ABSTRACT
In our study, the effects of water stress on photosynthesis and photosynthetic electron transport chain (PETC) were studied in several ways, including monitoring the change of gas exchange parameters, modulated chlorophyll fluorescence, rapid fluorescence induction kinetics, reactive oxygen species (ROS), antioxidant enzyme activities and D1 protein levels in apple leaves. Our results show that when leaf water potential (ψw) is above –1.5 MPa, the stomatal limitation should be the main reason for a drop of photosynthesis. In this period, photosynthetic rate (PN), stomatal conductance (Gs), transpiration rate (E) and intercellular CO2 concentration (Ci) all showed a strong positive correlation with ψw. Modulated chlorophyll fluorescence parameters related to photosynthetic biochemistry activity including maximum photochemical efficiency (Fv/Fm), actual photochemical efficiency of PSII (ΦPSII), photochemical quenching coefficient (qP) and coefficient of photochemical fluorescence quenching assuming interconnected PSII antennae (qL) also showed a strong positive correlation as ψw gradually decreased. On the other hand, in this period, Stern-Volmer type non-photochemical quenching coefficient (NPQ) and quantum yield of light-induced non-photochemical fluorescence quenching [Y(NPQ)] kept going up, which shows an attempt to dissipate excess energy to avoid damage to plants. When ψw was below –1.5 MPa, PN continued to decrease linearly, while Ci increased and a ‘V’ model presents the correlation between Ci and ψw by polynomial regression. This implies that, in this period, the drop in photosynthesis activity might be caused by non-stomatal limitation. Fv/Fm, ΦPSII, qP and qL in apple leaves treated with water stress were much lower than in control, while NPQ and Y(NPQ) started to go down. This demonstrates that excess energy might exceed the tolerance ability of apple leaves. Consistent with changes of these parameters, excess energy led to an increase in the production of ROS including H2O2 and O2•−. Although the activities of antioxidant enzymes like catalase (CAT), superoxide dismutase (SOD) and peroxidase (POD) increased dramatically and ascorbate peroxidase (APX) decreased in apple leaves with drought stress, it was still not sufficient to scavenge ROS. Consequently, the accumulation of ROS triggered a reduction of net D1 protein content, a core protein in the PSII reaction center. As D1 is responsible for the photosynthetic electron transport from plastoquinone A (QA) to plastoquinone B (QB), the capacity of PETC between QA and QB was considerably downregulated. The decline of photosynthesis and activity of PETC may result in the shortage of adenosine triphosphate (ATP) and limitation the regeneration of RuBP (Jmax), a key enzyme in CO2 assimilation. These are all non-stomatal factors and together contributed to decreased CO2 assimilation under severe water stress.
KEY WORDS: Photosynthetic electron transport chain, Antioxidant enzymes, D1 protein, Reactive oxygen species, Water stress
Summary: This research reports that drought-induced photosynthesis declines due to stomatal and non-stomatal limitation, which was PSII photoinhibition-dependent on D1 protein and over-reduces the electron transport chain.
INTRODUCTION
Water availability is an important factor affecting plant growth and yield in arid and semi-arid regions, where plants are often subjected to periods of drought (Chaves et al., 2003). Under drought stress conditions, many metabolic processes, including photosynthesis, are negatively affected. For instance, water deficiency damages basic organization structure, which inhibits carbon assimilation and damages photosynthetic apparatus (Ali and Ashraf, 2011; Golldack et al., 2011). Previous studies have illustrated the decrease in photosynthesis of leaves is usually caused by stomatal limitation under mild to moderate drought conditions and non-stomatal limitation under severe drought conditions (Degl'Innocenti et al., 2009; Misson et al., 2010).
Such a decrease in photosynthesis leads to plants absorbing more light energy than can be consumed by photosynthetic carbon fixation. This excess energy has the potential to trigger an increase in the production of reactive oxygen species (ROS) including O2•− and H2O2, which has been proven to hinder the synthesis of PSII core D1 (Murata and Takahashi, 2008). Consistent with the inhibition of D1 synthesis, the activity of photosynthetic electron transport chain (PETC) also downregulates.
Furthermore, some previous studies indicated the fixation of CO2 in the Calvin cycle is sensitive to environmental stresses including high-temperature stress, low-temperature stress (Greer et al., 1986) and salt stress (Altaweel et al., 2007). Under these environmental stresses, the inhibition of the synthesis of D1 protein due to interruption of the fixation of CO2 might be expected to accelerate the decrease in photosynthesis. Nevertheless, it remains unclear (1) how the drought stress impacts the turnover of D1 protein and activity of PETC and (2) how the photosynthesis and PETC interact especially in the non-stomatal limiting phase under drought stress conditions.
In the present study, leaf water potential (ψw) and gas exchange parameters including net photosynthetic rate (PN), intercellular CO2 concentration (Ci), transpiration rate (E) and stomatal conductance (Gs) are utilized to explore the main reason for the decrease in photosynthesis of apple leaves under different drought stress levels. Through analysis of chlorophyll a fluorescence and the determination of D1 protein content, we can assess the activity of photosynthetic apparatus, including PETC. The content of O2•− and H2O2 and antioxidant enzymes activities were also used for probing the damage level to photosynthesis of apple leaves brought from water deficiency. All of the above techniques were applied to this study in order to investigate how water stress impacts the turnover of D1 protein, activity of PETC and the relationship between photosynthesis and PETC, especially in the non-stomatal limiting phase under water stress conditions.
RESULTS
ψw and gas exchange
ψw was sensitive to drought conditions and affected by different intensities of drought stress and subsequent rehydration. The ψw of control plants was higher than those of plants subjected to slight stress (LS), moderate stress (MS) or severe stress (SS). On day 5 and 10, the ψw of LS plants was approximately equal to control and decreased significantly after 16 days. The ψw decreased significantly in MS and SS plants throughout the stress period. The ψw of SS plants dropped to −3.19 MPa on day 33 (Fig. 1), in which leaves wilted seriously and some leaf margin dried up. After rehydration, water status of all stressed plants recovered to control level and plants with different stress treatments showed different recovery rates; specifically, the ψw of LS plants recovered within 1 day while MS and SS plants took over 5 days.
Fig. 1.
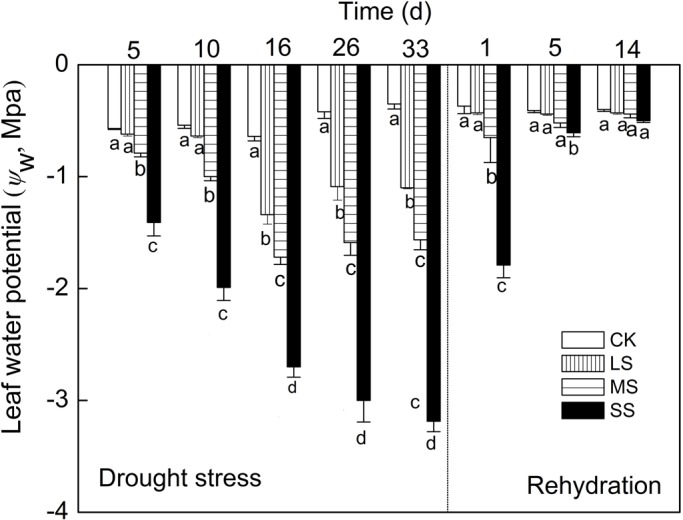
Responses of ψw to water stress and subsequent rehydration in apple leaves. Different letters indicate significant difference by Tukey tests at P<0.05. CK, control group. Values are means±s.e. (n=6).
