-treatment assay
HFF cells were grown in 96 well plates to confluency. 10 μM of respective compound was added to the wells (performed in triplicate). After 24 h of compound exposure, cells were then washed twice with D10 media. Cells were then infected with 20,000 RH tachyzoites/mL and parasite growth was quantified by fluorescence for 5 days post-infection.
To evaluate the effect of compound exposure to free tachyzoites, 10 μM of respective compound was added to RH-dTom tachyzoites isolated from culture and resuspended in D10 media at 10e6 tachzyoites/mL. Exposure to compound lasted 4 h at 37 °C. After treatment, tachyzoites were centrifuged and washed twice. Confluent HFF in 96 well plates were then infected with 2000 treated tachyzoites per well. Tachyzoite growth was quantified by fluorescence at 5 days post infection.
2.8. Calculated physicochemical properties
Polar surface area (PSA) and lipophilicity (cLogD) values were assesed by the ChemAxon chemistry cartridge via JChem for Excel Software. Aqueous solubility of the most potent three compounds was determined by dissolving in DMSO and then spiked into either pH 6.5 PBS or 0.01 M HCl (pH 2.0). Samples were then analyzed 30 min after nephelometry to determine a solubility range. Partition coefficient values were then analyzed at pH 7.4 through chromatographic retention properties against standard compounds with known values through gradient HPLC. Chromatography-based protein binding values (cPPB) were then determined by correlation of known chromatographic retention properties by using a human albumin column against a standard series of compounds with known cPPB values (Valko et al., 2003). To determine metabolic stability, compounds were incubated with liver microsomes at 1 μM from both humans and mice at 37 °C at a protein concentration of 0.4 mg/mL. Reactions were initiated by the addition of an NADPH-regenerating system. Reactions were then quenched at varying time points (2, 30, and 60 min) and compound concentrations were then determined through LCMS.
2.9. Efficacy in vivo
Female Swiss Webster mice were obtained from Charles' River. At 2 months old, mice were infected intraperitoneally with 5000 ME49 tachyzoites. Simultaneously, treatment began with experimental compounds at 5 mg/kg (n = 10), pyrimethamine at 5 mg/kg (n = 20) or solvent (n = 24) given once per day. Doses was determined for all three compounds by prior screening for toxicity. All experimental compounds and pyrimethamine were dissolved in 5% DMSO, 47.5% Propylene glycol (Sigma-Aldrich), and 47.5% Kollisolv (Sigma-Aldrich). Mice were weighed daily to assess overall health and to monitor weight changes indicative of infection. P values were obtained through the use of IBM STSS Statistics 22 software. All in vivo studies were institutionally approved and carried out under IACUC #12-062-10.
3. Results and discussion
3.1. Primary screen for RH strain T. gondii and human fibroblast (HFF) cytotoxicity
Thirteen derivatives of KG8 (Fig. 2) were initially screened against the RH strain T. gondii for their potency in growth inhibition, and in human foreskin fibroblasts for host cell cytotoxicity (Table 1). We first discovered that replacing the 4-pyridyl substructure with a phenyl (KGW15) did not lower potency against T. gondii. Further, compounds in which the para position of the phenyl substructure was substituted with electron-donating (KGW42, 43, 64) and electron-withdrawing (KGW44, 54) groups were also quite active with IC50s in the range of 0.20–3.8 μM; of these KGW64 and KGW44 were the most potent. Compound KGW65, the 3-pyridyl isostere of KG8, was marginally more potent than the parent compound. However, replacing the 4-pyridyl substructure with an imidazole (KGW45) or cyclohexyl (KGW66) significantly weakened or abolished activity, respectively. Removal of the acyl hydrazone carbonyl (KGW68) and adding a nitro group (KGW59) increased potency by an order of magnitude. Replacing the acyl hydrazone of KG8 with an acyl oxime ether to afford the poloxin analog KGW72 reduced potency 6-fold. KGW73, a saturated analog of the benzoquinone substructure, was inactive. Finally, none of the compounds decreased HFF viability at up to 100 μM concentration.
Fig. 2.
KGW structures.
