Keywords: nerve regeneration, migraine, rhesus monkey, neurogenic inflammation, cellular oncogene fos, neuronal nitric oxide synthase, calcitonin gene related peptide, trigeminal system, behavior, immunohistochemistry, neural regeneration
Abstract
Several animal models of migraine have been established, and those based on trigeminovascular system activation are widely accepted. However, most of these models have been established on lower animals, such as rodents, and involve only a single administration of a noxious stimulus. In this study, an inflammatory soup (10 μL), consisting of prostaglandin E2 (0.2 mM), serotonin (2 mM), bradykinin (2 mM) and histamine (2 mM), was injected into the dura mater of conscious rhesus monkeys through an indwelling catheter. The infusion started on day 8 and was repeated every 3 days, for a total of six administrations, to induce neurogenic inflammation. We performed behavioral assessments and measured the expression of the oncogene c-fos, neuronal nitric oxide synthase (nNOS) and calcitonin gene related peptide (CGRP) in the trigeminal system and in multiple brain regions involved in pain processing by immunohistochemical staining. Compared with monkeys in the control group, three of the four animals in the inflammatory soup group displayed decreased motor behaviors, and two showed increased ipsilateral nose and mouth secretions during the stimulus period. Higher expression levels of c-fos, nNOS and CGRP were found in various brain areas of experimental animals compared with controls, including the trigeminal nucleus caudalis, thalamus, hypothalamus, midbrain, pons and other areas involved in pain perception. These results suggest that repeated inflammatory soup stimulation of the dura activates the trigeminovascular system and produces migraine-like pathological changes and abnormal behaviors in conscious rhesus monkeys.
Chinese Library Classification No. R453; R338; R741
Introduction
Migraine is a common neurological disorder affecting 2.4–25% of females and 1.6–7.4% of males, often occurring over many years and even throughout life (Silberstein, 2008; Finocchi and Strada, 2014; Lipton and Silberstein, 2015). Despite numerous studies on migraine, the pathophysiological mechanisms are not fully understood. Moreover, current anti-migraine drugs do not work on some patients (Tfelt-Hansen and Olesen, 2012). To investigate the underlying mechanisms and to develop effective new drugs, a reliable animal model that mimics most of the features of migraine is required. Several animal models have been established based on various concepts of migraine pathology. Among these models, neurovascular models established by activating the trigeminovascular system are widely accepted (Eikermann-Haerter and Moskowitz, 2008).
It has been reported that noxious stimulation of the dura mater can induce neurogenic inflammation and activate the trigeminovascular system (Buture et al., 2016). For example, infusion of inflammatory soup (IS) into the dura of animals induces sterile inflammation and results in migraine-like symptoms and pathologic changes similar to those in migraine patients. However, while migraineurs experience repeated nociceptive activation with a resultant hyper-responsive state and central sensitization, most animal models are based on a single dural injection of a noxious stimulus (Woolf and Salter, 2000; Eikermann-Haerter and Moskowitz, 2008). Indeed, animal models of migraine generated by repeated dural stimulation exhibit pathologic changes that differ from those induced by a single stimulation (Moskowitz, 2007; Melo-Carrillo and Lopez-Avila, 2013). Thus, to obtain a better understanding of the mechanisms underlying migraine recurrence, an animal model of repeated dural stimulation is needed. Oshinsky and Gomonchareonsiri (2007) produced a chronic state of trigeminal hypersensitivity by repeatedly stimulating the dura of awake rats, and concluded that this model has the potential to elucidate the mechanisms of recurrent headache. However, this model was established in rodents, making it difficult to mimic the structural and functional changes that occur in human migraineurs. Therefore, an animal model based on the rhesus monkey, which has 93% genetic similarity with humans (Rhesus Macaque Genome Sequencing and Analysis Consortium et al., 2007), might be a better option. In addition, as clinical trials have failed to confirm the efficacy of drugs that are effective in animal experiments (Olesen and Jansen-Olesen, 2012), the rhesus monkey model may be a promising choice for preclinical experiments.