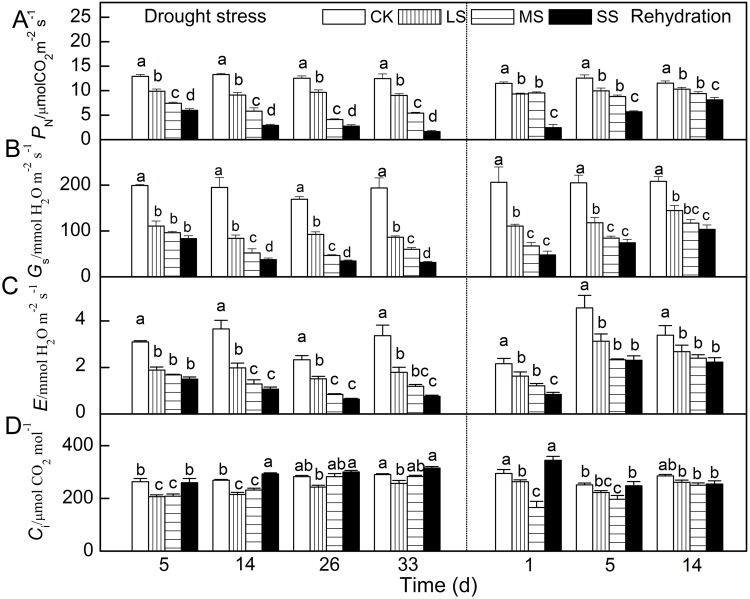
PN, Gs, E and Ci were also influenced differently by imposed drought stress and subsequent rehydration. In comparison with control, PN, Gs and E decreased gradually as stress proceeded (Fig. 2). After 33 days of drought stress treatments, when compared with control, PN, Gs and E of LS, MS and SS plants decreased 28%, 57% and 87% (LS); 56%, 69% and 84% (MS) and 47%, 65% and 78% (SS), respectively.
Fig. 2.
Responses of gas exchange parameters to water stress and subsequent rehydration in apple leaves. (A) PN. (B) Gs. (C) E. (D) Ci. Different letters indicate significant difference by Tukey tests at P<0.05. Values are means±s.e. (n=6).
Unlike the three parameters above, Ci of LS and MS plants went down after 5 days of treatment and showed a trend of increasing over time. Meanwhile, Ci of SS plants remained at a high level and steady state after 14 days of treatment. After rehydration, PN, Gs and E of all stressed plants gradually increased and recovered to levels of control to different extents. Specifically, PN, Gs and E of LS, MS and SS plants recovered to 90%, 82% and 71% (LS); 69%, 56% and 50% (MS) and 79%, 71% and 66% (SS) of control group after 14 days, respectively. However, after rehydration, Ci of LS, MS and SS plants all fell first and grew later, unlike PN, Gs and E.
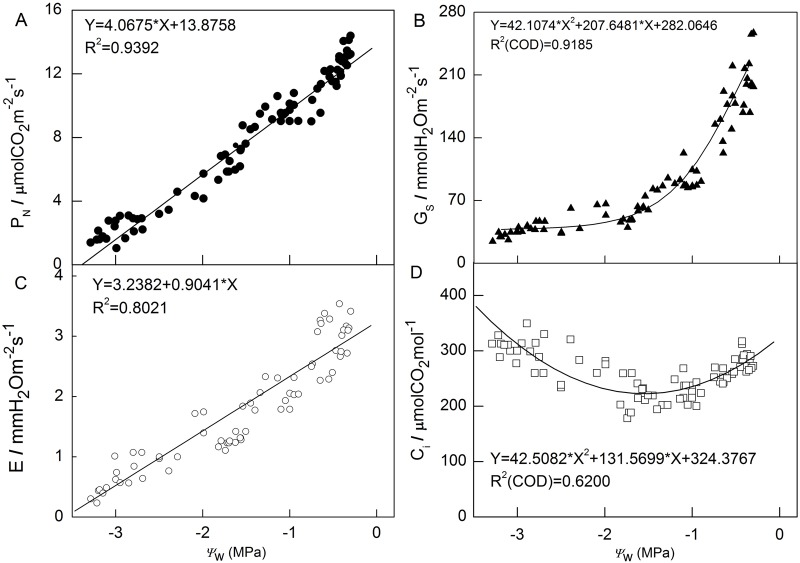
Furthermore, in order to analyze the relationship between drought stress and gas exchange parameters, we calculated correlation coefficients the between ψw and PN, Gs, E and Ci (Fig. 3). A positive linear regression correlation between PN and ψw was seen, with the coefficient reaching 0.9392. Similar correlation existed between E and ψw with a smaller coefficient 0.8021. The correlation coefficient of Gs between ψw was 0.9185 and that of Ci between ψw was 0.6200; their polynomial regression has the same turning point at approximately −1.40 MPa. In general, PN, Gs and E had a positive correlation with ψw, while Ci had a ‘V’ model correlation.
Fig. 3.
Correlation analysis between gas exchange parameters and ψw of apple tree leaves under water stress. (A) PN. (B) Gs. (C) E. (D) Ci. The coefficients of determination (R2) were calculated using the data from all treatments.
Because of this, we investigated whether Rubisco carboxylation and RuBP regeneration might be limiting during drought stress by measuring the PN/Ci response, and calculated the value of both the maximum velocity of Rubisco for carboxylation (Vcmax) and the maximum rate of electron transport (Jmax). On day 12, for control group, LS, MS and SS the Vcmax values were 76.55, 74.82, 51.96 and 23.24 µmol m−2 s−1, respectively; the Jmax values were 80.71, 70.97, 58.34 and 41.24 µmol m−2 s−1, respectively. In MS and SS, drought stress reduced Vcmax and Jmax significantly; these results suggest that MS and SS have a major impact on RuBP regeneration capacity and RuBP carboxylase activity, but LS has a lesser effect on RuBP carboxylase activity.