3.2. Screen for activity against T. gondii PRU strain
The five compounds (KGW64, 44, 54, 65, and 59) with IC50 values less than 2 μM against T. gondii strain RH were tested against strain PRU (Type II lineage) which is considered less virulent and more representative of natural infections (Howe and Sibley, 1995). All five compounds were less active against the PRU strain; notably, KGW65 was completely inactive and KGW64 was only weakly active. KGW44 (IC50 = 0.99 μM) was the most potent derivative, followed by KGW59 (IC50 = 1.4 μM). From these data, KGW44 and KGW59 were selected for further profiling.
3.3. Expanded mammalian cell cytotoxicity screen
KGW44 and KGW59 were tested against the U-2OS (osteosarcoma), HEK-293 (kidney), HC-04 (liver) and NR-9456 (macrophage) cell lines to further assess their selectivity against T. gondii (Table 2). Neither compound showed any effects on these cells at concentrations up to 100 μM.
Table 2.
Expanded mammalian cell viability screen results and point mutagenicity.
| Derivative ID | NR-9456 IC50 (μM) | U2OS IC50 (μM) | HEK293 IC50 (μM) | HCO4 IC50 (μM) | Ames Assay |
|---|---|---|---|---|---|
| KG81 | >100 | >100 | 0.97 | 28 | Negative |
| KGW44 | >100 | >100 | >100 | >100 | Negative |
| KGW59 | >100 | >100 | >100 | >100 | Negative |
Derivatives of KG8 (Sanford et al., 20181) that were identified as effective in the preliminary screen (Table 1) were further screened for possible cell viability inhibition in NR-9456, U2OS, and HEK293.
Potential point mutagenicity was also screened for with the use of a bacterial reversion assay (Ames Assay).
3.4. Bacterial reverse mutation assay
Compounds KGW44 and KGW59 were tested at 0.75 μM (ca. 3x average T. gondii IC50) for their capacity to induce DNA point mutations in a bacterial model (Ames assay) with Salmonella typhimurium strain TA100; neither compound was mutagenic.
3.5. Pre-treatment assays
To probe a possible mode of action, both HFF cells and tachyzoites were pre-treated with 10 μM of KGW44 and KGW59. KGW59 pre-treatment of HFF cells prior to infection with tachyzoites resulted in a decrease of parasite growth to 30.2% of control (p = 0.00007; Fig. 3). When tachyzoites were exposed to KGW59 prior to infection no significant change in growth was found. When HFF cells were treated with KGW44 no significant change in growth was measured. However, when tachyzoites were treated with KGW44 prior to infection, percent growth was reduced to 60.3% of control (p = 0.002; Fig. 3). From these data, it can be reasoned that KGW59 may be taken up by the host cell to effect T. gondii growth while KGW44 may elicit a direct effect on the extracellular parasite prior to invasion.
Fig. 3.
Host cell pre-treatment and extracellular tachyzoite exposure results. (a.) HFF cells were pretreated with compounds at 10 μM and allowed to incubate at 37 °C for 24 h. Cells were then washed and infected with 2000 RH-dTom tachyzoites. (b.) Isolated RH-dTom tachyzoites were pretreated with compounds at 10 μM for 4 h at room temperature, then 2000 parasites were used to infect HFF. Fluorescent readings were taken 5 days post-infection for both experiments. (p value = **0.00007; *0.002).
3.6. Physicochemical and in vitro ADME
Data in Table 3 indicate that KGW44 and KGW59 were more lipophilic and less soluble than KG8. The three compounds had calculated polar surface area (PSA) values ranging from 72 to 128 A2 indicating that polarity would not be a rate-limiting factor for membrane permeability (Palm et al., 1997). Plasma protein binding for KGW44 (84.3%) was considerably lower than that of KG8 which had a cPPB value of >99.5%. Metabolic stability testing in both human and mouse liver microsomes (Table 4) indicated that both KGW44 and KGW59 were considerably more stable than KG8. The intrinsic clearance (CLint) and predicted hepatic extraction ratio (EH) values indicate that all of the compounds were more rapidly metabolized in mouse vs. human liver microsomes. The metabolic instability of these compounds may be a consequence of their twin tert-butyl groups (Barnes-Seeman et al., 2013; Westphal et al., 2015) and electrophilic benzoquinone substructure.
Table 3.
