ABSTRACT
During zebrafish gastrulation the planar cell polarity (PCP) protein Vang-like 2 (Vangl2) regulates the polarization of cells that are engaged in directed migration. However, it is unclear whether Vangl2 influences membrane-protrusive activities in migrating gastrula cells and whether these processes require the fibronectin extracellular matrix. Here, we report that Vangl2 modulates the formation and polarization of actin-rich filopodia-like and large lamellipodia-like protrusions in ectodermal cells. By contrast, disrupted Glypican4/PCP signaling affects protrusion polarity but not protrusion number or directed migration. Analysis of fluorescent fusion protein expression suggests that there is widespread Vangl2 symmetry in migrating cells, but there is enrichment at membrane domains that are developing large protrusions compared with non-protrusive domains. We show that the fibronectin extracellular matrix is essential for cell-surface Vangl2 expression, membrane-protrusive activity and directed migration. Manipulation of fibronectin protein levels rescues protrusion and directed migration phenotypes in vangl2 mutant embryos, but it is not sufficient to restore either PCP, or convergence and extension movements. Together, our findings identify distinct roles for Vangl2 and Glypican4/PCP signaling during membrane protrusion formation and demonstrate that cell-matrix interactions underlie Vangl2-dependent regulation of protrusive activities in migrating gastrula cells.
KEY WORDS: Vangl2, Fibronectin, Protrusion, Polarity, Migration, Gastrulation, Zebrafish
Summary: The extracellular matrix protein fibronectin plays an important role in membrane protrusion formation and polarization in migrating gastrula cells, interacting with the planar cell polarity protein Vangl2.
INTRODUCTION
Establishment of planar cell polarity (PCP) is a complex process that involves many proteins functioning cell autonomously and/or non-autonomously. First described in reference to the orientation of insect hairs and bristles (Lawrence, 1966; Nubler-Jung et al., 1987), PCP and its associated proteins are linked to a variety of morphogenetic processes, including gastrulation movements (Goodrich and Strutt, 2011; Gray et al., 2011; Jessen and Solnica-Krezel, 2005). The six core PCP genes identified in fly include van gogh, prickle, dishevelled, frizzled, flamingo and diego (Strutt and Strutt, 2007; Wong and Adler, 1993; Wu and Mlodzik, 2009). Genetic evidence that a core PCP protein is required for vertebrate gastrulation came with the identification of vang-like 2 (vangl2), which encodes a four-pass transmembrane protein, as the defective gene in trilobite mutant zebrafish (Danio rerio) embryos (Jessen et al., 2002; Solnica-Krezel et al., 1996). Loss of the Vangl2 protein produces a strong convergence and extension gastrulation phenotype, characterized by shortened and broadened embryonic body axes. Underlying normal convergence and extension movements are several cell behaviors, among them directed migration and mediolateral intercalation. Here, PCP is defined as the elongation and mediolateral alignment (MLA) of ectodermal and mesodermal cells perpendicular to the dorsal embryonic axis. Loss of Vangl2 causes lateral cells to meander, moving dorsally along indirect trajectories (Jessen et al., 2002). How Vangl2 regulates directed migration during zebrafish gastrulation is not understood.
A growing body of data indicates that a non-canonical Wnt pathway controls PCP during gastrulation. With the aid of the glycosylphosphatidylinositol-anchored Glypican4 co-receptor, Wnt11 and Wnt5b bind Frizzled receptors to effect changes in the subcellular distribution of Dishevelled (Heisenberg et al., 2000; Kilian et al., 2003; Ohkawara et al., 2003; Topczewski et al., 2001). Downstream, Rho family small GTPases and their effector proteins (e.g. Rho kinase) are thought to stimulate changes in actin arrangement and cell polarization (Habas et al., 2003, 2001; Marlow et al., 2002). Vangl2 and its cytoplasmic binding partner, Prickle1a, somehow influence the Glypican4/PCP pathway (Carreira-Barbosa et al., 2003; Dohn et al., 2013; Jessen et al., 2002; Veeman et al., 2003), perhaps by regulating Dishevelled signaling (Seo et al., 2017). Zebrafish with null mutations in vangl2 and glypican4 (knypek) or vangl2 and wnt11 (silberblick) have convergence and extension phenotypes stronger than the individual mutants (Heisenberg and Nüsslein-Volhard, 1997; Marlow et al., 1998). These findings indicate that Vangl2 and Glypican4 have distinct functions during gastrulation movements.
A hallmark of PCP in fly epithelium is asymmetrical expression of certain core proteins (Strutt, 2002). In the wing, Van Gogh and Prickle localize to the proximal side of the cell, whereas Frizzled, Dishevelled and Diego are enriched distally in the area of actin-rich trichome formation. Asymmetrical protein-containing membrane domains on adjacent cells interact in order to regulate the global alignment of trichome polarity in relation to the wing axes. Unlike fly proteins, asymmetrical expression of vertebrate core PCP proteins has proven challenging to demonstrate. In polarized non-migrating neuroectoderm or axial mesoderm, fluorescent fusion proteins for Vangl2 and Prickle are enriched at anterior cell boundaries, whereas Dishevelled preferentially localizes to posterior membranes (Ciruna et al., 2006; Roszko et al., 2015; Yin et al., 2008). It is unknown whether PCP proteins are asymmetrically expressed in lateral ectodermal or mesodermal cells that are undergoing directed migration. Data from non-migrating cells suggest an anterior/posterior axis of PCP. However, migrating gastrula cells exhibit dynamic changes in cell shape, orientation and position relative to neighboring cells (Jessen et al., 2002; Roszko et al., 2015; Yin et al., 2009) and a plane of polarization may be difficult to discern. Whatever the subcellular distribution of PCP proteins, understanding their function in migrating gastrula cells remains a key issue.
For migrating cells, adhesion to the extracellular matrix (ECM) is usually required to generate the traction needed for membrane protrusion formation and cell body translocation (Friedl and Gilmour, 2009). During migration, vangl2 mutant cells lack directionality, which indicates a possible relationship between Vangl2, the ECM and membrane-protrusive activity. Experiments using the frog gastrula demonstrate that integrin α5β1 and fibronectin interactions suppress inappropriate membrane-protrusive activity (Davidson et al., 2006). Moreover, overexpression of frog Vangl2, Prickle or Frizzled7 disrupts fibronectin fibril assembly and organization, in a manner correlating with the severity of the PCP phenotype (Goto et al., 2005). Similar to frog gastrulation movements, zebrafish convergence and extension occur in the context of a fibronectin-containing ECM network (Boucaut and Darribere, 1983; Latimer and Jessen, 2010; Winklbauer and Keller, 1996). During gastrulation, a layer of fibronectin forms between the ectoderm and superficial mesoderm and another layer between the yolk and mesendoderm, with individual fibrils protruding between cells (Latimer and Jessen, 2010). Therefore, by late gastrulation, ectodermal and mesodermal cell migration is associated with a fibrillar ECM. Notably, whereas loss of either Vangl2 or Prickle1a results in reduced fibronectin, glypican4 mutant embryos exhibit increased fibronectin assembly (Dohn et al., 2013; Williams et al., 2012). These different effects on ECM structure further support the notion that Vangl2/Prickle1a and Glypican4/PCP signaling have distinct effects on cell behaviors. In addition, these data suggest that fibronectin may be necessary for certain aspects of Vangl2 function.
The major goal of this study was to determine how Vangl2 and fibronectin regulate membrane protrusion dynamics in migrating zebrafish gastrula cells. We used time-lapse imaging combined with mosaic expression of fluorescent fusion proteins to visualize protrusions in live embryos. We have shown that Vangl2 regulates distinct aspects of protrusion formation compared with Glypican4. We found GFP-VANGL2 expression to be generally symmetrical in migrating gastrula cells, but enriched in forming membrane protrusions compared with non-protrusive domains. Our work implicates fibronectin in the regulation of protrusion formation and polarization, and Vangl2 cell-surface expression. Finally, we have shown that increasing fibrillar fibronectin in vangl2 mutant embryos rescues the protrusion phenotype, but not PCP. These results uncover a previously unrecognized interaction between Vangl2, fibronectin and membrane-protrusive activity, which are required for the dorsal convergence of gastrula cells.
RESULTS
Vangl2 and Glypican4 differentially regulate membrane protrusion formation and directed migration
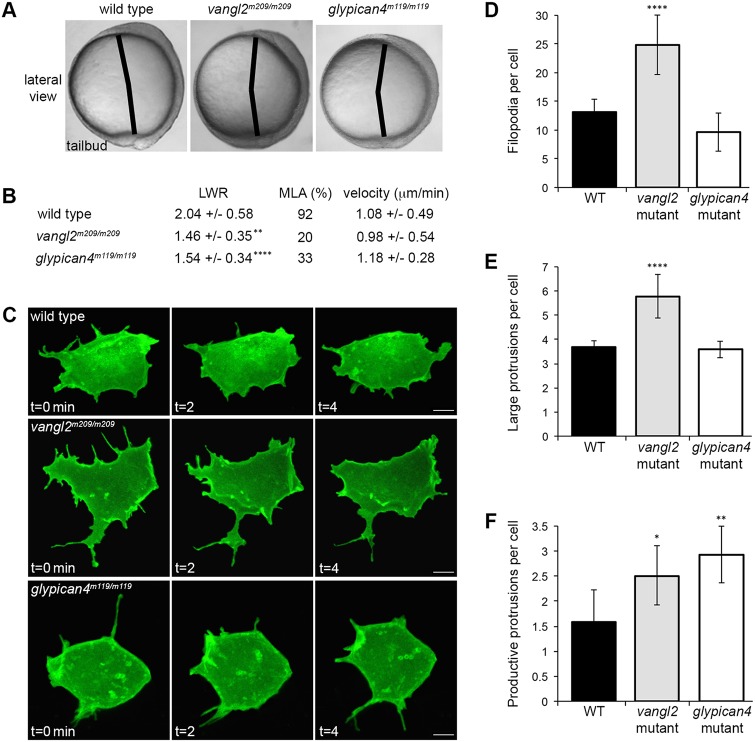
Multiple cell behaviors contribute to the processes of convergence and extension during zebrafish gastrulation, including the directed migration of lateral cells toward the dorsal body axis (Jessen and Solnica-Krezel, 2005). Whereas wild-type gastrula cells are elongated and mediolaterally aligned, this type of PCP is disrupted in vangl2 and glypican4 homozygous mutant embryos (Jessen et al., 2002; Topczewski et al., 2001). To better understand the mechanism whereby Vangl2 regulates dorsal convergence, we analyzed and compared membrane protrusion formation in vangl2m209/m209 and glypican4m119/m119 mutants. Time-lapse confocal microscopy was used to image late gastrula lateral ectodermal cells 40-60 degrees from the notochord (Fig. 1A). We achieved mosaic labeling by injecting single blastomeres from eight-cell-stage embryos with synthetic mRNA that encoded fluorescent fusion proteins. Lifeact-GFP and membrane-targeted RFP (memRFP) were used to assess the types of membrane protrusions that are formed by lateral ectodermal cells. Three distinct protrusions were identified: small spike-like actin-rich protrusions that resemble filopodia; large sheet-like actin-rich protrusions that resemble lamellipodia/filolamellipodia (henceforth large protrusions); and spherical bleb-like protrusions that are initially devoid of actin (Blaser et al., 2006) (Fig. 1B,C). Filopodia-like protrusions (henceforth filopodia) predominate, followed by large protrusions, and therefore were the focus of our study. Bleb-like protrusions are much less abundant and are often associated with dividing cells (Fig. 1C).