Modulated chlorophyll fluorescence
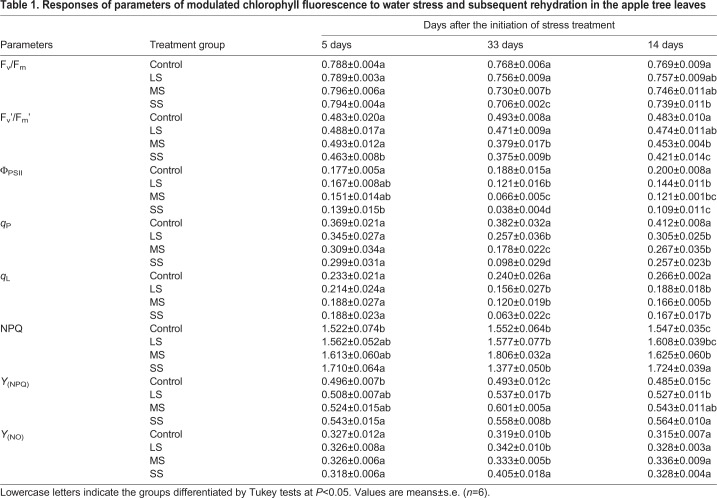
During drought stress conditions, maximum photochemical efficiency (Fv/Fm) and Fv′/Fm′ both decreased after 33 days of treatment (Table 1). After 5 days of water deficit, Fv′/Fm′ of SS plants was lower than others. Actual photochemical efficiency of PSII (ΦPSII) had similar trends throughout the experiments; it decreased significantly with increased intensity of water stress on day 33. ΦPSII of SS plants decreased to 24% of control group on day 33. Interestingly, similar trends existed in qP and coefficient of photochemical fluorescence quenching assuming interconnected PSII antennae (qL). In addition, Stern-Volmer type non-photochemical quenching coefficient (NPQ) and Y(NPQ) with drought treatments all increased on day day 5. But on day 33, Y(NPQ) of SS plants decreased compared with MS plants, and at the same time, NPQ of SS plants dropped to minimum in all plants.
Table 1.
Responses of parameters of modulated chlorophyll fluorescence to water stress and subsequent rehydration in the apple tree leaves
It is noteworthy that, although 14 days of rehydration made ψw recover to pre-drought stress levels, it was not sufficient for total recovery in parameters of chlorophyll fluorescence, especially in MS and SS plants. After 14 days of rehydration, compared with control group, chlorophyll fluorescence parameters in stressed plants recovered in different degrees.
Rapid fluorescence induction kinetics
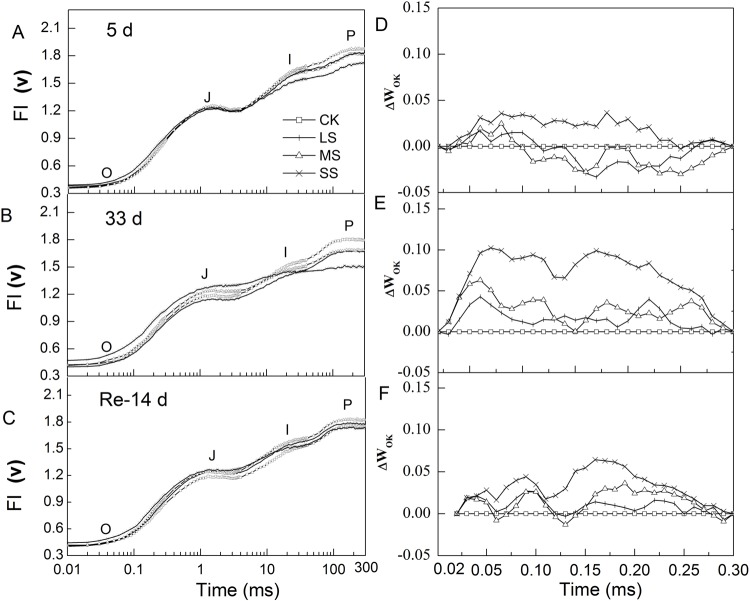
All rapid fluorescence induction kinetics exhibited a typical polyphasic OJIP curve, where O was original fluorescence (initial fluorescence, F0), J and I sites were intermediate transients, and P was the peak (maximal fluorescence, Fm) (Fig. 4). On the left column in Fig. 4, all transients had similar trends in Fig. 4A,C, while there were significant differences in Fig. 4B. F0 of SS plants significantly increased on day 33, while there was little difference between day 5 and after 14 days of rehydration. The fluorescence intensity (FI) of the J site in SS plants was significantly higher than those in control group and LS plants, while it was lower on the I site compared with control group and LS. On the right column in Fig. 4, OJIP curves with different treatments were normalized (L-band) between O and K (300 μs) sites. The value at about 150 μs in the L-band is an indicator of the energetic connectivity among PSII units and the high value means low connectivity. As shown in Fig. 4D, the L-band of SS plants had a positive value on day 5, while negative values were present in MS and LS plants. On day 33, all L-bands in the three stress treatments had positive values and the value was higher with increasing intensity of stress. After 14 days of rehydration, the L-bands of LS and MS plants recovered close to control group level, but that of SS plants was still remarkably higher than control group (Fig. 4F).
Fig. 4.
Responses of chlorophyll a fluorescence transient (OJIP) and L-Band to different water stress treatments. (A-F) Reactions for 5 days (A,D), 33 days (B,E) and then rehydration treatment for 14 days (C,F) in apple leaves. VOK=(Ft−FO)/(F300µs−FO), ΔVOK=VOK(treatment)-VOK(control). Values are means±s.e. (n=6).
Western blot analysis of D1 protein
To prove that the drought stress damaged key site of photosynthetic apparatus may be on the photosynthetic electron transport from QA to QB, western blot analysis with an antibody against the D1 protein was conducted (Fig. 5). A significant reduction was observed, and with the increase of the stress intensity and extension of the treatment time, the difference in D1 contents between drought treated plants and control group went up. After 14 days of rehydration the photosynthetic operation was improved due to elevated D1 synthesis, but not enough to recover to control group level.
Fig. 5.
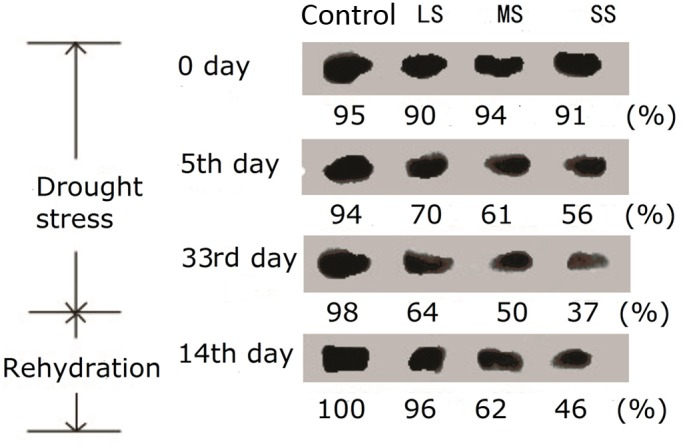
D1 protein contents with different water stress treatments for 0 day, 5 days, 33 days and rehydration treatment for 14 days. Quantitative analysis for the content of D1 protein is completed using gray analysis by Quantity One (Bio-Rad). And the content of D1 protein in the control with rehydration treatment for 14 days is chosen as the reference (100%).
Accumulation of ROS and change of antioxidant enzyme activities
Our results showed clearly that 33 days of drought stress induced a higher generation rate of O2•− and greater H2O2 contents (Table 2). With the enhancement of drought stress intensity, the contents of O2•− and H2O2 were significantly higher than in control group.
Table 2.
