Is there a need for small molecule neuroprotectants in inherited retinal degeneration (iRDs)? iRDs are a heterogeneous cluster of diseases which lead to blindness in 1 in every 2–3000 people. A plethora of causative genetic defects were revealed in the last 30 years. Current research focuses on development of pharmacological, biological or mechanical implant treatments. Gene therapy demonstrated success in patients with specific genetic mutations and Luxturna, recently approved by the FDA for RPE65 related disease, entered the market at a cost of $425,000 per eye (Russell et al., 2017). Given over 250 genes link to iRDs, gene-specific therapy for each gene is challenging and limits widespread applicability. Cell-based approaches aim to replace defective photoreceptor or retinal pigment epithelium (RPE) cells, however concerns remain over long-term benefit, safety and integration of transplanted photoreceptor cells (Santos-Ferreira et al., 2016). Human embryonic stem cell (hESC) derived RPE patches appear more promising when transplanted into an initial cohort of patients affected by age-related macular degeneration (AMD) (da Cruz et al., 2018). An alternative to replacing defective cells or genes is to identify neuroprotective factors preventing vision loss. Drug treatment has several advantages including: i) bypass permanent modifications and associated risks ii) ability to fine-tune an effective and safe dose for each patient, iii) can easily stop treatment if adverse effects encountered and iv) is relatively less expensive compared to gene- or cell-therapy.
Why are brain-derived neurotrophic factor (BDNF) mimetics good candidates? From identification of nerve growth factor by Rita Levi-Montalcini in the 1950s, neurotrophic factors emerged as candidate therapeutic agents to counteract neurodegenerative disorders. BDNF belongs to the neurotrophin family and it is involved in the regulation of synaptic plasticity, neuronal development and protection from oxidative stress and apoptosis (Figure 1). The therapeutic potential of BDNF for iRDs relates to activation of the membrane receptor tropomyosin related kinase B (TrkB). However, clinical limitations of BDNF include a short in vivo half-life and inability to cross the blood-retinal barrier. BDNF can also bind, with lower affinity, the p75NTR and sortilin receptors potentially leading to adverse effects. To overcome these pharmacokinetic limitations in research models, BDNF is administered to the eye via intravitreal injection, transplantation of genetically modified stem cells or by viral gene therapy. Recent efforts focused on the identification of small molecule BDNF-mimetics acting as TrkB agonists with better pharmacokinetic properties than recombinant BDNF. In 2010, LM22A-4 was the first small molecule identified to mimic the TrkB-interacting domain of BDNF, and displayed neuronal-protective effects in several in vitro models (Massa et al., 2010). Using cell-based screening, 7,8-dihydroxyflavone (7,8-DHF), a naturally occurring polyphenolic compound was identified as a TrkB agonist (Jang et al., 2010). 7,8-DHF exhibit a protective effects for retinal cells during excitotoxic and oxidative stress and against glucose-induced diabetic apoptosis (Gupta et al., 2013; Yu et al., 2018). Moreover, multiple intra-peritoneal administration of 5 mg/kg of 7,8-DHF results in long-term neuroprotective effects against hypoxic-ischemic injury in immature murine retina (Huang et al., 2018). Furthermore, 7,8-DHF shows neuroprotective activity in several CNS conditions. For example, chronic oral administration of 7,8-DHF at 30 mg/kg per day prevents progressive degeneration of midbrain dopaminergic neurons in a primate model of Parkinson’s disease (He et al., 2016). A key advantage of these small molecules is that structural optimization studies can improve their agonistic efficacy or generate prodrugs with increased bioavailability (Chen et al., 2018). Interestingly, while BDNF-mediated TrkB activation in primary neurons is followed by ubiquitination and subsequent degradation of the receptors, 7,8-DHF does not induce TrkB degradation resulting in a longer lasting signal (Liu et al., 2014). In 2017, a 7,8-DHF prodrug demonstrated in vitro and in vivo efficacy in mammalian models of Alzheimer’s disease. Chronic oral administration for 3 months of the 7,8-DHF prodrug at 7.25 mg/kg per day, improved cognitive function, inhibited the loss of hippocampal synapses, prevented amyloid β protein deposition inhibiting the pathological cleavage of amyloid precursor protein and Tau (Chen et al., 2018).
Figure 1.