Fig. 1.
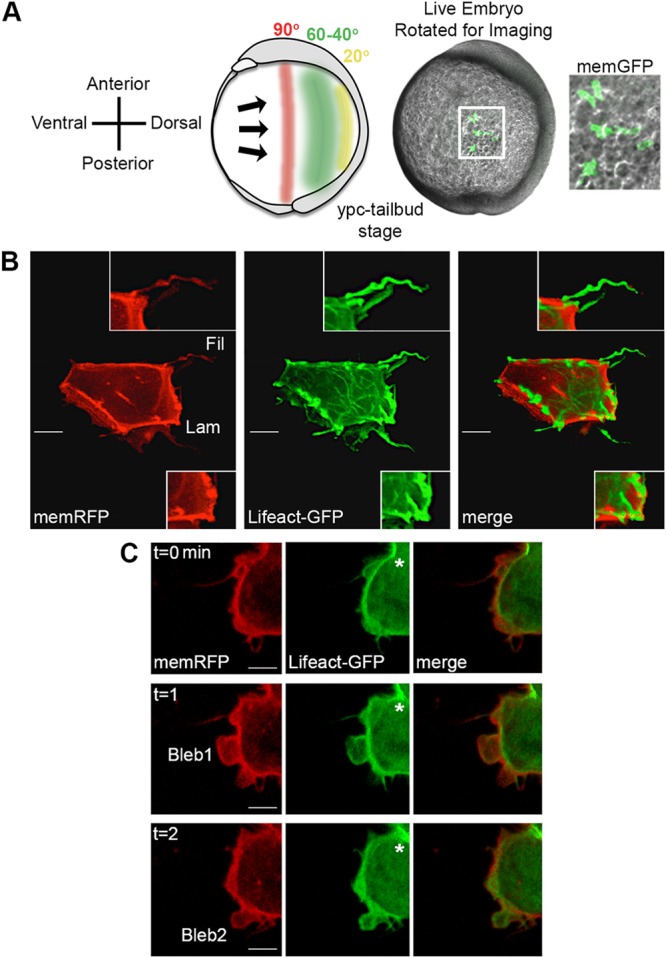
Time-lapse imaging of membrane protrusions. (A) Schematic (left) and live wild-type zebrafish embryo (right) highlighting the 40°-60° lateral region that was analyzed. Lateral ectodermal cells with mosaic memGFP expression from an embryo used for confocal imaging at 60° from dorsal (white boxed area, enlarged far right). ypc, yolk-plug closure. (B) Wild-type ectodermal cell expressing memRFP and Lifeact-GFP with filopodia (Fil) and large lamellipodia-like (Lam) membrane protrusions. Insets show enlarged images of protrusion classifications. (C) Wild-type ectodermal cell undergoing division. Asterisks indicate forming cleavage furrow. Two blebs are observed during the three time points. Scale bars: 5 µm.
For the analysis of membrane-protrusive activity, single blastomeres were injected with only memGFP, to minimize mRNA cytotoxicity. Homozygous vangl2 and glypican4 mutant embryos are identified by their strong convergence and extension phenotypes at tailbud stage (Fig. 2A) (Solnica-Krezel et al., 1996). First, we confirmed that mutant lateral ectodermal cells exhibited defective PCP, as indicated by a reduced length-width ratio (LWR) and MLA (Fig. 2B). We next quantified the number of protrusions that were formed by ectodermal cells. Wild-type embryos averaged 13.16 filopodia and 3.68 large protrusions per cell (Fig. 2C-E). In vangl2 embryos, these averages increased to 24.84 filopodia and 5.77 large protrusions per cell (Fig. 2C-E). By contrast, glypican4 mutant ectodermal cells showed no difference in the number of filopodia (9.58) or large protrusions (3.59) compared with wild type (Fig. 2C-E). From the total number of large protrusions, we determined the proportion that were productive, meaning protrusion formation in one direction is followed by cell body translocation in the same direction. We found the average number of productive protrusions formed by cells in vangl2 and glypican4 embryos to be increased (Fig. 2F; wild type=1.58, vangl2=2.50, glypican4=2.92).
Fig. 2.
Membrane protrusion formation in vangl2 and glypican4 mutant embryos. (A) Live embryo images at late yolk-plug closure/tailbud stage. Black lines denote the polster-tailbud angle. (B) PCP and migration velocity quantitation in the ectoderm. LWR and MLA values were obtained from: wild type (WT), n=50 cells, 13 embryos; vangl2m209/m209, n=10 cells, 8 embryos; glypican4m119/m119, n=49 cells, 5 embryos. PCP data for vangl2 mutant ectoderm was previously published (Jessen et al., 2002), which explains the lower n. (C) Time-lapse confocal images of ectodermal cells expressing memGFP over three time points. (D-F) Quantitation of the total numbers of filopodia (D), large protrusions (E) and productive protrusions (F) formed by WT (n=12 cells, 8 embryos), vangl2m209/m209 (n=10 cells, 7 embryos) and glypican4m119/m119 (n=12 cells, 5 embryos). Data are mean±s.d. *P<0.05, **P<0.01, ****P<0.0001; P values are versus WT; one-way ANOVA significance test followed by Tukey HSD post-hoc tests. Scale bars: 5 µm.
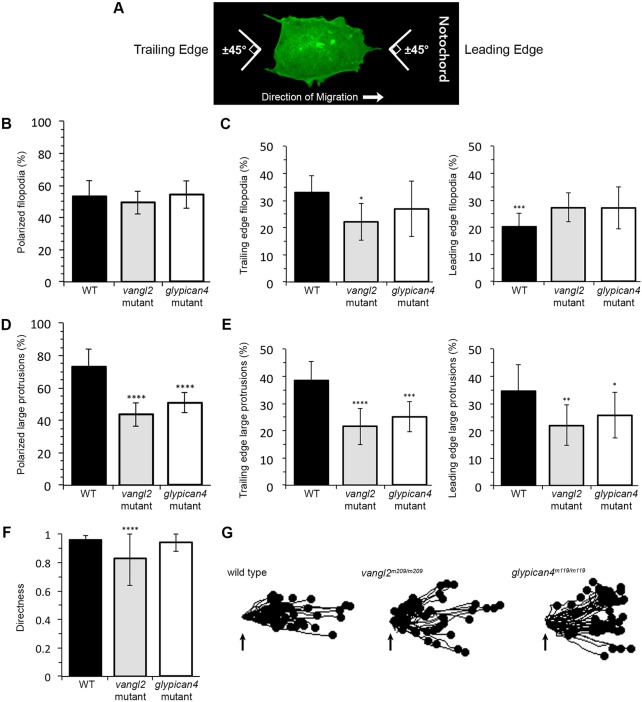
Given the difference in protrusion number between vangl2 and glypican4 mutant embryos, we examined the polarity of filopodia and large protrusions. Similar to MLA, protrusion polarity was determined in relation to the notochord, running anterior-posterior along the dorsal body axis. Protrusions were considered polarized if their base was oriented ±45° from the path of dorsal migration and included protrusions at both the leading and trailing edges (Fig. 3A). We did not detect a polarized distribution of filopodia in ectodermal cells (Fig. 3B; wild type=53.23%, vangl2=49.52%, glypican4=54.27% polarized protrusions). However, there was a significant difference between the numbers of leading versus trailing edge wild-type filopodia (Fig. 3C; 32.97% and 20.26%, respectively). We also found that a decreased number of vangl2 mutant filopodia localized to the trailing edge compared with in the wild type (Fig. 3C; vangl2=21.69%). Unlike filopodia, 73.10% of wild-type large protrusions are oriented along the path of dorsal migration (Fig. 3D). By contrast, a reduced number of polarized large protrusions are observed in ectodermal cells from both vangl2 and glypican4 mutant embryos (Fig. 3D; vangl2=43.76%, glypican4=50.97%). We detected no difference in the numbers of leading versus trailing edge large protrusions in wild-type or PCP mutant embryos (Fig. 3E). Rose diagrams indicate that vangl2 and glypican4 mutant ectodermal cells generate large protrusions at multiple positions around their circumference (Fig. S1). The reduced number of polarized large protrusions in vangl2 and glypican4 mutant ectoderm suggests that these cells have indirect migration trajectories. Migration directness values were calculated by dividing the Euclidean distance by the accumulated or total migration distance (Table S1); a value of 1 indicates a straight migration path. Whereas vangl2 mutant ectodermal cells have reduced directness (0.83) compared with wild-type cells (0.96), loss of Glypican4 function has a negligible effect (0.94) on directness (Fig. 3F). However, trajectory plots indicate that both mutations disrupt overall net dorsal convergence (Fig. 3G). These data imply that Vangl2 and Glypican4 are both necessary for PCP and polarized membrane-protrusive activity, but these proteins have distinct effects on protrusion formation and directed cell movement.
Fig. 3.
Membrane protrusion polarity and directed migration in vangl2 and glypican4 mutant embryos. (A) Wild-type (WT) ectodermal cell image illustrating polarized protrusion angles. (B,C) Quantitation of the total percentages of polarized filopodia (B) and filopodia localized at the leading versus trailing edge (C) in WT (n=11 cells, 8 embryos), vangl2m209/m209 (n=10 cells, 7 embryos) and glypican4m119/m119 (n=11 cells, 5 embryos). (D,E) Quantitation of polarized large protrusions (D) and large protrusions localized at the leading versus trailing edge (E) in WT (n=12 cells, 8 embryos), vangl2m209/m209 (n=10 cells, 7 embryos) and glypican4m119/m119 (n=12 cells, 5 embryos). (F) Directed migration values in WT (n=50 cells, 11 embryos), vangl2m209/m209 (n=51 cells, 13 embryos) and glypican4m119/m119 (n=50 cells, 8 embryos). (G) Schematic of the migration paths of individual ectodermal cells. Origins (arrows) standardized for comparison. Dorsal is to the right. Data are mean±s.d. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; P values are versus WT, except WT leading edge P value which is versus WT trailing edge; one-way ANOVA significance test followed by Tukey HSD post-hoc tests.