Contents of O2•− and H2O2 in the apple tree leaves after 33 days' drought stress
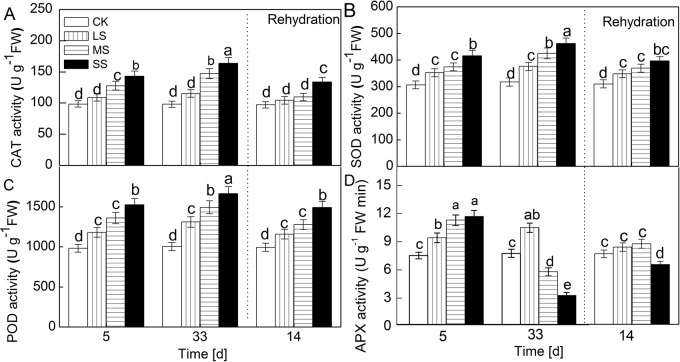
Significant increases were illustrated in the activities of antioxidant enzymes in drought-stressed plants (Fig. 6). Catalase (CAT) activity in the leaves put through drought stress treatments was much higher than that in control group. A similar response to drought was seen in the activities of superoxide dismutase (SOD) and peroxidase (POD). On the other hand, the change in ascorbate peroxidase (APX) activity was markedly different than those in antioxidant enzymes under MS and SS conditions; APX activity declined at day 33. After 14 days of rehydration, compared with control group, the activities of three antioxidant enzymes in stressed plants recovered by different degrees, but these parameters did not recover to control group level, especially in APX.
Fig. 6.
Change in the activity of CAT (A), SOD (B), POD (C) and APX (D) with different water stress treatments for 5 days, 33 days and rehydration treatment 14 days. Values are means±s.e. (n=6).
DISCUSSION
ψw can be regarded as an indicator to effectively assess water status of plants (Lima et al., 2002). In the present study, ψw decreased with the degree and duration of drought stress treatments (Fig. 1). Gas exchange, which was PN, Gs and E decreased significantly and they were closely related to the degree and duration of drought stress (Fig. 2). These parameters were all found to have a strong relationship with ψw (Fig. 3). Besides the linear correlation between PN and ψw (Fig. 3A; Šimpraga et al., 2011; Sun et al., 2013), a simple positive regression correlation was also found between Gs or E and ψw (Fig. 3B,C). At the earlier stage of drought stress, the plummet in Gs suggests that a reduction in stomatal conductance can have protective effects because it allows the plant to save water and to improve its efficient use (Chaves et al., 2009). As some studies indicated before, the decrease in photosynthesis is usually caused by stomatal limitation under mild to moderate drought condition when both Gs and Ci decline while non-stomatal limitation is the main reason for the decrease in photosynthesis when Ci increases and Gs reaches a minimum inflection point (Pérez-López et al., 2012; Zhou et al., 2013). In our study, when ψw was above −1.5 Mpa, accompanied with a decrease of Gs and E, the Ci also significantly decreased under moderate drought stress treatments for 5 days, demonstrating that stomatal limitation primarily led to decrease of PN in this period. As the degree of drought stress aggravated, when ψw was below −1.5, PN kept linearly decreasing while Ci increased and Gs remained stable at approximately 70 mmol H2O m−2 s−1. Ci even increased when PN continually went up linearly and a ‘V’ model is presented to describe the correlation between Ci and ψw (Fig. 3D). The changes of gas exchange parameters in this period implied the drop in photosynthesis activity might be caused by non-stomatal rather than stomatal limitation.
In order to further explore the relationship between drought stress and gas exchange parameters and photosynthetic activity, the rehydration treatment was conducted. The results showed that photosynthetic capacity impaired by drought stress can recover with different degrees after rehydration treatment. For instance, ψw and PN in slight and moderate treatments recovered almost to the control level, while Gs or E only had a slight increase and Ci decreased significantly after 1 day of rehydration. The reversibility was dependent on not only the duration time of rehydration but also the degree and duration time of drought (Gomes et al., 2012; Šircelj et al., 2007). After 1 day of rehydration, ψw and PN of apple leaves with severe stress treatment was still lower than that in slight and moderate treatments (Figs 2 and 3). However, the gap diminished significantly after 14 days' rehydration.
Drought stress significantly reduced CO2 assimilation rates at high Ci, while only with a certain degree of lowering PN rates at low Ci. According to the model of photosynthesis (Farquhar and Sharkey, 1982; Sharkey et al., 2007), these results suggest that drought stress had a major impact on Jmax, with less effect on Vcmax.
Fv/Fm, known as maximum quantum yield for primary photochemistry, could provide a simple and rapid way to evaluate when plants were exposed to stress environment (Henriques, 2009; Zai et al., 2012). Our study found Fv/Fm in all three treatments decreased significantly compared with control after 33 days' drought treatment (Table 1). After rehydration for 14 days, Fv/Fm of apple leaves under LS and MS stress can recover almost to control level while Fv/Fm under SS stress did not recover and was still significantly lower than in control (Table 1). In addition, ΦPSII decreased substantially, as well as qP and qL, showing the capability of photochemistry conversion and linear electron flux were both sensitive to the degree and duration time of drought stress. Beyond these parameters, the rise in NPQ and the decline in Fm suggested the increase in energy dissipation through the xanthophyll cycle, which is the protecting mechanism to maintain normal photosynthesis in plants (Demmig-Adams and Iii, 1996; Jahns and Holzwarth, 2012). Interestingly, although NPQ in LS and MS went up, NPQ in the severe drought stress dropped dramatically. As has been reported previously, the increase in Y(NPQ) expresses the attempt to dissipate excess energy while the increase in quantum yield of non-regulated heat dissipation and fluorescence emission [Y(NO)] signifies that excess energy fluxes are out of control and might produce photodamage to plants (Kramer et al., 2004). In our study, under drought conditions, the increase in both Y(NPQ) and Y(NO) compared with control also demonstrated the excess energy exceeded the regulatory ability of apple leaves and could not be effectively dissipated especially under SS. It might be a sign of irreversible cell dehydration and metabolism impairment (Kramer et al., 2004).
In the chlorophyll a fluorescence transient, the momentary maximum fluorescence intensity represents the subsequent kinetic bottlenecks of the electron transport chain (Strasser et al., 2010; Lazár et al., 2006). Schansker et al. (2005) reported that these limitations are the exchange of a reduced plastoquinone molecule with an oxidized one at the QB site (J-step) and the reoxidation of plastoquinol (PQH2, I-step). According to previous research on the OJIP-test, the change of chlorophyll fluorescence intensity in O-J, O-I, J-I phase can represent photosynthetic electron transport capacity between QA and QB, QA and photosystem I (PSI) and QB and PSI acceptors, respectively (Stirbet and Govindjee, 2011). In the present study, the relationship was studied between the electron transport capacity and ψw, the results implied the action side of drought stress was mainly on the electron transport from QA to QB for a higher correlation coefficient than that in the electron transport from QA to PSI and QB to PSI.
