Abstract
d-Limonene is a bioactive food component found in high concentration in citrus peel oil with anticancer effects in preclinical studies of mammary carcinogenesis. Extrapolation of preclinical data to human cancer is limited, in part, by inadequate information on the oral bioavailability and tissue disposition of d-limonene in humans. As a fat-soluble compound, d-limonene is more likely to deposit in fatty tissues such as the breast. To assess disposition of d-limonene in humans, we conducted a pilot study of oral d-limonene-rich lemonade. Following a 1-wk washout period devoid of citrus, healthy adults consumed 40 oz. of freshly prepared lemonade containing 500 to 600 mg d-limonene daily for 4 wk. On the first and last consumption days, blood and buttock fat biopsy were collected. Matched preintervention and postintervention fat biopsies (n = 7), and matched preintervention and postintervention plasma samples (n = 6), were analyzed for d-limonene levels using gas chromatography and mass spectrometry. There was a significant increase in d-limonene levels in the fat biopsies after 4 wk (P = 0.009); initial levels ranged from nondetectable to 7.79 μmol/kg tissue, and postintervention levels ranged from 53.6 to 294 μmol/kg tissue. Plasma d-limonene levels increased from 0.35 to 0.72 μmol/l initially to postintervention levels of 0.54 to 1.65 μmol/l (P = 0.016). Postintervention adipose d-limonene levels were 51.0 to 195 times higher than plasma levels (P = 0.009). Our results demonstrate accumulation of d-limonene in adipose tissue after oral dosing and support additional studies of d-limonene for chemoprevention in tissues such as the breast that are comprised of a significant fat fraction.
INTRODUCTION
Epidemiological evidence suggests that diets high in fruits and vegetables may be protective against certain cancers (1). This protective effect has been attributed to a number of compounds found in food that have pharmacological effects called bioactive food components (BAFC) (2). d-Limonene is a BAFC commonly found in high concentration in citrus peel oil. In animal models, d-limonene and structural analogs have demonstrated strong chemopreventive effects in lymphomas, mammary, gastric, liver, and lung cancers (3–7). Of these cancers, the preclinical evidence is strongest for a potential chemopreventive role in mammary carcinogenesis. For example, rats fed a 5% d-limonene diet before introduction of nitrosomethylurea (NMU) have demonstrated significantly reduced tumor size and tumor number compared to the control diet (8,9). In rats with 7,12-dimethylbenz(a)anthracene (DMBA) induced mammary cancer, d-limonene diets as low as 0.1 and 0.01% were effective in significantly increasing tumor latency (10,11). In mouse mammary gland organ culture, d-limonene demonstrated 78% inhibition of initiation of DMBA-induced tumors at a concentration of 1 × 10−8 mol/l (12), a concentration achievable with oral dosing. Although the anticancer mechanism of d-limonene is unclear, inhibition of the isoprenylation of a number of small GTP-ase proteins that have involvement in cell proliferation and motility, such as Ras and Rho, have been implicated (13,14).
Despite the body of evidence demonstrating d-limonene’s anticancer effects, research investigating its disposition in humans is limited. d-Limonene is bioavailable in humans after oral administration. The two major circulating metabolites of limonene are perillic acid and dihydroperillic acid (15). In a trial conducted in advanced cancer patients, peak plasma levels of limonene, perillic acid, and dihydroperillic acid were found to be around 10 to 12, 20 to 37, and 11 to 16 μmol/l, respectively, at the maximum tolerated dose (8 gm/m2) (16). Perillic acid and dihydroperillic acid have been shown to be more potent inhibitors of protein isoprenylation than limonene, and perillic acid is also a more potent inhibitor of cell growth (17,18). However, d-limonene is likely to have a favorable tissue distribution for exerting biological activities due to its high lipophilicity, whereas the tissue distribution of the oxygenated metabolites may be limited by their polarity.
