Abstract
Multiple sclerosis is a neurodegenerative and inflammatory disease, a hallmark of which is demyelinating lesions in the white matter. We hypothesized that alterations in white matter microstructures can be non-invasively characterized by advanced diffusion magnetic resonance imaging. Seven diffusion metrics were extracted from hybrid diffusion imaging acquisitions via classic diffusion tensor imaging, neurite orientation dispersion and density imaging, and q-space imaging. We investigated the sensitivity of the diffusion metrics in 36 sets of regions of interest in the brain white matter of six female patients (age 52.8 ± 4.3 years) with multiple sclerosis. Each region of interest set included a conventional T2-defined lesion, a matched perilesion area, and normal-appearing white matter. Six patients with multiple sclerosis (n = 5) or clinically isolated syndrome (n = 1) at a mild to moderate disability level were recruited. The patients exhibited microstructural alterations from normal-appearing white matter transitioning to perilesion areas and lesions, consistent with decreased tissue restriction, decreased axonal density, and increased classic diffusion tensor imaging diffusivity. The findings suggest that diffusion compartment modeling and q-space analysis appeared to be sensitive for detecting subtle microstructural alterations between perilesion areas and normal-appearing white matter.
Keywords: multiple sclerosis, hybrid diffusion imaging, NODDI, diffusion tensor imaging, q-space imaging
Chinese Library Classification No. R445; R742
Introduction
Multiple Sclerosis (MS) is a chronic inflammatory and neurodegenerative disease, characterized by widespread central nervous system damage that includes demyelinating white matter (WM) lesions and changes in normal-appearing white matter (NAWM) and gray matter. Conventional magnetic resonance imaging, including T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) and pre- and post-contrast T1-weighted imaging, aids in establishing the diagnosis of MS and monitoring disease progression in a safe, noninvasive manner (Filippi and Rocca, 2007; Polman et al., 2011). These forms of conventional MRI, however, provide limited information regarding the underlying microstructural changes in MS. The mechanisms of the observed alterations in T2-FLAIR maps may be elucidated by diffusion magnetic resonance imaging (dMRI) approaches, which allow for probing human brain microstructure by measuring water diffusion properties influenced by the biologic activity of the surrounding tissues. Thus, advanced microstructural imaging biomarkers are potentially more sensitive to MS-specific microstructural changes than conventional T1- or T2-weighted imaging.
Among dMRI techniques, diffusion tensor imaging (DTI) is most widely used to study MS. DTI-derived parameters are well established to differ in patients with MS compared to controls (Filippi et al., 2000, 2001; Rovaris and Filippi, 2007; Warlop et al., 2009; Liu et al., 2012; Bester et al., 2013; Bodini et al., 2013; Harrison et al., 2013; Sbardella et al., 2013; Vishwas et al., 2013; Banaszek et al., 2015; Rocca et al., 2016; Schneider et al., 2017). DTI, however, has inherent limitations. First, DTI uses a simplified mono-Gaussian diffusion model that limits the technique to the approximation of a single fiber at each voxel; thus, DTI-derived indices may be biased in WM tracts that contain crossing fibers (Wheeler-Kingshott and Cercignani, 2009; Kodiweera et al., 2016). Second, DTI is a second-order approximation of the complex diffusion function that comprises a mixture of diffusion compartments.
The finer granularity of diffusion compartments can be assessed using higher-order diffusion modeling. Diffusion signals in WM tissue are modeled with different approaches: fast and slow diffusion components (Clark and Le Bihan, 2000); anisotropic hindered and restricted compartments (Jespersen et al., 2010; Fieremans et al., 2011; De Santis et al., 2013); fast isotropic free water and anisotropic tissue compartments (Pasternak et al., 2009); three compartments of fast isotropic diffusion, slow restricted isotropic diffusion, and restricted anisotropic diffusion (Chiang et al., 2014); and three compartments of fast isotropic diffusion (e.g., CSF), anisotropic hindered diffusion (e.g., extracellular water), and highly restricted anisotropic diffusion (e.g., intra-axonal compartments) (Zhang et al., 2012). The output parameters from diffusion modeling are potentially less ambiguous than those of DTI for characterizing diffusion-weighted microstructures with increased specificity in clinical studies of the human brain.
