Abstract
The peripheral nervous system plays a major role in the maintenance of our physiology. Several peripheral nerves intimately regulate the state of the brain, spinal cord, and visceral systems. A new class of therapeutics, called bioelectronic medicines, are being developed to precisely regulate physiology and treat dysfunction using peripheral nerve stimulation. In this review, we first discuss new work using closed-loop bioelectronic medicine to treat upper limb paralysis. In contrast to open-loop bioelectronic medicines, closed-loop approaches trigger ‘on demand’ peripheral nerve stimulation due to a change in function (e.g., during an upper limb movement or a change in cardiopulmonary state). We also outline our perspective on timing rules for closed-loop bioelectronic stimulation, interface features for non-invasively stimulating peripheral nerves, and machine learning algorithms to recognize disease events for closed-loop stimulation control. Although there will be several challenges for this emerging field, we look forward to future bioelectronic medicines that can autonomously sense changes in the body, to provide closed-loop peripheral nerve stimulation and treat disease.
Keywords: spinal cord injury, stroke, plasticity, closed-loop, bioelectronic medicine, machine learning, nerve stimulation, vagus nerve
Bioelectronic Medicine Background
Bioelectronic medicine is an emerging field of therapeutics aiming to treat dysfunction and disease using peripheral nerve stimulation (Famm et al., 2013; Birmingham et al., 2014). Here nerve stimulation is generally used as a way to affect molecule release, organ function, central nervous system activity, and other physiological events. The vagus nerve is the one of the most common targets for a bioelectronic medicine therapy and has been targeted in over 200,000 patients to treat a wide array of diseases including epilepsy, depression, heart failure, and obesity (Guiraud et al., 2016). Vagus nerve stimulation (VNS) protocols generally follow a preprogrammed nerve stimulation schedule, otherwise known as open-loop stimulation. For example, open-loop VNS treatments for patients suffering from epilepsy routinely consist of preprogrammed stimulation schedules delivered throughout the day (e.g., 30 seconds of VNS “on” followed by 5 minutes of VNS “off”). Critically, there are several disease states that may require precise closed-loop stimulation to trigger rapid neural activation and achieve therapeutic effects (for a review comparing open-loop and closed-loop therapy systems: Sun and Morrell, 2014). Below we review recent work using closed-loop VNS to treat multiple forms of paralysis, where VNS is triggered during upper limb rehabilitation to enhance neural plasticity and recovery of upper limb function (Ganzer et al., 2018b; Meyers et al., 2018). Further, we also outline our perspective on future developments in the field of bioelectronic medicine, including potential non-invasive stimulation interfaces and machine learning based strategies for decoding physiological data to ‘close the loop’. We performed a PubMed literature search for articles published in the period of January 2013–August 2018 on VNS.
Closed-Loop Bioelectronic Medicine for Treating Paralysis
Spinal cord injury (SCI) and stroke are leading causes of paralysis, and commonly leave motor circuits extensively damaged (e.g., neural circuits innervating the musculature of a limb). Recent work demonstrates that these damaged circuits can be rewired to enable recovery using therapies that promote neural circuit change, otherwise known as neural plasticity (e.g., synaptic plasticity or axonal sprouting). Recent preclinical studies have used molecular therapies (Ganzer et al., 2013, 2016, 2018a; van den Brand et al., 2015; Manohar et al., 2017) or closed-loop bioelectronic medicines (Ganzer et al., 2018b; Meyers et al., 2018) combined with rehabilitation to facilitate neural plasticity and recovery following injury. Below we outline recent preclinical findings demonstrating enhanced neural plasticity and upper limb recovery following SCI and stroke enabled by closed-loop VNS.
VNS activates nuclei critical for plasticity enhancing neuromodulator release and engages several other plasticity promoting factors (Hays et al., 2013; Hays, 2016). Used in a closed-loop design (Hays et al., 2013; Hays, 2016; Khodaparast et al., 2016; Ganzer et al., 2018b; Meyers et al., 2018), brief bursts of VNS (0.5 second duration) are triggered by successful movements during post-injury upper limb rehabilitation (Figure 1A). Therefore, the neural activity responsible for these successful movements is paired with plasticity enhancing neuromodulators, to enable ‘targeted plasticity’ of these damaged circuits (Hays et al., 2013; Hays, 2016; Edgerton and Gad, 2018). Recent preclinical closed-loop VNS studies demonstrate that spared motor circuits following injury can be rewired and strengthened in models of SCI and stroke to significantly increase recovery of limb function (Ganzer et al., 2018b; Meyers et al., 2018). Over weeks of therapy and thousands of precise closed-loop VNS pairings, the spared upper limb motor circuits form new connections from the brain and spinal cord to the targeted upper limb musculature (Ganzer et al., 2018b; Meyers et al., 2018). This extensive neural plasticity is associated with significant recovery of upper limb function that importantly also generalizes to untrained motor tasks (Meyers et al., 2018). These results support the hypothesis that closed-loop VNS can promote the rewiring of spared motor circuits, and are the first studies to demonstrate that closed-loop VNS promotes post-injury motor system neural plasticity.