Neurogenic inflammation is the term given to a neurally mediated inflammatory response in meningeal tissue characterized by vasodilatation, leakage of plasma proteins from blood vessels and mast cell degranulation (Buture et al., 2016). Neuropeptides such as calcitonin-gene related peptide (CGRP) are released when trigeminal fibers or trigeminal ganglia are activated (Buture et al., 2016). Those neuropeptides have an important role in pathophysiology of migraine and other primary headaches (Moskowitz, 1984, 1993). The proto-oncogene c-fos, which regulates the transcription of target genes, is widely used as a marker of cell activation (Clayton et al., 1997; Bohár et al., 2013). Neuronal nitric oxide synthase (nNOS) is a calmodulin-dependent enzyme that produces nitric oxide, which is involved in the regulation of cerebral cranial blood flow and arterial diameter during headache (Olesen, 2008). Expression of CGRP, c-fos and nNOS in the central nervous system induced by electrical or chemical stimulation of the trigeminovascular system has been demonstrated to reflect migraine-like pathologic changes in animal models (Clayton et al., 1997; Knyihár-Csillik et al., 2001; Russell et al., 2014; Karsan and Goadsby, 2015).
In the present study, the dura was repeatedly stimulated in conscious rhesus monkeys to induce neurogenic inflammation and activate trigeminovascular nociceptors, potentially mimicking recurrent attacks of headache in humans. Immunohistochemistry was used to study CGRP, c-fos and nNOS expression in the trigeminal system and in multiple brain regions involved in pain processing, in an effort to elucidate the pathological mechanisms underlying migraine.
Materials and Methods
Animals
Six male rhesus monkeys (2–4 years old, weighing 3.5–5.2 kg, provided by Chengdu Pingan Research Base of Animal Breeding, Chengdu, China (license number: SCXK (Chuan) 2008-013), were raised according to the Chinese GB 14925-2010 criteria. The rhesus monkeys were allowed to acclimate to the experimental environment for 3 weeks prior to the procedures. During this period, we observed their behavior and performed neurological examinations to exclude abnormal subjects. All six rhesus monkeys were included in the experiments. Our study was approved by the Medical Ethics Committee, Sichuan University, China (approval number: K2018060). The monkeys were randomly divided into the IS (stimulation; n = 4) and control (sodium chloride; n = 2) groups.
Implantation of the cannula
After 12 hours of preoperative fasting and water-deprivation, the rhesus monkeys were anesthetized with ketamine (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China) for induction and given 30 mg/kg 1.5% pentobarbital (Shanghai Kefeng Chemical Reagent Co., Ltd., Shanghai, China) during surgery. An incision was performed to expose the anteromedian skull. The craniotomy (2 mm in diameter) was performed with an electric drill. Two holes, located on either side of the midline, 5 mm posterior to the junction of the coronal suture and midline, were drilled to expose the dura mater. To avoid burning and damage to the dura, normal saline irrigation was used to drop the temperature of the drill. Then, two sterile plastic cannulas (1.5 mm wide, 1 cm long) (Contec Medical Systems Co., Ltd., Qinhuangdao, China) were placed between the skull and the dura, with one open end positioned near the transverse sinus at the midline. Dental cement was applied, fixing the cannula to the surrounding skull, and sterile bone wax was applied around the cannula to prevent leakage. The incision was sutured and covered with a plastic cap.
The procedure was conducted under aseptic conditions at room temperature (25°C). After the operation, the monkeys were administered 100–300 mg/kg of ampicillin sodium per day (Livzon Pharmaceutical Group Inc., Zhuhai, China) to prevent infection, then allowed to resume oral food intake gradually. All animals had a 1-week recovery period before infusion.
Infusion of IS
IS was used to stimulate the dura mater by inducing sterile inflammation. The IS was composed of a mixture of prostaglandin E2 (0.2 mM), serotonin (2 mM), bradykinin (2 mM) and histamine (2 mM) (Chengdu Shuobo Elite Technology Co., Ltd., Chengdu, China). The IS is based on components found in inflamed human tissues and has been used extensively to induce migraine in animal models (Steen et al., 1995; Oshinsky and Gomonchareonsiri, 2007; Wieseler et al., 2012). A 10-µL volume of inflammatory soup was infused into the dura to induce inflammation through the indwelling catheter, with infusion lasting 2 minutes. The IS was prepared when needed. An equal volume of saline was administered in the control group. The infusion was started on day 8 after surgery and was repeated every 3 days for a total of six IS administrations.
Behavioral assessment
Behavioral assessment was performed during the stimulus and interstimulus periods. Because no behavioral criteria for rhesus monkeys had been published, it was difficult to perform an accurate assessment. Therefore, the assessment was conducted by referencing the behavioral criteria in rats (adapted from Melo-Carrillo and Lopez-Avila, 2013). During the stimulus, two investigators blinded to group assignment observed motor behaviors, such as walking, scratching, escape behaviors and grooming behaviors, 15 minutes prior to and 4 hours after each infusion. The daily activities were recorded at 8:00, 16:00 and 20:00 each day (between stimuli) for 1 hour each. In addition, behavioral assessments were conducted and daily activities were recorded once a day during the recovery period.
