ABSTRACT
Disorders of N-linked glycosylation are increasingly reported in the literature. However, the targets that are responsible for the associated developmental and physiological defects are largely unknown. Bone morphogenetic proteins (BMPs) act as highly dynamic complexes to regulate several functions during development. The range and strength of BMP activity depend on interactions with glycosylated protein complexes in the extracellular milieu. Here, we investigate the role of glycosylation for the function of the conserved extracellular BMP antagonist Short gastrulation (Sog). We identify conserved N-glycosylated sites and describe the effect of mutating these residues on BMP pathway activity in Drosophila. Functional analysis reveals that loss of individual Sog glycosylation sites enhances BMP antagonism and/or increases the spatial range of Sog effects in the tissue. Mechanistically, we provide evidence that N-terminal and stem glycosylation controls extracellular Sog levels and distribution. The identification of similar residues in vertebrate Chordin proteins suggests that N-glycosylation may be an evolutionarily conserved process that adds complexity to the regulation of BMP activity.
KEY WORDS: Glycosylation, Bone morphogenetic protein, Short gastrulation, Chordin, Morphogen, Drosophila
Summary: Altering the Short gastrulation glycosylation status could add great diversity to its function in regulating the pattern of bone morphogenetic protein activity in cells, wings and embryos during Drosophila development.
INTRODUCTION
Glycosylation of secreted proteins in multicellular organisms increases structural diversity, extending the possibilities of cell responses to the microenvironment. Protein glycosylation depends on a series of reactions taking place in different cellular compartments. Activated nucleotide-sugar precursors are synthesized in the cytoplasm and imported into the lumen of the endoplasmic reticulum (ER) and Golgi compartments, in which these sugar substrates are used as the building blocks. N-linked glycosylation is initiated in the ER by adding a conserved oligosaccharide en bloc from a dolichol-linked precursor oligosaccharide onto newly translated proteins. After an initial trimming in the ER, the oligosaccharide chain is processed and modified in the Golgi apparatus (Stanley et al., 2017). A series of glycosyltransferases and multiprotein complexes regulate these steps. Improper sugar trimming is linked to errors in protein folding and protein degradation in the ER. Notably, a series of congenital disorders of glycosylation have been reported, with the majority affecting primarily N-glycan assembly (Freeze et al., 2014).
A great challenge is to understand how glycosylation modulates the function of the molecular targets. Crucial targets of glycosylation are the bone morphogenetic proteins (BMPs) and their binding partners, which control embryonic development and tissue patterning (Bier and De Robertis, 2015). Extracellular proteoglycans and glycoproteins modulate BMP signaling in several species. For example, Xenopus and mammalian N-acetylgalactosaminyltransferases inhibit BMP signaling (Herr et al., 2008). In zebrafish, embryos that have been injected with morpholinos against beta-1,4-galactosyltransferase have reduced activation of the BMP-dependent transcription factors Smad1/5/8 (Machingo et al., 2006). Likewise, mutation of glycosylation sites in mouse twisted gastrulation (Tsg, also known as Twsg1), which is an important regulator of BMP activity, reduces its binding to BMPs (Billington et al., 2011). Glycosylation also controls the secretion and folding of human BMP2 (Hang et al., 2014), BMP receptor recognition, specificity and binding strength (Lowery et al., 2014; Saremba et al., 2008).
In Drosophila, activity of the BMPs Decapentaplegic (Dpp), Glass bottom boat (Gbb) and Screw (Scw) depends on glycan-based interactions. Early work has shown that genes involved in proteoglycan biosynthesis, such as division abnormally delayed (dally), sugarless (sgl) and sulfateless (sfl), regulate Dpp function (Häcker et al., 2005; Selleck, 2000). Furthermore, glycosylation can transform Drosophila Dpp from a long-range to a short-range signal (Humphreys et al., 2013). However, the role of N-linked glycosylation on Drosophila BMP function is less explored. N-linked glycans in Drosophila are less complex than in vertebrates (North et al., 2006; Aoki et al., 2007; Gagneux and Varki, 1999). Nonetheless, several enzymes in the N-glycosylation pathway have been reported to impair Drosophila development (Wandall et al., 2003; Tian and Ten Hagen, 2006; Yamamoto-Hino et al., 2015).
Dpp activity depends on the formation of protein complexes with Tsg proteins, Tolloid (Tld) metalloproteases and the dedicated antagonist Short gastrulation (Sog). The formation of these complexes is required to regulate the Dpp activity range during formation of the veins in the pupal wing and to specify the amnioserosa, the dorsal-most extra-embryonic tissue in the embryo. In the pupal wing, Dpp and Gbb ligands are transported from the longitudinal veins in a complex with Sog, the Tsg-family protein Crossveinless and the Tolloid-related (Tlr) metalloprotease, for signaling in the posterior crossvein (PCV) forming area (Serpe et al., 2005; Serpe et al., 2008; Ray and Wharton, 2001). Longitudinal veins also require Sog and Dpp antagonism (Yu et al., 1996) and may depend on shuttling peak amounts of BMPs to the center of the provein domains by the action of Sog, which is produced by the intervein cells (Araujo et al., 2003; Negreiros et al., 2010). During embryogenesis, laterally secreted Sog binds Dpp and Scw in a complex with Tsg that inhibits Dpp locally and facilitates long-range ligand diffusion towards the dorsal midline. Away from the source of Sog, Tld cleaves Sog, delivering peak BMP levels for receptor activation and dorsal amnioserosa formation (Peluso et al., 2011; Sawala et al., 2012; Mizutani et al., 2006; Umulis et al., 2006; Eldar et al., 2002). Sog is an N-glycosylated protein that presents evolutionarily conserved structure and function (François and Bier, 1995; Marqués et al., 1997). Sog and the vertebrate Chordin homologs regulate dorsal-ventral patterning across phyla by antagonizing BMP activity and regulating BMP spread (Bier and De Robertis, 2015). Furthermore, Sog (Srinivasan et al., 2002) and Chordin (Plouhinec et al., 2013) form morphogen gradients in the extracellular space in invertebrate and vertebrate embryos respectively. As Sog ultimately regulates the pattern of BMP activity in tissues, modifications that alter Sog binding and distribution will potentially have a great impact on BMP function. Here, we investigate whether Sog glycosylation is important for Sog function. We show that Sog glycosylation mutants alter BMP activity in cells, wings and embryos, consistent with a role in regulating BMP activity during Drosophila development.
RESULTS
BMP signaling is impaired in frc− wings
fringe connection (frc) encodes a UDP-sugar transporter that transports a broad range of UDP sugars for the synthesis of glycans, including N-linked types, glycosaminoglycans and mucins (Selva et al., 2001; Goto et al., 2001). frc is thus at the basis of glycoprotein and glucosaminoglycan synthesis. Loss-of-function frc alleles are pupal lethal and frc− wings of adult escapers show notches at the margin, because of impairment of the Notch pathway (Goto et al., 2001). frc− wings also display veins of uneven width, which may result from the additional impairment of other signaling pathways (Fig. 1A,B). Given the implication of the BMP pathway in wing vein patterning, we investigated whether frc regulates BMP pathway activity.
Fig. 1.
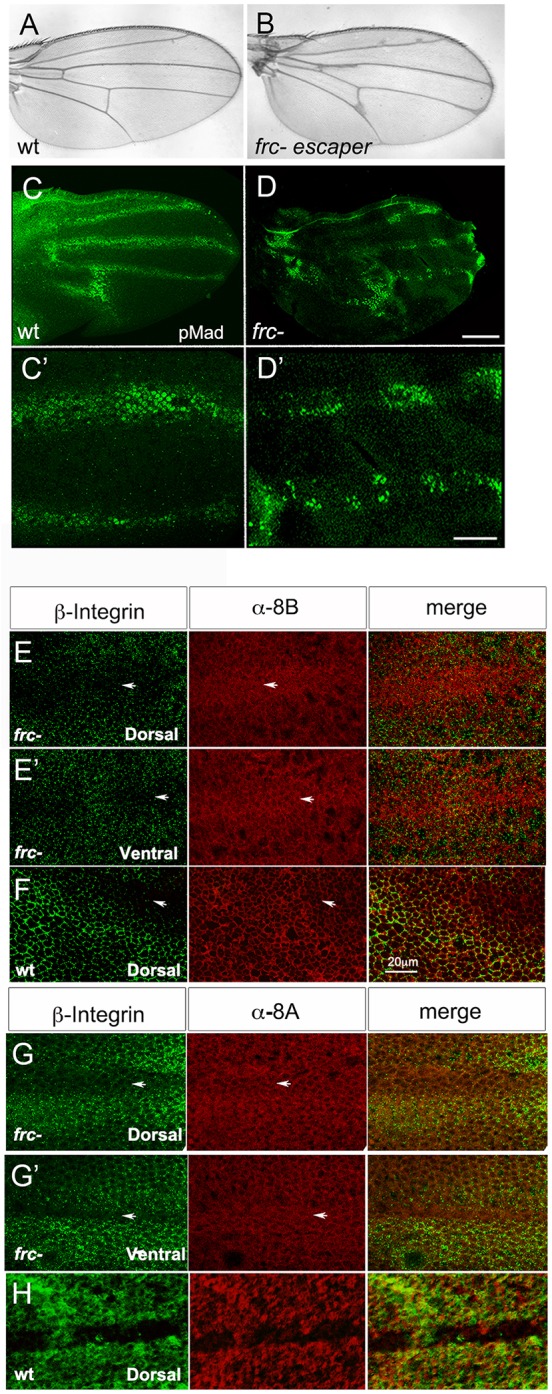
frc mutants affect BMP activity and modify Sog distribution in the pupal wing. (A,B) Wild-type (A) and frc− (B) adult wing. (C-D′) Wild-type (wt) (C,C′) and frc− (D,D′) 24 h APF wings stained for pMAD. High magnification shows continuity of pMAD staining in wt (C′) and a patchy pattern in frc− provein staining (D′). (E-H) Double immunolabeling for β-integrin and anti-8B (E-F) or anti-8A (G-H) Sog antisera in frc− (E,E′,G,G′) or wt (F,H) 24 h APF wings. Dorsal (E,F,G,H) and ventral (E′,G′) wing epithelia are shown. Note Sog staining inside the provein territory in both the dorsal and ventral wing epithelia in frc−. Arrows indicate the protein domain. Scale bars: 100 μm in C,D; 20 μm in C′,D′; 20 μm in E-H.
