Keywords: nerve regeneration, tethered cord syndrome, surgery, therapy, prognosis, children patients, surgical outcome, surgical methods, prophylactic surgery, spina bifida, neural regeneration
Abstract
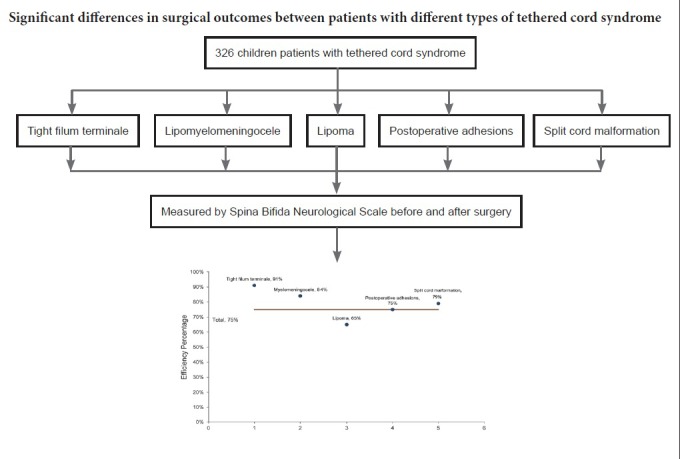
Tethered cord syndrome is a progressive disease with a typically insidious onset in infants and children, and which can lead to persistent progress of neurological deficits and a high rate of disability without timely intervention. The purpose of this study was to investigate the curative effect of microsurgery in children with different types of tethered cord syndrome. In this study, we analyzed 326 patients with tethered cord syndrome, aged from 2 months to 14 years old, who were followed for 3–36 months after microscopic surgery. Based on clinical manifestations and imaging findings, these patients were classified into five types: tight filum terminale (53 cases), lipomyelomeningocele (55 cases), lipomatous malformation (124 cases), postoperative adhesions (56 cases), and split cord malformation (38 cases). All patients underwent microsurgery. Curative effects were measured before and 3 months after surgery by Spina Bifida Neurological Scale based on sensory and motor functions, reflexes, and bladder and bowel function. The results showed that Spina Bifida Neurological Scale scores improved in all five types after surgery. Overall effective rates in these patients were 75%. Effective rates were 91% in tight filum terminale, 84% in lipomyelomeningocele, 65% in lipomatous malformation, 75% in postoperative adhesion, and 79% in split cord malformation. Binary logistic regression analysis revealed that types of tethered cord syndrome (lipoma-type or not) and symptom duration before surgery were independent influencing factors of surgical outcome. These results show that therapeutic effect is markedly different in patients with different types of tethered cord syndrome. Suitable clinical classification for tethered cord syndrome will be helpful in predicting prognosis and guiding treatment. This trial has been registered in the Chinese Clinical Trial Registry (registration number: ChiCTR1800016464).
Chinese Library Classification No. R459.9; R744
Introduction
Tethered cord syndrome (TCS) results from fixation of the spinal cord in the vertebral canal due to adhesions of either congenital or acquired origin, which ultimately lead to mechanical torsion and ischemia in the distal spinal cord including conus medullaris. Thus, motor-sensory deficits and associated urological and orthopedic complications are observed in TCS (Kang et al., 2009; Solmaz et al., 2011). Symptoms of TCS include bladder/bowel dysfunction, neuro-orthopedic abnormalities, weakness in the lower extremities, sensory anomalies, back and leg pain, and scoliosis (Vassilyadi et al., 2012). TSC is an important factor of neurological and urological problems in children (McLone, 1996; Solmaz et al., 2011; Ostling et al., 2012; Foster et al., 2014). Congenital TCS in children is usually occult (not readily discernible by signs or symptoms), with neurological dysfunction progressively increasing during childhood growth (Lew et al., 2007). In our clinical practice, we have found that symptoms in adults such as neuro-orthopedic abnormalities are usually more significant than in children, and can seriously affect the adults’ quality of life and are associated with high morbidity. Currently, there is no unified classification method for TCS. Consequently, in this study, we enrolled 326 children with different types of TCS and aimed to firstly determine the effect of surgical treatment, then identify differences in curative effect between various types of TCS, and finally discuss the clinical classification and surgical technique.
Subjects and Methods
Subjects
This is a retrospective case analysis. Data were available from 326 children (< 15 years old) who had undergone microsurgery between January 2006 and June 2014 in the Chinese People’s Liberation Army (PLA) General Hospital, China.
Included children were diagnosed with TCS before the age of 15 years old. Children were excluded because of: (1) simultaneous surgery for other spinal diseases, such as spinal dural arteriovenous fistula, neurenteric cyst, and sacral canal cyst; (2) simultaneous surgery for different types of TCS, for example, patients underwent surgery on both split cord malformation and lipoma; and (3) extra-dural surgery.