Data from animal studies has suggested that d-limonene might distribute preferentially into anatomical sites rich in fatty tissue such as breast. In female rats given an oral dose of radiolabeled d-limonene, the radioactivity concentrates in adipose and mammary tissues suggested accumulation of d-limonene and/or its derived metabolites (18). In the cancer therapeutic trial cited earlier (16), limonene and its metabolites were identified and quantified in metastatic lymph node tissue collected from two breast cancer patients receiving limonene 8 g/m2 per day for 21 days. The intratumoral d-limonene levels were found to exceed the corresponding plasma levels by 1.9-and 5.5-fold, whereas most metabolites of limonene were trace constituents in tissue with tissue-to-plasma ratios of limonene 4 to 10 times higher than its major metabolite, perillic acid (16). Based on these limited data, we hypothesize that d-limonene has favorable tissue distribution and will distribute extensively to anatomical sites rich in fatty tissue. To test this hypothesis, we conducted a pilot study to determine the systemic and adipose tissue disposition of d-limonene following consumption of limonene-rich lemonade.
MATERIALS AND METHODS
Study Participants
Seven healthy adults participated in this pilot study, six females and one male. Eligible participants had to be willing to consume 40 oz. of lemonade daily. The average age was 29 yr old (range = 18–38 yr), and the average body mass index (BMI) was 25.4 kg/m2 (range = 22.4–31.5 kg/m2). All participants were in general good health, were not pregnant or breast-feeding, had normal liver and renal function, and had no incidence of cancer within the last 5 yr or other serious or chronic diseases. The study was approved by the University of Arizona Human Subjects Committee, and written consent was obtained from all participants.
Study Design and Intervention
This study was a single arm, 4-wk, lemonade feeding trial conducted among healthy, free-living adults. Prior to baseline evaluation, participants were instructed to consume at least 3 glasses of water per day for 1 wk. During this time, participants were also required to refrain from any citrus or citrus products. At their baseline visit, participants were given breakfast with high d-limonene lemonade. The lemonade was freshly prepared by blending 2 whole lemons (with peel) with 40 oz (1,182 ml) of water. Six hours after the lemonade consumption, a blood sample and a needle fat biopsy from the buttock were collected. Participants were then provided with a 1-week supply of fresh lemons and instructions for the daily lemonade preparation. Subjects were required to prepare 40 oz. of lemonade fresh daily, and consumed the preparation each day in a single dose with food for 4 wk. Participants returned weekly for safety and adherence evaluation and brought samples of their homemade lemonade for d-limonene content analysis to determine individual d-limonene intakes. At the end of Week 4, participants returned to the clinic and consumed breakfast with 40 oz. of lemonade prepared fresh in the clinic. Six hours after the lemonade consumption, a second blood sample and a repeat fat biopsy from the buttock were collected. In addition, a fasting blood sample was collected before and after 4 wk of daily lemonade intervention for a complete blood count with differential leukocyte count and a comprehensive blood chemistry analysis for evaluation of safety. Lemonade aliquots, plasma, and buttocks adipose biopsy samples were stored at −80°C until d-limonene analysis.
Plasma d-Limonene Analysis
Plasma d-limonene concentrations were determined using a published assay (19) with minor modifications. Briefly, plasma samples or plasma calibration standards (100 μl) were mixed with an equal volume of the internal standard solution (1.2 mg/l of perillyl aldehyde in 100% acetonitrile) to precipitate the plasma proteins. After vortexing and centrifugation, the supernatant was removed and mixed with 100 μl hexane for d-limonene extraction. This mixture was then vortexed and centrifuged. One microliter of the hexane layer was injected into the GC-MS system with a splitless injection at 220°C. Chromatographic separation of d-limonene and internal standard was achieved on a high resolution GC DB-5MS fused silica capillary column with an initial oven temperature set at 60°C and increased to 140°C at 20°C per min. The mass spectrometer source was set at 280°C, with the mass analyte analyzer set at selective ion monitoring with the prominent masses of each analyte. d-Limonene and the internal standard elute at approximately 9 and 14 min, respectively. Calibration curves were constructed by plotting d-limonene to internal standard peak area ratios against the d-limonene concentration. The assay is linear over the d-limonene concentration range of 1 to 250 ng/ml. The interday and intraday variation for the assay was <10%. Extraction recovery of both d-limonene and internal standard were >80%.