Immunohistochemistry
The expression levels of c-fos, nNOS and CGRP were assessed by immunohistochemistry. One hour after the last infusion (the 23rd day after surgery), the rhesus macaques were deeply anesthetized with an overdose of pentobarbital (55 mg/kg) and perfused transcardially with 1 L of 0.9% NaCl solution, followed by 1 L of ice-cold 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4). Then, the brain and cervical spinal cord were immediately removed and postfixed for 12 hours in 4% paraformaldehyde at 4°C under constant gentle agitation. The brain and cervical spinal cord were then immersed in 30% sucrose solution in PBS for 24 hours. Tissue samples were cut from multiple brain areas, including the trigeminal nucleus caudalis, frontal lobe, parietal lobe, thalamus, hypothalamus, hippocampus, midbrain, pons, and the upper and lower segment of the medulla. Frozen coronal sections, 20-μm-thick, were then used for immunohistochemical staining. Briefly, sections were washed in xylene to remove the paraffin, rehydrated through serial dilutions of alcohol, and then washed with PBS (pH 7.2).
For antigen retrieval, sections were placed in a citrate solution (pH 6.0) and heated in a microwave for 5 minutes. Afterwards, the samples were incubated with primary rabbit anti-rhesus antibody (anti-c-fos, SYSY, Gottingen, Germany, 1:500 dilution; anti-nNOS, Millipore, Bayswater, VIC, Australia, 1:300 dilution; anti-CGRP, LSBio, Seattle, WA, USA, 1:200 dilution) for 60 minutes at 37°C and then overnight at 4°C. After washing, the sections were incubated with a biotinylated secondary goat anti-rabbit antibody (Beijing Zhongshan Jinqiao Biological Technology Co., Ltd., Beijing, China) at a concentration of 1:200 for 40 minutes at 37°C. The sections were thereafter incubated with streptavidin-peroxidase for 30 minutes at room temperature. Finally, sections were rinsed, treated with 3,3′-diaminobenzidine (Beijing Zhongshan Jinqiao Biological Technology Co., Ltd.) and counterstained with hematoxylin. Immunostaining was observed with a light microscope (LEICA DM500, Wetzlar, Germany), and representative images were captured. Cells expressing c-fos, nNOS or CGRP contained brownish-yellow granules under the microscope after staining. The proportion of positively-stained cells was determined by counting at 200× magnification by investigators blinded to the experiments.
Statistical analysis
Data, expressed as the mean ± SD, were analyzed using SPSS 20.0 software (SPSS Inc., IBM, Armonk, NY, USA). The expression of c-fos, nNOS and CGRP in rhesus monkeys with and without dural IS stimulation was compared using Student’s t-test. A value of P < 0.05 was considered statistically significant.
Results
Behavioral change in the recurrent migraine rhesus monkey model
No monkey died or suffered serious infection after surgery. Prior to the first infusion, no obvious behavioral differences were observed before or 7 days after cannula implantation. After infusion, the four monkeys in the IS group showed differences in motor activities, compared with the control group. All of the treated monkeys exhibited decreased motor behaviors, including reduced walking, climbing in the cage, eating and aggressive actions. They also displayed an increase in scratching behavior and more frequent changing of posture. During the period between stimuli, the monkeys in the IS group exhibited a reduced length of time spent on daily activities, but they also seemed to be more unsettled compared with the controls, similar to their behavior immediately after the stimuli. No neurological deficit was found in monkeys in either group. During the stimulus period, monkeys sometimes showed increased nose and mouth secretions, 34 to 165 (79.6 ± 50.2) minutes after a single stimulus, lasting for 17 to 51 (33.2 ± 14.3) minutes. This phenomenon was observed in two monkeys in the IS group (two and three times in each of the two monkeys), but was not observed in the control group.