During pupal wing development, dpp is expressed in the center of broad and irregular (4-7 cells wide) longitudinal-vein-competent or provein domains (Blair, 2007). Dpp is required for vein fate maintenance and refinement, and formation of the vein proper requires BMP activity to be restricted to the center of the proveins by the action of both its receptor Thickveins (Tkv) and Sog (de Celis, 1997; Yu et al., 1996). Sog is expressed in the adjacent intervein domains, from which it diffuses into the provein territory, likely to control the transport of Dpp outwards and along the veins (Yu et al., 1996; Araujo et al., 2003; Negreiros et al., 2010). BMP activity in the provein domains can be detected with anti-phosphorylated Mad (pMad) antisera. From 24 h after puparium formation (h APF) to wing expansion, pMad becomes restricted to quasi-continuous 3- or 4-cell-wide domains along the longitudinal veins, as well as in the PCV (Fig. 1C) (de Celis, 1997; Conley et al., 2000). We observed that, in frc− wings, pMad staining is patchy along longitudinal veins and enlarged at the PCV, consistent with the resulting veins of uneven width and extra PCV tissue in adult wings (Fig. 1D). This pattern could result from uneven BMP diffusion and/or uneven BMP receptor activation within the provein domain, based on modified interactions with glycosylated molecules.
Sog distribution is also altered in frc− wings. We have shown that Sog diffusion from intervein-producing cells into the provein domains takes place only at the dorsal surface of wild-type wings and is restricted to the basolateral domain of the dorsal epithelium (Negreiros et al., 2010). Furthermore, the use of antisera against N- and C-terminal Sog epitopes (8A and 8B, respectively) suggests that only C-terminal Sog fragments are able to enter the provein domain (Araujo et al., 2003; Negreiros et al., 2010) (Fig. 1F,H). However, in frc− wings, both the N- and C-terminal epitopes of Sog are detected in the provein domains (Fig. 1E,G). These results suggest that glycosylation is necessary to control the distribution of Sog between the intervein and vein-competent domains and that Sog interactions based on glycosylated residues could be modified in frc− mutants. As frc modifies the loss-of-vein phenotype of enhancer piracy sog lines (Fig. S1) and Sog is a glycoprotein (Marqués et al., 1997), we decided to investigate whether Sog glycosylation regulates BMP function.
Sog and Chordin putative glycosylation sites are conserved
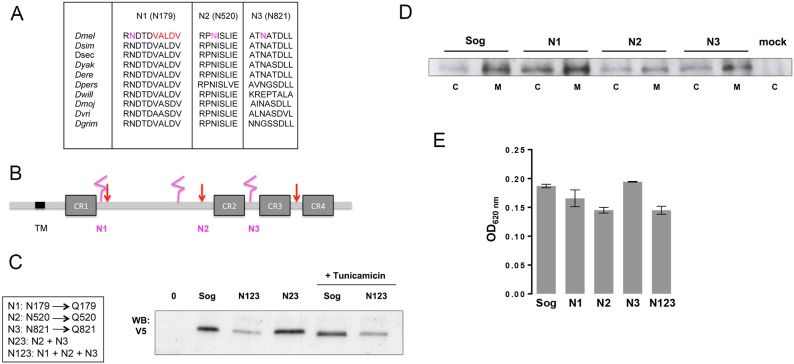
It has been known for a long time that Sog is a glycoprotein (Marqués et al., 1997). However, how glycosylation contributes to Sog function has not been investigated. The evolutionary conservation of putative glycosylation sites suggests a positive selection for these sites in protostome and deuterostome lineages. We have aligned Sog sequences from the 12 sequenced Drosophila species and found that, among the six predicted glycosylation sites, three are conserved in all the analyzed species (Fig. 2A; Fig. S2B). The first conserved site is located after the first cysteine rich (CR) domain, in close proximity to a Tld cleavage site (Peluso et al., 2011; Marqués et al., 1997) (Fig. 2B). The second and third sites lie in the stem and after the second CR domain, respectively (Fig. 2B). Putative glycosylation sites in locations that correspond to the second site are consistently detected in vertebrate species as well (Fig. S2B).
Fig. 2.
Drosophila sog has three conserved glycosylation sites. (A) Alignment of Sog sequences from Drosophila sp. shows that three putative glycosylation sites are conserved (Arg residue in pink in D. melanogaster). A Tld/Tlr cleavage site sequence close to N1 is shown in red. (B) Sog protein scheme with the location of the four conserved CR domains, Tld/Tlr cleavage sites (red arrows) and putative glycosylation sites (pink). TM, transmembrane domain. (C-E) Analysis of Sog constructs expressed in S2 cells. (C) Glycosylation mutants produced by site-directed mutagenesis and their effect on Sog migration in SDS-PAGE. N23 and N123 migrate faster than wild-type Sog. All mutants and wild-type Sog bear a C-terminal V5/His tag. Treatment with Tunicamycin decreases wild-type Sog Mw and confirms that Sog is glycosylated. (D) SDS-PAGE for Sog protein in cells (C) and extracellular medium (M) shows that Sog is secreted and that only the N2 mutation slightly decreases Sog secretion. (E) ELISA for S2 cell-secreted Sog confirms the analysis in D.
To investigate a functional role for Sog glycosylation, we generated single, double and triple Sog glycosylation mutant constructs, in which we abolished the conserved putative N-glycosylation sites (Asn-X-Ser/Thr) by mutating the Asn residues to Gln. We refer to these constructs as SogN mutants (N1, N2, N3, N23 and N123) (Fig. 2C). Expression of V5/His-tagged double and triple mutants in S2 cells revealed that these mutants have a reduced molecular weight when compared with wild-type Sog, as seen on SDS-PAGE (Fig. 2C). Treatment of cells with tunicamycin, a specific inhibitor of N-linked glycosylation, abolishes this difference (Fig. 2C). This strongly suggests that the identified sites are N-glycosylated and that a decrease in the number of sugar side chains is responsible for the differential migration pattern of SogN mutants.
As loss of glycosylation is frequently associated with impaired protein folding (Caramelo and Parodi, 2015), we tested whether SogN mutants are appropriately secreted. We transfected S2 cells with equivalent levels of wild-type or mutated epitope tagged sog constructs. After 48 h of induction, we collected cell (C) or medium (M) samples for western blot and ELISA. Secretion of Sog into the medium was slightly decreased only for constructs bearing the second mutated site (N2 and N123) and was unaffected for the other mutants (Fig. 2D,E). Therefore, eventual phenotypes resulting from sogN expression (see below) are unlikely to result from impaired protein secretion.
Sog glycosylation mutants display increased BMP binding
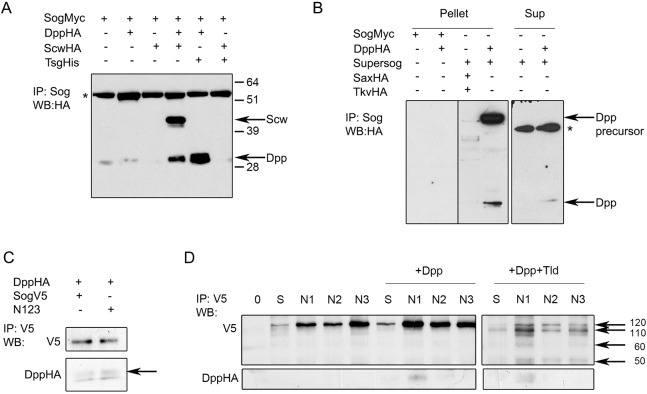
Next, we analyzed whether SogN mutants displayed differences in BMP binding properties and cleavage by metalloproteases. Full-length Sog binds both Dpp homodimers and heterodimers, but shows a greater affinity for Dpp heterodimers (Fig. 3A, compare lanes 2 and 4) (Shimmi et al., 2005). This preference is modified by the presence of Tsg, which increases the affinity of full-length Sog for Dpp homodimers (Fig. 3A, lanes 5 and 6). This behavior is distinct for the N-terminal Supersog cleavage product, which is able to bind Dpp alone (Fig. 3B, lanes 4 and 6). Interestingly, the Sog triple glycosylation mutant N123 appears to show a weak increase in binding to Dpp homodimers in the absence of Tsg (Fig. 3C), which suggests that Sog glycosylation may modulate Sog binding to BMPs. Among the single glycosylation mutants, SogN1 was the sole mutant showing some enhancement in binding to Dpp homodimers in the absence of another BMP or Tsg (Fig. 3D, lane 7).