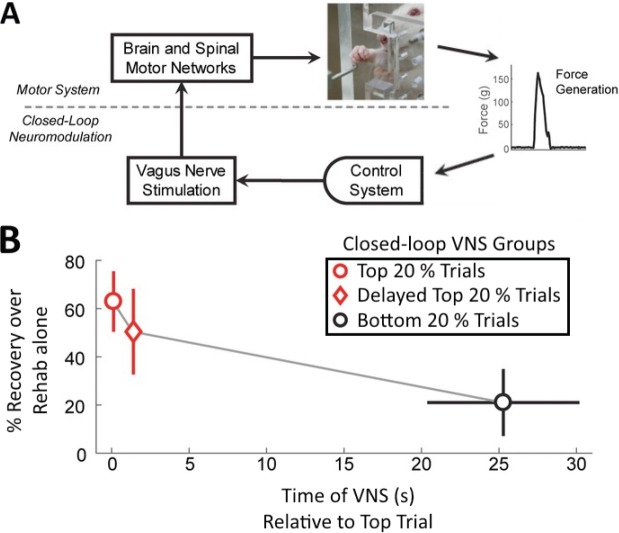
Figure 1.
Upper limb recovery decreases as closed-loop vagus nerve stimulation (VNS) is progressively delayed from successful movements.
(A) Schematic of closed-loop VNS. Appropriate VNS is triggered from forelimb force generation following injury. (B) Three groups received closed-loop VNS following spinal cord injury. O: VNS immediately after the ‘best’ movements (top 20%); ◊: VNS with 1.5 s delay from the ‘best’ movements (top 20%); O: VNS immediately after the ‘worst’ movements (bottom 20%). Upper limb recovery decreased as the onset of closed-loop VNS was progressively delayed from successful movements (i.e., ‘Top Trials’). Adapted with permission; please see Ganzer et al. (2018b) for further details. s: Second(s).
Closed-loop Vagus Nerve Stimulation: Timing Matters
Closed-loop bioelectronic medicines may be governed by critical stimulation timing rules that enable optimized therapeutic effects. We recently assessed the hypothesis that closed-loop VNS (Figure 1A) significantly enhances recovery following SCI when VNS is immediately paired with a successful movement, compared to VNS delivered with a 1.5 seconds or several seconds delay following a successful movement (Ganzer et al., 2018b). Upper limb recovery decreased progressively as the onset of closed-loop VNS became more delayed from successful movements (Figure 1B). Importantly, these therapy groups received the same dose of VNS. These results confirm that closed-loop VNS timing, and not dose, ultimately determined the extent of recovery.
These results are in agreement with the synaptic eligibility trace theory, a critical set of timing rules for enhancing synaptic plasticity (He et al., 2015). He et al. (2015) demonstrated that following sufficient coincident firing at a synapse, a synaptic eligibility trace is activated – a ‘synaptic memory’ decaying over several seconds that can be converted into synaptic strengthening given the correct molecular reinforcement (e.g., norepinephrine application and β2 receptor stimulation). During rehabilitation, activity within distributed sensorimotor circuits may enable multiple synaptic eligibility trace-like events for VNS to convert via the release of plasticity promoting factors (Hays et al., 2013; Hays, 2016). Although further investigation is warranted during other disease states, accumulating evidence indicates that closed-loop VNS may follow precise timing rules for maximizing targeted plasticity, mediated by synaptic eligibility trace mechanisms across a range of therapy conditions and synapses.