A total of 326 patients (128 males and 198 females) were included in this retrospective analysis. Mean age was 8.50 ± 3.94 years. Protocols were approved by the Ethics Committee of the Chinese PLA General Hospital of China (approval No. S2016-074-01). Clinical symptoms and signs included lumbosacral cutaneous neoplasm, hair tufts, pilonidal sinus (in 92 cases), urinary retention and urinary incontinence (in 121 cases), bowel disorder (in 38 cases), bilateral or unilateral lower limb muscle atrophy, weak muscle strength (in 52 cases), unilateral, bilateral clubfoot, or cavus deformity (in 46 cases), lower extremity, perineal numbness or hypoesthesia (in 36 cases), lumbosacral region and lower limb pain (in 25 cases), and perineal or foot skin ulcers (in 16 cases). Ultrasound examination showed renal pelvis dilatation or hydronephrosis in 15 cases. Diagnoses of TCS were made at 2–21 weeks (average 15.00 ± 5.89 weeks) before surgery.
Imaging findings and clinical types
Radiological examinations showed that all children with TCS exhibited different types of bone deformities. Spina bifida was the most common type, while sacrococcygeal bone dysplasia, scoliosis, vertebral insufficiency, and sub-section half-cone malformations were also found. All patients underwent magnetic resonance imaging (MRI) examination. MRI results showed conus below the S1 level in 216 cases, and conus in the normal position in two cases. As some patients had 4 or 6 lumbar vertebrae, the last lumbar vertebrae with a rectangular shape was defined as L5 (Paik et al., 2013).
Surgical methods
Patients received general anesthesia in the prone position. Longitudinal incisions were usually preferred to fully expose the operational field. The bony exposure had to be wide enough to expose the normal dura. Excess fat, fibrous tissue, and scarring were removed, which caused spinal cord and nerve compression. The dural sac was usually opened along the midline under a microscope. As much as possible, all factors that may stretch and compress the spinal cord (such as lipoma and bony spur) were removed. Finally, the filum terminale was cut to relieve tension in the lower spine and cauda equina.
Different operational points were used for TCS. (1) Tight filum terminale: the boundary between the spinal cord and filum terminale was identified and the lower end of the filum terminale was cut. (2) Lipomyelomeningocele: after separately expanding the upper and lower lamina of the bulging part, the boundary of normal dura was identified to free the bulging sac and protect the spinal cord (which may potentially bulge out). After freeing the adhesion nerves, the excess dura sac was removed to ensure the bulging cord and nerve roots sank into the dura sac. Lastly, 5-0 absorbable suture was used to remodel the lumbosacral epidural shape. The most important objective was to build an adequate barrier above the spinal defect to preserve remnant neural function and prevent infection (Dias, 1999; McLone, 2005; Mattogno et al., 2017). (3) Cicatricial adhesion-type: surgical removal of the scar and adhesion(s) were performed under a microscope with electrophysiological monitoring. (4) Lipoma type: there were several forms of this type, with the surgery being relatively complicated. Based on the relationship between lipoma and conus medullaris according to the study of Pang et al. (2013), lipomas could be divided into dorsal, transitional, terminal, and chaotic types. For the terminal type, the boundary between the spinal cord and filum terminale lipoma was intraoperatively identified and the lipoma resected. For dorsal and transitional types, it was necessary to identify the neural placode. Dissection began from the rostral end of the lipoma and was cut all the way along the neural placode using a Cavitron Ultrasonic Surgical Aspirator and neurophysiological monitoring. Total or near-total resection was achieved in most cases. For some chaotic types, only near-total excision was possible as it was difficult to identify the complete neural placode and the nerve root always traversed through the lipoma. After removal of the lipoma, and ensuring that nerve adhesions were completely released, the pia from both sides was sutured together with 6-0 absorbable thread to reconstruct the neural placode and reduce the risk of local adhesions. Finally, the dural sac was repaired using artificial dura mater. (5) Split cord malformation: the surgical approaches were different for types I and II of diastematomyelia deformities (Pang sub-types: type I reflects bone separation and type II reflects fiber or membrane separation) (Shang et al., 2010). For type I, after removing the bone spur, part of the dura mater was resected to turn two dural sacs into a single one. This achieved complete decompression of the spinal cord and cut the end of the filum terminale. For type II, the cause for the tethered cord was specially analyzed, and the adhesive spinal nerves loosened or the filum terminale resected.
Efficacy assessment
The Spina Bifida Neurological Scale (SBNS) recommended by Oi et al. (1992) was used for neurological scoring and grading. All patients were graded by SBNS pre-surgery and 3 months after surgery to evaluate efficacy. Scoring was based on: (1) motor function; (2) reflexes; and (3) bowel and bladder function. Scoring was respectively rated as 6 points, 4 points, 5 points, with a total score of 15 points regarding the level of spinal function from grade I to V. Grade I: No spinal neurological deficit; grade II: independent activity but unable to control urine; grade III: independent activity associated with general paresis; grade IV: patients are incapable of taking care of themselves; and grade V: completely bed-ridden.