Fig. 3.
Loss of Sog glycosylation modifies Dpp binding. (A) S2 cells transfected with wild-type Sog-myc and different combinations of Tsg and BMP ligands. Co-immunoprecipitation (Co-IP) with cell supernatants shows that Sog binds only BMP heterodimers in the absence of Tsg, but binds Dpp homodimers in the presence of Tsg. (B) S2 cells transfected with wild-type Sog-myc or the N-terminal Supersog fragment reveal that only Supersog binds Dpp alone using cell pellets (Pellet). That Supersog interacts with Dpp in the extracellular space is confirmed by using cell supernatants (Sup). The presence of Sax or Tkv in lane 3 does not change this pattern. (C) S2 cells transfected with wild-type sog-V5 or sogN123-V5, and dpp-HA. Co-IP for V5 suggests that binding of the N123 mutant to Dpp is weakly enhanced compared with wild-type Sog. (D) S2 cells transfected with wild-type sog (S) or single glycosylation mutants (N1, N2, N3) plus dpp-HA, or plus dpp-HA and tld-HA. Co-IP for V5. SogN1 binds weakly to Dpp alone, whereas other mutants and wild-type Sog do not. Arrows in D point to the different Sog fragments produced by the Tld metalloprotease and show that the cleavage pattern is similar among all constructs. Asterisks indicate nonspecific bands.
Sog glycosylation could also affect Sog cleavage by Tld metalloproteases and impact on the consequent release of BMPs for receptor binding. Unlike vertebrate Chordin, Sog cleavage by Tld metalloproteases relies on the presence of BMPs (Marqués et al., 1997; Peluso et al., 2011). We found that SogN mutants display a cleavage pattern similar to wild-type Sog (Fig. 3D, right panel and Yu et al., 2000). Therefore, loss of Sog glycosylation does not affect cleavage by Tld metalloproteases, although a decrease in cleavage efficiency cannot be ruled out in these experiments.
Loss of glycosylation sites enhances Sog function in vivo
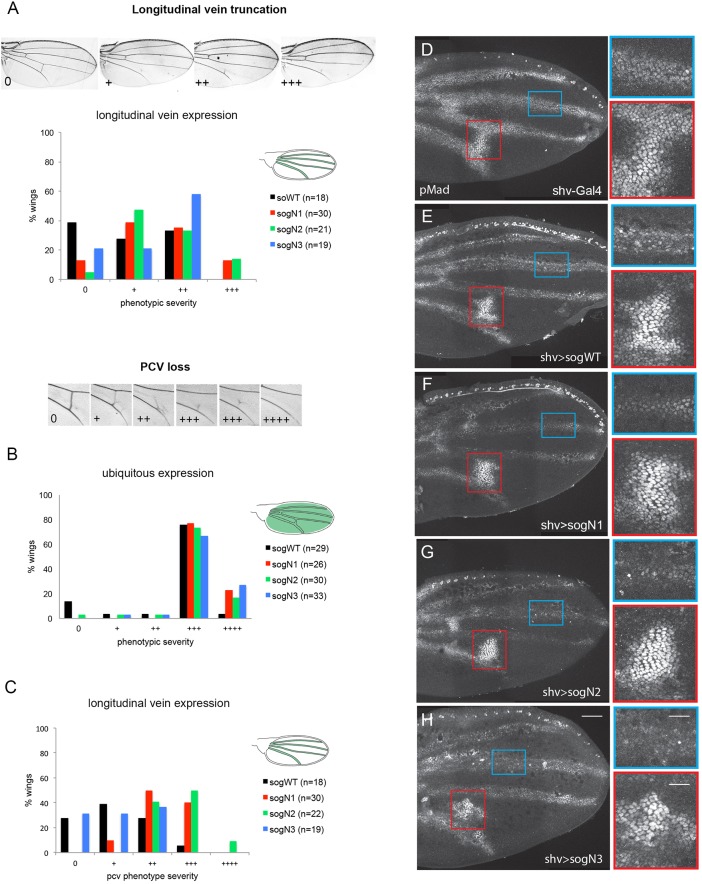
The differences in BMP binding described above suggested that loss of glycosylation could modify Sog function in vivo. To test this prediction, we assayed the effects of overexpressing sog glycosylation mutants during embryogenesis and pupal wing development. During pupal wing development, Dpp is expressed in the longitudinal veins, in which it establishes the vein proper domain (de Celis, 1997; Sotillos and De Celis, 2005). In addition, Dpp is transported from longitudinal veins to initiate BMP signals and formation of the PCV (Conley et al., 2000; Serpe et al., 2005; Ralston and Blair, 2005; Shimmi et al., 2005, 2005). Ubiquitous overexpression of wild-type sog in the wing leads to various degrees of longitudinal vein truncation and PCV loss (Yu et al., 1996; Serpe et al., 2005) (Fig. S3 and Fig. 4B). Vein-restricted sog overexpression, using the shortvein Gal4 driver (shv-Gal4) (Sotillos and De Celis, 2005), also results in longitudinal vein truncation to various degrees, as well as partial or complete loss of PCV (Fig. 4A,C). We observed that overexpression of sogN mutants leads to more severe longitudinal vein truncation phenotypes when compared with wild-type sog (sogWT), with sogN1 and sogN2 showing the greatest effect (Fig. 4A). Consistent with the inhibition of the BMP pathway that is exerted by sog, overexpression of sogWT and sogN also increases the severity of longitudinal vein truncation in the dppshv background (Fig. S4).
Fig. 4.
Loss of glycosylation sites enhances Sog function in the wing. (A-C) The adult wing venation pattern produced by Gal4/UAS expression of wild-type Sog or Sog glycosylation mutants was quantified. Expression induced by the shv-Gal4 driver in the longitudinal veins (A,C) or ubiquitous expression in the wing with the MS1096-Gal4 driver (B) leads to different degrees of longitudinal vein loss (A) or posterior crossvein (PCV) phenotypes (B,C). Increasing severity of phenotype is indicated by increasing number of + symbols. (D-H) 24 h APF control pupal wing (D) or wings expressing wild-type sog (E) or sogN mutants (F-H), driven by shv-Gal4 and stained for pMad. The blue and red boxed areas correspond to the enlarged images on the right. Scale bars: 50 μm and 20 μm in left and right panels, respectively.
sog overexpression also results in PCV loss. We find that ubiquitous overexpression of sogN mutants in the wing (with a MS1096-Gal4 driver) increases PCV loss when compared with sogWT overexpression (Fig. 4B). This difference is exacerbated when the overexpression is restricted to the longitudinal proveins by using the shv-Gal4 driver, with sogN1 and sogN2 again showing the strongest effects (Fig. 4C). These different effects on the PCV produced by local (ubiquitous, MS1096-Gal4) versus at-a-distance (longitudinal proveins, shv-Gal4) overexpression of sog mutants indicate that the transport of BMP signaling complexes in the extracellular space is affected in the sogN1 and sogN2 mutants.
Accordingly, overexpression of sogWT and sogN mutants in longitudinal proveins affects the pMad pattern in the pupal wing. In the longitudinal proveins, pMad staining is decreased and uneven in sogWT overexpression wings when compared with control wings (Fig. 4D,E), consistent with the inhibition of BMP signaling by Sog in these domains. In contrast, the PCV appears to be enlarged, with increased pMad levels (Fig. 4E), indicating an increase in BMP transport from the longitudinal proveins to the PCV domain. Overexpression of sogN mutants appears to exacerbate these defects, with the PCV often appearing detached from the longitudinal proveins (Fig. 4F-H), consistent with the phenotypes observed in adult wings (Fig. 4A,C) and with increased BMP binding (Fig. 3D). Interestingly, the pMad pattern that results from sog overexpression (decreased and non-uniform levels in longitudinal proveins and enlarged PCV) is reminiscent of the pMad pattern that is observed in frc− wings (compare Fig. 4F-H with Fig. 1C,D).
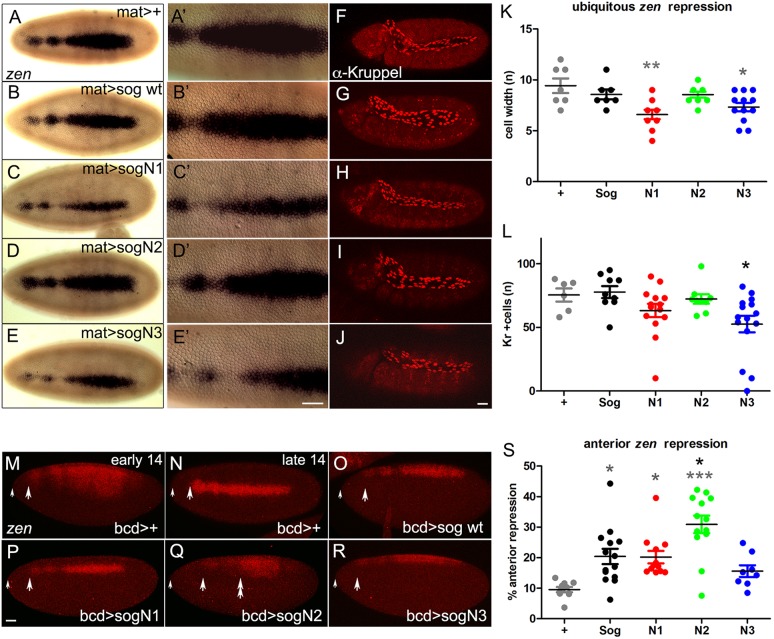
During embryogenesis, sog is expressed in the lateral neuroectoderm, in which it inhibits dpp from auto-activating (Biehs et al., 1996). Sog also diffuses dorsally to concentrate BMP activity to the dorsal-most region of the embryo (Mizutani et al., 2005; Shimmi et al., 2005; Ashe and Levine, 1999; Decotto and Ferguson, 2001). Peak BMP activity leads to dorsal expression of zen, RACE (also known as ANCE) and rho, as well as the formation of the amnioserosa, which is absent in loss-of-function sog mutants (Ray et al., 1991; Yu et al., 2000). After gastrulation, amnioserosal cells can be detected with antibodies against the Kruppel (Kr) transcription factor. To assay the effects of sogN mutants in the embryo we drove early ubiquitous expression of sogWT and sogN mutants with a maternal Gal4 driver (matα-Gal4). sogWT overexpression does not significantly alter the domain of zen expression or the number of Kr+ cells, as was previously shown (Yu et al., 2000) (Fig. 5A,B,F,G). However, overexpression of sogN1 and sogN3 reduces the domain of zen expression as well as the number of Kr+ cells (Fig. 5C,E,H,J,K,L), indicating that these mutants exert an inhibitory effect on BMP signaling. sogN2 had no effect (Fig. 5D,I).