Adipose d-Limonene Analysis
Adipose d-limonene concentrations were analyzed according to a method developed in our laboratory (20). Briefly, adipose biopsies were weighed and incubated at 37°C in a water bath for 2.5 h with 200 μl of 30% potassium hydroxide and 1 ml ethanol to induce saponification. Calibration standards, prepared by spiking different concentrations of limonene to a fixed amount of pork fat, were saponified using the same procedure. After cooling to room temperature, 3 ml hexane, 1 ml purified H2O, and 30 μl internal standard solution (9.5 mg/l of perillyl aldehyde in methanol) were added to the saponified samples and calibration standards. Samples were vortexed and then centrifuged at room temperature. The hexane layer was then removed and concentrated to 0.3 ml under a stream of nitrogen on a prefrozen metal block. One microliter of the concentrate was injected into the GC-MS system with a splitless injection at 220°C. Chromatographic separation of d-limonene and internal standard was achieved on a high resolution GC DB-5MS fused silica capillary column with an initial oven temperature held at 70°C for 10 min, ramping 15°C/min up to 300°C and held for 5 min. The mass spectrometer source temperature was set to 250°C, with the mass analyzer set to selected ion monitoring mode with a positive polarity for the prominent masses of each analyte. Limonene and the internal standard elute at approximately 8 and 15 min, respectively. Calibration curves were constructed by plotting d-limonene to internal standard peak area ratios against the amount of d-limonene spiked. The assay is linear between 79.0 and 2,529 ng d-limonene. Within- and between-day assay variations were <10%. Extraction recovery of both d-limonene and internal standard were >80%.
Lemonade d-Limonene Analysis
The analysis of d-limonene content in the lemonade was performed using a reversed-phase high-performance liquid chromatography (HPLC) procedure as previously described (21). An aliquot of the lemonade was mixed and diluted with the mobile phase before injecting into the HPLC. Chromatographic separation was achieved using a Supelco LC-ABZ column (150 × 4.6 mm; Supelco, Bellefonte, PA), and a mobile phase consisted of acetonitrile and sodium acetate buffer [25 mM (pH 5.0)] in the ratio of 70:30. The flow rate of the mobile phase was 1.1 ml/min. The column eluent was monitored with a UV detector at a wavelength of 230 nm. d-Limonene contents were quantified using calibration curves prepared with d-limonene standards diluted with the mobile phase. The calibration curve was linear over the concentration range of 0.5 to 100 mg/ml.
Data Analysis
The differences between patient-matched preintervention and postintervention adipose d-limonene levels were compared using a paired, 2-tailed, Student’s t-test. A paired, 2-tailed, Student’s t-test was also used to compare patient-matched preintervention and postintervention plasma d-limonene levels. The differences between time-matched plasma and adipose d-limonene levels were also compared using a paired, 2-tailed t-test. A P value <0.05 was considered statistically significant. Pearson’s correlation was used to determine if d-limonene juice levels were associated with d-limonene deposition in adipose and/or plasma and was also used to determine if initial or postintervention serum levels were correlated to the time-matched adipose samples.
RESULTS
Analysis of the d-limonene content of repeat lemonade samples showed d-limonene levels ranging between 480 and 790 mg d-limonene per 40 oz. of lemonade. Compliance was determined by the return of unused lemons and was 100%. Assuming participants consumed the full 40 oz. of lemonade per day, the average levels measured in the weekly lemonade samples for each patient would represent their d-limonene consumption. The consumption of study lemonade was well tolerated with no clinically significant changes in hematology or blood chemistry.
Consuming the lemonade with a meal alleviated gastrointestinal distress and maximized d-limonene absorption.
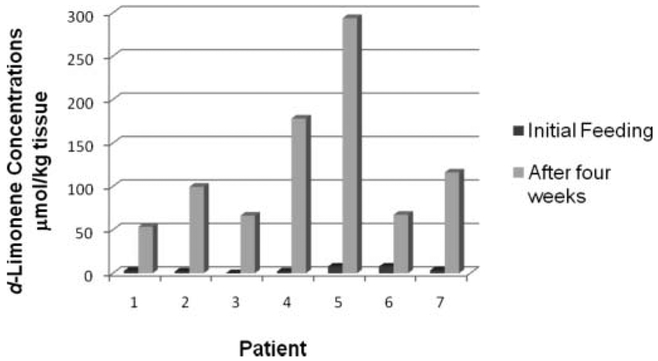
Figure 1 shows the relative d-limonene adipose concentrations after a single dose and again after repeat daily dosing of high d-limonene lemonade for 4 wk for individual study subjects (n = 7). As illustrated in the figure, d-limonene concentrations were low or undetectable in the adipose tissue 6 h after the initial lemonade consumption for all subjects and greatly increased after 4 wk of daily consumption. Table 1 summarizes the average and range of d-limonene concentrations of all adipose samples collected after the initial single dose of high d-limonene lemonade and after 4 wk of daily consumption. Data are presented in molar concentrations for comparisons of concentrations used in prior studies. Initial adipose d-limonene concentrations ranged from not detectable to 7.79 μmol/kg tissue (mean = 3.79 μmol/kg). On average, postintervention adipose d-limonene levels increased 44-fold to 53.6 to 294 μmol/kg-tissue (mean = 137 μmol/kg), and this was statistically significantly higher than the initial levels (P = 0.009).