Treatment efficacy was evaluated based on the following method. Marked effect: one or more items of postoperative sensory, motor, or urine symptoms were dramatically improved. Stable: postoperative symptoms improved slightly. For those that were not remarkably improved, the symptoms and signs did not become worse with aging. Invalid: postoperative symptoms were not relieved, and the symptoms and signs gradually retrogressed with aging. Aggravated: lower extremity and/or bowel and bladder dysfunction retrogressed postoperatively compared with preoperation. Patients were unable to recover within one year.
Effective rate was calculated as follows: Efficiency (%) = (marked effect + stable) / total number of cases followed-up.
Statistical analysis
Data were analyzed using SPSS 20.0 software (IBM, Armonk, NY, USA). The results of scoring were considered to be distributed normally. Measurement data were described as the mean ± SD. Binomial dichotomized data were compared by chi-square test. Mean values between groups were compared using the paired t-test. Binary logistic regression analysis was performed to assess factors that independently influence treatment efficiency. P values < 0.05 were considered statistically significant.
Results
Quantitative analysis of participants
No patient was lost from the study during follow-up. A total of 326 patients were included in this retrospective analysis (Figure 1).
Figure 1.
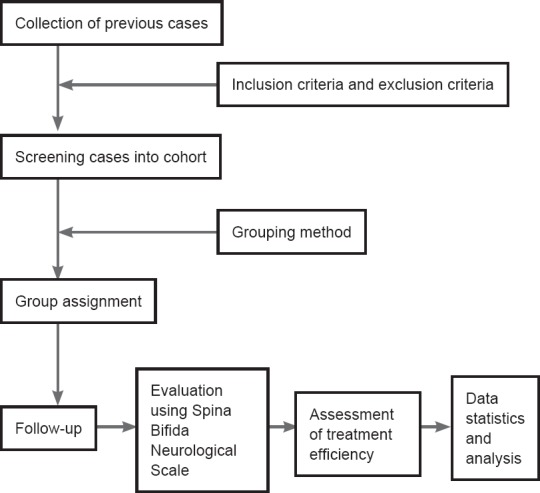
Study flowchart.
Baseline analysis of participants
All patients were evaluated by SBNS immediately after admission. Diagnoses of TCS were made at 2–21 weeks (15 weeks on average) before surgery. Surgery was performed at 1–12 days (4 days on average) after admission. Post-surgery scores were collected at final observation points (at least 3 months after surgery).
Outcome analysis
Patients were followed up from 3 months to 3 years. Preoperative and postoperative SBNS functional classification and statistical analysis results are shown in Figure 2. According to SBNS grading scores and combined preoperative and postoperative sensory, motor, reflex, bowel, and bladder situations, clinical efficacy was classified as: marked effect, stable, ineffective, and worse. Postoperative efficacy results for different types of TCS are shown in Figures 3 and 4. Since untreated TCS showed a high deterioration rate, current symptoms were considered valid or effective depending on whether they were improved or stable after operation. The efficiency of different types of TCS preoperatively and postoperatively was: tight filum terminale, 91%; lipomyelomeningocele, 84%; lipoma-type, 65%; postoperative adhesion-type, 75%, and split cord malformation, 79%. The overall effective rate in this group was 75%. According to univariate analysis results (Table 1), we found that patients without lipoma (P < 0.01) and with short symptom duration (P < 0.01) had a high efficiency rate. To eliminate mutual interference between isolated variables, we performed binary logistic regression analysis. Four variables including age, gender, symptom duration, and type of TCS (lipoma type or not) were included. In this model, lipoma type was coded as 1 and non-lipoma type as 0. Males were represented by 1 and females by 0. The results showed that type of TCS (OR = 2.621, 95% CI: 1.555–4.418, P < 0.01) and length of symptom duration before surgery (OR = 1.451, 95% CI: 1.302–1.573, P < 0.05) remained independent predictors. Typical cases are exhibited in (Figures 5 and 6).
Figure 2.
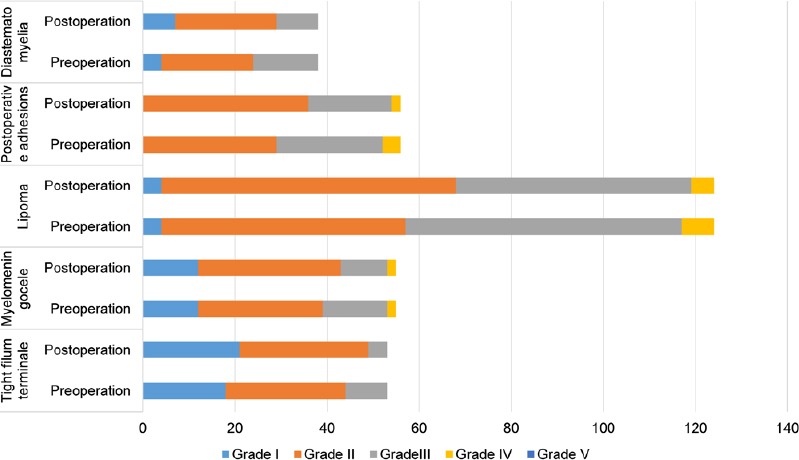
Spina Bifida Neurological Scale (SBNS) functional classification of children with different types of tethered cord syndrome before surgery and 3 months after surgery (n = 326).