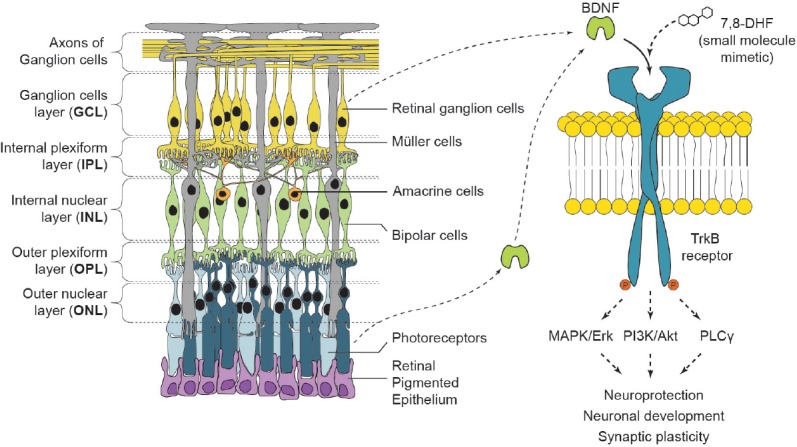
Overview of brain-derived neurotrophic factor (BDNF)/tropomyosin related kinase B (TrkB) signaling in the retina.
Depending on the organism, BDNF is produced by distinct cell types in the retina to act in an autocrine/paracrine fashion. The TrkB-BDNF axis exerts its function in the regulation of synaptic plasticity, neuronal development and neuroprotection through the activation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (Erk), and phospholipase C γ (PLCγ) pathways. Several small molecules, including 7,8-dihydroxyflavone (7,8-DHF) have been shown to act as TrkB agonist and induce a neuroprotective effect in both retina and central nervous system.
What are the potential limitations of BDNF mimetics? It is important to consider and distinguish adverse responses reported for biological BDNF versus BDNF mimetics. BDNF and TrkB have widespread expression in the brain particularly in the neocortex, hippocampus, striatum and brainstem (Liu et al., 2016). Scharfman et al. reported in 1997 that 25–100 ng/mL of BDNF induced neuronal hyperexcitability through overactivation of TrkB signaling in rat entorhinal and hippocampal slices (Scharfman, 1997). This hyperexcitability induces learning and memory impairment at 2–3 and seizures at 6 months of age. Notably, the author later comments: “Pathologic levels of BDNF-dependent synaptic plasticity may contribute to conditions such as epilepsy and chronic pain sensitization, whereas application of the trophic properties of BDNF may lead to novel therapeutic options in neurodegenerative diseases”. Another publication correlates higher levels of BDNF with breast cancer, however, there is no evidence that BDNF is a cancer driver (Patani et al., 2011). In relation to the safety of BDNF mimetics, a 3 week treatment of 7,8-DHF at 5 mg/kg did not induce pathological changes in murine kidney, liver, lung, muscle, spleen, cortex, hippocampus, heart, intestine or testis but did promote neuroprotection and improved function (Liu et al., 2016). In monkey, 11 months of oral 7,8-DHF treatment at 30 mg/kg per day did not demonstrate toxicity on blood, liver, heart, muscle, kidney or pancreas, however, body weight was reduced by about 15% compared to controls (He et al., 2016). Controversy remains regarding BDNF and nociception. Contradicting reports suggest intrathecal administration of BDNF produces a pro-nociceptive or anti-nociceptive effect and microglial-derived BDNF was connected with development of neuropathic pain (Liu et al., 2016). Notably, effects of 7,8-DHF on pain are unknown. Likewise, binding of BDNF to p75NTR or sortilin (low affinity receptors for mature BDNF and high affinity for pro-BDNF) activates apoptotic pathways but whether 7,8-DHF is an agonist of these receptors is unknown (Liu et al., 2016). Interestingly, 7,8-DHF is consumed by humans as a nootropic without any reported undesirable effects.