Expression of c-fos, nNOS and CGRP in different brain areas of the recurrent migraine rhesus monkey model c-fos
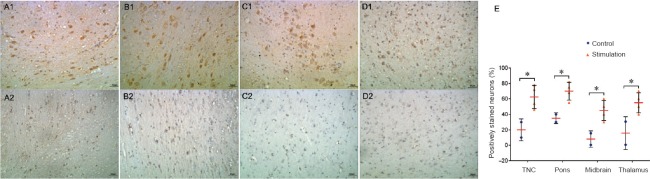
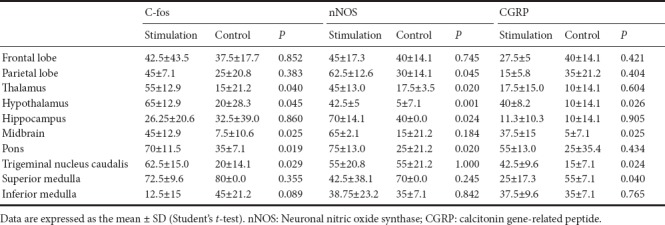
The number of c-fos-positive cells in the trigeminal nucleus caudalis was significantly more in the IS group than in the control group (P = 0.029). Additionally, c-fos-positive cells were more numerous in the IS group in most other tested brain regions involved in pain perception compared with the control group, and the areas with statistically significant differences included the thalamus (P = 0.040), hypothalamus (P = 0.045), midbrain (P = 0.025) and pons (P = 0.019) (Figure 1 and Table 1).
Figure 1.
c-fos immunoreactivity in different brain regions of a rhesus monkey model of recurrent migraine (immunohistochemical staining).
Cells expressing c-fos contained brownish-yellow granules under the microscope after staining. c-fos-positive cells were stained brown primarily in the cytoplasm. Repeated IS stimulation of the dura in rhesus monkeys increased c-fos immunoreactivity in the TNC (A1), pons (B1), midbrain (C1) and thalamus (D1). (A2–D2) Images of the same brain regions from controls. Original magnification, 200×. (E) Plot of the counts of c-fos-positive cells in these four brain regions in the IS and control animals (*P < 0.05). Scale bars: 50 μm. IS: Inflammatory soup; TNC: trigeminal nucleus caudalis.
Table 1.
Ratio (%) of cells positive for c-fos, nNOS or CGRP in each brain region
nNOS
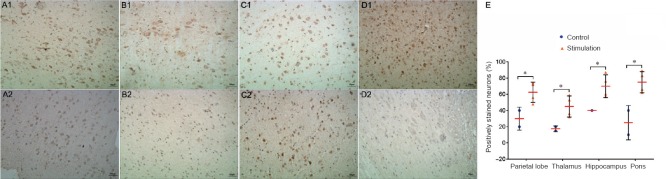
Similarly, nNOS-positive cells in most brain regions were more numerous in the IS group than in the control group, and the following areas showed significant differences between the groups: parietal lobe (P = 0.045), thalamus (P = 0.02), hypothalamus (P = 0.001), hippocampus (P = 0.024) and pons (P = 0.02) (Figure 2 and Table 1).
Figure 2.
n-NOS immunoreactivity in different brain regions of the rhesus monkey model of recurrent migraine (immunohistochemical staining).
Cells containing nNOS showed brownish-yellow granules under the microscope after staining. nNOS-positive cells were stained brown yellow in the nucleus. Repeated IS stimulation of the dura in rhesus monkeys increased the immunoreactivity of n-NOS in the parietal lobe (A1), thalamus (B1), hippocampus (C1) and pons (D1). (A2–D2) Images of the same brain regions from controls. Original magnification, 200×. (E) Plot of the counts of nNOS-positive cells in these four brain regions in the IS and control groups (*P < 0.05). Scale bars: 50 μm. nNOS: Neuronal nitric oxide synthase; IS: inflammatory soup.
CGRP
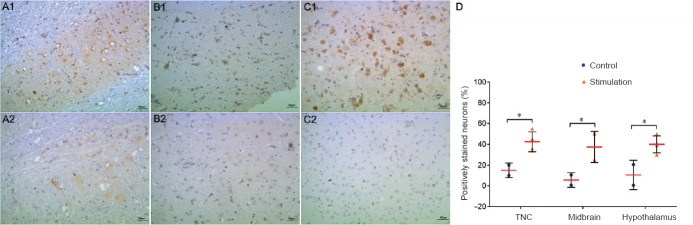
The number of CGRP-positive cells was significantly greater in the hypothalamus (P = 0.026), midbrain (P = 0.025), superior medulla (P = 0.040) and trigeminal nucleus caudalis (P = 0.024) in the IS group compared with the control group (Figure 3 and Table 1).
Figure 3.
CGRP immunoreactivity in different brain regions of the rhesus monkey model of recurrent migraine (immunohistochemical staining).