Fig. 5.
Loss of glycosylation enhances Sog function in the embryo. (A-L) Embryonic pattern produced by expression of wild-type sog or sog glycosylation mutants sogN1, sogN2 or sogN3, with the ubiquitous matα-GAL4 driver. Effects were analyzed by dorsal zen expression (A-E), quantified in K, or by Kruppel protein in amnioserosal cells (F-J), quantified in L. (M-S) Embryonic pattern produced by localized expression of wild-type sog or sog glycosylation mutants sogN1, sogN2 or sogN3 with the anteriorly restricted bcd-GAL4/GCN driver. Effects were analyzed by dorsal zen expression. The domain of zen repression from the anterior tip of the embryo was measured and quantified in (S). A small arrow points to the anterior tip of each embryo. Large arrows point to the most anterior region of zen expression detected. Double arrow in Q shows the region in which zen is expanded relative to wild-type sog by anterior sogN2 expression. *P≤0.05, **P≤0.01, ***P≤0.001 (Student's t-test). +, control. Gray asterisks indicate comparison with +; black asterisks indicate comparison with Sog. Scale bars: 30 μm.
Taking into account that, in the wing, sogN mutants displayed different effects locally versus at-a-distance, we performed a similar analysis in the embryo. We induced localized expression of sogN mutants in the anterior tip of the embryo using the bicoid-Gal4 driver (bcdGCN-Gal4) (Yu et al., 2000) (Fig. 5M-R). Anterior overexpression of sogN1 and sogN3 decreases the domain of zen expression to the same extent as sogWT (Fig. 5O,P,R). However, sogN2 inhibits zen expression further away from the anterior tip (Fig. 5O,Q,S). In addition, in bcd>sogN2 the zen domain is broad in the middle of the embryo, which suggests that SogN2 shuttles the active BMP complex towards the posterior end to a greater extent than wild-type Sog (Fig. 5Q).
Altogether, the analysis of sog overexpression in the wing and the embryo shows that the effects of individual mutations in putative Sog glycosylation sites are context-dependent, with SogN1 and SogN2 showing the greatest effects in the wing, and SogN2 in the embryo. Nevertheless, it is important to point out that, in all contexts, the loss of putative glycosylation sites increases the well-established effects of Sog to inhibit and/or shuttle BMPs.
Glycan removal inhibits Sog retrieval
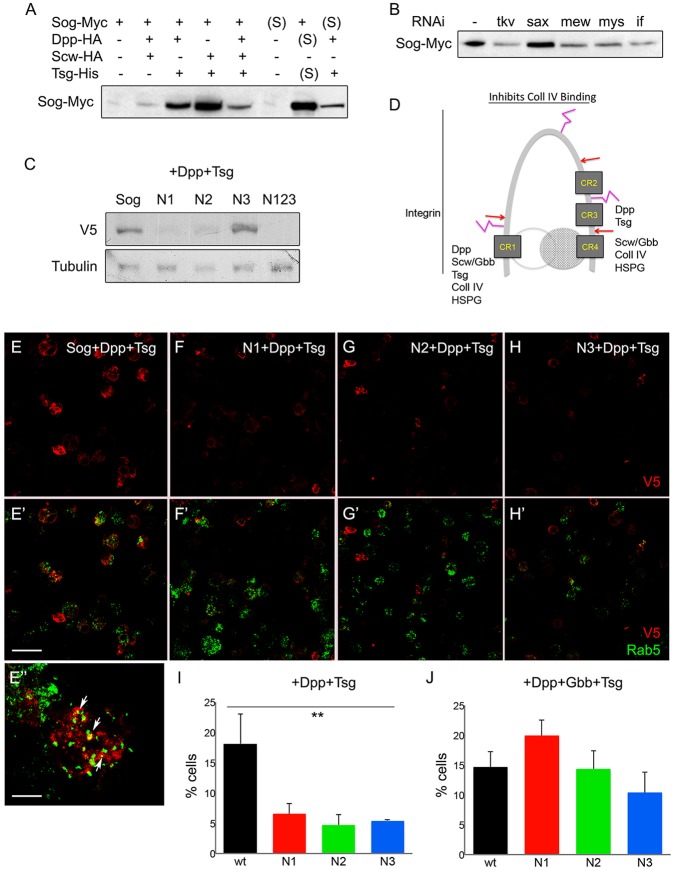
Precise morphogen activity requires the control of the levels and spatial distribution of extracellular morphogens and their antagonists. In Drosophila, endocytosis controls the level of Sog protein in the extracellular space and thus BMP activity (Srinivasan et al., 2002; Negreiros et al., 2010). Sog suppresses the action of excess Gbb in the wing and Scw in the embryo, but not Dpp (Neul and Ferguson, 1998; Nguyen et al., 1998; Yu et al., 2000). Accordingly, extracellular Sog is able to bind to the BMPs Scw and Gbb and, with lower affinity, to Dpp. However, in the presence of Tsg, Sog binds to and antagonizes both Dpp heterodimers and homodimers (Ross et al., 2001; Shimmi et al., 2005). Importantly, Sog retrieval from the extracellular space also increases in the presence of Tsg (Fig. 6A), regardless of the BMP involved. Sog retrieval from the extracellular space is dependent on BMP and integrin receptors [BMP receptor type I Tkv, βPS integrin receptor Myospheroid (Mys), αPS1 integrin receptor Multiple edematous wings (Mew) and αPS2 receptor Inflated (If)], as tkv, mys, mew and if knockdowns decrease intracellular Sog amount in S2 cells (Fig. 6B). This indicates that Sog retrieval in the presence of Tsg requires an interaction with Dpp homo- or heterodimers and with integrin receptors.
Fig. 6.
Sog levels are controlled by glycan and receptor-based retrieval from the extracellular space. (A) S2 cells transfected with wild-type sog-myc construct and different combinations of constructs producing Tsg and BMP ligands. Cellular levels of Sog are detected with anti-myc antiserum. Sog amounts increase in the presence of Dpp plus Tsg. These cellular levels correspond to Sog retrieved from the medium, as equivalent intracellular levels are observed when medium (S) from construct-expressing cells is added to cells that do not express sog-myc. (B) Intracellular Sog levels retrieved from Sog-myc+Dpp-HA+Tsg-His medium decrease by knocking down the Dpp receptor gene tkv or αPS1 (mew), αPS2 (if) or βPS (mys) integrin expression. (C) S2 cells transfected with wild-type sog-V5 or sogN1-V5, sogN2-V5, sogN3-V5 or triple sogN123-V5 mutant constructs in the presence of constructs producing Tsg and Dpp. Sog retrieval decreases by mutating the N1 and N2 sites. (D) Predicted conformation of Sog protein with regions depicted for binding to established extracellular partners. Red arrows indicate Tld/Tlr cleavage sites; pink represents glycosylated residues; gray shaded and open circles indicate BMPs. (E-J) Medium from S2 cells transfected with dpp-HA and tsg-His plus wild-type sog-V5, or sogN1-V5, sogN2-V5 or sogN3-V5 was added to naive cells and immunolabeled with anti-V5 (red) and anti-Rab5 (green) (E-H′). (E′′) High magnification for wild-type Sog-V5 and Rab5 shows partial colocalization, indicative of Sog endocytosis. Arrows indicate V5+Rab5+ punctae. Quantification of these cells (I) shows that loss of N1, N2 and N3 glycosylation sites decreases the number of cells with V5+Rab5+ endocytic punctae. (J) In cells expressing dpp-HA, gbb-His and tsg-His plus wild-type (wt) sog-V5, or sogN1-V5, sogN2-V5 or sogN3-V5, the number of cells with V5+Rab5+ punctae is equivalent. **P≤0.01 (Student's t-test). Scale bars: 20 μm in E′; 10 μm in E″.