Vangl2 overexpression affects membrane-protrusive activity, similar to the loss of Vangl2
Another hallmark of core PCP protein function is that excess activity results in cell polarity defects (Yang and Mlodzik, 2015). Previously published work has not reported Vangl2 overexpression relative to Vangl2 loss-of-function cellular phenotypes. Therefore, we performed Vangl2 gain-of-function studies to substantiate its role in membrane protrusion formation and polarity. We verified with immunofluorescence (Fig. S2) and western blot (Fig. S3) that vangl2 mRNA injection at the single-cell stage increases protein expression. We used live imaging and whole-mount in situ hybridization to visualize the severity of the convergence and extension phenotype at the end of gastrulation. Compared with controls, vangl2-injected wild-type embryos are broadened mediolaterally and have shorter anterior-posterior axes (Fig. S3). Next, using mosaic memGFP expression and time-lapse imaging, we analyzed PCP and membrane protrusions in vangl2-injected embryos. Again, vangl2 mRNA injection was done at the single-cell stage to promote ubiquitous protein expression. The LWRs and MLA of vangl2 overexpressing lateral ectodermal cells are reduced compared with wild type (Fig. S3). Increased Vangl2 causes lateral ectodermal cells to average twice as many filopodia (27.63) and large protrusions (6.44) as control cells (Fig. S3). Vangl2 overexpression also disrupts filopodium and large protrusion polarity (Fig. S1; Fig. S3; 40.74% and 49.64% polarized protrusions, respectively). Lastly, increasing Vangl2 protein levels has a strong effect on directed ectodermal cell migration (Fig. S3; wild type=0.96, vangl2 mRNA=0.69). These results indicate that a specific level of cell-surface Vangl2 expression is essential for PCP, membrane-protrusive activity, and directed migration.
Vangl2 expression is enriched in forming membrane protrusions
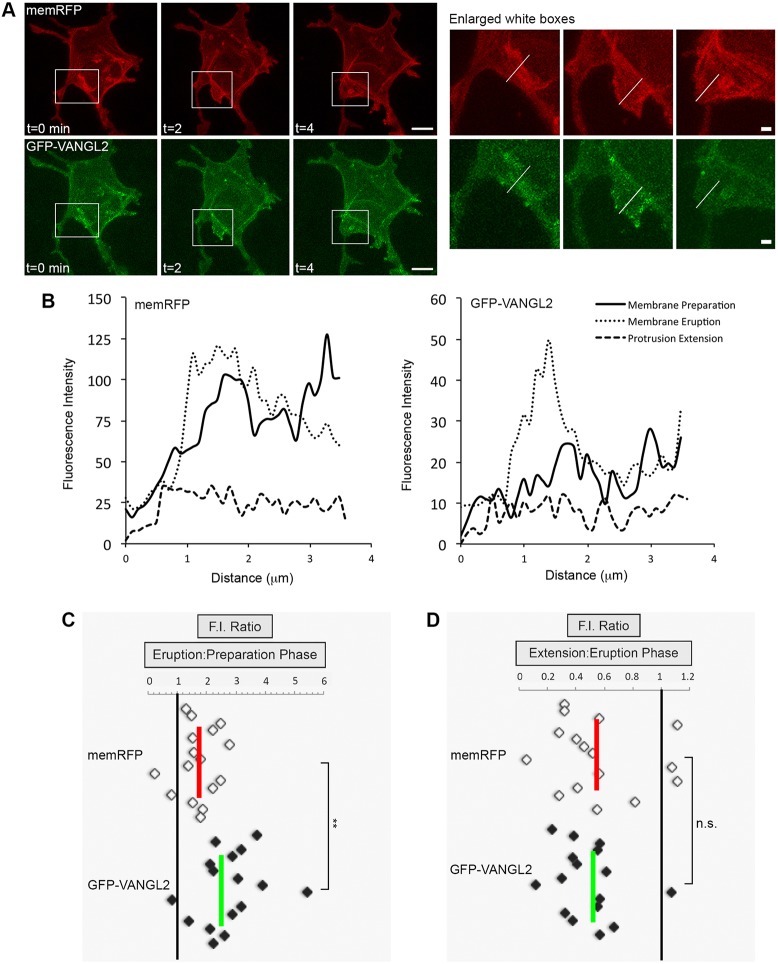
Previous work using polarized zebrafish notochord cells has demonstrated asymmetrical anterior-membrane-biased Vangl2 protein expression at post-gastrulation stages (Roszko et al., 2015). Axial cells are unlike the lateral gastrula cells that are engaged in directed migration, in terms of dynamic changes in membrane-protrusive activity and position relative to neighboring cells. Therefore we examined the distribution of a human GFP-VANGL2 fusion protein (Roszko et al., 2015) in anterior, posterior, leading edge and trailing edge plasma membrane domains of wild-type lateral ectodermal cells. Here, anterior/posterior refers to the cell axis perpendicular to the path of dorsal migration, whereas the leading edge/trailing edge axis is parallel to the migration path (Fig. S4). Single blastomeres from eight-cell-stage embryos were injected with synthetic mRNA that encoded GFP-VANGL2 to generate mosaic expression, and cells were imaged at the tailbud-1-somite stage. We injected GFP-VANGL2 at low doses so as not to perturb cell polarity (Roszko et al., 2015). Blastomeres were co-injected with memRFP to control for plasma membrane variations and changes in fluorescence intensity due to membrane thinning or thickening. Consistent with published data (Roszko et al., 2015), GFP-VANGL2 localizes in a punctate pattern at the plasma membrane and within putative vesicular compartments (Fig. S4, Fig. 4A). By analyzing the fluorescence intensity ratios at multiple positions along the anterior to posterior membrane domains, we concluded that GFP-VANGL2 expression is not broadly asymmetrical in polarized lateral ectodermal cells (Fig. S4). Similar results were obtained for the leading edge and trailing edge membrane domains (Fig. S4).
Fig. 4.
Increased Vangl2 expression in forming membrane protrusions. (A) Time-lapse confocal images of wild-type lateral ectodermal cells expressing memRFP and GFP-VANGL2 (three time points shown). White boxes highlight a forming large protrusion (enlarged images on the right; white lines approximate the regions analyzed). (B) Plot profiles of fluorescence intensity (F.I.) across the plasma membrane of an individual large protrusion expressing memRFP (left) and GFP-VANGL2 (right) before protrusion formation (preparation), after formation (eruption) and during extension. (C,D) memRFP and GFP-VANGL2 F.I. ratios for individual large protrusions [eruption phase/preparation phase (C), n=16 protrusions, 6 embryos; extension phase/eruption phase (D), n=16 protrusions, 6 embryos]. Vertical red and green lines indicate the averages. **P<0.01; two-tailed paired t-test. n.s., not significant. Scale bars: 5 µm in A, left; 1 µm in A, right.
The increased number of membrane protrusions in vangl2 mutant cells suggests that Vangl2 may normally function to inhibit protrusive activity, or limit protrusion formation or lifespan at specific membrane domains. Therefore, we quantified GFP-VANGL2 protein expression during the formation of large protrusions. Large protrusions were chosen because they are associated with migratory behaviors, such as cell body translocation. We quantified fluorescence intensity at three subjectively named time points: before protrusion formation (preparation phase); during early protrusion development (eruption phase); and after protrusion elongation or remodeling (extension phase). Blastomeres were injected with a combination of GFP-VANGL2 and memRFP, and time-lapse imaging was performed near the tailbud stage (Fig. 4A). We report a significant increase in GFP-VANGL2 protein localization within erupting membrane protrusions (Fig. 4B,C), that becomes reduced as protrusions extend or remodel (Fig. 4B,D). GFP-VANGL2 expression within erupting protrusions is enriched compared with non-protrusive membrane domains (Fig. S5). However, our studies found that there was no difference in GFP-VANGL2 localization between polarized and non-polarized protrusions (Fig. S5). In conclusion, although GFP-VANGL2 does not exhibit widespread asymmetrical membrane expression in migrating gastrula cells, our data demonstrate protein enrichment in membrane domains that are associated with protrusive activity.
Fibronectin is required for membrane protrusion formation and polarity
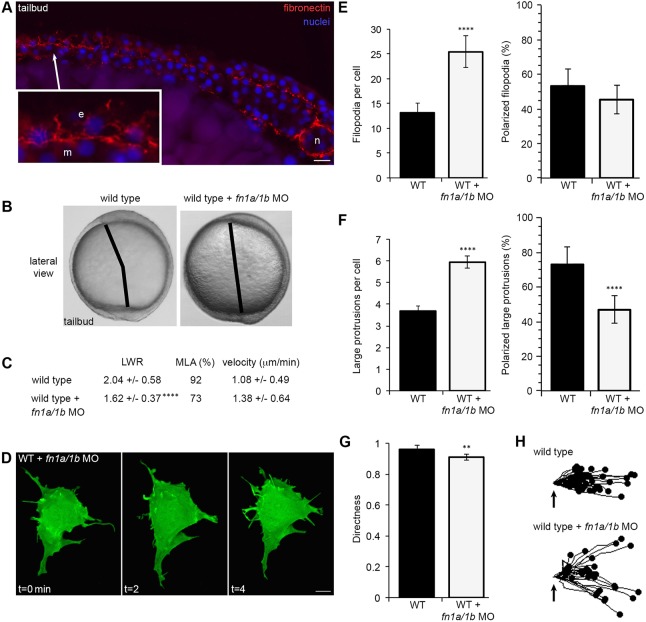
Our published data report that vangl2 mutant embryos and prickle1a morphants have reduced fibronectin (Dohn et al., 2013; Williams et al., 2012). Experiments using the frog gastrula indicate that integrin-fibronectin interactions are essential for polarized membrane protrusion formation (Davidson et al., 2006). Therefore we hypothesized that loss of fibronectin disrupts protrusion formation and, perhaps, PCP in lateral ectodermal cells. Zebrafish express two fibronectin genes (fn1a and fn1b) during gastrulation (Sun et al., 2005; Thisse et al., 2001; Trinh and Stainier, 2004). Correlating with the establishment of PCP, fibronectin protein expression and fibril assembly increase near the end of gastrulation (Fig. 5A) (Latimer and Jessen, 2010; Roszko et al., 2015). We used a combination of fn1a and fn1b antisense morpholino oligonucleotides to knockdown protein expression, which produced a mild convergence and extension phenotype in wild-type embryos (Fig. 5B) (Jülich et al., 2005; Latimer and Jessen, 2010). These morpholinos reduce fibronectin expression during gastrulation, a phenotype that is rescued by injection of fn1a/1b mRNA (Fig. S6) (Latimer and Jessen, 2010). Fibronectin knockdown alters LWRs and MLA compared with wild type, but does not reduce PCP to the extent that was observed during Vangl2 loss-of-function (Fig. 5C). Reduced fibronectin expression also causes ectodermal cells to have excess numbers of filopodia and large protrusions (Fig. 5D-F; 25.42 and 5.94, respectively), similar to those found in vangl2 mutant cells. Unlike the filopodia of fn1a/1b morphant ectodermal cells, the percentage of polarized large protrusions is markedly lower (Fig. S1; Fig. 5E,F; 46.88% polarized protrusions). Further, loss of fibronectin disrupts the directed migration of ectodermal cells (Fig. 5G,H; wild type=0.96, fn1a/1b MO=0.91). These data provide evidence that fibronectin supports proper membrane-protrusive activity, but that the ECM plays a lesser role during the establishment of PCP and convergence and extension movements.