Cells containing CGRP showed brownish-yellow granules under the microscope after staining. CGRP-positive cells were stained brown primarily in the cytoplasm. Repeated IS stimulation of the dura in rhesus monkeys increased the immunoreactivity of CGPR in the TNC (A1), midbrain (B1) and hypothalamus (C1). (A2–C2) Images of the same brain regions from controls. Original magnification, 200×. (D) Plot of the counts of CGRP-positive cells in these three brain regions in the IS and control groups (*P < 0.05). Scale bars: 50 μm. CGRP: Calcitonin gene-related peptide; IS: inflammatory soup; TNC: trigeminal nucleus caudalis.
These results show that in the thalamus, hypothalamus, midbrain and pons, all three neurogenic inflammatory markers (c-fos, nNOS and CGRP) were more highly expressed in the IS group than in the control group (Table 1).
Discussion
In this study, a model of recurrent migraine was established in conscious rhesus monkeys using repeated inflammatory dural stimulations. Behaviors and c-fos expression levels in the trigeminal nucleus caudalis suggest that this model mimics, at least in part, the pathologic changes in patients with recurrent headache such as migraines. Immunohistochemical analysis suggests pathological changes throughout the whole brain.
The IS group exhibited abnormal behaviors both immediately after the stimulus and during the interstimulus periods. The decreased motor behaviors observed in our experimental monkeys might mimic the reduction in routine physical activities in humans with headache, and the increased scratching and slow movements might reflect discomfort in their heads and unsettled feelings caused by pain. Similar increases in resting behavior have also been found in rat models of chronic headache, which might parallel the reduction in daily activities in human patients (Goadsby et al., 2002). However, the increased nose and mouth secretions during the stimulus period in the present study are not commonly observed in migraineurs. This response might occur because of the hyperactivation of the trigeminovascular system after chemical stimulation of the dura. Thus, this model based on neurogenic inflammation only partially mimics the changes in migraine patients, and further investigation is needed to improve the model. Because no behavioral criteria for rhesus monkeys have been proposed to date, it is difficult to perform a reliable assessment. Thus, it is unclear whether these abnormal behaviors reflect migraine-related symptoms, requiring further investigation.
The trigeminovascular system and neurogenic inflammation have been shown to play important roles in neurovascular headaches, such as migraine. Neurogenic inflammation is a sterile inflammation found in different diseases, including psoriasis, asthma and migraine. It can be induced by the release of inflammatory neuropeptides and exogenous electrical or chemical stimuli (Woolf and Salter, 2000; Eikermann-Haerter and Moskowitz, 2008; Filipović et al., 2014). Several laboratory markers have been proposed to reflect trigeminal stimulation and neurogenic inflammation, including plasma protein extravasation, expression of c-fos mRNA and protein in the trigeminal nucleus caudalis, and blood flow. Most importantly, c-fos immunoreactivity and mRNA levels may more closely reflect pain than assessments of vasoconstriction, protein extravasation and closure of arteriovenous shunts (Hunt et al., 1987). In 1987, for the first time, Hunt et al. reported that stimulation of rat primary sensory neurons increases c-fos-protein-like immunoreactivity in the nuclei of postsynaptic neurons in the dorsal horn of the spinal cord. Furthermore, increased expression of c-fos was found in the brainstem and thalamus in migraine models. In contrast, Clayton et al. (1997) observed an attenuation of fos-like immunoreactivity in the trigeminal nucleus caudalis following trigeminovascular activation in the anesthetized guinea pig. In the present study, we found remarkably increased expression of c-fos in the trigeminal nucleus caudalis, which suggested a migraine-like pathologic change in our models. Moreover, this immunoreactivity is reduced by anti-migraine drugs, such as triptans and dihydroergotamine (Hoskin et al., 1996a, b). Thus, our rhesus monkey model needs to be investigated for its responsiveness to drugs.
c-fos has a unique ability to respond to polysynaptic activation, which allows mapping of functional pathways. This property made it possible to map neuronal activation from dural stimulation to other brain structures involved in the control of migraine pain, including the superior salivatory nucleus, periaqueductal gray and hypothalamus (Akerman et al., 2013). Here, we also found increased expression of c-fos in several other brain regions that are part of the pain matrix, including the thalamus, hypothalamus, midbrain and pons. Nitric oxide, which is generated from inducible nitric oxide synthase, dilates vessels and induces inflammation. After administering nitroglycerin subcutaneously to rats, Knyihár-Csillik et al. (2001) observed an increase in nitric oxide synthase-immunoreactive nerve fibers in the supratentorial cerebral dura mater and the superior sagittal sinus. Nitric oxide is a key molecule in migraine, and the increased expression of nNOS in brain regions involved in nociceptive processing, as shown in the current study, suggests that our animal model might mimic migraine-like headache.