The loss of putative glycosylation sites decreases Sog retrieval in S2 cells (Fig. 6C). In the presence of Dpp and Tsg, SogWT-V5 is retrieved from the medium by cells that do not express V5-tagged Sog. In the same condition, intracellular levels of SogN1, SogN2 and the triple mutant SogN123 decrease compared with SogWT (Fig. 6C). This indicates that glycosylated residues at the Sog N-terminus and stem are required for an interaction with membrane receptors that retrieve Sog from the extracellular space. Furthermore, Sog retrieval results in internal vesicle-bound Sog. Co-localization analyses shows that a significant amount of intracellular Sog+ punctae correspond to Rab5+ endocytic vesicles (Fig. 6E). Notably, the percentage of cells displaying Sog that is associated with endocytic vesicles decreases by mutating any of the three putative glycosylation sites N1, N2 or N3 (Fig. 6F-I). Therefore, a likely interpretation of these results is that loss of Sog glycosylation leads to a decrease in receptor-based Sog endocytosis, thus increasing the level of extracellular Sog. Interestingly, retrieval of wild-type and mutant Sog is equivalent in the presence of Tsg+Dpp+Gbb (Fig. 6J).
DISCUSSION
N-linked glycosylation modulates Sog function
Here, we have undertaken a biochemical and functional analysis of glycosylation in the BMP antagonist Sog. Our results confirm that Sog is glycosylated and show that three target sites for N-glycosylation are evolutionarily conserved. While appropriate glycosylation may be important for protein folding in general (Caramelo and Parodi, 2015; Jayaprakash and Surolia, 2017), and thus may impact the total amount of active glycoprotein produced, our results indicate that Sog glycosylation controls a higher level of Sog function. Our in vivo functional analysis shows that the loss of conserved glycosylation sites enhances extracellular Sog activity in all the contexts tested. Specifically, the mutation of conserved glycosylation sites increases BMP antagonism in the wing (N1 and N2) and embryo (N1 and N3), consistent with an increase in Dpp binding in S2 cells (N1). Furthermore, the loss of Sog glycosylation sites also increases the range of Sog effects in the pupal wing (N1 and N2) and embryo (N2), and decreases the retrieval of Sog protein from the extracellular space in S2 cells (N1 and N2, and to a smaller extent N3). Therefore, our results consistently point to a role for the first glycosylation site (N1) in dampening BMP antagonism and for the first and second glycosylation sites (N1 and N2) in restricting BMP shuttling by Sog.
The presence of glycans on extracellular proteins frequently alters the degree and quality of interactions with the extracellular matrix and with other secreted proteins (Tauscher et al., 2016; Dennis, 2017; Jayaprakash and Surolia, 2017). By analyzing the position of the putative Sog glycosylation sites one might gain insight to explain how glycan addition may modify Sog binding to interacting partners. It has been suggested that Chordin and Sog assume a horseshoe-like conformation that enables cooperative BMP binding (Troilo et al., 2015; Larraín et al., 2000; Shimmi et al., 2005). In this arrangement, specific N- and C-terminal regions interact with BMPs (CR1, CR3 and CR4) (Sawala et al., 2012; Troilo et al., 2015), Tsg proteins (CR1 and surrounding) (Yu et al., 2004), heparan sulfate proteoglycans (CR1 and CR4) (Jasuja et al., 2004), integrin receptors (N-terminal) (Araujo et al., 2003; Larraín et al., 2000) and collagen (CR1 and CR4) (Sawala et al., 2012) (Fig. 6D). Taking into account that most glycans are present at the external face of Drosophila glycoproteins, it is unlikely that Sog glycosylation directly alters BMP dimer binding at internal sites (Jayaprakash and Surolia, 2017). Alternatively, glycosylation could modify Sog conformation, indirectly changing the interaction with BMPs. Another possibility is that Sog glycosylation provides the structural basis for interaction of the Sog/BMP complex with the extracellular matrix and transmembrane receptors. These interactions could secondarily affect BMP binding and metalloprotease cleavage dynamics as well as movement of the BMP complex in the tissue. Structural analysis of the Sog protein should allow for testing of this prediction in the future.
The most consistent results gathered in this manuscript concern the N1 and N2 sites. The N-terminal Cysteine Repeat 1 (CR1)-containing Sog domain harbors a Tld/Tlr site proximal to N1 (Marqués et al., 1997). Metalloprotease cleavage at this site generates a Supersog N-terminal fragment, which is a stronger BMP antagonist than full-length Sog (Yu et al., 2000) and is able to bind Dpp homodimers, unlike full-length Sog that only binds BMP heterodimers (Yu et al., 2000; Shimmi et al., 2005) (Fig. 3). Therefore, one mechanism by which SogN1 could increase BMP antagonism would be to favor the generation of Supersog fragments. However, our results contradict this interpretation, as loss of this first glycosylation site does not favor N-terminal Sog cleavage or modify the Sog cleavage pattern. Alternatively, loss of N1 glycosylation may modify interactions that take place near this site. For example, by favoring BMP binding, either by increasing binding strength or decreasing the requirement for accessory proteins such as Tsg, ultimately resulting in less BMP available for receptor activation. This interpretation is consistent with the ability of SogN1 to bind Dpp in the absence of Tsg, unlike wild-type Sog that requires either a BMP heterodimer for binding or the presence of Tsg to bind Dpp homodimers.
Stem (N2) Sog glycosylation controls the BMP activity range
An essential and conserved feature of Sog and its vertebrate Chordin homologs is the ability to form highly dynamic complexes with BMP, Tsg and Tld proteins. During embryonic dorsal-ventral patterning, Sog and Chordin form multiprotein complexes that shuttle BMPs, concentrating BMP activity away from the site of Sog or Chordin synthesis in a source-and-sink model of morphogen gradient formation (Bier and De Robertis, 2015). This model relies on the ability of Sog and Chordin to diffuse in the extracellular space (Srinivasan et al., 2002; Plouhinec et al., 2013) and to interact with BMP, Tsg and Tld proteins (Mizutani et al., 2005; Umulis et al., 2006; Plouhinec et al., 2013; Reversade and De Robertis, 2005; Ambrosio et al., 2008; Inomata et al., 2008). In the pupal wing, formation of similar Sog/BMP complexes and shuttling BMPs away from the Sog source is also important for PCV formation (Serpe et al., 2008).
By altering the N2 Sog glycosylation site we observe BMP inhibition in the embryo along a greater extension relative to wild-type Sog. A slight increase in zen expression away from the source of anterior sogN2 expression is also observed, indicating that BMPs are shuttled further away from the SogN2 source in the process (Fig. 5Q). This effect is strongest in the wing, in which sogN2 expression in the provein territory leads to inhibition of PCV formation close to the source (generating detached PCV and pMAD staining) and increased pMAD staining away from the source (Fig. 4G). These results can be interpreted as indication that Sog glycosylation at the N2 site restricts Sog diffusion and consequently how far from the source it is able to shuttle BMPs. Remarkably, the N2 site is the best conserved among Chordins (Fig. S2B), indicating that the vertebrate BMP activity range may depend on Chordin stem glycosylation as well.
Interestingly, in frc− wings Sog distribution is abnormal, with high levels entering the provein domain, away from the intervein Sog-producing source. An uneven pattern of pMAD inside the provein territory is also observed in frc− pupal wings, akin to the effect of overexpressing sogN inside the provein. Therefore, despite the generalized decrease in glycosylation expected in frc− wings, if the altered pMAD pattern in frc− results in great part from non-glycosylated Sog entering the provein domain, then an important function of Sog glycosylation would be to control the even spread of BMPs along the center of the provein domain, leading to the formation of straight, continuous veins (Yu et al., 1996).
Other developmental processes that require the precise control of BMP spread have been reported to depend on glycan residues. In the female germline stem cell (GSC) niche, the collagen IV Viking and the proteoglycan Dally bind to extracellular Dpp near anterior GSCs and prevent it from spreading further to the posterior, allowing the differentiation of cystoblasts (Guo and Wang, 2009; Wang et al., 2008; Hayashi et al., 2009; Harris and Ashe, 2011). Similarly, during embryonic dorsal closure, mummy, which encodes a key enzyme during synthesis of O- and N-linked glycans, is required to define a highly restricted BMP activity field (Humphreys et al., 2013; Schimmelpfeng et al., 2006; Tonning et al., 2006). Therefore, glycosylated Sog, Viking, Dally and Mummy may be classified as a novel type of BMP regulators that can transform BMP from a long-range to a short-range signal.
Glycans control extracellular Sog levels
The resident time of a secreted morphogen in the extracellular milieu has a direct impact on the range over which it is able to exert its effects (Gonzalez-Gaitan and Jülicher, 2014). As a secreted morphogen, extracellular Sog levels are regulated by endocytosis. This has proven important for Sog spread during embryogenesis (Srinivasan et al., 2002) and wing development (Araujo et al., 2003; Negreiros et al., 2010). Our S2 cell results show that Sog retrieval from the extracellular space requires Dpp (Tkv) and αPSβPS integrin receptors (Fig. 6B). Accordingly, intracellular Sog levels are low inside mew and mys clones, implying that αPS1βPS integrin receptors regulate Sog levels in the pupal wing (Negreiros et al., 2010). By mutating Sog glycosylation sites N1 and N2 we show that the amount of Sog retrieved from the extracellular space reduces. These sites are located inside the region of the Sog molecule responsible for integrin binding (Araujo et al., 2003). Interestingly, integrin receptors bind Xenopus Chordin, controlling the amount of Chordin that is internalized (Larraín et al., 2000). Taking into account the structural similarities between Sog and Chordin, the possibility of a conserved mechanism of glycan-based Sog and Chordin endocytosis should be investigated.