Fig. 5.
Fibronectin regulates membrane protrusion dynamics. (A) Cryosection through the dorsolateral region of a wild-type (WT) tailbud-stage embryo showing fibronectin and nuclei. Inset shows an enlarged image illustrating the relationship between ECM, ectodermal (e) and mesodermal (m) cells. n, notochord. (B) Live embryo images at late yolk-plug closure/tailbud stage in WT and fn1a/1b morpholino (MO)-injected WT embryos. Black lines denote the polster-tailbud angle. (C) PCP and migration velocity quantitation of ectodermal cells. LWR and MLA values were obtained from: WT, n=50 cells, 13 embryos; fn1a/1b MO-injected WT, n=48 cells, 10 embryos. (D) Representative ectodermal cell expressing memGFP over three time points from time-lapse data. (E,F) Quantitation of the average total number (left) and the total percentage of polarized (right) filopodia (E) and large protrusions (F) in WT (n=12 cells, 8 embryos) and fn1a/1b morphant (n=10 cells, 7 embryos) embryos. (G) Directed migration values in WT (n=50 cells, 11 embryos) and fn1a/1b morphants (n=32 cells, 10 embryos). (H) Schematic of the migration paths of individual ectodermal cells. Origins (arrows) standardized for comparison. Dorsal is to the right. Data are mean±s.d. **P<0.01, ****P<0.0001; P values are versus WT; two-tailed unpaired t-test. Scale bars: 20 µm in A; 5 µm in D.
Fibronectin regulates cell-surface Vangl2 expression
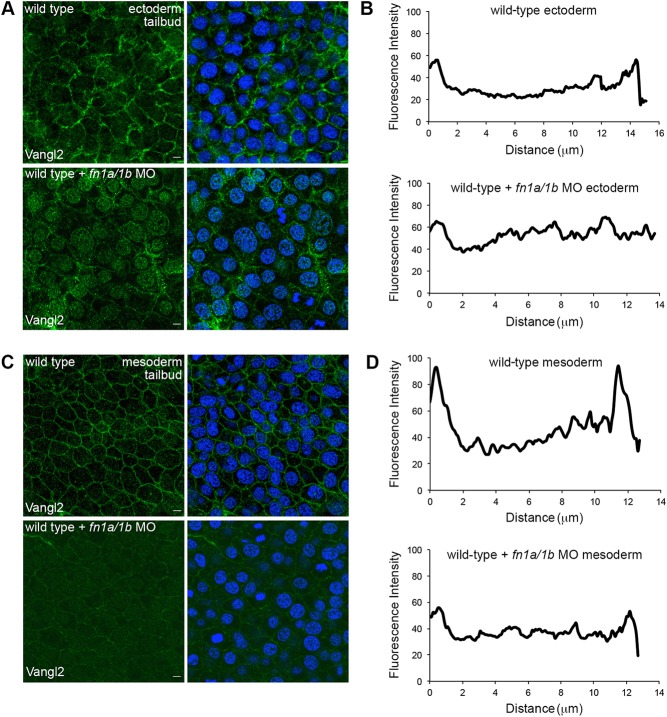
Vangl2 protein expression at the plasma membrane increases at mid-gastrulation, immediately before the onset of Vangl2-dependent PCP and the appearance of the vangl2 mutant convergence and extension phenotype (Roszko et al., 2015; Sepich et al., 2000). At mid-gastrulation, loss of Vangl2 increases the formation of large membrane protrusions but does not affect protrusion polarity (Fig. S7). Vangl2 membrane localization, and therefore cell-surface function, is regulated by Prickle1a and an unknown extracellular signal or signals (Dohn et al., 2013; Roszko et al., 2015). As the late gastrula undergoes Vangl2-dependent changes in cell behavior, fibrillar fibronectin increases (Latimer and Jessen, 2010). Therefore, we reasoned that fibronectin may control Vangl2 membrane accumulation. To determine the role of fibronectin in Vangl2 translocation to the cell surface, wild-type embryos were injected with fn1a/fn1b morpholinos and fixed for Vangl2 immunolabeling at two stages: early gastrulation (60% epiboly) and the end of gastrulation (tailbud). Vangl2 is primarily cytoplasmic during early gastrulation (Fig. S8) and translocates to the plasma membrane at mid-gastrulation stages (Roszko et al., 2015). Here, we used a polyclonal antibody that recognizes an epitope near the Vangl2 N-terminus (Fig. S2). Vangl2 expression at 60% epiboly is similar between morphant and control embryos (Fig. S8). However, by the tailbud stage, Vangl2 does not localize effectively to the plasma membranes of ectodermal (Fig. 6A,B) or mesodermal cells (Fig. 6C,D) in fn1a/1b morphants. Though the immunofluorescence images suggest that reduced cell-surface Vangl2 expression could be attributed to protein loss, western blot analyses indicate that morphant embryos have near-normal levels of Vangl2 protein (Fig. S9). Together, these data are evidence that fibronectin is necessary for Vangl2 translocation to the plasma membrane and suggest that the fn1a/1b morphant phenotype may partially be because of the loss of Vangl2 at the cell surface.
Fig. 6.
Fibronectin is required for cell-surface Vangl2 expression. (A,C) Zebrafish Vangl2 protein expression in wild type and fn1a/1b morpholino (MO)-injected tailbud-stage embryos without (left) and with (right) DAPI nuclear labeling. Immunofluorescence labeling of Vangl2 in the ectoderm (A) and mesoderm (C). (B,D) Representative plot profiles showing average fluorescence intensities across single ectodermal cells in wild type (n=30 cells, 3 embryos) and fn1a/1b morphant embryos (n=30 cells, 3 embryos) in ectoderm (B) and mesoderm (D). Scale bars: 5 µm.
Fibronectin rescues membrane-protrusive activity but not PCP in vangl2 mutant embryos
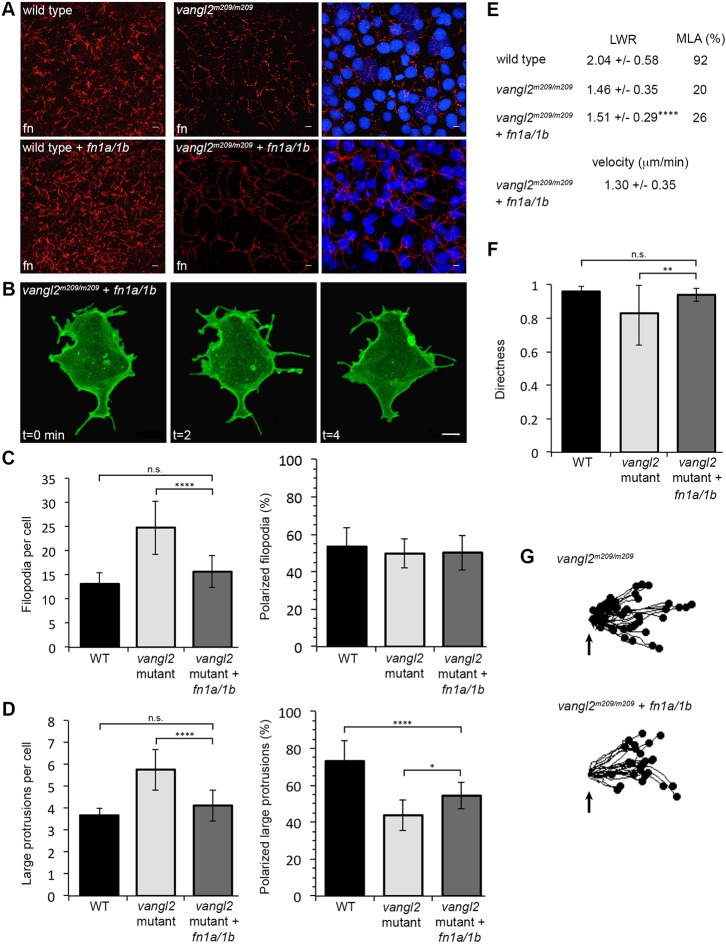
The ability of fibronectin to promote cell-surface Vangl2 expression indicates a complex relationship between ECM, PCP and the formation of polarized protrusions. This is supported by our in vitro data that implicates human VANGL2 in the regulation of integrin-mediated cell adhesion to multiple ECM proteins (Jessen and Jessen, 2017). Loss of Vangl2 decreases fibronectin protein and fibril assembly (Williams et al., 2012) and morpholino knockdown of fibronectin in vangl2 mutant embryos does not enhance the protrusion phenotypes (Fig. S10). Similarly, vangl2 mRNA overexpression in fn1a/1b morphant embryos does not enhance the individual protrusion phenotypes (Fig. S11). To further elucidate the relationship between fibronectin and Vangl2, we tested whether an increase in fibronectin suppresses the protrusion phenotypes in vangl2 mutants. We injected single-cell-stage embryos with an equal mixture of synthetic mRNA that encoded fn1a and fn1b. Both fn1a/1b mRNA-injected vangl2 mutant and wild-type embryos showed increased fibronectin, but vangl2 mutants, in particular, had increased fibrillogenesis (Fig. 7A). In addition to fn1a/1b mRNA, embryos were injected with memGFP at the eight-cell-stage to achieve mosaic labeling and cells were imaged using time-lapse microscopy. Ectodermal cells from fn1a/1b-injected vangl2 mutants have near wild-type numbers of filopodia and large membrane protrusions (Fig. 7B-D; 15.69 and 4.13, respectively). Despite the rescue of protrusion number, injected vangl2 mutant embryos retain the convergence and extension phenotype that is seen in control embryos (Fig. S12). Therefore, we further examined membrane protrusion polarity and the PCP of ectodermal cell bodies. Total filopodia polarity is unaffected in vangl2 mutant embryos, and remains unchanged in fn1a/1b-injected mutants (Fig. 7C). Defective polarization of large protrusions in vangl2 mutants was partially rescued by increasing fibronectin expression (Fig. S1; Fig. 7D; 54.37% polarized protrusions). By contrast, neither the elongation nor orientation of vangl2 mutant ectodermal cells was improved (Fig. 7E). Nonetheless, increasing fibronectin in vangl2 mutant embryos rescued the directed migration phenotype (Fig. 7F,G). These data confirm that PCP is required for convergence and extension movements and illustrate that ECM supports proper membrane-protrusive activity and directed cell migration. Fibronectin however, is not sufficient for establishment of PCP.