Horizontal axis shows the number of patients. SBNS scores pre- and post-operation were measured by paired t-test (P < 0.05). P-values of each type are: tight filum terminale-type, 0.004; lipomyelomeningocele-type, 0.044; lipoma-type, 0.008; postoperative adhesion-type, 0.011; and split cord malformation-type, 0.003. Differences were statistically significant.
Figure 3.
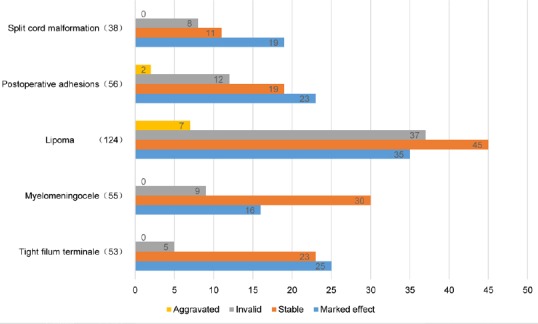
Efficacy analysis of different types of tethered cord syndrome postoperatively.
Horizontal axis shows the number of patients (n = 326).
Figure 4.
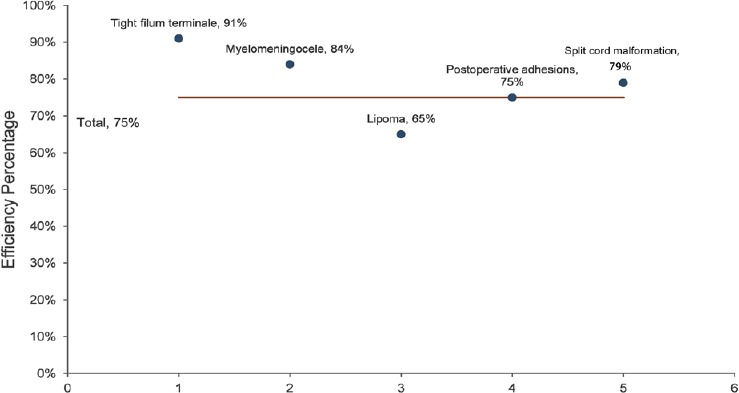
Efficacy percentage of different types of tethered cord syndrome postoperatively.
Efficiency = (marked effect + stable)/total number of cases followed up (n = 326).
Table 1.
Results of univariate analysis of factors that may affect treatment efficiency
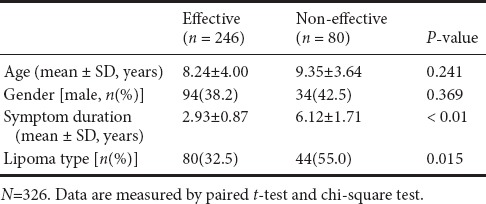
Figure 5.
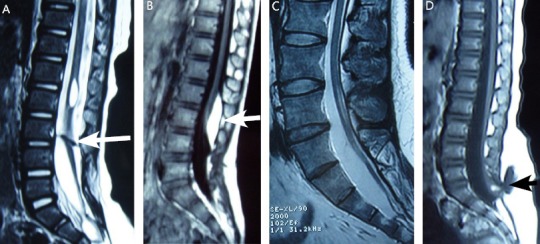
Typical cases.
(A) A female patient aged 12 years was diagnosed with diastematomyelia type I, T2-weighted. The white arrow shows a bone fissure. (B) A female patient aged 5 years was diagnosed with dorsal lipoma, T1-weighted. The white arrow shows a lipoma. (C) A male patient aged 14 years was diagnosed with tight filum terminale, T2-weighted. (D) A female patient aged 2 years was diagnosed with lipomyelomeningocele, T1-weighted. The arrow shows a bulging mass.
Figure 6.
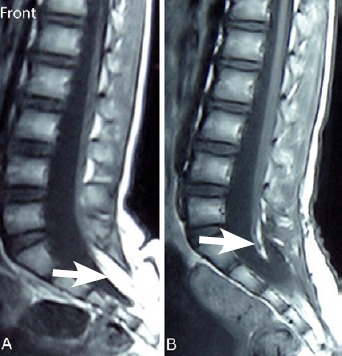
Typical case.
(A) T1-weighted magnetic resonance image of a 7-year-old female patient with transitional lipoma at S1–3 level. White arrow shows lipoma. (B) T1-weighted magnetic resonance image scanned 6 months after surgery. White arrow shows that the lipoma was almost completely removed.