Physiochemical parameters, solubility, and plasma protein binding of top KGW compounds.
| Identifier | cPPBa (% Bound) | PSAb (A2) | gLogD (pH 7.4) | Sol6.5c(ug/mL) |
|---|---|---|---|---|
| KG81 | >99.5 | 71.4 | 3.9 | 3.1–6.3 |
| KGW44 | 84.3 | 101.7 | 4.9 | <1.6 |
| KGW59 | ND | 127.7 | >5.3 | <1.6 |
(ND: No chromatographic peak under cPPB conditions) (Sanford et al., 20181).
Plasma protein binding was estimated using a chromatographic method.
PSA values were calculated through the ChemAxon chemistry cartridge with JChem from Excel.
Kinetic solubility was determined by nephelometry after 30 min at room temperature.
Table 4.
Metabolic stability of KGW44 and KGW59.
| Compound ID | Microsome species | T1/2 (min) | CLint, in vitro (μL/min/mg protein) | Predicted EH |
|---|---|---|---|---|
| KG81 | Human | 9 | 129 | 0.88 |
| Mouse | <2 | >886 | N/A | |
| KGW44 | Human | 83 | 21 | 0.45 |
| Mouse | 9 | 99 | N/A | |
| KGW59 | Human | 111 | 16 | 0.38 |
| Mouse | 37 | 46 | 0.50 |
Metabolic stability was assessed in both human and mouse liver microsomes. The half-life (T1/2), intrinsic clearance in vitro (CLint), and the predicted hepatic extraction ratios (EH) were determined (Sanford et al., 20181).
3.7. Efficacy in vivo
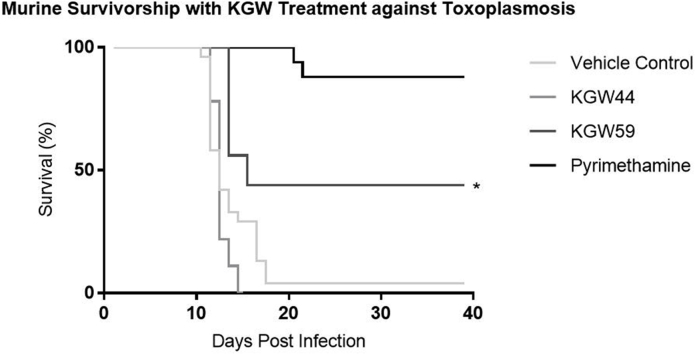
KGW44 and KGW59 were tested for their capacity to increase survivorship in Swiss-Webster mice following a lethal challenge with 5000 ME49 strain T. gondii tachyzoites (Fig. 4). Compounds were administered IP at 5.0 mg/kg/day for 10 days post-infection. Pyrimethamine was included as a positive control and treated mice displayed 90% survivorship, while 45% of KGW59-treated mice survived, and 0% of KGW44-treated mice survived.
Fig. 4.
In vivo survival following compound treatment in a murine model. Swiss-Webster mice were infected with 5000 ME49 T. gondii tachyzoites and then subsequently treated with respective compound for 10 days at 5 mg/kg. Survival was quantified over 40 d using a Kaplan-Meier curve. (p value = 0.004*).
4. Conclusion
To define the potential of benzoquinone acyl hydrazone KG8 as a new lead against T. gondii, we performed a baseline structure-activity relationship for this new chemotype. Several derivatives displayed IC50's comparable to that of pyrimethamine with little to no cytotoxicity. The best of these, KGW44 and KGW59, had higher metabolic stability than KG8. In an in vivo T. gondii murine model, KGW59 significantly increased survivorship. This work provides new insights for optimization of this novel chemotype.
Funding statement
This report was supported by the following NIH grants: GM103427 (PHD) and AI116723-01 (JLV). Additionally, the following support is acknowledged: the Nebraska Research Initiative (PHD) and the University of Nebraska at Omaha FUSE (AGS) and GRACA (TTS).
Conflicts of interest
The authors declare that no competing interests exist.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2018.11.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Detailed methods for synthesis of experimental compounds are included as supplementary material.