Fig. 7.
Fibronectin expression rescues the vangl2 membrane protrusion phenotype but not PCP. (A) Fibronectin expression in tailbud-stage wild type (WT) and vangl2m209/m209 mutant control embryos (top) or WT and mutant embryos injected with fn1a/1b synthetic mRNA (bottom). Nuclei labeled with DAPI (right). fn, fibronectin. (B) Representative ectodermal cell expressing memGFP over three time points from time-lapse data. (C,D) Quantitation of the average total number of protrusions and the total percentage of polarized protrusions in WT (n=12 cells, 8 embryos), vangl2m209/m209 mutant control embryos (n=10 cells, 7 embryos) and vangl2m209/m209 mutants injected with fn1a/1b mRNA (n=10 cells, 7 embryos). (E) PCP quantitation in the ectoderm. LWR and MLA values obtained from: vangl2m209/m209 PCP and migration velocity quantitation in the ectoderm. LWR and MLA values were obtained from: WT, n=50 cells, 13 embryos; vangl2m209/m209, n=10 cells, 8 embryos; fn1a/1b-injected vangl2m209/m209 mutants, n=50 cells, 8 embryos. (F) Directed migration values in WT (n=50 cells, 11 embryos), vangl2m209/m209 (n=51 cells, 13 embryos) and fn1a/1b-injected vangl2m209/m209 mutants (n=26 cells, 8 embryos). (G) Schematic of the migration paths of individual ectodermal cells. Origins (arrows) standardized for comparison. Dorsal is to the right. Data are mean±s.d. *P<0.05, **P<0.01, ****P<0.0001; P values are versus WT, except where indicated on the graphs; one-way ANOVA significance test followed by Tukey HSD post-hoc tests. n.s., not significant. Scale bars: 5 µm.
DISCUSSION
PCP proteins are linked to several morphogenetic processes, including the gastrulation movements of convergence and extension (Gray et al., 2011). At ∼80% epiboly lateral gastrula cells engage in directed migration behaviors, which continue through the tailbud stage (Sepich et al., 2005). As they migrate, round cells elongate and align mediolaterally (Roszko et al., 2015). This event is accompanied by a straightening of migration trajectories toward the dorsal axis, a process that fails to occur in vangl2 mutant embryos (Jessen et al., 2002). In the fly wing, core PCP proteins regulate the production and asymmetrical positioning of a single actin-rich trichome in each epithelial cell (Strutt and Strutt, 2007; Taylor et al., 1998). Thus regulation of actin rearrangement is thought to underlie the establishment of PCP in vertebrate tissues. However, it has been unclear whether PCP proteins regulate actin-rich membrane protrusions in migrating lateral gastrula cells. Here, we have systematically analyzed formation and polarization of protrusions in cells with disrupted Vangl2 function and compared these phenotypes with those obtained after manipulation of Glypican4/PCP signaling. We defined cellular domains of Vangl2 protein expression and report that Vangl2 enrichment at the cell surface requires fibronectin. We have established a role for the ECM during protrusion formation and polarization, and link fibronectin with Vangl2 function.
Regulation of membrane-protrusive activity by PCP proteins
During early zebrafish gastrulation, before the onset of PCP-dependent cell movements, dorsal prechordal plate progenitor cells use a combination of blebs and actin-containing filopodia and large lamellipodia-like protrusions (Diz-Muñoz et al., 2010). Here, large protrusions are associated with directed migration and blebs are associated with cell tumbling and meandering (Diz-Muñoz et al., 2016). Using time-lapse imaging and mosaic memGFP expression, we examined for the first time the dynamic membrane-protrusive activity that occurs as polarized lateral ectodermal cells undergo directed migration. Our analysis identified filopodia and large protrusions as the predominant protrusions produced by ectodermal cells. Blebs are less abundant, except in dividing cells, in which they form at cell poles to stabilize cell shape (Sedzinski et al., 2011).
Unlike glypican4 mutant ectodermal cells, vangl2 mutant cells have an increased number of filopodia and large protrusions. Glypican4 likely acts as a Wnt co-receptor to activate downstream proteins and, subsequently, actin regulators (Roszko et al., 2009). By contrast, Vangl2 interacts with Prickle1a to potentially contain or limit Glypican4/PCP signaling within specific cellular domains. Although the vangl2 and glypican4 mutant convergence and extension phenotypes are similar, our data assert that these proteins have distinct effects on the ECM. In glypican4 mutant embryos, fibrillar fibronectin is enhanced because of increased cell-surface cadherin expression and cell adhesion (Dohn et al., 2013). Conversely, vangl2 mutant and prickle1a morphant embryos exhibit decreased fibronectin expression and assembly, possibly because of increased matrix metalloproteinase activity and/or decreased cadherin expression (Dohn et al., 2013; Williams et al., 2012). We propose that fibronectin is necessary for proper formation and polarization of membrane protrusions (see further discussion below). Consequently, although it is increased in vangl2 mutant embryos, protrusion number is not higher in glypican4 mutants, as the fibronectin ECM remains intact. The ability of fibronectin to function as a suppressor of excess protrusion formation is supported by previous work in frog (Davidson et al., 2006).
Notably, vangl2- and glypican4-deficient ectodermal cells have excess non-polarized large protrusions, suggesting a relationship between protrusion polarity and the establishment of PCP. Previous work reports that vangl2 mutant mesodermal cell MLA is slightly biased along the anterior/posterior axis (Roszko et al., 2015). Although rose diagrams suggest a slight anterior/posterior bias in the polarization of large protrusions from vangl2 and glypican4 mutant ectodermal cells, in general, protrusions were detected around the entire cell circumference. These data support the notion that loss of protrusion polarity may represent an expansion of membrane domains that are capable of forming protrusions. A bias in wild-type filopodia polarity was not detected, indicating that these thin protrusions may play a sensory role. After surveying the extracellular environment for specific signals, we hypothesize that filopodia promote the formation of polarized large lamellipodia-like protrusions (Faix and Rottner, 2006). When wild-type lateral gastrula cells migrate dorsally, they generate more cell-cell contacts as they align and pack. One assumption is that mediolaterally oriented protrusions aid cell packing and thus directed movement. As a result, individual cell behaviors would affect morphogenesis at the tissue level (Davidson et al., 2010). In addition, manipulation of Vangl2 function interferes with ectodermal cell directed movement. Despite having a protrusion polarity phenotype similar to that found in vangl2 mutant embryos, glypican4 mutant cells migrate along relatively straight trajectories, indicating that polarized-protrusive activity may not be sufficient for directed migration. Unlike vangl2 mutant embryos however, glypican4 mutants have normal numbers of membrane protrusions, and both increased cadherin-mediated cell adhesion and increased fibronectin fibrillogenesis (Dohn et al., 2013). We hypothesize that these features contribute to more efficient directed migration of ectodermal cells in glypican4 mutant embryos compared with vangl2 mutants. The mechanism behind Glypican4/PCP signaling and its effects on cell packing and migration trajectories need to be further explored, as directed mesodermal cell migration is also Vangl2 dependent (Jessen et al., 2002).
Vangl2 expression in migrating ectodermal cells
A feature of fly PCP is that certain core proteins are asymmetrically expressed in the wing epithelium (Strutt and Strutt, 2009). Fluorescent fusion proteins and cell transplantation methods have allowed the identification of an anterior/posterior axis of asymmetrical PCP protein expression in non-migratory polarized axial cells. Here, Vangl2 and Prickle are enriched at anterior membranes, whereas Dishevelled localizes posteriorly (Ciruna et al., 2006; Roszko et al., 2015; Yin et al., 2008). It is important to note that axial cells do not exhibit Vangl2 or Prickle asymmetry in gastrula-stage embryos (Roszko et al., 2015; Yin et al., 2008). Based on our examination of lateral ectodermal cells that are engaged in directed migration, GFP-VANGL2 does not localize asymmetrically along either the anterior/posterior or leading edge/trailing edge cell axes. These cells undergo rapid changes in morphology and cohesiveness as they migrate and, because Vangl2 expression is dynamic in migrating cells, an asymmetry may remain undetected. It is possible that subtle differences in Vangl2 protein stability exist at certain regions of the membrane (Chien et al., 2015). Our data do confirm that GFP-VANGL2 is enriched at the plasma membrane domains that are engaged in protrusive activity, compared with non-protrusive domains. Specifically, GFP-VANGL2 expression exhibits a transient increase within newly developed large protrusions before protrusion elongation or remodeling. These data are reminiscent of zebrafish hindbrain motor neurons, in which GFP-Vangl2 expression localizes to filopodia tips before protrusion retraction (Davey et al., 2016). Our study did not find a difference in GFP-VANGL2 expression between polarized and non-polarized protrusions. Wild-type ectodermal cells form protrusions around their circumference, but more polarized protrusions are formed along the path of migration. By contrast, vangl2 mutant cells have both an increased number of protrusions and an increased number of non-polarized protrusions. We hypothesize that, rather than acting as a general inhibitor of membrane protrusion formation, Vangl2 acts to destabilize, restrict or limit protrusive activity. Further work is needed to determine how Vangl2 expression and function are regulated at distinct membrane domains and how this translates into polarized cell behaviors. For example, Prickle1a may influence subtle differences in Vangl2 expression or stability at polarized versus non-polarized protrusions. Notably, vangl2 mRNA overexpression does not inhibit protrusion formation, which suggests that a balanced level of core PCP protein expression is necessary for proper protrusive activity and directed migration. Perhaps Vangl2-dependent communication of signals within and between cells regulates localization of other PCP proteins to membrane protrusions (Strutt and Strutt, 2009).