Complications
Cerebrospinal fluid leakage was observed in seven cases, six of which belonged to lipomatous malformation-type and one belonged to postoperative adhesion-type. They were all cured after conservative treatment. Urinary retention occurred in six patients (lipoma) during the early postoperation period. Nine cases experienced lower extremity numbness, and three cases had decreased distal limb muscle strength. Delayed wound healing occurred in two cases.
Discussion
Clinical features and imaging performance of primary TCS are complicated. Currently, there is no unified consensus on the classification of primary TCS (Table 2). Considering the relationship between cause of primary TCS and spina bifida, Lew and Kothbauer (2007) divided TCS into occult spinal dysraphism and spina bifida aperta based on a genetic point of view. Prior-type contains thickened filum terminale, dermal sinus, lumbosacral lipoma, lipomyelomeningocele, split cord malformation, and neuroenteric cyst. Post-type contains spinal meningocele, myelomeningocele, myeloschisis, and hemimyelomeningocele. However, this approach is too complicated for the clinic and can easily be confused. Meanwhile, van Leeuwen et al. (2001) divided adult TCS into four types: postrepair myelomeningocele, filum terminale lipoma and tight filum terminale, lipomyelomeningocele and conus lipoma, and split cord malformation. Later, Lee et al. (2006) divided TCS into postrepair myelomeningocoele, tight filum terminale, lipomatous malformation, split cord malformation, and arachnoidal adhesions. In China, Zhou et al. (1994) divided TCS into thickened filum terminale, lipoma type, cicatricial adhesions, spinal cord or cauda equina tumor, and mixed type. Meanwhile, Li et al. (2005) divided TCS into six types according to spinal cord morphology and changes in pathological anatomy: filum terminale tethered (including A and B subtypes), spinal cord adhesions, spinal lipoma, cyst, diastematomyelia, and static lesions.
Table 2.
Synthesis of studies on classification of tethered cord syndrome
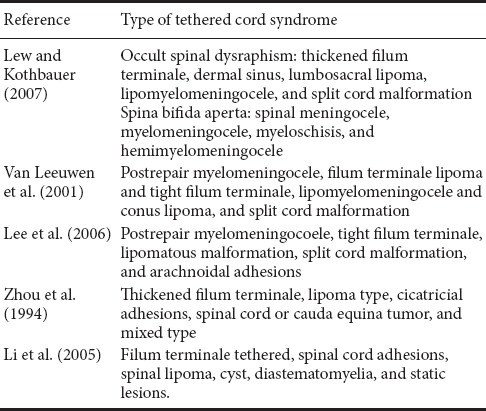
We tend towards agreeing with the classification method of Lee et al. Nonetheless, some adjustments are necessary in the case of children. For example, as a common form of childhood TCS, lipomyelomeningocele is used as a separate clinical type since its clinical and imaging features are specific. Arachnoid adhesions may exist in many types of TCS, and although this complication can also be caused by inflammation and trauma, arachnoid adhesions should not be considered a separate type to avoid confusion. For children with congenital TCS, we have adopted a classification method in the clinic that is mainly based on etiology, clinical features, and especially MRI manifestations. This method is simple, explicit, and helpful in guiding treatment and predicting prognosis.
After analyzing the data for our group of patients, we believe there are many factors that may affect curative effect and prognosis, including TCS type and length of symptom duration. Among the different types of TCS, patients with tight filum terminale type resulted in optimal curative effective, with less complications than in other types due to reduced degree of structural changes and milder symptoms. Surprisingly, lipomyelomeningocele-type achieved a high level of surgical efficacy. This may be due to early surgical treatment (an average of 11 months after diagnosis) of the significant lumbosacral bulging mass. Split cord malformation-type also reached a relatively good efficacy rate of 79%. Although the anatomical structure of lipomyelomeningocele-type and split cord malformation-type was substantially more complicated than tight filum terminale-type, high structural integrity of the spinal cord in these two types had good results. Because of possible structural damage due to previous surgeries and existence of cicatricial adhesions (which increase the difficulty of retethering), postoperative adhesion-type had a low rate of efficacy. Compared with other types, lipoma-type had the worst efficacy due to its complex anatomical lesions, which resulted in considerable structural damage. Increasing evidence shows that aggressive and early prophylactic surgery performed on lipomas can have good long-term results (Pang et al., 2009; da Rosa et al., 2016; Bai et al., 2018). We also observed three cases of self-growth lipoma, which were strongly associated with substantial weight changes (Gao et al., 2016). Therefore, to prevent TCS aggravation, it is important to perform surgical treatment early.