FIG. 1.
Individual subject d-limonene concentrations in matched needle buttock biopsies 6 h after initial high-limonene lemonade consumption and after 4 wk of repeat daily dosing.
TABLE 1.
Average d-limonene levels determined in time-matched adipose and plasma samples collected after a single dose of high-limonene lemonade consumption and after 4 wk of daily consumptiona
| Adipose d-Limonene Concentration, μmol/kgb |
Plasma d-Limonene Concentration, μmol/lc |
Adipose/Plasma d-Limonene Ratioc,d |
Post 4 Wk/ Initial Adipose d-Limonene Ratiob |
Post 4 Wk/ Initial Plasma d-Limonene Ratioc |
|
|---|---|---|---|---|---|
| Initial | |||||
| Mean (SD) | 3.79 (3.26)e | 0.48 (0.21) | 7.6 (7.5) | — | — |
| Range | ND–7.79 | 0.35–0.72 | ND–20.0 | — | — |
| Post 4 wk | |||||
| Mean (SD) | 137 (87.2)f,g | 1.12 (0.42)h | 111.0 (51.5) | 44.5 (29.3) | 2.6 (1.1) |
| Range | 53.6–293.9 | 0.54–1.65 | 51.0–195 | 8.6–89.3 | 1.1–4.2 |
Abbreviation is as follows: ND, not detectable.
n = 7.
n = 6.
Calculated by assuming the fat density of 0.9 g/ml (kg/l).
Initial adipose and plasma levels were not significantly different (P = 0.157).
Significantly higher than the initial adipose samples (P = 0.009).
Post 4-wk intervention adipose levels were significantly higher than plasma levels (P = 0.009).
Significantly higher than the initial plasma samples (P = 0.016).
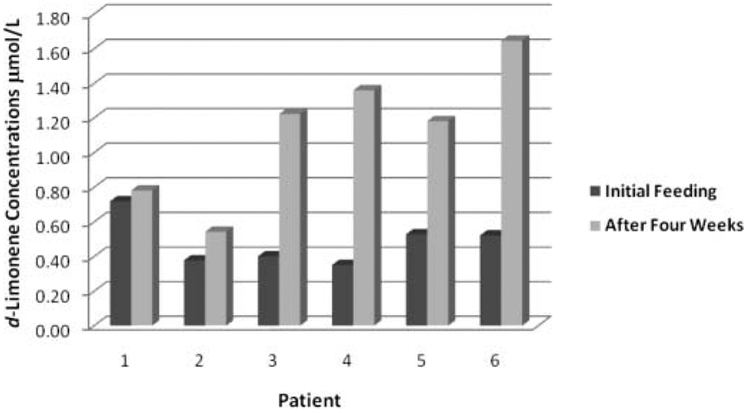
Figure 2 shows the change in plasma d-limonene concentration for the individual study subjects (n = 6) after the singledose and subject-matched postrepeated lemonade dosing daily for 4 wk. All subjects demonstrated measurable d-limonene in plasma 6 h after the initial lemonade consumption, although at concentrations in the low μmol/l range. As shown in Table 1, initial plasma levels were 0.35 to 0.72 μmol/l (mean = 0.48 μmol/l), and this was not significantly different from initial adipose levels (P = 0.157). There was a small but statistically significant increase in postintervention plasma d-limonene levels (P = 0.016), with postintervention levels ranging from 0.54 to 1.65 μmol/l (mean = 1.12 μmol/l). We compared the adipose and plasma d-limonene concentrations by assuming that the adipose tissue has a density of 0.9 g/ml (kg/l). Postintervention adipose d-limonene levels were found to be significantly higher than the postintervention plasma d-limonene concentrations (P = 0.009) with an adipose-to-plasma concentration ratio of 51 to 195 (mean = 111).