At early gastrulation, Vangl2 protein localizes within presumptive cytoplasmic vesicles but enriches at the plasma membrane by mid-gastrulation. Vangl2 cell-surface expression at mid-gastrulation precedes the Vangl2-dependent onset of PCP and protrusion polarization. Our previous work demonstrates Prickle1a can control Vangl2 expression (Dohn et al., 2013), suggesting that prickle1a morphant phenotypes may be partially because of disrupted Vangl2 function. Interestingly, disruption of other polarity proteins (Glypican4, Frizzled7, Dishevelled, and Scribble1) or tissue patterning regulators does not significantly affect cell-surface Vangl2 expression (Roszko et al., 2015). Because of a temporal correlation between the requirement for Vangl2 and fibronectin fibrillogenesis at late gastrulation (Latimer and Jessen, 2010; Sepich et al., 2000), we hypothesized that the ECM influences plasma membrane levels of Vangl2. We report that loss of fibronectin inhibits translocation to the membrane, but whether fibronectin impacts Vangl2 expression directly or indirectly is unclear. Human VANGL2 interacts with integrin αv and promotes cell adhesion to ECM substrates, including fibronectin (Jessen and Jessen, 2017). We hypothesize that integrin-fibronectin interactions may affect Vangl2 protein stability and function at the plasma membrane. Other signaling pathways or post-translational modifications may also contribute to Vangl2 intracellular trafficking and expression (Gao et al., 2011; Merte et al., 2009).
Interaction of fibronectin and Vangl2 during protrusion formation and polarization
Fibronectin has long been recognized for its roles in vertebrate gastrulation. Among these, fibronectin affects cell polarity and protrusive activity, as well as providing a substrate or tracks for migration (Darribère and Schwarzbauer, 2000; Davidson et al., 2006; Winklbauer and Keller, 1996). However, the requirement for fibronectin during zebrafish gastrulation and its relationship with PCP protein function are still emerging. Lateral ectodermal cells interact with a network of fibronectin and laminin, which forms a boundary at the ectoderm/mesoderm interface (Latimer and Jessen, 2010). Laminin mutant embryos do not have obvious gastrulation cell movement phenotypes (Parsons et al., 2002). Assembly of fibronectin fibrils relies on actin mechanotension, which is generated by integrin-mediated cell-matrix interactions and cadherin-mediated adhesion (Schwarzbauer and DeSimone, 2011). Here, we show that fn1a/1b morphant embryos have defects in PCP and membrane protrusion number and polarity. Although the protrusion phenotypes are similar to those of vangl2 mutant embryos, loss of fibronectin has a lesser effect on MLA and directed cell migration. We hypothesize that the ECM is not essential for all cell behaviors underlying dorsal convergence and, further, reduced fibronectin in vangl2 mutant gastrulae is not sufficient to explain the severity of the vangl2 phenotype. Supporting this notion, neither zygotic fn1a/natter mutant embryos (Trinh and Stainier, 2004) nor fn1a/1b morphants (Latimer and Jessen, 2010) exhibit strong convergence and extension defects. Moreover, the vangl2 cell elongation, MLA and gastrulation phenotypes cannot be rescued by increasing fibronectin. Loss of Vangl2 increases matrix metalloproteinase activity and decreases cell-surface cadherin expression (Cantrell and Jessen, 2010; Dohn et al., 2013; Jessen and Jessen, 2017; Williams et al., 2012), either of which compromises the ability to assemble a normal ECM. Although fibronectin expression cannot be restored to wild-type levels in vangl2 mutant embryos, fn1a/fn1b mRNA rescues protrusion number, directed migration and, to a lesser extent, protrusion polarity. It is unclear how a uniform fibronectin ECM promotes polarized protrusive activity, given that GFP-VANGL2 localizes to both polarized and non-polarized protrusions. It will be important to understand in more detail the dynamics of Vangl2 expression in polarized gastrula cells and to determine the localization patterns of other core PCP proteins. Our results indicate that fibronectin is required to suppress excess protrusion formation. Moreover, we argue that fibronectin regulates protrusion number and polarity directly, but by inhibiting cell-surface Vangl2 expression it influences PCP indirectly. The ability of Vangl2 to affect fibronectin proteolysis and cadherin expression (Dohn et al., 2013; Williams et al., 2012) provides possible mechanisms to regulate ECM assembly and, subsequently, membrane-protrusive activity. Future studies will further address the complex relationships between Vangl2 function, Glypican4/PCP signaling and the fibronectin ECM.
MATERIALS AND METHODS
Zebrafish lines and husbandry
Adult zebrafish (Danio rerio) 1-2 years of age were maintained following standard procedures (Solnica-Krezel et al., 1994). Embryos were collected after natural spawning, grown at 28.5°C in egg water (purified water with 60 mg/l Instant Ocean) and staged according to morphology (Kimmel et al., 1995). Embryo ages were between 6 and 10.5 h postfertilization. Strains used in this study included: wild type (AB*, TL and WIK), vangl2/trilobitem209 (Jessen et al., 2002; Solnica-Krezel et al., 1996), vangl2/trilobitevu7 (Jessen et al., 2002) and glypican4/knypekm119 (Solnica-Krezel et al., 1996; Topczewski et al., 2001). The vangl2m209 and glypican4m119 alleles behave like null mutations, whereas the vangl2vu7 allele is a chromosomal deletion lacking the vangl2 gene.
The Middle Tennessee State University Institutional Animal Care and Use Committee approved the animal research described in this paper. All procedures were conducted following approved guidelines. The Office of Laboratory Animal Welfare assurance number is A4701-01.
Morpholinos, synthetic mRNA and embryo microinjection
Antisense morpholino oligonucleotides were obtained from Gene Tools. The following morpholinos, which have been previously published, were used: fn1a (5′-TTTTTTCACAGGTGCGATTGAACAC-3′), fn1b (5′-TACTGACTCACGGGTCATTTTCACC-3′ and 5′-GCTTCTGGCTTTGACTGTATTTCGG-3′) and vangl2 (5′-AGTTCCACCTTACTCCTGAGAGAAT-3′) (Dohn et al., 2013; Jülich et al., 2005; Latimer and Jessen, 2010; Trinh and Stainier, 2004; Veeman et al., 2003; Williams et al., 2012). Each morpholino was injected at a dose of 3-10 ng/embryo. The fn1a/1b morpholino combination reduces fibronectin protein expression (Fig. S3) and produces the reported anterior somite defect (Jülich et al., 2005; Koshida et al., 2005; Latimer and Jessen, 2010). The vangl2 morpholino produces convergence and extension phenotypes (Dohn et al., 2013; Williams et al., 2012) and late gastrulation ectodermal cell protrusion phenotypes similar to the vangl2m209/m209 mutant (24.60 filopodia and 5.77 large protrusions on average per cell). None of the morpholinos used in this study stimulates obvious p53-dependent cell death.
DNA constructs encoding either Vangl2, the F-actin marker Lifeact-GFP (Riedl et al., 2008), memGFP, memRFP, GFP-VANGL2, Fibronectin1a or Fibronectin1b were cloned in the pCS2 vector and linearized by NotI restriction endonuclease. The fibronectin clones are not targeted by the fn1a/1b morpholinos. Synthetic mRNA was made using the mMESSAGE mMACHINE™ SP6 Transcription Kit as directed by the manufacturer (Ambion) and purified by Sephadex G-50 columns (Millipore-Sigma). Microinjection into single-cell-stage or eight-cell-stage embryos was performed following standard methods (Gilmour et al., 2002). Synthetic mRNA was injected at the following doses: vangl2 (100-300 pg); Lifeact-GFP (100 pg), memGFP and memRFP (100 pg); GFP-VANGL2 (50 pg); fn1a and fn1b (200 pg).
Whole-mount in situ hybridization, immunofluorescence and antibodies
Staged embryos were fixed overnight in 4% paraformaldehyde in PBS and washed with PBS that contained 0.1% Tween-20 before high resolution in situ hybridizations were performed as described (Thisse and Thisse, 2008). Antisense RNA probes were generated by in vitro transcription and purified following standard methods (Westerfield, 2000). For immunofluorescence, embryos were fixed in either 4% paraformaldehyde/PBS/sucrose fix buffer [1:1 mixture of 8% paraformaldehyde/PBS and 2× sucrose buffer; 8.0 g sucrose, 0.15 ml 0.2 M CaCl2, 90 ml 0.2 M Na2HPO4 buffer (pH 7.3)] overnight or in Prefer Fixative (Anatech) for 2 h, and then manually dechorionated and permeabilized for 30 min at room temperature (5% normal donkey serum, 0.5% Triton X-100, PBS). Embryos were blocked for 2 h at room temperature [5% bovine serum albumin, 1% dimethyl sulfoxide (DMSO), 0.1% Triton X-100, PBS] with gentle rocking. Vangl2 or fibronectin primary antibodies (see below) were diluted in block solution and applied before incubating embryos overnight at 4°C with gentle rocking. Embryos were washed for 6×15 min in PBST with DMSO (0.1% Triton X-100, 1% DMSO, PBS). Embryos were treated with secondary antibodies (711-545-152 and 711-585-152, Jackson ImmunoResearch) diluted 1:500 in 50% block solution and 50% PBST (0.1% Triton X-100, PBS), and incubated for 2 h at room temperature under UV protection with gentle rocking. For nuclei labeling, embryos were washed with PBS before 30 min incubation in 2-(4-amidinophenyl)-1H-indole-6-carboxamidine (DAPI). Embryos were washed 3× with PBS and stored at 4°C. Sections were obtained by embedding fibronectin immunolabeled/DAPI stained embryos in 1.2% agarose in a 5% sucrose solution followed by cryosectioning at a thickness of 10 µm. Fibronectin was detected using a rabbit polyclonal antibody generated against an epitope within the human protein (1:100, F3648, Millipore-Sigma). An affinity purified rabbit polyclonal antibody capable of recognizing an epitope near the N-terminus (NH2-RSKSRDSSSRGDKSC-COOH) of zebrafish Vangl2 was generated (1:100, ProSci). This antibody will not bind Vangl1 because of lack of epitope sequence homology, and Vangl1 is not expressed during gastrulation (Jessen and Solnica-Krezel, 2004). We validated the antibody by immunolabeling embryos overexpressing vangl2 mRNA and embryos with a vangl2 chromosomal deletion (Fig. S2).