Herein, we performed a feasibility study using one of the three-compartment models, neurite orientation dispersion and density imaging (NODDI). The NODDI axonal density index in WM was almost identical to the values obtained from electron microscopy of ex vivo mouse brain and is in good agreement with previously reported fiber densities of human brain using electron microscopy (Sepehrband et al., 2015); and the neurite density index in gray matter correlates with tau pathology in an animal model of Alzheimer’s disease (Colgan et al., 2015). The NODDI model was validated in ex vivo spinal cord with MS pathology in a study concluding that the NODDI-derived diffusion metrics match their histologic counterparts (Grussu et al., 2017). Other NODDI applications include in vivo human studies of MS (Schneider et al., 2017), classic galactosemia (Timmers et al., 2015), neonatal encephalopathy (Lally et al., 2014), WM development in the newborn (Kunz et al., 2014), brain aging (Billiet et al., 2015; Nazeri et al., 2015; Kodiweera et al., 2016), and mild traumatic brain injury (Mustafi et al., 2016), and has yielded valuable microstructural information on neurologic disorders of the human brain.
Alternatively, microstructural changes in WM may be studied by a non-parametric analysis, q-space imaging. The q-space approach estimates the water diffusion function, the probability density function (PDF), also called mean apparent propagator (MAP) (Ozarslan et al., 2013) or ensemble average propagator (EAP) (Descoteaux et al., 2011; Hosseinbor et al., 2013), through a Fourier transform relationship (Callaghan, 1991): P ( , Δ)=FT–1 [EΔ(
, Δ)=FT–1 [EΔ( )], where P is PDF;
)], where P is PDF;  is the displacement vector; Δ is the diffusion time; E is the normalized q-space signal; and
is the displacement vector; Δ is the diffusion time; E is the normalized q-space signal; and  is the q-space wavevector determined by the diffusion gradient strength (
is the q-space wavevector determined by the diffusion gradient strength ( ) and the duration (δ),
) and the duration (δ),  =γ
=γ δ. The zero displacement probability, P0, is the return to origin probability (P0 = P (
δ. The zero displacement probability, P0, is the return to origin probability (P0 = P ( = 0, Δ)), and represents the probability of those water molecules having no net diffusion within the diffusion time Δ. P0 is often interpreted as a measure of restricted diffusion and cellularity (Assaf et al., 2000; Wu and Alexander, 2007; Ozarslan et al., 2013). In animal studies of dysmyelination, P0 exhibited high sensitivity to myelination and brain maturation (Biton et al., 2006; Wu et al., 2011), consistent with other studies of P0 in demyelination of the human brain with MS (Assaf et al., 2002, 2005). These studies show decreased P0 associated with myelin-deficient WM and NAWM in MS.
= 0, Δ)), and represents the probability of those water molecules having no net diffusion within the diffusion time Δ. P0 is often interpreted as a measure of restricted diffusion and cellularity (Assaf et al., 2000; Wu and Alexander, 2007; Ozarslan et al., 2013). In animal studies of dysmyelination, P0 exhibited high sensitivity to myelination and brain maturation (Biton et al., 2006; Wu et al., 2011), consistent with other studies of P0 in demyelination of the human brain with MS (Assaf et al., 2002, 2005). These studies show decreased P0 associated with myelin-deficient WM and NAWM in MS.
In the present study, we used hybrid diffusion imaging (HYDI) (Wu and Alexander, 2007), a flexible and versatile dMRI technique, to acquire multishell diffusion data in patients with MS and clinically isolated syndrome (first episode of neurologic symptoms consistent with MS). The HYDI approach conveniently enables complementary data-processing strategies, such as model-fitting and non-parametric analyses, using one diffusion dataset. Here, the microstructural changes were characterized by the above-mentioned dMRI metrics in MS lesions, perilesion areas (hereafter referred to as perilesions), and NAWM. Statistical comparisons between types of tissues were performed for individual sets of regions of interest (ROIs) including a lesion, a matched perilesion, and NAWM (36 sets), and also at the group level.