References
- Barnes-Seeman D., Jain M., Bell L., Ferreira S., Cohen S., Chen X.-H., Amin J., Snodgrass B., Hatsis P. Metabolically stable tert-butyl replacement. ACS Med. Chem. Lett. 2013;4:514–516. doi: 10.1021/ml400045j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi T., Kanai S., Kakinuma K., Okazaki T., Mizoue K. Synthesis of NG-061 and its analogs, and their biological evaluation as an enhancer of nerve growth factor. Chem. Pharm. Bull. 2000;48:1470–1473. doi: 10.1248/cpb.48.1470. [DOI] [PubMed] [Google Scholar]
- Fung H.B., Kirschenbaum H.L. Treatment regimens for patients with toxoplasmic encephalitis. Clin. Therapeut. 1996;18(6):1037–1056. doi: 10.1016/s0149-2918(96)80059-2. [DOI] [PubMed] [Google Scholar]
- Guiguemde W.A., Shelat A.A., Bouck D., Duffy S., Crowther G.J., Davis P.H., Smithson D.C., Connelly M., Clark J., Zhu F., Jiménez-Díaz M.B., Martinez M.S., Wilson E.B., Tripathi A.K., Gut J., Sharlow E.R., Bathurst I., El Mazouni F., Fowble J.W., Forquer I., McGinley P.L., Castro S., Angulo-Barturen I., Ferrer S., Rosenthal P.J., Derisi J.L., Sullivan D.J., Lazo J.S., Roos D.S., Riscoe M.K., Phillips M.A., Rathod P.K., Van Voorhis W.C., Avery V.M., Guy R.K. Chemical genetics of Plasmodium falciparum. Nature. 2010;465(7296):311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe D.K., Sibley L.D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 1995;172(6):1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- Kamau E.T., Srinivasan A.R., Brown M.J., Fair M.G., Caraher E.J., Boyle J.P. A focused small-molecule screen identifies 14 compounds with distinct effects on Toxoplasma gondii. Antimicrob. Agents Chemother. 2012;56(11):5581–5590. doi: 10.1128/AAC.00868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C., Park J.E., Park Bang, J.K., Nicklaus M.C., Lee K.S. Probing binding modes of small molecule inhibitors to the polo-box domain of human polo-like kinase 1. ACS Med. Chem. Lett. 2010;1:110–114. doi: 10.1021/ml100020e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland M.M., Zach S.J., Wang X., Potluri L.P., Neville A.J., Vennerstrom J.L., Davis P.H. Review of experimental compounds demonstrating anti-toxoplasma activity. Antimicrob. Agents Chemother. 2016;21(12):7017–7034. doi: 10.1128/AAC.01176-16. 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S., Ghatak S., Patil N., Dandawate P., Ambike V., Adsule S., Unni D., Venkateswara Swamy K., Padhye S. Novel dual cyclooxygenase and lipoxygenase inhibitors targeting hyaluronan-CD44v6 pathway and inducing cytotoxicity in colon cancer cells. Bioorg. Med. Chem. 2013;21:2551–2559. doi: 10.1016/j.bmc.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville A.J., Zach S.J., Wang X., Larson J.J., Judge A.K., Davis L.A., Vennerstrom J.L., Davis P.H. Clinically available medicines demonstrating anti-toxoplasma activity. Antimicrob. Agents Chemother. 2015;59(12):7161–7169. doi: 10.1128/AAC.02009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm K., Stenberg P., Luthman K., Artursson P. Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm. Res. (N. Y.) 1997;14:568–571. doi: 10.1023/a:1012188625088. [DOI] [PubMed] [Google Scholar]
- Sanford A.G., Schulze T.T., Potluri L.P., Hemsley R.M., Larson J.J., Judge A.K., Zach S.J., Wang X., Charman S.A., Vennerstrom J.L., Davis P.H. Novel Toxoplasma gondii inhibitor chemotypes. Parasitol. Int. 2018;67(2):107–111. doi: 10.1016/j.parint.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 2000;30(12–13):1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko K., Nunhuck S., Bevan C., Abraham M.H., Reynolds D.P. Fast gradient HPLC method to determine compounds binding to human serum albumin. Relationships with octanol/water and immobilized artificial membrane lipophilicity. J. Pharmacol. Sci. 2003;92(11):2236–2248. doi: 10.1002/jps.10494. [DOI] [PubMed] [Google Scholar]
- Westphal M.V., Wolfstädter B.T., Plancher J.-M., Gatfield J., Carreira E.M. Evaluation of tert-butyl Isosteres: case studies of physicochemical and pharmacokinetic properties, efficacies, and activities. ChemMedChem. 2015;10:461–469. doi: 10.1002/cmdc.201402502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.