Currently, most clinicians believe that patients with symptomatic TCS should have early surgical treatment (Von Koch et al., 2002; Kumar and Singh, 2003). For asymptomatic patients (low or TSC), whether or not to adopt prophylactic surgery is still controversial (Pang et al., 2010; Usami et al., 2016), but the safety of prophylactic surgery is supported by some proponents (van der Meulen et al., 2002). Nevertheless, although symptoms in adults are generally more serious than in children, we do not agree that untethering operations in adults are more difficult. It should also be noted that for the surgical approach of lipomyelomeningocele, simply adopting a bulging mass excision repair operation often causes postoperative local scar formation and critical retethering. Therefore, for lipomyelomeningocele, we aim to thoroughly release all factors that may cause spinal cord nerve adhesions under the microscope. Lipoma-type is more complicated compared with other types of TCS, while dorsal, transitional, and terminal types of lipoma can be totally or near-totally resected, the effect of which is good. However, for the chaotic-type lipoma of TCS, difficulties are encountered in the operational procedure. Yet with using electrophysiological monitoring and a Cavitron Ultrasonic Surgical Aspirator, most of the lipoma can be resected and nerve adhesions released.
Degree of intraoperative neurolysis is also an important factor that can ultimately affect efficacy. Prognosis of the operation is affected by both irreversible structural changes at a tethering level and more cranial segments of the spinal cord. Thus, radical untethering is necessary (Sysoev et al., 2018). It is difficult to completely release adhesive nerves for some lipoma-types, which is a main reason for an unsatisfactory curative effect. In addition, incomplete neurolysis may increase the risk of retethering, which is an important reason for postoperative deterioration or repeated condition. The previously reported incidence is approximately 5–50% (Kang et al., 2003; Lew and Kothbauer 2007), and the retethering rate in this group of three-years-old is 6%. We reconstructed the conus medullaris by suturing spinal pia mater from both sides of the neural placode, and then completing an expanded repair of the dura sac using artificial spinal dura mater to reduce the incidence of retethering.
Because the level of medullary conus does not considerably increase in the majority of cases, indication of retethering is not obvious by MRI. Consequently, there are limitations for evaluating the curative effect of TCS with imaging methods alone. Analyzing clinical symptoms and signs combined with different objective examinations can alleviate this limitation. For example, properties of neurogenic bladder and urethra dysfunction can objectively be reflected by urodynamic results. This method can predict damage to the upper urinary tract and provide an objective clinical index.
In addition, electromyography and surface somatosensory evoked potentials of the lower extremities and perineum are helpful for determining the severity extent of lipoma and thickened filum terminale, which cause TCS. These methods provide certain significance for surgical planning and evaluation of therapeutic effects. We also believe that the use of state-of-the-art neurophysiological monitoring during surgery is essential. Intraoperative electrophysiological monitoring has become sine qua non in TCS surgery. With the help of intraoperative electromyographic monitoring, motor roots and functional spinal cord can be accurately identified during the untethering operation. Needles are commonly inserted into the sartorius (L1), rectus femoris (L2–3), anterior tibialis (L4–5), and extensor hallucius (L5). Gastrocnemius (S1) and external anal sphincter (S2–4) activities can also be recorded. To monitor conduction of L4–S1 cord segments in some cases, posterior tibial nerve somatosensory evoked potentials and motor conductive velocities (posterior tibial nerve somatosensory evoked potential and motor conductive velocity examination of the posterior tibial and common peroneal nerves) were applied during the operation. Our results show a delayed cortical potential latency with low or absent amplitude. A slight to moderate decrease or complete block was detected in motor conductive velocity, which showed a decreased active potential amplitude. This indicates that changes in motor conductive velocity correlate with clinical neurological changes. Although pudendal somatosensory evoked potentials were used to monitor S2–4 cord segments, some infants are susceptible to inhalation anesthetics, while evoked cortical tracings are unstable, thereby limiting the value of intraoperative electromyographic monitoring when applied in children younger than 1 year of age (Fone et al., 1997; Giddens et al., 1999; Pang et al., 2009). Recently, we used far-infrared thermography technology to analyze revascularization of the lower extremities by comparing temperature changes post- and pre-operation. In all cases, temperature of the lower extremities elevated at different levels, which may provide objective evaluation and be a good indicator for lower extremity sensory function. After observing this group, we found marked improvement in nutritional status after operation. In total, 16 TCS patients had foot or perineal refractory ulcers, and all were healed by 3 months after surgery. We detected blood flow and temperature of the distal lower extremities and found improvements with the help of far-infrared thermography technology.
In this study, efficacy of refractory ulcers in the lower limbs was best: all ulcers were healed (100%), with pain relieved (92%), bowel and bladder function improved (68%), and lower extremity muscle strength improved (35%). Muscle strength improvements mainly manifested in the dorsiflexion and plantarflexion strength of ankles and toes. Patients who suffered from muscle atrophy and bone deformities were unable to fully recover, but surgical operation prevented the deformity from worsening.
Taken together, early diagnosis and microsurgical operation are key to treating TCS. Several factors are associated with operational effect and prognosis, including TCS type, length of course, timing of operation, symptom severity, neural neurolysis, and retethering. Suitable clinical classification for TCS is helpful in predicting prognosis and guiding treatment.
Additional files: Open peer review reports 1 (48.3KB, pdf) and 2 (7.3KB, pdf) .