Addition of sugar side chains as a general mechanism to modify BMP function
Several molecules that interact with BMP ligands are modified by glycans, and BMPs themselves are subject to glycan addition (Hang et al., 2014; Lowery et al., 2014; Tauscher et al., 2016). Among these molecules are proteoglycans (Guo and Wang, 2009; Häcker et al., 2005; Selleck, 2000), Tsg family proteins (Billington et al., 2011), as well as Sog/Chordin (Marqués et al., 1997). Therefore, disorders of glycosylation or changes in glycan availability will have a compounded impact on BMP function that increases in complexity with an increase in the number of glycosylated partners in a given biological context. Here, we have shown that Sog function is modified at specific evolutionarily conserved glycosylation sites. The slight variability of effects observed in the different contexts is in consonance with the fact that the population of sugars attached to each glycosylated asparagine in a mature glycoprotein depends on the cell type, and the physiological and metabolic status of the cell in which the glycoprotein is expressed (Dennis et al., 2009; Dennis, 2017). Therefore, altering the Sog glycosylation status could add great diversity to Sog glycoprotein function and, consequently, BMP activity during development.
MATERIALS AND METHODS
Lines used in this study were loss-of-function frc00073, crossed into a TM6-Tb balancer to identify homozygous pupae, dppshv, and the MS1096- and matα-Gal4 drivers, obtained from the Bloomington Indiana Stock Center. The shv-GAL4 driver was a gift from José DeCelis (Centro de Biologia Molecular Severo Ochoa, University of Madrid, Spain). All drivers have been described previously (Yu et al., 1996; Sotillos and De Celis, 2005).
Constructs for S2 cell expression
A Drosophila melanogaster sog cDNA cloned into pBluescript (Yu et al., 1996) was mutated at codons 179 (N1), 520 (N2) or 821 (N3), replacing Asn for Gln (CAA) (Retrogen). Production of the double (N23) mutated construct was performed by pBS-SogN1 and pBS-SogN2 digestion with SmaI/Xba and the triple (N123) mutated construct was performed by pBS-SogN1 and pBS-SogN23 digestion with SmaI/NotI. Wild-type and mutant sog sequences were amplified and inserted into KpnI/NotI sites in pMT-V5/HisC (Invitrogen) to produce C-terminal tagged inducible constructs.
Transgenic constructs
Constructs for Gal4/UAS mediated expression in flies were generated in pTiger, a derivative of pUASp (Ferguson et al., 2012). For the pTiger-SogWT construct, pBS-SogWT and pTiger were digested with KpnI and XbaI and ligated using T4 DNA ligase (New England Biolabs). For the pTiger-SogN1, N2 and N3 constructs, SogN1, N2 and N3 sequences were amplified by PCR from pBS-SogN1, N2 and N3, digested with KpnI and XbaI and introduced into KpnI/XbaI-digested pTiger using T4 DNA ligase (New England Biolabs). pTiger-Sog constructs were sent to Bestgene for injection and integrated into the attPVK00027-docking site using the ΦC31 system (Markstein et al., 2008). At least two lines for each construct were generated and presented consistent effects.
All constructs were sequenced before use in cell transfection or transformation. See supplementary Materials and Methods and Table S1 for further details and primers used in the production of S2 cell and transgenic constructs.
S2 cell culture and transfection
S2 cells were cultured in Schneider's cell medium (Gibco) containing 10% heat inactivated fetal bovine serum (FBS). Cells were transfected with CellFectin (Invitrogen) according to manufacturer's instructions. For pMT-sog-V5/His constructs, protein expression was induced by treatment of 0.7 mM CuSO4 24 h after transfection. Sog protein was harvested from medium 48 h after CuSO4 treatment. Vectors for sog-myc and tsg-His expression are described in (Yu et al., 2000). tld-HA, dpp-HA (Marqués et al., 1997), gbb-FLAG and Mad-FLAG (Ross et al., 2001) were a generous gift from M. O'Connor. Transfection product proteins were produced in the absence of glycosylation by incubating with tunicamycin (10 µg/ml). RNA interference for mys, mew, if, sax and tkv was performed as in Kang and Bier (2010), with double stranded RNA synthesized from primers listed in Table S2.
ELISA
96-well plates (Nunc) were sensitized with 100 μl/well (4 μg/ml) of anti-His (372900, Invitrogen), the capture antibody, overnight at 4°C. Wells were washed 3× with PBS (pH 7.2); Tween 20 0.025% (PBST) and blocked with PBS supplemented with 10% FBS in 200 μl/well. Plates were allowed to stand for 2 h at 37°C and washed 3× with PBST. Subsequently, 50 μl of supernatants from S2 cell cultures were added to the wells. Plates were covered and incubated overnight at 4°C. After washing as plates 4× with PBST, 100μl (4 μg/ml) of the detection antibodies (anti-V5, R90025, Novex, with Life Technologies) was added to the wells. Plates were incubated for 1 h at room temperature and washed 6× with PBST, and then 100 μl of streptoavidin-alkaline phosphatase (1 μg/ml) diluted in PBS supplemented with 10% FBS was added to each well. Plates were incubated for 3 h at room temperature, plates were washed 8× with PBST and 100 μl of the 1.0 mg/ml solution substrate bis-azine ethyl benzothiazole sulfonic acid (Sigma), diluted in 20 mM Tris and 100 mM MgCl2, was added to each well. Readings were performed on the Beckman Coulter AD340 reader with 405 nm filter.
Immunoblotting and immunoprecipitation
Medium or cell lysates from transfected S2 cells were collected 72 h after transfection. Cells were harvested and lysed with 200 µl ice-cold lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.1% NP-40, protease inhibitor cocktail-Complete (Behringer Manheim)] and centrifuged at 10,000 g for 20 min at 4°C to remove cell debris. After preclearing of total cell lysates with protein-G Sepharose beads for 20 min, the lysates were incubated with 30 µl of antibody-bound agarose beads (crosslinked with monoclonal anti-V5 antibody, Invitrogen) at 4°C for 3 h. Beads were washed 3× with lysis buffer, then boiled in SDS-PAGE sample buffer at 95°C for 5 min and electrophoresed on 10% Bis-Tris gel and detected by immunoblotting onto PVDF membranes using anti-V5 (1:2000).
Immunofluorescence and in situ hybridization in pupal wings and embryos
24-48 h pupal wings were fixed for 10 min in 4% PFA in PBS (pH 7.0) and permeabilized in PBST buffer (PBS+0.1% Tween 20), blocked with 5% normal goat serum, incubated with primary antibody overnight at 4°C (rabbit anti-phospho-Smad1/5, Cell Signaling Technology, 41D10, 1:500; mouse anti-β integrin, 1:500, Developmental Studies Hybridoma Bank; anti-Sog 8A or 8B antisera, 1:500; Srinivasan, 2002) and subsequently incubated with Alexa Fluor secondary antibodies. Embryos were fixed and devitelinized in methanol using standard procedures, following dechorionation in bleach. Embryos were blocked with 5% normal goat serum in PBST for 1 h and incubated with the primary anti-Kr antibodies (1:1000, kind donation of Francisco Lopes) overnight at 4°C. Secondary antibodies used were Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 568 goat anti-mouse (1:500; Thermo Fisher Scientific, A-11008 and A-11004, respectively), incubated for 1 h at room temperature. Hoechst 33342 was used at 1 µg/ml for nuclear stains. The fluorescence was detected by confocal microscopy using a Leica SP5-AOBS microscope. In situ hybridization was performed as previously described (Araujo and Bier, 2000).
Protein sequence analysis
Sog homolog protein sequences were downloaded from Flybase or NCBI for the 12 Drosophila sequenced genomes, using the 2015 release. Sequence accession numbers are listed in Supplementary Material and Methods. Protein sequences were aligned using ClustalW in the MacVector (MacVector) program. Glycosylation sites were predicted in each protein using NetNGlyc 1.0.
Rab5/Sog colocalization analysis
Sog endocytosis was analyzed by adding medium from control, wild-type Sog or N-mutant Sog-expressing cells, grown in the presence of dpp-HA and tsg-His, to non-transfected cells. Rab5 and Sog (wild type or mutant) were detected in the receiving cells with rabbit anti-Rab5 (1:500, ab31261, Abcam) and mouse anti-V5 (1:500) antisera and subsequently incubated with Alexa Fluor secondary antibodies as above. Colocalization was analyzed with ImageJ software in single confocal optical sections. The number of cells with at least 2 Rab5+V5+ punctae was quantified for each condition.
Supplementary Material
Acknowledgements
We are indebted to Mihaela Serpe and Michael O'Connor for kindly providing BMP pathway constructs and flies. We thank Attilio Pane and members of the Araujo and Todeschini labs for suggestions and critical reading of the manuscript. Monoclonal antibodies against integrin subunits were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa (Department of Biology, Iowa City, IA 52242, USA). Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: E.N., E.B., A.T., H.A.; Formal analysis: E.N., S.H., K.-H.K., A.C., W.B.D., K.C., H.A.; Investigation: E.N., S.H., K.-H.K., A.C., K.C., H.A.; Writing - original draft: S.H., H.A.; Writing - review & editing: E.N., S.H., K.-H.K., K.C., E.B., A.T., H.A.; Supervision: E.N., W.B.D., E.B., A.T., H.A.; Project administration: E.B., A.T., H.A.; Funding acquisition: E.B., A.T., H.A.