So what does it change inside photosynthetic apparatus and how does it lead to the decrease of electron transport capacity between QA to QB? D1 protein has been proved to undertake biological function transferring photosynthetic electron from QA to QB (Roffey et al., 1994). In our study, D1 protein content decreased with the degree of water stress aggravated and the duration of water stress prolonged. After rehydration, D1 protein content recovered to some extent (Fig. 5). Previous research has shown that, to prevent the accumulation of photodamaged D1 and PSII, plants developed a repair process consisting of several steps as follows: proteolytic degradation of the D1 protein; synthesis of the precursor to the D1 protein (pre-D1); insertion of the newly synthesized precursor into the thylakoid membrane concomitant with the assembly of other PSII proteins; maturation of the D1 protein by C-terminal processing of pre-D1; and finally, assembly of the oxygen-evolving machinery (Aro et al., 1993, 2005). Under normal conditions, D1 protein content remains at a certain level by the balance between the damage and repair of D1 (Baena-González and Aro, 2002). Environmental stresses like salt stress and high temperature negatively influence the D1 content in PSII through inhibiting the repair as well as accelerating the damage. ROS was reported to be involved in the inhibition of repair (Takahashi and Murata, 2008). ROS generated by abiotic stresses blocks PSII repair by suppressing the transcription and translation of psbA genes encoding D1 (Nishiyama et al., 2001, 2004; Suleyman and Allakhverdiev, 2002).
Due to suppression of ROS on the transcription of psbA gene and translation D1 protein, the concentrations of H2O2 via O2•− were probed in order to confirm if more ROS was induced by water stress. Apple leaves accumulated more ROS with different water stress treatments for 33 days than in control (Table 2). Since fixation of CO2 in the Calvin cycle is sensitive to environmental stress (Murata et al., 2007), it is likely to result in the limitation of photosynthesis and apple leaves absorbing more light energy than can be consumed through photosynthetic carbon fixation. The limitation of the photosynthetic fixation of CO2 decreases the utilization of NADPH, with a resultant decline in the level of NADP+ (Murata and Takahashi, 2008). Given that NADP+ is a major acceptor of electrons in PSI, depletion of NADP+ accelerates the transport of electrons from PSI to molecular oxygen with generation of H2O2 via O2•− (Asada, 1999). Although plants have some protecting mechanisms that can dissipate excess energy such as non-photochemical quenching (Pieters and Tezara, 2003; Nabe et al., 2007), photorespiration (Cornic and Fresneau, 2002) and the Mehler reaction (Asada, 1999), the amount of energy dissipated by these mechanisms is still limited. When the degree and duration of water stress exceed the tolerance of plants, excess energy will lead to an increase in the production of ROS including O2•− and H2O2.
During evolution, a series of antioxidant enzymes were developed to scavenge ROS induced by adverse environments. For instance, SOD plays a central role in the enzymatic defense system in removing O2•− (Bowler et al., 1992) and CAT is indispensable in ROS-detoxification for its potential to directly dismutate H2O2 into H2O and O2•− under stressed conditions (Garg and Manchanda, 2009). H2O2 is converted to water and oxygen via the ascorbate (AsA)-glutathione cycle and antioxidative enzymes (Blokhina et al., 2003). The ascorbate-glutathione cycle involves APX, which uses AsA as an electron donor to scavenge H2O2, so APX is also a key enzyme. Our results showed that antioxidant enzymes including CAT, SOD and POD activity increased as the degree of water stress aggravated and the duration of water stress prolonged this, but APX activity decreased under SS (Fig. 6). These results suggest that the AsA-glutathione cycle may not have a main role in clearing H2O2 in severe drought condition. Despite the fact that there were three antioxidant enzymes with higher activity, apple leaves under stress still accumulated more ROS than in control. This response to a water deficient environment indicates an insufficient protective mechanism in apple plants to clear excess ROS under stress for a long time. Consequently, the excess accumulation of ROS does harm to plant proteins, lipids, carbohydrates, DNA and ultimately results in irreversible damage and cell death (Apel and Hirt, 2004; Gill and Tuteja, 2010).
CONCLUSION
Water deficiency in arid and semi-arid regions in northwestern China severely influences apple production. It is urgent to investigate how drought impacts the yield of apples and find a new understanding regarding this. As one of the most important biochemical reactions and the foundation of apple yield, photosynthesis decreases dramatically in drought environment.
After analysis of indicators and exploring their relationships among each other, it is concluded that photosynthetic activities are closely related to ψw and the response of photosynthetic apparatus to drought stress can be separated to two stages, and ψw with −1.5 MPa is the point to split the two stages.
In the first stage, the decline of photosynthetic CO2 assimilation under low drought stress was due to stomatal limitation, nevertheless, Vcmax decreased slightly. Together with stomatal close, the consumption of NADPH and Jmax declined and caused a series of biochemical changes including overproduction of ROS, inhibition of D1 protein repair and eventual impairment of the electron transport chain.
In the second stage, the decline of photosynthetic CO2 assimilation under SS was due to non-stomatal limitation. After drought induced stomatal closure and inhibited CO2 assimilation, it then caused further PSII photoinhibition, dependent on the turnover of D1 protein, and over-reduced the electron transport chain, which increased the production of ROS (H2O2 and O2•−). The over-accumulated ROS inhibited the turnover of D1 protein and reduced electron QA to QB. NADP+ and end electron acceptors may also both decline and in turn limit the synthesis of adenosine triphosphate (ATP) and the regeneration of RuBP (Lawlor and Tezara, 2009; Lin et al., 2009; Campos, et al., 2014). Thus, to interrupt QA to QB, ATP shortage and low regeneration of RuBP we should impair the electron transport chain and the main non-stomatal factors under SS.
MATERIALS AND METHODS
Plant materials and drought stress treatments
The experiments were conducted in Northwest A&F University (NWAFU), Yangling, Shaanxi, China, located at 34°17′N, 108°04′E. Annual highest temperature was 36°C while the lowest was −11°C. The potted substrate was composed of soil mixture and organic matter (2:1, v/v; pH 7.5) with slow release organic-mineral fertilizer in growing season. The soil was collected from the top layer to 20 cm. The field capacity (FC) of potted substrate was 44.5%.
Three-year-old apple (Malus domestica Borkn. cv. Red Fuji) trees on M26 rootstocks were grown in plastic pots (245 mm diameter and 280 mm high). All the potted young trees were normally irrigated for 24 weeks under field conditions before drought stress was imposed. A plastic greenhouse (20 m×8 m×4 m) was utilized as the shelter to protect apple trees from the rain. The soil relative water content in control group was approximately 80% of maximal FC. Apple trees with LS, MS and SS were installed at 80%, 60% and 40% of the soil relative water content in control group. Four groups were arranged in a completely randomized design with eight replications.
ψw measurements
For each treatment, six sunlight-exposed mature leaves were used. Referring to previous studies (Gomes-Laranjo et al., 2006; Jones, 2007; Šircelj et al., 2005), the ψw was measured with a pressure-bomb (Model 3000, Corp Santa Barbara, USA) between 8:00 h and 9:00 h.