FIG. 2.
Individual subject d-limonene concentrations in matched plasma samples after initial high-limonene lemonade feeding and after 4 wk of repeat daily dosing.
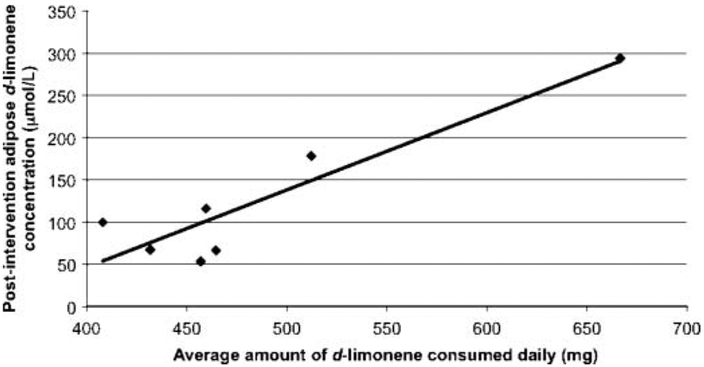
There was a positive correlation between the average amount of d-limonene measured in the study lemonade and the postintervention (4 wk) adipose d-limonene concentration (ρ = 0.91; P = 0.003; Fig. 3). There was no significant correlation between average measured d-limonene content in lemonade and change in d-limonene deposition in adipose, plasma d-limonene levels at either time point, or adipose-to-plasma d-limonene ratios. Importantly, d-limonene levels in adipose and plasma were not correlated at either the initial feeding (ρ = 0.26; P = 0.30) or after 4 wk of repeat daily dosing (ρ = 0.31; P = 0.87). There were no significant associations between BMI and d-limonene final adipose or plasma levels or changes in d-limonene deposition from baseline. Nevertheless, these exploratory correlative analyses would need to be interpreted with cautions due to the small sample size.
FIG. 3.
Correlation between average amount of d-limonene consumption and adipose d-limonene concentration after 4 wk of daily feeding. R2 = .84; ρ = 0.91; P = 0.003.
DISCUSSION
The primary objective of this pilot feeding study was to determine whether d-limonene partitions extensively to human adipose tissue after oral consumption. Among 7 healthy adults consuming high-limonene lemonade, d-limonene did distribute preferentially to adipose tissue as compared to plasma. After the single dose of lemonade, adipose d-limonene levels were zero-to 20-fold (mean 7.6–fold) higher compared to time-matched plasma samples. The postintervention adipose d-limonene levels ranged from 51 to 195 (mean = 111) times of that in corresponding plasma samples. The data suggest significant deposition of d-limonene in adipose as compared to plasma and wide individual variability in exposure with lemonade feeding. Our findings are consistent with data from animal studies. A study by Crowell et al. (18) showed that in female rats, d-limonene and its derived metabolites (nonspecific assay) depot in anatomical sites rich in fatty tissue. In that study, a single dose of 1g/kg d-limonene resulted in peak adipose tissue levels of d-limonene/metabolite that were 6.6 times greater than those achieved in plasma, whereas the peak level in mammary tissue was 5 times greater than the plasma levels. Although the d-limonene exposure was much higher in these rodents than this study, the adipose-to-plasma ratio deposition pattern is comparable to this study, where we found that the adipose biopsy d-limonene levels following the initial dose were on average 7.6 times higher than the plasma levels. In addition to the preferential distribution to the adipose tissue, we showed that d-limonene accumulates extensively in adipose tissue following repeated dosing. On average, there was a 44-fold increase in postintervention adipose d-limonene levels. This extent of accumulation of d-limonene was not observed in plasma samples (2.6-fold increase was observed in plasma). Based on general pharmacokinetic principles, the 2.6-fold increase in plasma d-limonene concentration following repeated dosing is not unexpected when the agent is administered approximately every elimination half-life. The differential accumulation of adipose and plasma d-limonene suggests that measurement of circulating d-limonene levels in humans may significantly underestimate the d-limonene concentrations in adipose tissue.