Funding
This research was funded by the Ministério da Ciência, Tecnologia, Inovações e Comunicações (CNPq/Brazil) (477157/2013-0 to H.A.; 302088/2017-2 to A.T.; CsF Joven Talento Fellowship to S.H.), the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (E26/010.001/2015 to H.A.; E-26/202.940/2016 to A.T.; fellowship to E.N.), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (88881.068099/2014 to A.T.; fellowship to A.C.), the National Institutes of Health (R01 NS29870 and R01 GM117321 to E.B.). E.B. is the recipient of a Paul G. Allen Family Foundation Distinguished Investigators Award. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.167338.supplemental
References
- Ambrosio A. L., Taelman V. F., Lee H. X., Metzinger C. A., Coffinier C. and De Robertis E. M. (2008). Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev. Cell 15, 248-260. 10.1016/j.devcel.2008.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K., Perlman M., Lim J.-M., Cantu R., Wells L. and Tiemeyer M. (2007). Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J. Biol. Chem. 282, 9127-9142. 10.1074/jbc.M606711200 [DOI] [PubMed] [Google Scholar]
- Araujo H., and Bier E. (2000). sog and dpp exert opposing maternal functions to modify toll signaling and pattern the dorsoventral axis of the Drosophila embryo. Development 127, 3631-3644. 10.1016/j.celrep.2015.10.079 [DOI] [PubMed] [Google Scholar]
- Araujo H., Negreiros E. and Bier E. (2003). Integrins modulate Sog activity in the Drosophila wing. Development 130, 3851-3864. 10.1242/dev.00613 [DOI] [PubMed] [Google Scholar]
- Ashe H. L. and Levine M. (1999). Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature 398, 427-431. 10.1038/18892 [DOI] [PubMed] [Google Scholar]
- Biehs B., François V. and Bier E. (1996). The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 10, 2922-2934. 10.1101/gad.10.22.2922 [DOI] [PubMed] [Google Scholar]
- Bier E. and De Robertis E. M. (2015). EMBRYO DEVELOPMENT. BMP gradients: a paradigm for morphogen-mediated developmental patterning. Science 348, aaa5838 10.1126/science.aaa5838 [DOI] [PubMed] [Google Scholar]
- Billington C. J., Fiebig J. E., Forsman C. L., Pham L., Burbach N., Sun M., Jaskoll T., Mansky K., Gopalakrishnan R., O'Connor M. B. et al. (2011). Glycosylation of twisted gastrulation is required for BMP binding and activity during craniofacial development. Front. Physiol. 2, 59 10.3389/fphys.2011.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair S. S. (2007). Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu. Rev. Cell Dev. Biol. 23, 293-319. 10.1146/annurev.cellbio.23.090506.123606 [DOI] [PubMed] [Google Scholar]
- Caramelo J. J. and Parodi A. J. (2015). A sweet code for glycoprotein folding. FEBS Lett. 589, 3379-3387. 10.1016/j.febslet.2015.07.021 [DOI] [PubMed] [Google Scholar]
- Conley C. A., Silburn R., Singer M. A., Ralston A., Rohwer-Nutter D., Olson D. J., Gelbart W. and Blair S. S. (2000). Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127, 3947-3959. [DOI] [PubMed] [Google Scholar]
- de Celis J. F. (1997). Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development 124, 1007-1018. [DOI] [PubMed] [Google Scholar]
- Decotto E. and Ferguson E. L. (2001). A positive role for Short gastrulation in modulating BMP signaling during dorsoventral patterning in the Drosophila embryo. Development 128, 3831-3841. [DOI] [PubMed] [Google Scholar]
- Dennis J. W. (2017). Genetic code asymmetry supports diversity through experimentation with posttranslational modifications. Curr. Opin. Chem. Biol. 41, 1-11. 10.1016/j.cbpa.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Dennis J. W., Lau K. S., Demetriou M., and Nabi I. R. (2009). Adaptive regulation at the cell surface by N-glycosylation. Traffic 10, 1569-1578. 10.1111/j.1600-0854.2009.00981.x [DOI] [PubMed] [Google Scholar]
- Eldar A., Dorfman R., Weiss D., Ashe H., Shilo B.-Z. and Barkai N. (2002). Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature 419, 304-308. 10.1038/nature01061 [DOI] [PubMed] [Google Scholar]
- Ferguson S. B., Blundon M. A., Klovstad M. S. and Schupbach T. (2012). Modulation of gurken translation by insulin and TOR signaling in Drosophila. J. Cell Sci. 125, 1407-1419. 10.1242/jcs.090381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- François V. and Bier E. (1995). Xenopus chordin and Drosophila short gastrulation genes encode homologous proteins functioning in dorsal-ventral axis formation. Cell 80, 19-20. 10.1016/0092-8674(95)90446-8 [DOI] [PubMed] [Google Scholar]
- Freeze H. H., Chong J. X., Bamshad M. J. and Ng B. G. (2014). Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am. J. Hum. Genet. 94, 161-175. 10.1016/j.ajhg.2013.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneux P. and Varki A. (1999). Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology 9, 747-755. 10.1093/glycob/9.8.747 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M. and Jülicher F. (2014). The role of endocytosis during morphogenetic signaling. Cold Spring Harb. Perspect Biol. 6, a016881 10.1101/cshperspect.a016881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S., Taniguchi M., Muraoka M., Toyoda H., Sado Y., Kawakita M. and Hayashi S. (2001). UDP-sugar transporter implicated in glycosylation and processing of Notch. Nat. Cell Biol. 3, 816-822. 10.1038/ncb0901-816 [DOI] [PubMed] [Google Scholar]
- Guo Z. and Wang Z. (2009). The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development 136, 3627-3635. 10.1242/dev.036939 [DOI] [PubMed] [Google Scholar]
- Häcker U., Nybakken K. and Perrimon N. (2005). Heparan sulphate proteoglycans: the sweet side of development. Nat. Rev. Mol. Cell Biol. 6, 530-541. 10.1038/nrm1681 [DOI] [PubMed] [Google Scholar]
- Hang Q., Zhou Y., Hou S., Zhang D., Yang X., Chen J., Ben Z., Cheng C. and Shen A. (2014). Asparagine-linked glycosylation of bone morphogenetic protein-2 is required for secretion and osteoblast differentiation. Glycobiology 24, 292-304. 10.1093/glycob/cwt110 [DOI] [PubMed] [Google Scholar]
- Harris R. E. and Ashe H. L. (2011). Cease and desist: modulating short-range Dpp signalling in the stem-cell niche. EMBO Rep. 12, 519-526. 10.1038/embor.2011.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Kobayashi S. and Nakato H. (2009). Drosophila glypicans regulate the germline stem cell niche. J. Cell Biol. 187, 473-480. 10.1083/jcb.200904118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr P., Korniychuk G., Yamamoto Y., Grubisic K. and Oelgeschläger M. (2008). Regulation of TGF-(beta) signalling by N-acetylgalactosaminyltransferase-like 1. Development 135, 1813-1822. 10.1242/dev.019323 [DOI] [PubMed] [Google Scholar]
- Humphreys G. B., Jud M. C., Monroe K. M., Kimball S. S., Higley M., Shipley D., Vrablik M. C., Bates K. L. and Letsou A. (2013). Mummy, A UDP-N-acetylglucosamine pyrophosphorylase, modulates DPP signaling in the embryonic epidermis of Drosophila. Dev. Biol. 381, 434-445. 10.1016/j.ydbio.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata H., Haraguchi T. and Sasai Y. (2008). Robust stability of the embryonic axial pattern requires a secreted scaffold for chordin degradation. Cell 134, 854-865. 10.1016/j.cell.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Jasuja R., Allen B. L., Pappano W. N., Rapraeger A. C. and Greenspan D. S. (2004). Cell-surface heparan sulfate proteoglycans potentiate chordin antagonism of bone morphogenetic protein signaling and are necessary for cellular uptake of chordin. J. Biol. Chem. 279, 51289-51297. 10.1074/jbc.M408129200 [DOI] [PubMed] [Google Scholar]
- Jayaprakash N. G. and Surolia A. (2017). Role of glycosylation in nucleating protein folding and stability. Biochem. J. 474, 2333-2347. 10.1042/BCJ20170111 [DOI] [PubMed] [Google Scholar]
- Kang K. H., and Bier E. (2010). dHIP14-dependent palmitoylation promotes secretion of the BMP antagonist Sog. Dev. Biol. 346, 1-10. 10.1016/j.ydbio.2010.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larraín J., Bachiller D., Lu B., Agius E., Piccolo S. and De Robertis E. M. (2000). BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development 127, 821-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery J. W., Amich J. M., Andonian A. and Rosen V. (2014). N-linked glycosylation of the bone morphogenetic protein receptor type 2 (BMPR2) enhances ligand binding. Cell. Mol. Life Sci. 71, 3165-3172. 10.1007/s00018-013-1541-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machingo Q. J., Fritz A. and Shur B. D. (2006). A beta1,4-galactosyltransferase is required for Bmp2-dependent patterning of the dorsoventral axis during zebrafish embryogenesis. Development 133, 2233-2241. 10.1242/dev.02378 [DOI] [PubMed] [Google Scholar]
- Markstein M., Pitsouli C., Villalta C., Celniker S. E. and Perrimon N. (2008). Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat. Genet. 40, 476-483. 10.1038/ng.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués G., Musacchio M., Shimell M. J., Wünnenberg-Stapleton K., Cho K. W. and O'Connor M. B. (1997). Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell 91, 417-426. 10.1016/S0092-8674(00)80425-0 [DOI] [PubMed] [Google Scholar]