Embryo protein extraction, western blot and antibodies
For zebrafish embryo whole-cell lysates, pools of ∼20-50 embryos were manually dechorionated, deyolked, lysed in RIPA [50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, mammalian protease inhibitor cocktail] and clarified by centrifugation. Whole-embryo lysates were boiled for 10 min following addition of Laemmli sample buffer (1× final concentration). Protein extracts were separated by 10% SDS-PAGE and transferred to a PVDF membrane using a Trans-Blot Turbo Transfer System following the manufacturer's instructions (Bio-Rad). Non-specific binding to membranes was blocked with 5% non-fat milk in TBS-Tween [50 mM Tris (pH 7.4), 150 mM NaCl, 0.1% Tween-20] and membranes were incubated overnight with rabbit polyclonal Vangl2 antibody (1:1000) in block solution at 4°C with gentle rocking. Membranes were incubated with a peroxidase-conjugated secondary antibody (1:5000, 711-035-152, Jackson ImmunoResearch), developed using Clarity ECL substrate (Bio-Rad) and imaged using a GelDoc-It Imaging System (UVP). During each experiment, blots were stripped at room temperature for 15 min using 25 mM glycine and 1% SDS (pH 2.0) and re-probed in a 1:1000 dilution of rabbit β-actin antibody (#4967, Cell Signaling Technology) or a 1:1000 dilution of mouse GAPDH antibody (AM4300, ThermoFisher). GAPDH was also detected using a peroxidase-conjugated secondary antibody (715-035-150, Jackson ImmunoResearch). Densitometry was performed on western blots with UVP Visionworks software.
Microscopic imaging
Staged and dechorionated live or fixed embryos were mounted as previously described (Roszko et al., 2015) and imaged with a Zeiss LSM700 confocal microscope with a 63× oil-immersion objective (N.A. 1.4). Time-lapse images were collected for 20 min at 1-2 min intervals with a 0.39 or 0.50 µm z-axis step size. Embryo orientation was documented for each time-lapse series by taking low-magnification (10×) reference images under transmitted light. Yolk-plug closure/tailbud stage live embryo images were obtained with an Olympus SZX16 stereomicroscope equipped with a Q-Color5 CCD camera. Sectioned embryo images were acquired using an Olympus IX83 inverted microscope equipped with a 20× objective and a Hamamatsu Flash 4.0 CMOS camera.
Quantitation of cell shape, alignment, velocity and directness
Cell LWRs and MLA analyses were performed using Fiji software (fiji.sc) (Schindelin et al., 2012) as described (Jessen et al., 2002). To obtain cell migration velocity and directionality data, we tracked cell movement during our 20 min time-lapse image series (10 frames) using Fiji's Manual Tracking tool. This tool outputs data points that are distributed as scaled latitude and longitude values, which were exported to Ibidi's Chemotaxis and Migration Tool standalone software (ibidi.com) to generate plot diagrams and directness and velocity statistics.
Analysis of membrane protrusions
We quantified and annotated membrane protrusions using Fiji software. Protrusion number and polarity were quantified as the number formed during a 20 min time interval (10 image frames). A spike-like structure distinguished actin-rich filopodia from other types of protrusions. Large actin-rich protrusions were subjectively classified by their irregular shapes and base widths >2.5 μm and included lamellipodia-like protrusions and filolamellipodia. Characterized by their spherical appearance, blebs were rare in lateral ectodermal cells. Productive protrusions were quantified as the number of large protrusions formed that were associated with cell body translocation during a 20 min time interval (10 image frames). Protrusions were classified as polarized if they had a base to tip orientation within ±45° of the path of migration. Rose diagrams were generated using the angles of individual ectodermal cell large protrusions.
GFP-VANGL2 and Vangl2 localization
Time-lapse confocal image series were collected at the tailbud-1-somite stage as described above. Before analysis, we subtracted the image background and created sum slice projections. For the GFP-VANGL2 localization studies, we used Fiji's Plot Profile tool to quantify fluorescence intensities along the length of a line and generate an output of distance in µm (x-value) and grey value (y-value). To test for asymmetrical membrane expression bias, we drew a line through each cell from either anterior to posterior or leading edge to trailing edge membranes before producing a plot profile. During our membrane domain fluorescence intensity analysis, we avoided membrane-protrusive domains. We selected peak values at multiple positions for each membrane domain to generate anterior/posterior and leading edge/trailing edge fluorescence intensity ratios for memRFP and GFP-VANGL2. To assess GFP-VANGL2 expression dynamics in large membrane protrusions, we used Fiji's Plot Profile tool to gather fluorescence intensity data. Drawing a line inward from the plasma membrane at three time points, the Plot Profile tool produced plot profile data similar to that of our Vangl2 asymmetry study. Because membrane protrusion cycles of gastrula cells are highly dynamic in space and time, we examined GFP-VANGL2 expression at subjective time points associated with membrane protrusion preparation, eruption and extension. We selected the eruption plot apex, and the fluorescence intensity values for the corresponding distance points (x-values) were collected for the preparation and extension phases. These data points produced eruption/preparation and extension/eruption fluorescence intensity ratios for both memRFP and GFP-VANGL2. We used the eruption phase of large protrusion formation for comparison of GFP-VANGL2 expression between protrusive and non-protrusive domains and between polarized and non-polarized protrusions. To analyze endogenous zebrafish Vangl2 expression, we collected confocal z-stack images of epiblast cells at 60% epiboly and ectodermal and mesodermal cells at tailbud stage. We drew a line across the center of each cell from anterior to posterior membranes, and using Fiji's Plot Profile tool we generated a grey value plot profile, representative of fluorescence intensities along the length of the drawn line. As we used Vangl2 antibody labeling and not mosaic expression, we could not discern adjacent cell membranes. For consistency, we used congruent techniques when performing plot profile analyses on control and experimental cells. The fluorescence intensities for each distance point along the drawn line were averaged to generate a mean grey value plot profile.
Statistics
Data were exported to Microsoft Excel for graphing and StatCrunch software used for statistical analysis. The statistical tests performed and significance values obtained are indicated in each figure legend. The data presented are normally distributed.
Supplementary Material
Acknowledgements
We thank Alba Diz-Muñoz and Martin Bergert for the Lifeact-GFP plasmid, Anna Parnell for technical assistance and Tammy Jessen for excellent zebrafish facility maintenance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.R.J.; Methodology: A.M.L., J.R.J.; Validation: A.M.L.; Formal analysis: A.M.L., J.R.J.; Investigation: A.M.L., D.J.P.; Resources: J.R.J.; Writing - original draft: A.M.L., J.R.J.; Writing - review & editing: A.M.L., D.J.P., J.R.J.; Visualization: A.M.L., J.R.J.; Supervision: J.R.J.; Project administration: J.R.J.; Funding acquisition: J.R.J.
Funding
This study was funded by a grant from the National Institutes of Health (GM102356 to J.R.J.). Certain equipment items used to conduct this research were provided by Middle Tennessee State University. J.R.J., A.M.L. and D.J.P. acknowledge support from the Molecular Biosciences Ph.D. Program. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.165472.supplemental
References
- Blaser H., Reichman-Fried M., Castanon I., Dumstrei K., Marlow F. L., Kawakami K., Solnica-Krezel L., Heisenberg C.-P. and Raz E. (2006). Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev. Cell 11, 613-627. 10.1016/j.devcel.2006.09.023 [DOI] [PubMed] [Google Scholar]
- Boucaut J.-C. and Darribere T. (1983). Fibronectin in early amphibian embryos. Migrating mesodermal cells contact fibronectin established prior to gastrulation. Cell Tissue Res. 234, 135-145. 10.1007/BF00217407 [DOI] [PubMed] [Google Scholar]
- Cantrell V. A. and Jessen J. R. (2010). The planar cell polarity protein Van Gogh-Like 2 regulates tumor cell migration and matrix metalloproteinase-dependent invasion. Cancer Lett. 287, 54-61. 10.1016/j.canlet.2009.05.041 [DOI] [PubMed] [Google Scholar]
- Carreira-Barbosa F., Concha M. L., Takeuchi M., Ueno N., Wilson S. W. and Tada M. (2003). Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development 130, 4037-4046. 10.1242/dev.00567 [DOI] [PubMed] [Google Scholar]
- Chien Y.-H., Keller R., Kintner C. and Shook D. R. (2015). Mechanical strain determines the axis of planar polarity in ciliated epithelia. Curr. Biol. 25, 2774-2784. 10.1016/j.cub.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B., Jenny A., Lee D., Mlodzik M. and Schier A. F. (2006). Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439, 220-224. 10.1038/nature04375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darribère T. and Schwarzbauer J. E. (2000). Fibronectin matrix composition and organization can regulate cell migration during amphibian development. Mech. Dev. 92, 239-250. 10.1016/S0925-4773(00)00245-8 [DOI] [PubMed] [Google Scholar]
- Davey C. F., Mathewson A. W. and Moens C. B. (2016). PCP signaling between migrating neurons and their planar-polarized neuroepithelial environment controls filopodial dynamics and directional migration. PLoS Genet. 12, e1005934 10.1371/journal.pgen.1005934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L. A., Marsden M., Keller R. and Desimone D. W. (2006). Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr. Biol. 16, 833-844. 10.1016/j.cub.2006.03.038 [DOI] [PubMed] [Google Scholar]
- Davidson L. A., Joshi S. D., Kim H. Y., von Dassow M., Zhang L. and Zhou J. (2010). Emergent morphogenesis: elastic mechanics of a self-deforming tissue. J. Biomech. 43, 63-70. 10.1016/j.jbiomech.2009.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A., Krieg M., Bergert M., Ibarlucea-Benitez I., Muller D. J., Paluch E. and Heisenberg C.-P. (2010). Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 8, e1000544 10.1371/journal.pbio.1000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A., Romanczuk P., Yu W., Bergert M., Ivanovitch K., Salbreux G., Heisenberg C.-P. and Paluch E. K. (2016). Steering cell migration by alternating blebs and actin-rich protrusions. BMC Biol. 14, 74 10.1186/s12915-016-0294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohn M. R., Mundell N. A., Sawyer L. M., Dunlap J. A. and Jessen J. R. (2013). Planar cell polarity proteins differentially regulate extracellular matrix organization and assembly during zebrafish gastrulation. Dev. Biol. 383, 39-51. 10.1016/j.ydbio.2013.08.027 [DOI] [PubMed] [Google Scholar]