Participants and Methods
Participants
Six female patients (age 52.8 ± 4.3 years), diagnosed and classified according to internationally accepted criteria (Kurtzke, 1983; Polman et al., 2011), participated in this observational study from Dartmouth-Hitchcock Medical Center (Table 1). Four had relapsing-remitting MS, one had secondary progressive MS, and one had clinically isolated syndrome. Neurologic disability level, measured using the Expanded Disability Status Scale (EDSS) (Kurtzke, 1983), was mild to moderate, with scores ranging from 1.5 to 3.5 (out of 10). All of the participants were at least 1 month post-exacerbation and post-steroid therapy, and had had no change in disease-modifying therapy within the preceding month. Patients with other neurologic disorders or a current substance use disorder were excluded from the study. All participants provided informed consent approved by the guidelines of the institutional review board of the Geisel School of Medicine at Dartmouth on October 17, 2013 (No. 11482) and all procedures were in accordance with an institutional review board-approved study plan.
Table 1.
Demographics and characteristics of all the included patients

Image acquisition
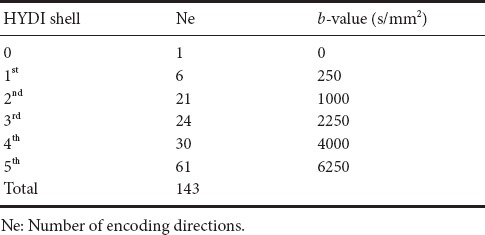
Images were acquired using a Philips 3T Achieva scanner (Cleveland, OH, USA) with an 8-channel head coil. T1-weighted images were acquired using a 3D magnetization-prepared rapid gradient echo (MPRAGE) sequence with echo time (TE) = 3.3 ms, repetition time (TR) = 6.8 ms, field of view (FOV) = 240 mm, voxel size = 1.2 × 1 × 1 mm, and 140 sagittal slices. Two-dimensional axial T2-FLAIR scans were obtained with: inversion time = 2800 ms, TE/TR = 125/11,000 ms, FOV = 240 mm, in-plane resolution of 0.94 mm, and 44 slices with a 3-mm slice thickness. The HYDI sequence used single-shot spin-echo echo-planar imaging (SS SE EPI) with five diffusion-weighting b-value shells (b = 250, 1000, 2250, 4000, and 6250 s/mm2) and 143 diffusion-weighting gradient directions (Table 2). HYDI MR parameters were: TE/TR = 114.24/3590 ms, diffusion gradient duration (δ) = 46 ms and separation (Δ) = 58.4 ms, 2-mm in-plane resolution, 40 axial slices with 3-mm slice thickness, and a parallel imaging SENSE factor of 2. The total scan time for HYDI was 24 minutes.
Table 2.
Hybrid diffusion imaging (HYDI) encoding scheme
HYDI data analyses
Pre-processing
All the diffusion data were visually inspected for severe scanner-related artifacts or motion artifacts prior to entering the pre-processing pipeline. The HYDI data pre-processing pipeline included denoising with a local principal component analysis approach (Manjon et al., 2013) and eddy current and motion corrections using the eddy_correct tool from the diffusion processing toolbox in the FMRIB Software Library (FSL) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki) (Jenkinson et al., 2002). All of the images, including T1-weighted and T2-FLAIR images, were transformed to the diffusion space where the b0 image (i.e., the first image in HYDI with zero diffusion weighting) served as a common reference. Linear registration was performed to align the T1-weighted and T2-FLAIR images with the b0 image using the FLIRT toolbox in FSL with seven parameters, including six rigid-body parameters and one global rescaling parameter. The same b0 image was also used as the reference image in FSL eddy_correct for registering the remaining diffusion-weighted images.