Plasma CGRP is elevated in migraine patients, and sumatriptan reduces it to normal levels. Studies on animal models of migraine induced by dural electrical stimulation also show an elevated release of CGRP and other vasoactive intestinal peptides in the trigeminal nucleus caudalis (Zagami et al., 1990; Zhao et al., 2017). CGRP participates in migraine through both central and peripheral pathways (Russell et al., 2014; Karsan and Goadsby, 2015). Peripherally, the CGRP released at trigeminal nerve endings dilates vessels and induces neurogenic inflammation. However, because CGRP does not excite or sensitize meningeal nociceptors, the peripheral pathway does not seem to be as important as previously thought. Thus, the role of CGRP in central systems might be underestimated. Storer et al. (2004) found a change in nociceptive trigeminovascular transmission following microiontophoresis of CGRP onto neurons in the trigeminocervical complex of the cat. Furthermore, another study showed that CGRP may be associated with allodynia and central sensitization (Sun et al., 2003). To better understand the central role of CGRP, we investigated its expression throughout the brain. The broad expression of CGRP suggests an important role in the central nervous system. Previous studies suggest that CGRP expression may be related to migraine complications, such as photophobia (Noseda and Burstein, 2011). Further studies are needed to elucidate the function of CGRP in various parts of the central nervous system.
The wide central expression of c-fos, nNOS and CGRP suggests that multiple brain regions are involved in migraine. Magnetic resonance imaging studies suggest that, in addition to the spinal trigeminal nucleus and thalamus, the primary and secondary somatosensory cortex (S1, S2), insula, anterior cingulate cortex and prefrontal cortex comprise a neural network that modulates pain (Boly et al., 2008) and might also participate in migraine (Bashir et al., 2013). In addition to imaging studies, anatomical and electrophysiologic findings suggest the participation of these brain regions in pain processing. For example, Marini et al. (1996) identified fibers projecting to the anterior cingulate cortex from the midline nuclei, intralaminar nuclei and ventrobasal nucleus of the thalamus, which receive nociceptive signals from the spinothalamic tract, indicating that the anterior cingulate cortex may also receive and process nociceptive signals (Marini et al., 1996). Electrophysiologic studies also identified pain-related neurons in the human anterior cingulate cortex (Hutchison et al., 1999). Therefore, we profiled immunoreactivity changes in a total of ten brain areas, to provide insight into the regions involved in the modulation of pain in migraine patients.
Repeated stimulation was performed because it seems to better simulate the various clinical and pathophysiological characteristics of migraine than a single stimulation. A previous study showed that rats given more than eight IS infusions display a very large increase in extracellular glutamate and a very long-lasting decrease in periorbital pressure thresholds in response to nitroglycerin compared with IS-naive rats or rats given only a few IS infusions (Oshinsky and Gomonchareonsiri, 2007). These different responses to nitroglycerin in rats can also be observed in patients (Christiansen et al., 1999). Furthermore, chronic and transformed migraine patients also present unique clinical characteristics. For example, while the frequency of attacks increases, headache intensity decreases, and associated symptoms become less obvious. Because it takes years for migraine episodes to transform into daily headaches, it is nearly impossible to test the effects of acute migraine medications before transformation (Buchgreitz et al., 2006). Furthermore, long-term follow-up is needed to assess the effectiveness of preventive drugs. In addition, repeated headaches decrease the effectiveness of acute medication. These observations suggest that repeated dural stimulation better reflects the pathophysiology of migraine. Therefore, our repeated stimulation model should help further our understanding of migraine progression and transformation.
A small number of c-fos-positive cells were found in the control group, which may be explained by anesthesia. Anesthetics can inhibit the pain response, interfere with metabolism in the cortex and other brain regions, and alter the neuronal expression of proteins such as c-fos (Stenberg et al., 2005). Anesthesia can help in performing invasive procedures. However, migraine patients are conscious during an attack. This might lead to observational discrepancies. We also found increased expression of c-fos and nNOS in glial cells. Activated neurons might transmit signals to surrounding cells through gap junctions and by paracrine mechanisms to induce glial expression of these proteins. Glial activation may play an important role in the induction of migraine complications (Thalakoti et al., 2007).