Gas exchange measurements
A portable photosynthesis system (LI-6400T, Li-Cor Inc., USA) with a 6400-02B light source (blue and red diode) was used to measure the photosynthetic gas exchange parameters including PN, Ci, E and Gs in vivo on sunny days between 8:00 h and 9:00 h. Measurements were made under an artificial irradiance of 1000 μmol (photons) m–2 s–1 at a temperature of 25°C using the fifth completely expanded leaf from the top of each plant. CO2 concentration and ambient water vapor pressure were kept at 350 μmol mol−1 and 1.30±0.15 kPa, respectively. To produce the PN/Ci curve, the CO2 concentration was set at 380 (for ambient leaves), 250, 200, 150, 100, 50, 350, 450, 550, 650 and 750 µmol mol–1 in turn, and the PPFD was kept at 1200 µmol (photons) m–2 s–1. The apparent carboxylation efficiency of Rubisco was estimated as the slope of the initial linear portion of each PN/Ci curve (Farquhar and Sharkey, 1982). Vcmax and Jmax were calculated according to Sharkey et al. (2007). When PN is Rubisco-limited, the response of PN to Ci can be described using the following equation:
where Vcmax is the maximum velocity of Rubisco for carboxylation, Ci is the intercellular CO2 concentration, Γ* is carbon dioxide compensation point, KC is the Michaelis constant of Rubisco for carbon dioxide, O is the partial pressure of oxygen at Rubisco and KO is the inhibition constant (usually taken to be the Michaelis constant) of Rubisco for oxygen, Rd is respiration rate. When PN is limited by RuBP regeneration,
Based on the number of electrons required for NADP+ reduction, the conservative values of 4 and 8 are used here. Leaf temperature was 25±1°C by the temperature control system of leaf chamber.
Chlorophyll fluorescence measurements
The same leaf was used for chlorophyll a fluorescence measurements right after gas exchange measurements. And measurements were conducted in vivo on sunny days (9:30 h to 11:30 h), with pulse amplitude modulation fluorometer (PAM-2500, Walz, Effeltrich, Germany).
Slow phase chlorophyll fluorescence transients (PSMT)
After a dark-adapted period (20 min) with dark leaf clip (DLC-8), the minimum fluorescence (F0) and maximum fluorescence (Fm) were determined respectively using measure light [<1 µmol(photons) m−2 s−1] and a 0.8 s saturating pulse at 6000 µmol (photons) m−2 s−1. Actinic light of 619 μmol (photons) m−2 s−1 drives photosynthesis and gives F. After about 5 min, the steady state value of fluorescence (Fs) was thereafter recorded and a second saturating pulse at 6000 µmol (photons) m−2 s−1 was imposed to determine Fm in the light adapted state (Fm′). F0′ was basal fluorescence after 5 μmol (photons) m−2 s−1 of far-red irradiation at 720-730 nm for 4 s, which excites PSI and oxidizes the plastoquinone and QA pools associated with PSII. Also, Fv/Fm, actual photochemical efficiency (Fv′/Fm′), ΦPSII, qP, qL, NPQ, Y(NPQ) and Y(NO) were obtained from the measured report.
Chlorophyll a fluorescence transient (OJIP-test) was induced by a red light with a saturating light pulse of 3000 μmol (photons) m−2 s−1 using light-emitting diodes (LEDs), and fluorescence values were recorded for 350 ms with a time resolution of 10 μs. All of the leaves were dark-adapted for 20 min before measuring. The fluorescence intensity at 20 μs (considered as F0), 2 ms (FJ) and 30 ms (FI) are intermediate levels, and maximum fluorescence or Fm (approximately 200 ms) was collected and used to calculate the parameters from JIP-test (Ceppi et al., 2011; Redillas et al., 2011).
Determination of ROS
The H2O2 content and O2•− generation rate were determined as described by Bai et al. (2010). Frozen tissues were homogenized in acetone at a ratio of 1.0 g sample to 2 ml ice-cold acetone. Titanium reagent (2% TiSO4) was added to a known volume of extract supernatant to give a Ti concentration of 2%. The Ti-H2O2 complex, together with unreacted Ti, was then precipitated by adding 0.2 ml of 17 M ammonia solution for every 1.0 ml of extract. The precipitate was washed five times with ice-cold acetone by resuspension, then drained and dissolved in 3 ml of 2.0 M H2SO4. Absorbance of the solution was measured at 410 nm against blanks that had been prepared similarly but without including plant tissue.
For evaluating the generation rate of O2•−, 1.0 g tissue was ground with 4.0 ml 65.0 mM phosphate buffer solution (PBS; pH 7.8) and centrifuged at 5000 g for 10 min. Afterward, 1.0 ml of supernatant was mixed with 0.9 ml 65 mM PBS (pH 7.8) and 0.1 ml 10.0 mM hydroxylamine hydrochloride, then incubated at 25°C for 20 min. Afterward, 17.0 mM sulfanilamide and 7 mM α-naphthylamine were added to the above mixture, which was then incubated at 25°C for 20 min. Light absorbance was measured at 530 nm. A standard curve with the nitrogen dioxide radical (NO2−) was used to calculate the production rate of O2•−.
Extraction and assay of activities by CAT, SOD, POD and APX
Fresh tissue samples (0.1 g each) were homogenized with 5% (w/v) polyvinylpyrrolidone and homogenized with 1.8 ml of 100 mM potassium phosphate buffer (pH 7.0) containing 1.0 mM EDTA and 0.3% Triton X-100. The homogenates were centrifuged at 13,000 g for 20 min at 4°C and the supernatants were used for enzyme assays.
CAT activity was determined by monitoring the decrease in absorbance at 240 nm due to decomposition of H2O2 (Chance and Maehly, 1955). The 1.0 ml reaction mixture contained 39 mM potassium phosphate buffer (pH 7.0), 10 mM H2O2 and 20.0 μl of enzyme extract. This reaction was initiated by adding H2O2.
SOD activity was assayed by monitoring the inhibition of the photochemical reduction of nitro blue tetrazolium (NBT) according to the methods of Dhindsa et al. (1981). The 1.0 ml reaction mixture contained 50.0 mM potassium phosphate buffer (pH 7.8), 6.5 mM methionine, 50.0 μM NBT, 10.0 μM EDTA, 20.0 μM riboflavin, and 20.0 μl of enzyme extract. A reaction mixture lacking enzyme served as the control. All mixtures were stirred under darkness in small glass test tubes, and then irradiated for 5 min by fluorescent lamps [160 μmol (photons) m−2 s−1]. After the reaction mixture turned from yellow to blue-black, its absorbance was measured at 560 nm. The mixture that lacked enzyme and had not been irradiated was used to zero the absorbance at 560 nm. One unit of SOD was defined as the amount of enzyme that produced 50% inhibition of NBT reduction under assay conditions.
POD activity was determined by monitoring the increase in absorbance at 470 nm based on oxidation reaction of guaiacol. The 1.0 ml reaction mixture contained 39.75 mM potassium phosphate buffer (pH 7.0), 10.0 mM H2O2, 10.0 mM guaiacol and 5 μl enzyme extract.
APX activity was measured by monitoring the decrease in absorbance at 290 nm. The mixture of 5 ml contained 50 mM Hepes-KOH (pH 7.6), 0.1 mM EDTA, 0.2 mM H2O2, 0.5 mM reduced AsA and enzyme extract. The reaction was initiated by adding H2O2. One unit of activity was the amount of APX that catalyzed the oxidation of 1 mmol ascorbate per min.