What are crucial milestones in development of BDNF mimetics for iRDs? BDNF mimetics, e.g. 7,8-DHF, display potential to treat iRDs based on preclinical efficacy and safety in neurodegeneration models. 7,8-DHF significantly restored cone photoreceptor visual function in a zebrafish model of inherited blindness (Daly et al., 2017). It is unknown, however, if 7,8-DHF protects rod photoreceptors or visual function in mammalian iRD models. 7,8-DHF administered orally crosses the blood brain barrier but there is no equivalent evidence reporting distribution across the blood-retinal barrier. The clinical safety profile of 7,8-DHF (or prodrug) is unknown and more studies are necessary to profile any adverse effects following systemic or local ocular delivery and to identify which genetic forms of iRD best respond to 7,8-DHF. Many promising retinal neuroprotective therapeutics including memantine, brimonidine or ciliary neurotrophic factor have failed in clinical trial. By optimizing the appropriate drug formulation, route of delivery, dosing regime and most relevant iRD target diseases 7,8-DHF has potential to be a novel, safe and effective treatment for iRD.
This project has received funding from the Science Foundation Ireland/Enterprise Ireland (SFI/EI) Technology Innovation Development Award (TIDA) grant No. 17/TIDA/4993 and the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 734907 (RISE/3D-NEONET project).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Annagrazia Adornetto, University of Calabria, Italy.
P-Reviewer: Adornetto A; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Chen C, Wang Z, Zhang Z, Liu X, Kang SS, Zhang Y, Ye K. The prodrug of 7, 8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease. Proc Natl Acad Sci U S A. 2018;115:578–583. doi: 10.1073/pnas.1718683115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, Gooljar SB, Carr AF, Vugler A, Ramsden CM, Bictash M, Fenster M, Steer J, Harbinson T, Wilbrey A, Tufail A, et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36:328–337. doi: 10.1038/nbt.4114. [DOI] [PubMed] [Google Scholar]
- 3.Daly C, Shine L, Heffernan T, Deeti S, Reynolds AL, O’Connor JJ, Dillon ET, Duffy DJ, Kolch W, Cagney G, Kennedy BN. A brain-derived neurotrophic factor mimetic is sufficient to restore cone photoreceptor visual function in an inherited blindness model. Sci Rep. 2017;7:11320. doi: 10.1038/s41598-017-11513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta VK, You Y, Li JC, Klistorner A, Graham SL. Protective effects of 7, 8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J Mol Neurosci. 2013;49:96–104. doi: 10.1007/s12031-012-9899-x. [DOI] [PubMed] [Google Scholar]
- 5.He J, Xiang Z, Zhu X, Ai Z, Shen J, Huang T, Liu L, Ji W, Li T. Neuroprotective effects of 7, 8-dihydroxyflavone on midbrain dopaminergic neurons in MPP+-treated monkeys. Sci Rep. 2016;6:34339. doi: 10.1038/srep34339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang HM, Huang CC, Tsai MH, Poon YC, Chang YC. Systemic 7, 8-dihydroxyflavone treatment protects immature retinas against hypoxic-ischemic injury via Müller glia regeneration and MAPK/ERK activation. Invest Ophthalmol Vis Sci. 2018;59:3124–3135. doi: 10.1167/iovs.18-23792. [DOI] [PubMed] [Google Scholar]
- 7.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7, 8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Chan CB, Ye K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl Neurodegener. 2016;5:2. doi: 10.1186/s40035-015-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Obianyo O, Chan CB, Huang J, Xue S, Yang JJ, Zeng F, Goodman M, Ye K. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7, 8-dihydroxyflavone in the binding and activation of the TrkB receptor. J Biol Chem. 2014;289:27571–27584. doi: 10.1074/jbc.M114.562561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN, Nehama D, Rajadas J, Longo FM. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patani N, Jiang WG, Mokbel K. Brain-derived neurotrophic factor expression predicts adverse pathological & clinical outcomes in human breast cancer. Cancer Cell Int. 2011;11:23. doi: 10.1186/1475-2867-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, Wittes J, Pappas J, Elci O, McCague S, Cross D, Marshall KA, Walshire J, Kehoe TL, Reichert H, Davis M, Raffini L, George LA, Hudson FP, Dingfield L, et al. (2017) Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos-Ferreira T, Llonch S, Borsch O, Postel K, Haas J, Ader M. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat Commun. 2016;7:13028. doi: 10.1038/ncomms13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol. 1997;78:1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Liu Q, Wang X, Liu H, Wang Y. 7,8-Dihydroxyflavone ameliorates high-glucose induced diabetic apoptosis in human retinal pigment epithelial cells by activating TrkB. Biochem Biophys Res Commun. 2018;495:922–927. doi: 10.1016/j.bbrc.2017.11.007. [DOI] [PubMed] [Google Scholar]