Acknowledgments:
We would like to express our gratitude to Professor Dachling Pang (MD, Professor of Pediatr Neurosurg, University of California, Davis, Chief of Pediatr Neurosurg) for his pioneering work on TCS.
Footnotes
Conflicts of interest: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support: This study was supported by the Science Foundation of Military Medical Research and Clinical Research Foundation of PLA General Hospital in China, No. 2016FC-CXYY-1006 (to AJS); the Application of Clinical Features of Capital City of Science and Technology Commission in China, No. Z171100001017140 (to AJS). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The study was approved by the ethical review board of PLA General Hospital in January 2016 (approval No. S2016-074-01). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Reporting statement: This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of PLA General Hospital in Beijing of China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be in particular shared. Study protocol form will be available. The data will be available immediately following publication without end date. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Mitsuhiro Enomoto, Tokyo Medical and Dental University, Japan; Brandon Miller, University of Kentucky, USA.
Funding: This study was supported by the Science Foundation of Military Medical Research and Clinical Research Foundation of PLA General Hospital in China, No. 2016FC-CXYY-1006 (to AJS); a grant from the Application of Clinical Features of Capital City of Science and Technology Commission in China, No. Z171100001017140 (to AJS).
P-Reviewers: Enomoto M, Miller B; C-Editor: Zhao M; S-Editors: Yu J, Wang J, Li CH; L-Editors: James R, Hindle A, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Bai S, Tao B, Wang L, Yu X, Xu B, Shang A. Aggressive resection of congenital lumbosacral lipomas in adults: indications, techniques and outcomes in 122 patients. World Neurosurg. 2018;112:e331–e341. doi: 10.1016/j.wneu.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 2.da Rosa SP, Scavarda D, Choux M. Results of the prophylactic surgery of lumbosacral lipomas 20 years of experience in the paediatric neurosurgery department La Timone enfants hospital, Marseille, France. Childs Nerv Syst. 2016;32:2205–2209. doi: 10.1007/s00381-016-3198-8. [DOI] [PubMed] [Google Scholar]
- 3.Dias MS. Myelomeningocele repair in utero. Pediatr Neurosurg. 1999;30:108. doi: 10.1159/000028773. [DOI] [PubMed] [Google Scholar]
- 4.Fone P, Vapnek J, Litwiller S, Couillard D, McDonald C, Boggan J, Stone A. Urodynamic findings in the tethered spinal cord syndrome: does surgical release improve bladder function. J Urol. 1997;157:604–609. [PubMed] [Google Scholar]
- 5.Foster K, Lam S, Lin Y, Greene S. Putative height acceleration following tethered cord release in children. J Neurosurg Pediatr. 2014;14:626–634. doi: 10.3171/2014.9.PEDS1417. [DOI] [PubMed] [Google Scholar]
- 6.Gao J, Kong X, Li Z, Wang T, Li Y. Surgical treatments on adult tethered cord syndrome: a retrospective study. Medicine (Baltimore) 2016;95:e5454. doi: 10.1097/MD.0000000000005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giddens JL, Radomski SB, Hirshberg ED, Hassouna M, Fehlings M. Urodynamic findings in adults with the tethered cord syndrome. J Urol. 1999;161:1249–1254. [PubMed] [Google Scholar]
- 8.Kang J, Yoon K, Ha S, Lee I, Jeun S, Kang S. Surgical management and outcome of tethered cord syndrome in school-aged children, adolescents, and young adults. J Korean Neurosurg Soc. 2009;46:468–471. doi: 10.3340/jkns.2009.46.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang JK, Lee KS, Jeun SS, Lee IW, Kim MC. Role of surgery for maintaining urological function and prevention of retethering in the treatment of lipomeningomyelocele: experience recorded in 75 lipomeningomyelocele patients. Childs Nerv Syst. 2003;19:23–29. doi: 10.1007/s00381-002-0674-0. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Singh S. Spinal dysraphism: trends in northern India. Pediatr Neurosurg. 2003;38:133–145. doi: 10.1159/000068819. [DOI] [PubMed] [Google Scholar]
- 11.Lee GY, Gong GW, Paradiso G, Fehlings MG. Adult tethered cord syndrome: clinical considerations and surgical management. Neurosurg Q. 2006;16:55–66. [Google Scholar]