Western blot analysis
Total protein extracts were obtained by grinding 100.0 mg of leaf tissue in 3.0 ml of protein extraction buffer [0.5 M Tris-HCl, pH 6.8, 5 M urea, 8% (w/v) SDS, and 20% β-mercaptoethanol]. Samples were centrifuged at 13,000 g for 10 min, and the supernatant was subjected to SDS-PAGE. For detection of the D1 protein, the samples were separated on a 15% polyacrylamide gel in Tris-Gly buffer and electroblotted onto a nitrocellulose membrane. Blots were reacted with a commercially available antibody generated against D1 protein (Agrisera, Vännäs, Sweden), diluted 1:5000, and an anti-chicken horseradish peroxidase-conjugated secondary antibody, diluted 1:5000.
Statistical analysis
The data obtained from measurements of selected photosynthetic parameters of plant leaves were statistically processed with Microsoft Excel 2007. Differences were evaluated by one-way ANOVA with the Statistical Program for Social Science 19 (SPSS, Chicago, USA). Only ANOVA Tukey results are presented in the paper. Graphs were plotted with Origin pro 7.5.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Resources: H.S., L. Ma; Data curation: Z.W., G.L.; Writing - original draft: G.L., Y.G.; Writing - review & editing: Z.W., Y.G.; Funding acquisition: Y.G., Z.Z., H.G., L. Mei.
Funding
This work was financially supported by the National Key Research and Development Program of China (2016YFD0201131), the National Key Technology R&D Program of China (2014BAD16B06) and the project of the China Agriculture Research System (CARS-28).
References
- Ali Q. and Ashraf M. (2011). Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. J. Agron. Crop Sci. 197, 258-271. 10.1111/j.1439-037X.2010.00463.x [DOI] [Google Scholar]
- Altaweel K., Iwaki T., Yabuta Y., Shigeoka S., Murata N. and Wadano A. (2007). A bacterial transgene for catalase protects translation of d1 protein during exposure of salt-stressed tobacco leaves to strong light. Plant Physiol. 145, 258-265. 10.1104/pp.107.101733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K. and Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373-399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- Aro E.-M., Virgin I. and Andersson B. (1993). Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143, 113-134. 10.1016/0005-2728(93)90134-2 [DOI] [PubMed] [Google Scholar]
- Aro E. M., Suorsa M., Rokka A., Allahverdiyeva Y., Paakkarinen V., Saleem A., Battchikova N. and Rintamäki E. (2005). Dynamics of photosystem II: a proteomic approach to thylakoid protein complexes. J. Exp. Bot. 411, 347-356. 10.1093/jxb/eri041 [DOI] [PubMed] [Google Scholar]
- Asada K. (1999). The water water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 50, 601-639. 10.1146/annurev.arplant.50.1.601 [DOI] [PubMed] [Google Scholar]
- Baena-González E. and Aro E.-M. (2002). Biogenesis, assembly and turnover of photosystem II units. Philos. Trans. R. Soc. B. Biol. Sci. 357, 1451-1460. 10.1098/rstb.2002.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T., Li C., Ma F., Feng F. and Shu H. (2010). Responses of growth and antioxidant system to root-zone hypoxia stress in two Malus species. Plant Soil. 327, 95-105. 10.1007/s11104-009-0034-x [DOI] [Google Scholar]
- Blokhina O., Virolainen E. and Fagerstedt K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 91, 179-194. 10.1093/aob/mcf118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Montagu V. M. and Inze D. (1992). Superoxide dismutase and stress tolerance. Annu. Rev. Plant Biol. 43, 83-116. 10.1146/annurev.pp.43.060192.000503 [DOI] [Google Scholar]
- Campos H., Trejo C., Peña-Valdivia C. B., García-Nava R., Conde-Martínez F. V. and Cruz-Ortega M. R. (2014). Stomatal and non-stomatal limitations of bell pepper (Capsicum annuum L.) plants under water stress and re-watering: delayed restoration of photosynthesis during recovery. Environ. Exp. Bot. 98, 56-64. 10.1016/j.envexpbot.2013.10.015 [DOI] [Google Scholar]
- Ceppi M. G., Oukarroum A., Çiçek N., Strasser R. J. and Schansker G. (2011). The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: A study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol. Plant 144, 277-288. 10.1111/j.1399-3054.2011.01549.x [DOI] [PubMed] [Google Scholar]
- Chance B. and Maehly A. C. (1955). Assay of catalase and peroxidases. Methods Enzymol. 2, 764-775. 10.1016/S0076-6879(55)02300-8 [DOI] [Google Scholar]
- Chaves M. M., Maroco J. P. and Pereira J. S. (2003). Understanding plant responses to drought—from genes to the whole plant. Funct. Plant Biol. 30, 239-264. 10.1071/FP02076 [DOI] [PubMed] [Google Scholar]
- Chaves M. M., Flexas J. and Pinheiro C. (2009). Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 103, 551-560. 10.1093/aob/mcn125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornic G. and Fresneau C. (2002). Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Ann. Bot. 89, 887-894. 10.1093/aob/mcf064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degl'Innocenti E., Hafsi C., Guidi L. and Navari-Izzo F. (2009). The effect of salinity on photosynthetic activity in potassium-deficient barley species. J. Plant Physiol. 166, 1968-1981. 10.1016/j.jplph.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B. and Iii W. W. A. (1996). The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1, 21-26. 10.1016/S1360-1385(96)80019-7 [DOI] [Google Scholar]
- Dhindsa R. S., Plumb-Dhindsa P. and Thorpe T. A. (1981). Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32, 93-101. 10.1093/jxb/32.1.93 [DOI] [Google Scholar]
- Farquhar G. D. and Sharkey T. D. (1982). Stomata1 conductance and photosynthesis. Ann. Rev. Plant Physiol. 33, 317-346. 10.1146/annurev.pp.33.060182.001533 [DOI] [Google Scholar]
- Garg N. and Manchanda G. (2009). ROS generation in plants: boon or bane? Plant Biosyst. 143, 81-96. 10.1080/11263500802633626 [DOI] [Google Scholar]
- Gill S. S. and Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909-930. 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Golldack D., Lüking I. and Yang O. (2011). Plant tolerance to drought and salinity: Stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 30, 1383-1391. 10.1007/s00299-011-1068-0 [DOI] [PubMed] [Google Scholar]
- Gomes M. T. G., Luz A. C. D., Santos M. R. D., Batitucci M. D. C. P., Silva D. M. and Falqueto A. R. (2012). Drought tolerance of passion fruit plants assessed by the OJIP chlorophyll a fluorescence transient. Sci. Hortic-Amsterdam. 142, 49-56. 10.1016/j.scienta.2012.04.026 [DOI] [Google Scholar]
- Gomes-Laranjo J., Coutinho J. P., Galhano V. and Cordeiro V. (2006). Responses of five almond cultivars to irrigation: Photosynthesis and leaf water potential. Agr. Water Manage. 83, 261-265. 10.1016/j.agwat.2005.11.007 [DOI] [Google Scholar]