Our study has some limitations. First, the sample size was small, which might impact the statistical analyses. However, primates are expensive and not readily available, and therefore in this pilot study, we could only use a small number of animals to identify tendencies and trends. In future studies, a relatively large sample population will be considered. Second, because no behavioral criteria for rhesus monkeys have been proposed to date, it was difficult to perform an accurate phenotypic assessment. Third, only immunohistochemistry was performed to examine the change in expression of various protein markers, without accurate quantitative assays, such as western blot assay or polymerase chain reaction. Additional assay techniques will be used in upcoming studies to rigorously investigate the pathophysiologic changes in various brain areas to confirm the immunohistochemical findings.
In conclusion, repeated IS stimulation of the dura activates the trigeminovascular system and produces migraine-like pathological changes and abnormal behaviors in conscious rhesus monkeys. Our analysis suggests broad histopathological changes throughout the entire pain matrix. Therefore, this novel model might be useful for preclinical studies on migraine.
Footnotes
Conflicts of interest: All authors declared no conflicts of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81500959 (to NC). Funders had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Institutional review board statement: The study was approved by the Medical Ethics Committee, Sichuan University, China (approval number: K2018060).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81500959 (to NC).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Patel B, Maxwell R; T-Editor: Liu XL
References
- 1.Akerman S, Holland PR, Hoffmann J. Pearls and pitfalls in experimental in vivo models of migraine: dural trigeminovascular nociception. Cephalalgia. 2013;33:577–592. doi: 10.1177/0333102412472071. [DOI] [PubMed] [Google Scholar]
- 2.Bashir A, Lipton RB, Ashina S, Ashina M. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology. 2013;81:1260–1268. doi: 10.1212/WNL.0b013e3182a6cb32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohár Z, Fejes-Szabó A, Tar L, Varga H, Tajti J, Párdutz Á, Vécsei L. Evaluation of c-Fos immunoreactivity in the rat brainstem nuclei relevant in migraine pathogenesis after electrical stimulation of the trigeminal ganglion. Neurol Sci. 2013;34:1597–1604. doi: 10.1007/s10072-013-1292-1. [DOI] [PubMed] [Google Scholar]
- 4.Boly M, Faymonville ME, Schnakers C, Peigneux P, Lambermont B, Phillips C, Lancellotti P, Luxen A, Lamy M, Moonen G, Maquet P, Laureys S. Perception of pain in the minimally conscious state with PET activation: an observational study. Lancet Neurol. 2008;7:1013–1020. doi: 10.1016/S1474-4422(08)70219-9. [DOI] [PubMed] [Google Scholar]
- 5.Buchgreitz L, Lyngberg AC, Bendtsen L, Jensen R. Frequency of headache is related to sensitization: a population study. Pain. 2006;123:19–27. doi: 10.1016/j.pain.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Buture A, Gooriah R, Nimeri R, Ahmed F. Current understanding on pain mechanism in migraine and cluster headache. Anesth Pain Med. 2016;6:e35190. doi: 10.5812/aapm.35190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia. 1999;19:660–667. doi: 10.1046/j.1468-2982.1999.019007660.x. [DOI] [PubMed] [Google Scholar]
- 8.Clayton JS, Gaskin PJ, Beattie DT. Attenuation of Fos-like immunoreactivity in the trigeminal nucleus caudalis following trigeminovascular activation in the anaesthetised guinea-pig. Brain Res. 1997;775:74–80. doi: 10.1016/s0006-8993(97)00930-x. [DOI] [PubMed] [Google Scholar]
- 9.Eikermann-Haerter K, Moskowitz MA. Animal models of migraine headache and aura. Curr Opin Neurol. 2008;21:294–300. doi: 10.1097/WCO.0b013e3282fc25de. [DOI] [PubMed] [Google Scholar]
- 10.Filipović B, Matak I, Lacković Z. Dural neurogenic inflammation induced by neuropathic pain is specific to cranial region. J Neural Transm. 2014;121:555–563. doi: 10.1007/s00702-013-1144-4. [DOI] [PubMed] [Google Scholar]
- 11.Finocchi C, Strada L. Sex-related differences in migraine. Neurol Sci. 2014;35(Suppl 1):207–213. doi: 10.1007/s10072-014-1772-y. [DOI] [PubMed] [Google Scholar]
- 12.Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 13.Hoskin KL, Kaube H, Goadsby PJ. Sumatriptan can inhibit trigeminal afferents by an exclusively neural mechanism. Brain. 1996a;119:1419–1428. doi: 10.1093/brain/119.5.1419. [DOI] [PubMed] [Google Scholar]