Another important finding of our study is that consumption of dietary lemonade made from citrus peel gave rise to high d-limonene concentrations in human adipose tissue. An average adipose tissue d-limonene concentration of 137 μmol/kg was achieved from consuming two whole lemons or on average of 575 mg d-limonene daily. These pilot data suggest that d-limonene concentrations might reach biologically relevant levels with a dietary lemonade intervention because d-limonene at a concentration of 1 × 10−8 mol/l demonstrated 78% inhibition of initiation of DMBA-induced tumors in mouse mammary gland organ culture (12). However, d-limonene concentrations in the low mmol/l range were required to inhibit mammary tumor growth in cell culture experiments (22) and to inhibit G protein prenylation (23,24). Intervention with higher doses of oral d-limonene products may be required to achieve low mmol/l tissue concentration and thus modulate these specific chemopreventive responses.
Our data provide indirect evidence that daily lemonade interventions could result in d-limonene deposition at biologically relevant levels in other high adipose tissue besides the buttocks, such as the breast. It is of interest that the most compelling antitumor activity of d-limonene has been observed in models of mammary carcinogenesis (8,12,25) as compared to other tumor sites. Adipose tissue is an active organ producing and secreting adipokines and other growth hormones (26), which might have direct or indirect effects on mammary carcinogenesis through endocrine-, paracrine-, and autocrine-mediated pathways (27) as well as through effects on the tumor microenvironment (28–30). Further studies are needed to determine whether d-limonene would affect the expression and secretion of adipose-derived cytokines and hormones in response to a daily high d-limonene lemonade intervention.
In this study, we showed that daily dietary lemonade intervention was met with high adherence and was well tolerated. Additionally, high d-limonene intake for 4 wk did not affect body weight, blood chemistry, or hematology. Study participants consumed between 480 and 790 mg (mean = 574 mg) of d-limonene each day. These levels are much higher than commercially available lemonade or orange juice, which contain roughly 3 mg/l and 20 to 73 mg/l d-limonene, respectively (31). It is worth noting that the amount consumed was strongly correlated with the adipose d-limonene concentration but was not correlated with the plasma concentration. Additionally, adipose to plasma ratios were not correlated with d-limonene consumption after either the initial feeding or after repeat daily dosing. Our study further illustrates the importance of measuring agent levels in the target tissue (or surrogate target) because plasma concentrations may not always reflect the target tissue distribution and accumulation. Our data suggests that d-limonene may accumulate in the breast, given the high adiposity of breast tissue. Further research is needed to determine the effects of d-limonene on the expression and secretion of adipose-derived cytokines and hormones and its effects in breast tissue and thus its potential as a cancer preventive agent.
ACKNOWLEDGMENT
This work was partially supported by an NCI Grant (KO7-CA-76009), the Arizona Cancer Center Core Grant (P30-CA-23074), and the DOD Idea Award (BC061529).
REFERENCES
- 1.Riboli E and Norat T: Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr 78(3 Suppl), 559S–569S, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Stan SK, Kar S, Stoner GD, and Singh SV: Bioactive food components and cancer risk reduction. J Cell Biochem 104, 339–356, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Gould MN: Prevention and therapy of mammary cancer by monoterpenes. J Cell Biochem Suppl 22, 139–144, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Haag JD, Lindstrom MJ, and Gould MN: Limonene-induced regression of mammary carcinomas. Cancer Res 52, 4021–4026, 1992. [PubMed] [Google Scholar]
- 5.Lu XG, Zhan LB, Feng BA, Qu MY, Yu LH, et al. : Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by d-limonene. World J Gastroenterol 10, 2140–2144, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowell PL and Gould MN: Chemoprevention and therapy of cancer by d-limonene. Crit Rev Oncog 5, 1–22, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Del Toro-Arreola S, Flores-Torales E, Torres-Lozano C, Del Toro-Arreola A, Tostado-Pelayo K, et al. : Effect of d-limonene on immune response in BALB/c mice with lymphoma. Int Immunopharmacol 5, 829–838, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Maltzman TH, Hurt LM, Elson CE, Tanner MA, and Gould MN: The prevention of nitrosomethylurea-induced mammary tumors by d-limonene and orange oil. Carcinogenesis 10, 781–783, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Chander SK, Lansdown AG, Luqmani YA, Gomm JJ, Coope RC, et al. : Effectiveness