Perspective on Electrode Technology for Non-invasive Bioelectronic Medicines
Future closed-loop bioelectronic medicines can be developed to treat an array of diseases including gastric dysfunction, cardiopulmonary pathology, metabolic disorders, and many other conditions. Several peripheral nerves are potential targets for closed-loop stimulation-based treatments. Interestingly, some critical nerve branches for therapy are accessible via transcutaneous stimulation (Mercante et al., 2018). Therefore, non-invasive transcutaneous stimulation is one potential avenue for targeting peripheral nerves in a closed-loop stimulation paradigm. Although promising, there are several challenges facing non-invasive closed-loop bioelectronic medicines, including the identification of a sufficiently robust electrode material for stimulation.
For a ‘take home’ closed-loop bioelectronic medicine, the device will need to be equipped with electrodes that are high performance and capable of delivering non-invasive stimulation without material degradation. Dry electrode technology is one potential candidate electrode material for delivering non-invasive neural stimulation. Dry electrodes developed by our group at Battelle Memorial Institute are comprised of a mixed ionic electronic conducting (MIEC) electrode material that offers several attractive features needed for a non-invasive stimulation interface (Shqau and Heintz, 2017). First, the MIEC electrode is a very flexible elastic material that is light weight and can be molded easily to fit several form factors and interface geometries. This should allow for personalized bioelectronic interfaces to mold to body parts of the patient allowing for enhanced comfort and electrode adherence. Secondly, the MIEC material robustly electrically couples to the skin. The MIEC interface uses carbon nanotubes as the electronic conductor and hyaluronic acid as the ionic conductor interspersed in a polymer matrix. This allows for low interfacial resistances and efficient flow of current from a traditional metallic interface, to the MIEC, and through the skin for stimulation. Lastly, the MIEC interface prevents the need for a hydrogel or conductive lotion, and is robust to temperature and humidity fluctuations over time (Shqau and Heintz, 2017). Further research needs to be performed to assess this promising material’s capability as a non-invasive bioelectronic medicine stimulation interface. This MIEC interface and future generations of novel electrode technologies should enable access to larger patient populations for maximizing the application of non-invasive bioelectronic medicines.
Perspective on the Role of Machine Learning in Bioelectronic Medicines
Future closed-loop bioelectronic medicines can utilize multimodal data streams to intelligently trigger and automatically control therapeutic stimulation (Figure 2). ‘Closing the loop’ can be achieved using limb function monitoring as highlighted above (Hays et al., 2013; Hays, 2016; Khodaparast et al., 2016; Ganzer et al., 2018b; Meyers et al., 2018), or using distributed multimodal data streams collected from multiple sensors (e.g., molecular sensing: Ferguson et al., 2013; Arroyo-Currás et al., 2017; or recording activity from peripheral nerves: Sevcencu et al., 2017; Zanos et al., 2018; Figure 2B). This stream of sensor data will be a critical component of future bioelectronic medicines, and will be a significant challenge for developing closed-loop interfaces. ‘Real time’ triggering of closed-loop neural stimulation will require advanced statistical approaches that can integrate sufficiently complex data streams, decipher patterns in the data, and decode when the patient’s physiology is changing.
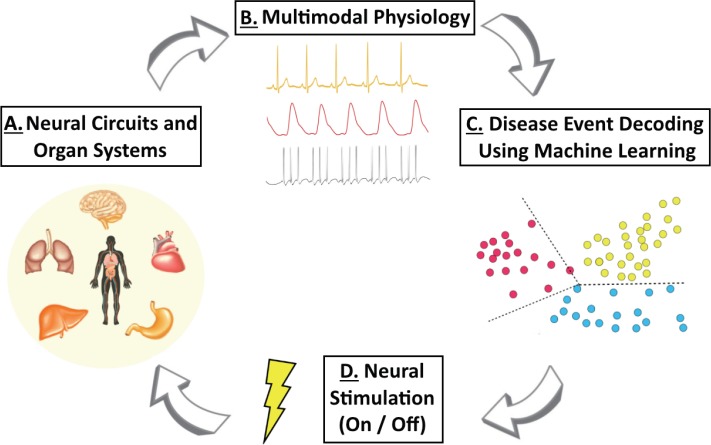
Figure 2.
Schematic of closed-loop bioelectronic medicine.