- Faix J. and Rottner K. (2006). The making of filopodia. Curr. Opin. Cell Biol. 18, 18-25. 10.1016/j.ceb.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Friedl P. and Gilmour D. (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445-457. 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- Gao B., Song H., Bishop K., Elliot G., Garrett L., English M. A., Andre P., Robinson J., Sood R., Minami Y. et al. (2011). Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev. Cell 20, 163-176. 10.1016/j.devcel.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour D. T., Jessen J. R. and Lin S. (2002). Manipulating gene expression in the zebrafish. In Zebrafish, A Practical Approach, Vol. 261 (ed. Nusslein-Volhard C. and Dahm R.), pp. 121-143. New York: Oxford University Press. [Google Scholar]
- Goodrich L. V. and Strutt D. (2011). Principles of planar polarity in animal development. Development 138, 1877-1892. 10.1242/dev.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Davidson L., Asashima M. and Keller R. (2005). Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr. Biol. 15, 787-793. 10.1016/j.cub.2005.03.040 [DOI] [PubMed] [Google Scholar]
- Gray R. S., Roszko I. and Solnica-Krezel L. (2011). Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev. Cell 21, 120-133. 10.1016/j.devcel.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R., Kato Y. and He X. (2001). Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107, 843-854. 10.1016/S0092-8674(01)00614-6 [DOI] [PubMed] [Google Scholar]
- Habas R., Dawid I. B. and He X. (2003). Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17, 295-309. 10.1101/gad.1022203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg C.-P. and Nüsslein-Volhard C. (1997). The function of silberblick in the positioning of the eye anlage in the zebrafish embryo. Dev. Biol. 184, 85-94. 10.1006/dbio.1997.8511 [DOI] [PubMed] [Google Scholar]
- Heisenberg C.-P., Tada M., Rauch G.-J., Saúde L., Concha M. L., Geisler R., Stemple D. L., Smith J. C. and Wilson S. W. (2000). Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76-81. 10.1038/35011068 [DOI] [PubMed] [Google Scholar]
- Jessen T. N. and Jessen J. R. (2017). VANGL2 interacts with integrin alphav to regulate matrix metalloproteinase activity and cell adhesion to the extracellular matrix. Exp. Cell Res. 361, 265-276. 10.1016/j.yexcr.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen J. R. and Solnica-Krezel L. (2004). Identification and developmental expression pattern of van gogh-like 1, a second zebrafish strabismus homologue. Gene Expr. Patterns 4, 339-344. 10.1016/j.modgep.2003.09.012 [DOI] [PubMed] [Google Scholar]
- Jessen J. R. and Solnica-Krezel L. (2005). Morphogenetic cell movements shaping the zebrafish gastrula. In Planar Cell Polarization during Development, Vol. 14 (ed. Mlodzik M.), pp. 131-165. San Diego: Elsevier Press. [Google Scholar]
- Jessen J. R., Topczewski J., Bingham S., Sepich D. S., Marlow F., Chandrasekhar A. and Solnica-Krezel L. (2002). Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 4, 610-615. 10.1038/ncb828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jülich D., Geisler R. and Holley S. A. (2005). Integrinalpha5 and delta/notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev. Cell 8, 575-586. 10.1016/j.devcel.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Kilian B., Mansukoski H., Barbosa F. C., Ulrich F., Tada M. and Heisenberg C.-P. (2003). The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech. Dev. 120, 467-476. 10.1016/S0925-4773(03)00004-2 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. and Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Koshida S., Kishimoto Y., Ustumi H., Shimizu T., Furutani-Seiki M., Kondoh H. and Takada S. (2005). Integrinalpha5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev. Cell 8, 587-598. 10.1016/j.devcel.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Latimer A. and Jessen J. R. (2010). Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biol. 29, 89-96. 10.1016/j.matbio.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A. (1966). Development and determination of hairs and bristles in the milkweed bug, Oncopeltus fasciatus (Lygaeidae, Hemiptera). J. Cell Sci. 1, 475-498. [DOI] [PubMed] [Google Scholar]
- Marlow F., Zwartkruis F., Malicki J., Neuhauss S. C. F., Abbas L., Weaver M., Driever W. and Solnica-Krezel L. (1998). Functional interactions of genes mediating convergent extension, knypek and trilobite, during the partitioning of the eye primordium in zebrafish. Dev. Biol. 203, 382-399. 10.1006/dbio.1998.9032 [DOI] [PubMed] [Google Scholar]
- Marlow F., Topczewski J., Sepich D. and Solnica-Krezel L. (2002). Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr. Biol. 12, 876-884. 10.1016/S0960-9822(02)00864-3 [DOI] [PubMed] [Google Scholar]
- Merte J., Jensen D., Wright K., Sarsfield S., Wang Y., Schekman R. and Ginty D. D. (2009). Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat. Cell Biol. 12, 41-46; sup pp 1-8 10.1038/ncb2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nubler-Jung K., Bonitz R. and Sonnenschein M. (1987). Cell polarity during wound healing in an insect epidermis. Development 100, 163-170. [DOI] [PubMed] [Google Scholar]
- Ohkawara B., Yamamoto T. S., Tada M. and Ueno N. (2003). Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development 130, 2129-2138. 10.1242/dev.00435 [DOI] [PubMed] [Google Scholar]
- Parsons M. J., Pollard S. M., Saude L., Feldman B., Coutinho P., Hirst E. M. and Stemple D. L. (2002). Zebrafish mutants identify an essential role for laminins in notochord formation. Development 129, 3137-3146. [DOI] [PubMed] [Google Scholar]
- Riedl J., Crevenna A. H., Kessenbrock K., Yu J. H., Neukirchen D., Bista M., Bradke F., Jenne D., Holak T. A., Werb Z. et al. (2008). Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605-607. 10.1038/nmeth.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszko I., Sawada A. and Solnica-Krezel L. (2009). Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin. Cell Dev. Biol. 20, 986-997. 10.1016/j.semcdb.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszko I., Sepich D. S., Jessen J. R., Chandrasekhar A. and Solnica-Krezel L. (2015). A dynamic intracellular distribution of Vangl2 accompanies cell polarization during zebrafish gastrulation. Development 142, 2508-2520. 10.1242/dev.119032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E. and DeSimone D. W. (2011). Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 3, a005041 10.1101/cshperspect.a005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedzinski J., Biro M., Oswald A., Tinevez J.-Y., Salbreux G. and Paluch E. (2011). Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature 476, 462-466. 10.1038/nature10286 [DOI] [PubMed] [Google Scholar]
- Seo H.-S., Habas R., Chang C. and Wang J. (2017). Bimodal regulation of Dishevelled function by Vangl2 during morphogenesis. Hum. Mol. Genet. 26, 2053-2061. 10.1093/hmg/ddx095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepich D. S., Myers D. C., Short R., Topczewski J., Marlow F. and Solnica-Krezel L. (2000). Role of the zebrafish trilobite locus in gastrulation movements of convergence and extension. Genesis 27, 159-173. [DOI] [PubMed] [Google Scholar]
- Sepich D. S., Calmelet C., Kiskowski M. and Solnica-Krezel L. (2005). Initiation of convergence and extension movements of lateral mesoderm during zebrafish gastrulation. Dev. Dyn. 234, 279-292. 10.1002/dvdy.20507 [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L., Schier A. F. and Driever W. (1994). Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics 136, 1401-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnica-Krezel L., Stemple D. L., Mountcastle-Shah E., Rangini Z., Neuhauss S. C., Malicki J., Schier A. F., Stainier D. Y., Zwartkruis F., Abdelilah S. et al. (1996). Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development 123, 67-80. [DOI] [PubMed] [Google Scholar]
- Strutt D. I. (2002). The asymmetric subcellular localisation of components of the planar polarity pathway. Semin. Cell Dev. Biol. 13, 225-231. 10.1016/S1084-9521(02)00041-1 [DOI] [PubMed] [Google Scholar]
- Strutt D. and Strutt H. (2007). Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev. Biol. 302, 181-194. 10.1016/j.ydbio.2006.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H. and Strutt D. (2009). Asymmetric localisation of planar polarity proteins: mechanisms and consequences. Semin. Cell Dev. Biol. 20, 957-963. 10.1016/j.semcdb.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Sun L., Zou Z., Collodi P., Xu F., Xu X. and Zhao Q. (2005). Identification and characterization of a second fibronectin gene in zebrafish. Matrix Biol. 24, 69-77. 10.1016/j.matbio.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Taylor J., Abramova N., Charlton J. and Adler P. N. (1998). Van Gogh: a new Drosophila tissue polarity gene. Genetics 150, 199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C. and Thisse B. (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59-69. 10.1038/nprot.2007.514 [DOI] [PubMed] [Google Scholar]
- Thisse B., Pflumio S., Furthauer M., Loppin B. Heyer V., Degrave A., Woehl R. Lux A., Steffan T., Charbonnier X. Q. et al. (2001). Expression of the zebrafish genome during embryogenesis (NIH R01 RR15402). ZFIN direct data submission (zfin.org).
- Topczewski J., Sepich D. S., Myers D. C., Walker C., Amores A., Lele Z., Hammerschmidt M., Postlethwait J. and Solnica-Krezel L. (2001). The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev. Cell 1, 251-264. 10.1016/S1534-5807(01)00005-3 [DOI] [PubMed] [Google Scholar]
- Trinh L. A. and Stainier D. Y. R. (2004). Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev. Cell 6, 371-382. 10.1016/S1534-5807(04)00063-2 [DOI] [PubMed] [Google Scholar]
- Veeman M. T., Slusarski D. C., Kaykas A., Louie S. H. and Moon R. T. (2003). Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 13, 680-685. 10.1016/S0960-9822(03)00240-9 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. A Guide for the Laboratory use of Zebrafish (Danio rerio). Eugene: University of Oregon Press. [Google Scholar]
- Williams B. B., Cantrell V. A., Mundell N. A., Bennett A. C., Quick R. E. and Jessen J. R. (2012). VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration. J. Cell Sci. 125, 2141-2147. 10.1242/jcs.097964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbauer R. and Keller R. E. (1996). Fibronectin, mesoderm migration, and gastrulation in Xenopus. Dev. Biol. 177, 413-426. 10.1006/dbio.1996.0174 [DOI] [PubMed] [Google Scholar]
- Wong L. L. and Adler P. N. (1993). Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J. Cell Biol. 123, 209-221. 10.1083/jcb.123.1.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. and Mlodzik M. (2009). A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 19, 295-305. 10.1016/j.tcb.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. and Mlodzik M. (2015). Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt). Annu. Rev. Cell Dev. Biol. 31, 623-646. 10.1146/annurev-cellbio-100814-125315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C., Kiskowski M., Pouille P.-A., Farge E. and Solnica-Krezel L. (2008). Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J. Cell Biol. 180, 221-232. 10.1083/jcb.200704150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C., Ciruna B. and Solnica-Krezel L. (2009). Convergence and extension movements during vertebrate gastrulation. Curr. Top. Dev. Biol. 89, 163-192. 10.1016/S0070-2153(09)89007-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.