of combined limonene and 4-hydroxyandrostenedione in the treatment of NMU-induced rat mammary tumours. Br J Cancer 69, 879–882, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elegbede JA, Elson CE, Qureshi A, Tanner MA, and Gould MN: Inhibition of DMBA-induced mammary cancer by the monoterpene d-limonene. Carcinogenesis 5, 661–664, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Russin WA, Hoesly JD, Elson CE, Tanner MA, and Gould MN: Inhibition of rat mammary carcinogenesis by monoterpenoids. Carcinogenesis 10, 2161–2164, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Mehta RG and Moon RC: Characterization of effective chemopreventive agents in mammary gland in vitro using an initiation-promotion protocol. Anticancer Res 11, 593–596, 1991. [PubMed] [Google Scholar]
- 13.Gould MN, Moore CJ, Zhang R, Wang B, Kennan WS, et al. : Limonene chemoprevention of mammary carcinoma induction following direct in situ transfer of v-Ha-ras. Cancer Res 54, 3540–3543, 1994. [PubMed] [Google Scholar]
- 14.Crowell PL, Chang RR, Ren ZB, Elson CE, and Gould MN: Selective inhibition of isoprenylation of 21-26-kDa proteins by the anticarcinogen d-limonene and its metabolites. J Biol Chem 266, 17679–17685, 1991. [PubMed] [Google Scholar]
- 15.Crowell PL, Elson CE, Bailey HH, Elegbede A, Haag JD, and Gould MN: Human metabolism of the experimental cancer therapeutic agent d-limonene. Cancer Chemother Pharmacol 35, 31–37, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Vigushin DM, Poon GK, Boddy A, English J, Halbert GW, et al. : Phase I and pharmacokinetic study of d-limonene in patients with advanced cancer: Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother Pharmacol 42, 111–117, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Bardon S, Picard K, and Martel P: Monoterpenes inhibit cell growth, cell cycle progression, and cyclin D1 gene expression in human breast cancer cell lines. Nutr Cancer 32, 1–7, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Crowell PL, Lin S, Vedejs E, and Gould MN: Identification of metabolites of the antitumor agent d-limonene capable of inhibiting protein isoprenylation and cell growth. Cancer Chemother Pharmacol 31, 205–212, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Chen Y, Gao Z, Xiong M, Zhong Z, et al. : Gas chromatographic-mass spectrometric analysis of d-limonene in human plasma. J Pharm Biomed Anal 44, 1095–1099, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Miller JA, Hakim IA, Thomson C, Thompson P, and Chow HH: Determination of d-limonene in adipose tissue by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 870, 68–73, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow HH, Salazar D, and Hakim IA: Pharmacokinetics of perillic acid in humans after a single dose administration of a citrus preparation rich in d-limonene content. Cancer Epidemiol Biomarkers Prev 11, 1472–1476, 2002. [PubMed] [Google Scholar]
- 22.Karlson J, Borg-Karlson AK, Unelius R, Shoshan MC, Wilking N, et al. : Inhibition of tumor cell growth by monoterpenes in vitro: evidence of a Ras-independent mechanism of action. Anticancer Drugs 7, 422–429, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Hardcastle IR, Rowlands MG, Barber AM, Grimshaw RM, Mohan MK, et al. : Inhibition of protein prenylation by metabolites of limonene. Biochem Pharmacol 57, 801–809, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Crowell PL, Ren Z, Lin S, Vedejs E, and Gould MN: Structure-activity relationships among monoterpene inhibitors of protein isoprenylation and cell proliferation. Biochem Pharmacol 47, 1405–1415, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Elson CE, Maltzman TH, Boston JL, Tanner MA, and Gould MN: Anti-carcinogenic activity of d-limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis. Carcinogenesis 9, 331–332, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, et al. : Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J Biol Chem 281, 26320–26328, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Vona-Davis L and Rose DP: Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 14, 189–206, 2007. [DOI] [PubMed] [Google Scholar]
- 28.McSherry EA, Donatello S, Hopkins AM, and McDonnell S: Molecular basis of invasion in breast cancer. Cell Mol Life Sci 64, 3201–3218, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, et al. : Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66, 11238–11246, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Lewis CE and Hughes R: Inflammation and breast cancer: microenvironmental factors regulating macrophage function in breast tumours: hypoxia and angiopoietin-2. Breast Cancer Res 9, 209, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hakim IA, Hartz V, Graver E, Whitacre R, and Alberts D: Development of a questionnaire and a database for assessing dietary d-limonene intake. Public Health Nutr 5, 939–945, 2002. [DOI] [PubMed] [Google Scholar]