- 12.Lew SM, Kothbauer KF. Tethered cord syndrome: an updated review. Pediatr Neurosurg. 2007;43:236–248. doi: 10.1159/000098836. [DOI] [PubMed] [Google Scholar]
- 13.Li JL, Chen YL, Chen WX. A study on surgical pathophysicology of myelodysplasia. Zhonghua Xiaoer Waike Zazhi. 2005;26:586–588. [Google Scholar]
- 14.Mattogno PP, Massimi L, Tamburrini G, Frassanito P, Di Rocco C, Caldarelli M. Myelomeningocele repair: surgical management based on a 30-year experience. Acta Neurochir. 2017;Suppl 124:138–148. doi: 10.1007/978-3-319-39546-3_22. [DOI] [PubMed] [Google Scholar]
- 15.McLone D. The adult with a tethered cord. Clin Neurosurg. 1996;43:203–209. [PubMed] [Google Scholar]
- 16.McLone DG. Spinal dysraphism: impact of technique and technology on expectations. Clin Neurosurg. 2005;52:261. [PubMed] [Google Scholar]
- 17.Oi S, Matsumoto S. A proposed grading and scoring system for spina bifida: Spina Bifida Neurological Scale (SBNS) Childs Nerv Syst. 1992;8:337–342. doi: 10.1007/BF00296565. [DOI] [PubMed] [Google Scholar]
- 18.Ostling L, Bierbrauer K, Kuntz C. Outcome, reoperation, and complications in 99 consecutive children operated for tight or fatty filum. World Neurosurg. 2012;77:187–191. doi: 10.1016/j.wneu.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Paik NC, Lim CS, Jang HS. Numeric and morphological verification of lumbosacral segments in 8280 consecutive patients. Spine. 2013;38:E573–578. doi: 10.1097/BRS.0b013e31828b7195. [DOI] [PubMed] [Google Scholar]
- 20.Pang D, Zovickian J, Oviedo A. Long-term outcome of total and near-total resection of spinal cord lipomas and radical reconstruction of the neural placode: part I-surgical technique. Neurosurgery. 2009;65:511–529. doi: 10.1227/01.NEU.0000350879.02128.80. [DOI] [PubMed] [Google Scholar]
- 21.Pang D, Zovickian J, Oviedo A. Long-term outcome of total and near-total resection of spinal cord lipomas and radical reconstruction of the neural placode, part II: outcome analysis and preoperative profiling. Neurosurgery. 2010;66:253–273. doi: 10.1227/01.NEU.0000363598.81101.7B. [DOI] [PubMed] [Google Scholar]
- 22.Pang D, Zovickian J, Wong S, Hou Y, Moes G. Surgical treatment of complex spinal cord lipomas. Childs Nerv Syst. 2013;29:1485–1513. doi: 10.1007/s00381-013-2187-4. [DOI] [PubMed] [Google Scholar]
- 23.Shang AJ, Zhang YZ, Qiao GY. Surgical treatment of split cord malformation. Junyi Jinxiu Xueyuan Xuebao. 2010;4:307–311. [Google Scholar]
- 24.Solmaz I, Izci Y, Albayrak B, Cetinalp E, Kural C, Sengul G, Gocmez C, Pusat S, Tuzun Y. Tethered cord syndrome in childhood: special emphasis on the surgical technique and review of the literature with our experience. Turk Neurosurg. 2011;21:516–521. [PubMed] [Google Scholar]
- 25.Sysoev K, Tadevosyan A, Samochernykh K, Khachatryan W. Prognosis of surgical treatment of the tethered cord syndrome in children. Childs Nerv Syst. 2018;34:305–310. doi: 10.1007/s00381-017-3630-8. [DOI] [PubMed] [Google Scholar]
- 26.Usami K, Lallemant P, Roujeau T, James S, Beccaria K, Levy R, Di Rocco F, Sainte-Rose C, Zerah M. Spinal lipoma of the filum terminale: review of 174 consecutive patients. Childs Nerv Syst. 2016;32:1265–1272. doi: 10.1007/s00381-016-3072-8. [DOI] [PubMed] [Google Scholar]
- 27.van der Meulen WD, Hoving EW, Staal-Schreinemacher A, Begeer JH. Analysis of different treatment modalities of tethered cord syndrome. Childs Nerv Syst. 2002;18:513–517. doi: 10.1007/s00381-002-0611-2. [DOI] [PubMed] [Google Scholar]
- 28.van Leeuwen R, Notermans NC, Vandertop WP. Surgery in adults with tethered cord syndrome: outcome study with independent clinical review. J Neurosurg Spine. 2001;94:205–209. doi: 10.3171/spi.2001.94.2.0205. [DOI] [PubMed] [Google Scholar]
- 29.Vassilyadi M, Tataryn Z, Merziotis M. Retethering in children after sectioning of the filum terminale. Pediatr Neurosurg. 2012;48:335–341. doi: 10.1159/000353477. [DOI] [PubMed] [Google Scholar]
- 30.Von Koch CS, Quinones-Hinojosa A, Gulati M, Lyon R, Peacock WJ, Yingling CD. Clinical outcome in children undergoing tethered cord release utilizing intraoperative neurophysiological monitoring. Pediatr Neurosurg. 2002;37:81–86. doi: 10.1159/000065109. [DOI] [PubMed] [Google Scholar]
- 31.Zhou GC, Xu JM, Zhou TJ. Relationship of MRI images and surgical findings on the tethered cord syndrome. Zhongguo Jizhu Jisui Zazhi. 1994;4:145–148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