- 14.Hoskin KL, Kaube H, Goadsby PJ. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiological study. Brain. 1996b;119:249–256. doi: 10.1093/brain/119.1.249. [DOI] [PubMed] [Google Scholar]
- 15.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 16.Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex. Nat Neurosci. 1999;2:403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- 17.Karsan N, Goadsby PJ. Calcitonin gene-related peptide and migraine. Curr Opin Neurol. 2015;28:250–254. doi: 10.1097/WCO.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 18.Knyihár-Csillik E, Tajti J, Chadaide Z, Csillik B, Vécsei L. Functional immunohistochemistry of neuropeptides and nitric oxide synthase in the nerve fibers of the supratentorial dura mater in an experimental migraine model. Microsc Res Tech. 2001;53:193–211. doi: 10.1002/jemt.1084. [DOI] [PubMed] [Google Scholar]
- 19.Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55(Suppl 2):103–122. doi: 10.1111/head.12505_2. [DOI] [PubMed] [Google Scholar]
- 20.Marini G, Pianca L, Tredici G. Thalamocortical projection from the parafascicular nucleus to layer V pyramidal cells in frontal and cingulate areas of the rat. Neurosci Lett. 1996;203:81–84. doi: 10.1016/0304-3940(95)12266-4. [DOI] [PubMed] [Google Scholar]
- 21.Melo-Carrillo A, Lopez-Avila A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia. 2013;33:1096–1105. doi: 10.1177/0333102413486320. [DOI] [PubMed] [Google Scholar]
- 22.Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984;16:157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- 23.Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43:S16–20. [PubMed] [Google Scholar]
- 24.Moskowitz MA. Pathophysiology of headache--past and present. Headache. 2007;47(Suppl 1):S58–63. doi: 10.1111/j.1526-4610.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- 25.Noseda R, Burstein R. Advances in understanding the mechanisms of migraine-type photophobia. Curr Opin Neurol. 2011;24:197–202. doi: 10.1097/WCO.0b013e3283466c8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther. 2008;120:157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Olesen J, Jansen-Olesen I. Towards a reliable animal model of migraine. Cephalalgia. 2012;32:578–580. doi: 10.1177/0333102412441719. [DOI] [PubMed] [Google Scholar]
- 28.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhesus Macaque Genome Sequencing and Analysis Consortium. Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 30.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silberstein SD. Recent developments in migraine. Lancet. 2008;372:1369–1371. doi: 10.1016/S0140-6736(08)61569-X. [DOI] [PubMed] [Google Scholar]
- 32.Steen KH, Steen AE, Reeh PW. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J Neurosci. 1995;15:3982–3989. doi: 10.1523/JNEUROSCI.15-05-03982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenberg C, Ovlisen K, Svendsen O, Lauritzen B. Effect of local anaesthesia on neuronal c-fos expression in the spinal dorsal horn and hypothalamic paraventricular nucleus after surgery in rats. Basic Clin Pharmacol Toxicol. 2005;96:381–386. doi: 10.1111/j.1742-7843.2005.pto_07.x. [DOI] [PubMed] [Google Scholar]
- 34.Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol. 2004;142:1171–1181. doi: 10.1038/sj.bjp.0705807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. doi: 10.1016/s0304-3959(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 36.Tfelt-Hansen P, Olesen J. Taking the negative view of current migraine treatments: the unmet needs. CNS Drugs. 2012;26:375–382. doi: 10.2165/11630590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wieseler J, Sprunger D, Ellis A, Maier SF, Watkins LR. Indwelling supradural catheters for induction of facial allodynia: surgical procedures, application of inflammatory stimuli, and behavioral testing. Methods Mol Biol. 2012;851:99–107. doi: 10.1007/978-1-61779-561-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 40.Zagami AS, Goadsby PJ, Edvinsson L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides. 1990;16:69–75. doi: 10.1016/0143-4179(90)90114-e. [DOI] [PubMed] [Google Scholar]
- 41.Zhao LP, Liu L, Pei P, Qu ZY, Zhu YP, Wang LP. Electroacupuncture at Fengchi (GB20) inhibits calcitonin gene-related peptide expression in the trigeminovascular system of a rat model of migraine. Neural Regen Res. 2017;12:804–811. doi: 10.4103/1673-5374.206652. [DOI] [PMC free article] [PubMed] [Google Scholar]