(A) Closed-loop bioelectronic medicines can record from and interface with numerous sources, including neural circuits and organ systems. (B) Ongoing activity acquired from one or more sensors can be utilized to assess physiological states (e.g., cardiopulmonary activity or neural activity from peripheral nerves). (C) Events or changes in these data streams can then be decoded using machine learning, in order to dynamically trigger closed-loop bioelectronic medicine when needed (e.g., during a detected cardiopulmonary disease event). (D) Neural stimulation modulates physiology providing therapeutic benefit, and is turned on and off by decoded physiological events. These cycles continue throughout treatment to provide closed-loop bioelectronic medicine and promote recovery of function.
Machine learning is a method of data analysis that uses algorithms to decode patterns in complex data. Machine learning is at the heart of several common technologies, such as financial forecasting or speech recognition software. For example, speech recognition software uses machine learning to take an input (speech), decode it using an algorithm (confirming that it is your voice), and then trigger an action (open an app on your phone).
Machine learning can also be used in future closed-loop bioelectronic medicines, where an input (sensor data; Figure 2B) is decoded (to decipher a disease event; Figure 2C) to trigger an action (neural stimulation; Figure 2D). Machine learning is currently used in several biomedical applications including brain machine interfaces (Bouton et al., 2016; Friedenberg et al., 2017; Schwemmer et al., 2018). Here an intended movement is decoded from a complex motor cortex signal, to trigger stimulation of the participant’s paralyzed arm muscles and activate the intended movement. There are several robust machine learning approaches for decoding complex physiological data, including support vector machines (Bouton et al., 2016), support vector regressions (Friedenberg et al., 2017), or other algorithms (e.g., deep neural networks; Schwemmer et al., 2018). For future closed-loop bioelectronic medicines, the decoding approach should be sufficiently accurate, fast, selective, and readily retrainable across several weeks of therapy. A personalized decoding algorithm trained on a subject’s physiological data should allow for improvements in therapeutic efficacy via personalized closed-loop stimulation schedules. Further, neural stimulation timing triggered by decoding algorithms may also need to be further tuned to meet physiological timing rules (e.g., synaptic eligibility trace theory timing requirements (He et al., 2015; Ganzer et al., 2018b) or modification of nerve stimulation parameters during different phases of diurnal cycles). We look forward to the future of closed-loop bioelectronic medicines, where advanced technology and data driven systems autonomously regulate therapy to promote recovery from debilitating disease.
Acknowledgments:
Thanks to the Battelle Memorial Institute team for contribution to figure generation and manuscript preparation.
Footnotes
Conflicts of interest: None declared.
Financial support: This work was supported by Battelle Memorial Institute.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by Battelle Memorial Institute.
C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Arroyo-Currás N, Somerson J, Vieira PA, Ploense KL, Kippin TE, Plaxco KW. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc Natl Acad Sci U S A. 2017;114:645–650. doi: 10.1073/pnas.1613458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birmingham K, Gradinaru V, Anikeeva P, Grill WM, Pikov V, McLaughlin B, Pasricha P, Weber D, Ludwig K, Famm K. Bioelectronic medicines: a research roadmap. Nat Rev Drug Discov. 2014;13:399–400. doi: 10.1038/nrd4351. [DOI] [PubMed] [Google Scholar]
- 3.Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, Sharma G, Sederberg PB, Glenn BC, Mysiw WJ, Morgan AG, Deogaonkar M, Rezai AR. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533:247–250. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- 4.Edgerton VR, Gad P. Is the vagus nerve our neural connectome? Elife. 2018;7:e35592. doi: 10.7554/eLife.35592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: a jump-start for electroceuticals. Nature. 2013;496:159–161. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson BS, Hoggarth DA, Maliniak D, Ploense K, White RJ, Woodward N, Hsieh K, Bonham AJ, Eisenstein M, Kippin TE, Plaxco KW, Soh HT. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Sci Transl Med. 2013;5:213ra165. doi: 10.1126/scitranslmed.3007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedenberg DA, Schwemmer MA, Landgraf AJ, Annetta NV, Bockbrader MA, Bouton CE, Zhang M, Rezai AR, Mysiw WJ, Bresler HS, Sharma G. Neuroprosthetic-enabled control of graded arm muscle contraction in a paralyzed human. Sci Rep. 2017;7:8386. doi: 10.1038/s41598-017-08120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganzer PD, Moxon KA, Knudsen EB, Shumsky JS. Serotonergic pharmacotherapy promotes cortical reorganization after spinal cord injury. Exp Neurol. 2013;241:84–94. doi: 10.1016/j.expneurol.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganzer PD, Manohar A, Shumsky JS, Moxon KA. Therapy induces widespread reorganization of motor cortex after complete spinal transection that supports motor recovery. Exp Neurol. 2016;279:1–12. doi: 10.1016/j.expneurol.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Ganzer PD, Beringer CR, Shumsky JS, Nwaobasi C, Moxon KA. Serotonin receptor and dendritic plasticity in the spinal cord mediated by chronic serotonergic pharmacotherapy combined with exercise following complete SCI in the adult rat. Exp Neurol. 2018a;304:132–142. doi: 10.1016/j.expneurol.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganzer PD, Darrow MJ, Meyers EC, Solorzano BR, Ruiz AD, Robertson NM, Adcock KS, James JT, Jeong HS, Becker AM, Goldberg MP, Pruitt DT, Hays SA, Kilgard MP, Rennaker RL., 2nd Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife. 2018b;7:e32058. doi: 10.7554/eLife.32058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guiraud D, Andreu D, Bonnet S, Carrault G, Couderc P, Hagège A, Henry C, Hernandez A, Karam N, Le Rolle V, Mabo P, Maciejasz P, Malbert CH, Marijon E, Maubert S, Picq C, Rossel O, Bonnet JL. Vagus nerve stimulation: state of the art of stimulation and recording strategies to address autonomic function neuromodulation. J Neural Eng. 2016;13:041002. doi: 10.1088/1741-2560/13/4/041002. [DOI] [PubMed] [Google Scholar]
- 13.Hays SA. Enhancing rehabilitative therapies with vagus nerve stimulation. Neurotherapeutics. 2016;13:382–394. doi: 10.1007/s13311-015-0417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013;207:275–299. doi: 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He K, Huertas M, Hong SZ, Tie X, Hell JW, Shouval H, Kirkwood A. Distinct eligibility traces for LTP and LTD in cortical synapses. Neuron. 2015;88:528–538. doi: 10.1016/j.neuron.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, Rennaker RL, 2nd, Hays SA. Vagus nerve stimulation during rehabilitative training improves forelimb recovery after chronic ischemic stroke in rats. Neurorehabil Neural Repair. 2016;30:676–684. doi: 10.1177/1545968315616494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manohar A, Foffani G, Ganzer PD, Bethea JR, Moxon KA. Cortex-dependent recovery of unassisted hindlimb locomotion after complete spinal cord injury in adult rats. Elife. 2017;6:e23532. doi: 10.7554/eLife.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercante B, Deriu F, Rangon CM. Medicines. Basel, Switzerland: 2018. Auricular neuromodulation: the emerging concept beyond the stimulation of vagus and trigeminal nerves. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL, 2nd, Kilgard MP, Hays SA. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke. 2018;49:710–717. doi: 10.1161/STROKEAHA.117.019202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwemmer MA, Skomrock ND, Sederberg PB, Ting JE, Sharma G, Bockbrader MA, Friedenberg DA. Meeting brain-computer interface user performance expectations using a deep neural network decoding framework. Nat Med. 2018 doi: 10.1038/s41591-018-0171-y. doi:10.1038/s41591-018-0171-y. [DOI] [PubMed] [Google Scholar]
- 21.Sevcencu C, Nielsen TN, Struijk JJ. A neural blood pressure marker for bioelectronic medicines for treatment of hypertension. Biosens Bioelectron. 2017;98:1–6. doi: 10.1016/j.bios.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Shqau K, Heintz A. Design of Medical Devices Conference. Minneapolis, MN, USA: 2017. Mixed ionic electronic conductors for improved charge transport in electrotherapeutic devices. [Google Scholar]
- 23.Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics. 2014;11:553–563. doi: 10.1007/s13311-014-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Brand R, Mignardot JB, von Zitzewitz J, Le Goff C, Fumeaux N, Wagner F, Capogrosso M, Martin Moraud E, Micera S, Schurch B, Curt A, Carda S, Bloch J, Courtine G. Neuroprosthetic technologies to augment the impact of neurorehabilitation after spinal cord injury. Ann Phys Rehabil Med. 2015;58:232–237. doi: 10.1016/j.rehab.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Zanos TP, Silverman HA, Levy T, Tsaava T, Battinelli E, Lorraine PW, Ashe JM, Chavan SS, Tracey KJ, Bouton CE. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc Natl Acad Sci U S A. 2018;115:E4843–4852. doi: 10.1073/pnas.1719083115. [DOI] [PMC free article] [PubMed] [Google Scholar]