- Mizutani C. M., Nie Q., Wan F. Y. M., Zhang Y.-T., Vilmos P., Sousa-Neves R., Bier E., Marsh J. L. and Lander A. D. (2005). Formation of the BMP activity gradient in the Drosophila embryo. Dev. Cell 8, 915-924. 10.1016/j.devcel.2005.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani C. M., Meyer N., Roelink H. and Bier E. (2006). Threshold-dependent BMP-mediated repression: a model for a conserved mechanism that patterns the neuroectoderm. PLoS Biol. 4, e313 10.1371/journal.pbio.0040313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negreiros E., Fontenele M., Câmara A. R. and Araujo H. (2010). alphaPS1betaPS integrin receptors regulate the differential distribution of Sog fragments in polarized epithelia. Genesis 48, 31-43. 10.1002/dvg.20579 [DOI] [PubMed] [Google Scholar]
- Neul J. L. and Ferguson E. L. (1998). Spatially restricted activation of the SAX receptor by SCW modulates DPP/TKV signaling in Drosophila dorsal-ventral patterning. Cell 95, 483-494. 10.1016/S0092-8674(00)81616-5 [DOI] [PubMed] [Google Scholar]
- Nguyen M., Park S., Marqués G. and Arora K. (1998). Interpretation of a BMP activity gradient in Drosophila embryos depends on synergistic signaling by two type I receptors, SAX and TKV. Cell 95, 495-506. 10.1016/S0092-8674(00)81617-7 [DOI] [PubMed] [Google Scholar]
- North S. J., Koles K., Hembd C., Morris H. R., Dell A., Panin V. M. and Haslam S. M. (2006). Glycomic studies of Drosophila melanogaster embryos. Glycoconj. J. 23, 345-354. 10.1007/s10719-006-6693-4 [DOI] [PubMed] [Google Scholar]
- Peluso C. E., Umulis D., Kim Y.-J., O'Connor M. B. and Serpe M. (2011). Shaping BMP morphogen gradients through enzyme-substrate interactions. Dev. Cell 21, 375-383. 10.1016/j.devcel.2011.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhinec J.-L., Zakin L., Moriyama Y. and De Robertis E. M. (2013). Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo. Proc. Natl. Acad. Sci. USA 110, 20372-20379. 10.1073/pnas.1319745110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston A. and Blair S. S. (2005). Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev. Biol. 280, 187-200. 10.1016/j.ydbio.2005.01.018 [DOI] [PubMed] [Google Scholar]
- Ray R. P. and Wharton K. A. (2001). Context-dependent relationships between the BMPs gbb and dpp during development of the Drosophila wing imaginal disk. Development 128, 3913-3925. [DOI] [PubMed] [Google Scholar]
- Ray R. P., Arora K., Nüsslein-Volhard C. and Gelbart W. M. (1991). The control of cell fate along the dorsal-ventral axis of the Drosophila embryo. Development 113, 35-54. [DOI] [PubMed] [Google Scholar]
- Reversade B. and De Robertis E. M. (2005). Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123, 1147-1160. 10.1016/j.cell.2005.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. J., Shimmi O., Vilmos P., Petryk A., Kim H., Gaudenz K., Hermanson S., Ekker S. C., O'Connor M. B. and Marsh J. L. (2001). Twisted gastrulation is a conserved extracellular BMP antagonist. Nature 410, 479-483. 10.1038/35068578 [DOI] [PubMed] [Google Scholar]
- Saremba S., Nickel J., Seher A., Kotzsch A., Sebald W. and Mueller T. D. (2008). Type I receptor binding of bone morphogenetic protein 6 is dependent on N-glycosylation of the ligand. FEBS J. 275, 172-183. 10.1111/j.1742-4658.2007.06187.x [DOI] [PubMed] [Google Scholar]
- Sawala A., Sutcliffe C. and Ashe H. L. (2012). Multistep molecular mechanism for bone morphogenetic protein extracellular transport in the Drosophila embryo. Proc. Natl. Acad. Sci. USA 109, 11222-11227. 10.1073/pnas.1202781109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmelpfeng K., Strunk M., Stork T. and Klämbt C. (2006). Mummy encodes an UDP-N-acetylglucosamine-dipohosphorylase and is required during Drosophila dorsal closure and nervous system development. Mech. Dev. 123, 487-499. 10.1016/j.mod.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Selleck S. B. (2000). Proteoglycans and pattern formation: sugar biochemistry meets developmental genetics. Trends Genet. 16, 206-212. 10.1016/S0168-9525(00)01997-1 [DOI] [PubMed] [Google Scholar]
- Selva E. M., Hong K., Baeg G.-H., Beverley S. M., Turco S. J., Perrimon N. and Häcker U. (2001). Dual role of the fringe connection gene in both heparan sulphate and fringe-dependent signalling events. Nat. Cell Biol. 3, 809-815. 10.1038/ncb0901-809 [DOI] [PubMed] [Google Scholar]
- Serpe M., Ralston A., Blair S. S. and O'Connor M. B. (2005). Matching catalytic activity to developmental function: tolloid-related processes Sog in order to help specify the posterior crossvein in the Drosophila wing. Development 132, 2645-2656. 10.1242/dev.01838 [DOI] [PubMed] [Google Scholar]
- Serpe M., Umulis D., Ralston A., Chen J., Olson D. J., Avanesov A., Othmer H., O'Connor M. B. and Blair S. S. (2008). The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev. Cell 14, 940-953. 10.1016/j.devcel.2008.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmi O., Umulis D., Othmer H. and O'Connor M. B. (2005). Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120, 873-886. 10.1016/j.cell.2005.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillos S. and De Celis J. F. (2005). Interactions between the Notch, EGFR, and decapentaplegic signaling pathways regulate vein differentiation during Drosophila pupal wing development. Dev. Dyn. 232, 738-752. 10.1002/dvdy.20270 [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Rashka K. E. and Bier E. (2002). Creation of a Sog morphogen gradient in the Drosophila embryo. Dev. Cell 2, 91-101. 10.1016/S1534-5807(01)00097-1 [DOI] [PubMed] [Google Scholar]
- Stanley P., Taniguchi N., Aebi M. (2017). N-Glycans. In Essentials of Glycobiology, 3rd edn (ed. Varki A., Cummings R. D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., Schnaar R. L., Seeberger P. H.). Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- Tauscher P. M., Gui J. and Shimmi O. (2016). Adaptive protein divergence of BMP ligands takes place under developmental and evolutionary constraints. Development 143, 3742-3750. 10.1242/dev.130427 [DOI] [PubMed] [Google Scholar]
- Tian E. and Ten Hagen K. G. (2006). Expression of the UDP-GalNAc: polypeptide N-acetylgalactosaminyltransferase family is spatially and temporally regulated during Drosophila development. Glycobiology 16, 83-95. 10.1093/glycob/cwj051 [DOI] [PubMed] [Google Scholar]
- Tonning A., Helms S., Schwarz H., Uv A. E. and Moussian B. (2006). Hormonal regulation of mummy is needed for apical extracellular matrix formation and epithelial morphogenesis in Drosophila. Development 133, 331-341. 10.1242/dev.02206 [DOI] [PubMed] [Google Scholar]
- Troilo H., Barrett A. L., Wohl A. P., Jowitt T. A., Collins R. F., Bayley C. P., Zuk A. V., Sengle G. and Baldock C. (2015). The role of chordin fragments generated by partial tolloid cleavage in regulating BMP activity. Biochem. Soc. Trans. 43, 795-800. 10.1042/BST20150071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umulis D. M., Serpe M., O'Connor M. B. and Othmer H. G. (2006). Robust, bistable patterning of the dorsal surface of the Drosophila embryo. Proc. Natl. Acad. Sci. USA 103, 11613-11618. 10.1073/pnas.0510398103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandall H. H., Pedersen J. W., Park C., Levery S. B., Pizette S., Cohen S. M., Schwientek T. and Clausen H. (2003). Drosophila egghead encodes a beta 1,4-mannosyltransferase predicted to form the immediate precursor glycosphingolipid substrate for brainiac. J. Biol. Chem. 278, 1411-1414. 10.1074/jbc.C200619200 [DOI] [PubMed] [Google Scholar]
- Wang X., Harris R. E., Bayston L. J. and Ashe H. L. (2008). Type IV collagens regulate BMP signalling in Drosophila. Nature 455, 72-77. 10.1038/nature07214 [DOI] [PubMed] [Google Scholar]
- Yamamoto-Hino M., Muraoka M., Kondo S., Ueda R., Okano H. and Goto S. (2015). Dynamic regulation of innate immune responses in Drosophila by Senju-mediated glycosylation. Proc. Natl. Acad. Sci. USA 112, 5809-5814. 10.1073/pnas.1424514112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Sturtevant M. A., Biehs B., François V., Padgett R. W., Blackman R. K. and Bier E. (1996). The Drosophila decapentaplegic and short gastrulation genes function antagonistically during adult wing vein development. Development 122, 4033-4044. [DOI] [PubMed] [Google Scholar]
- Yu K., Srinivasan S., Shimmi O., Biehs B., Rashka K. E., Kimelman D., O'Connor M. B. and Bier E. (2000). Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development 127, 2143-2154. [DOI] [PubMed] [Google Scholar]
- Yu K., Kang K. H., Heine P., Pyati U., Srinivasan S., Biehs B., Kimelman D. and Bier E. (2004). Cysteine repeat domains and adjacent sequences determine distinct bone morphogenetic protein modulatory activities of the Drosophila Sog protein. Genetics 166, 1323-1336. 10.1534/genetics.166.3.1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.