- Greer D. H., Berry J. A. and Björkman O. (1986). Photoinhibition of photosynthesis in intact bean leaves: role of light and temperature, and requirement for chloroplast-protein synthesis during recovery. Planta 2, 253-260. [DOI] [PubMed] [Google Scholar]
- Henriques F. S. (2009). Leaf chlorophyll fluorescence: Background and fundamentals for plant biologists. Bot. Rev. 75, 249-270. 10.1007/s12229-009-9035-y [DOI] [Google Scholar]
- Jones H. G. (2007). Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2, 119-130. [DOI] [PubMed] [Google Scholar]
- Kramer D. M., Johnson G., Kiirats O. and Edwards G. E. (2004). New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 79, 209-218. 10.1023/B:PRES.0000015391.99477.0d [DOI] [PubMed] [Google Scholar]
- Lawlor D. W. and Tezara W. (2009). Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann. Bot. 103, 561-579. 10.1093/aob/mcn244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazár D., Sušila P. and Nauš J. (2006). Early detection of plant stress from changes in distributions of chlorophyll a fluorescence parameters measured with fluorescence imaging. J. Fluoresc. 16, 173-176. 10.1007/s10895-005-0032-1 [DOI] [PubMed] [Google Scholar]
- Lima A. L. S., Damatta F. M., Pinheiro H. A., Totola M. R. and Loureiro M. E. (2002). Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environ. Exp. Bot. 47, 239-247. 10.1016/S0098-8472(01)00130-7 [DOI] [Google Scholar]
- Lin Z.-H., Chen L.-S., Chen R.-B., Zhang F.-Z., Jiang H.-X. and Ning T. (2009). CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol. 9, 43 10.1186/1471-2229-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misson L., Limousin J. M., Rodriguez R. and Letts M. G. (2010). Leaf physiological responses to extreme droughts in Mediterranean Quercus ilex forest. Plant Cell Environ. 33, 1898-1910. 10.1111/j.1365-3040.2010.02193.x [DOI] [PubMed] [Google Scholar]
- Murata N. and Takahashi S. (2008). How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 4, 178-182. 10.1016/j.tplants.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Murata N., Takahashi S., Nishiyama Y. and Allakhverdiev S. I. (2007). Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 1767, 414-421. 10.1016/j.bbabio.2006.11.019 [DOI] [PubMed] [Google Scholar]
- Nabe H., Funabiki R., Kashino Y., Koike H. and Satoh K. (2007). Responses to desiccation stress in bryophytes and an important role of dithiothreitol-insensitive non-photochemical quenching against photoinhibition in dehydrated states. Plant Cell Physiol. 48, 1548-1557. 10.1093/pcp/pcm124 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Yamamoto H., Allakhverdiev S. I., Inaba M., Yokota A. and Murata N. (2001). Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 20, 5587-5594. 10.1093/emboj/20.20.5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Li Y., Nagai Y., Miyata K., Yoshizumi M., Kagami S., Kondo S., Kiyomoto H., Shokoji T. and Kimura S. (2004). Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 43, 841 10.1161/01.HYP.0000118519.66430.22 [DOI] [PubMed] [Google Scholar]
- Pérez-López U., Robredo A., Lacuesta M., Mena-Petite A. and Muñoz-Rueda A. (2012). Elevated CO2 reduces stomatal and metabolic limitations on photosynthesis caused by salinity in Hordeum vulgare. Photosynth. Res. 111, 269-283. 10.1007/s11120-012-9721-1 [DOI] [PubMed] [Google Scholar]
- Pieters A. J. and Tezara W. A. (2003). Operation of the xanthophyll cycle and degradation of D1 protein in the inducible CAM plant, Talinum triangulare, under water deficit. Ann. Bot. 92, 393-399. 10.1093/aob/mcg153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redillas M. C. F. R., Strasser R. J., Jin S. J., Kim Y. S. and Kim J.-K. (2011). The use of JIP test to evaluate drought-tolerance of transgenic rice overexpressing OsNAC10. Plant Biotechnol. Rep. 5, 169-175. 10.1007/s11816-011-0170-7 [DOI] [Google Scholar]
- Roffey R. A., Kramer D. M., Govindjee, and Sayre R. T. (1994). Lumenal side histidine mutations in the D1 protein of photosystem II affect donor side electron transfer in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1185, 257-270. 10.1016/0005-2728(94)90240-2 [DOI] [PubMed] [Google Scholar]
- Schansker G., Tóth S. Z. and Strasser R. J. (2005). Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in Chl a fluorescene rise OJIP. Biochim. Biophys. Acta 1706, 250-261. 10.1016/j.bbabio.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Sharkey T. D., Bernacchi C. J., Farquhar G. D. and Singsaas E. L. (2007). Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 30, 1035-1040. 10.1111/j.1365-3040.2007.01710.x [DOI] [PubMed] [Google Scholar]
- Šimpraga M., Verbeeck H., Demarcke M., Joó É., Pokorska O., Amelynck C., Schoon N., Dewulf J., Van Langenhove H. and Heinesch B. (2011). Clear link between drought stress, photosynthesis and biogenic volatile organic compounds in Fagus sylvatica L. Atmos. Environ. 45, 5254-5259. 10.1016/j.atmosenv.2011.06.075 [DOI] [Google Scholar]
- Šircelj H., Tausz M., Grill D. and Batič F. (2005). Biochemical responses in leaves of two apple tree cultivars subjected to progressing drought. J. Plant Physiol. 162, 1308-1318. 10.1016/j.jplph.2005.01.018 [DOI] [PubMed] [Google Scholar]
- Šircelj H., Tausz M., Grill D. and Batič F. (2007). Detecting different levels of drought stress in apple trees (Malus domestica Borkh.) with selected biochemical and physiological parameters. Sci. Hortic-Amsterdam. 113, 362-369. 10.1016/j.scienta.2007.04.012 [DOI] [Google Scholar]
- Stirbet A. and Govindjee, (2011). On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 104, 236-257. 10.1016/j.jphotobiol.2010.12.010 [DOI] [PubMed] [Google Scholar]
- Strasser R. J., Tsimilli-Michael M., Sheng Q. and Goltsev V. (2010). Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 1797, 1313-1326. 10.1016/j.bbabio.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Suleyman I. and Allakhverdiev Y. N. S. M. (2002). Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in synechocystis. Plant Physiol. 130, 1443-1453. 10.1104/pp.011114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Gu J., Zeng J., Han S., Song A., Chen F., Fang W., Jiang J. and Chen S. (2013). Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci. Hortic-Amsterdam 161, 249-258. 10.1016/j.scienta.2013.07.015 [DOI] [Google Scholar]
- Zai X. M., Zhu S. N., Qin P., Wang X. Y., Che L. and Luo F. X. (2012). Effect of Glomus mosseae on chlorophyll content, chlorophyll fluorescence parameters, and chloroplast ultrastructure of beach plum (Prunus maritima) under NaCl stress. Photosynthetica 50, 323-328. 10.1007/s11099-012-0035-5 [DOI] [Google Scholar]
- Zhou S., Duursma R. A., Medlyn B. E., Kelly J. W. G. and Prentice I. C. (2013). How should we model plant responses to drought? An analysis of stomatal and non-stomatal responses to water stress. Agr. Forest Meteorol. 182-183, 204-214. 10.1016/j.agrformet.2013.05.009 [DOI] [Google Scholar]