DTI
DTI indices, including axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD), and fractional anisotropy (FA), were computed using inner HYDI shells with b-values of 0, 250, and 1000 s/mm2 (Table 2). The DTI analysis used the dtifit command in the FSL software package (Behrens et al., 2003). AD is the major eigenvalue of the diffusion tensor; RD is defined as the mean of the medium and minor eigenvalues of the diffusion tensor; MD is the mean of the three eigenvalues; and FA is the normalized variation of the three eigenvalues (Basser et al., 1994).
NODDI
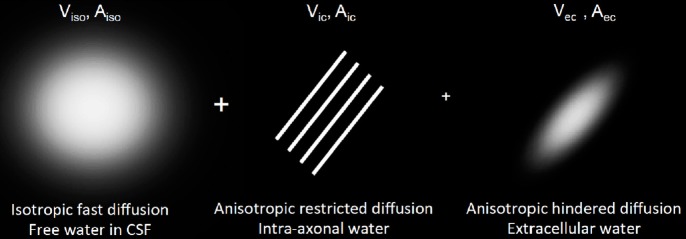
NODDI metrics were computed with the whole HYDI dataset. The NODDI formalism describes the detectable normalized diffusion signal A = VisoAiso + (1 – Viso) [VicAic + VecAec] (Figure 1), where Viso and Vic are the volume fractions of fast isotropic diffusion of CSF and intracellular water, respectively. The extracellular volume fraction is Vec = (1–Vic), a complementary value to Vic (Zhang et al., 2012). The symbols Aiso, Aic, and Aec represent the signal contributions from the fast isotropic diffusion (e.g., CSF), intracellular (intra-axonal), and extracellular (extra-axonal) compartments, respectively. Using the Watson distribution, NODDI analysis also provides an estimate of the orientation dispersion index (ODI) describing an averaged dispersion of axons within an imaging voxel in WM. NODDI metrics were computed on a voxel-by-voxel basis with nonlinear fits coded in a MATLAB toolbox provided by the Microstructure Imaging Group at University College London (http://mig.cs.ucl.ac.uk/index.php?n=Tutorial.NODDIMATLAB). For NODDI fitting, the initial conditions for isotropic free-water diffusivity and the intrinsic diffusivity of the neural tissue were set to 3 × 10–3 mm2/s and 1.7 × 10–3 mm2/s, respectively, as suggested previously (Zhang et al., 2012).
Figure 1.
Visualization of diffusion compartments in the NODDI model.
Viso and Vic are the volume fraction of the free isotropic diffusion and intra-axonal compartment, respectively. The extracellular volume fraction is Vec = (1 – Vic), a complement number to Vic. Aiso, Aic, and Aec are the normalized diffusion signal contributed from individual diffusion compartments. Aec: Diffusion signal from extra-axonal water; Aic: diffusion signal from intra-axonal water; Aiso: diffusion signal from isotropic fast diffusion; NODDI: neurite orientation dispersion and density imaging; Vec: volume fraction for extra-cellular water; Vic: volume fraction for intra-axonal water; Viso: volume fraction for isotropic fast diffusion; CSF: cerebrospinal fluid.
q-Space analyses
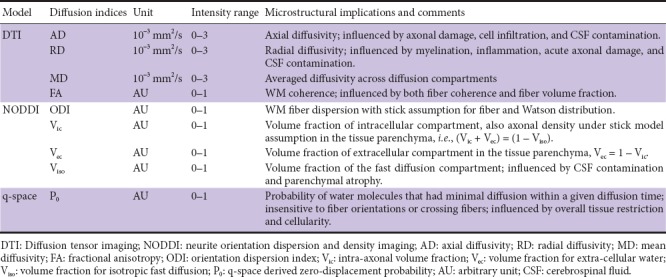
P0 was computed using the whole HYDI data set with a q-space analysis approach using in-house MATLAB code (Wu and Alexander, 2007; Wu et al., 2008). Table 3 summarizes the diffusion metrics’ acronyms, units, intensity ranges, and microstructural implications.
Table 3.
Abbreviations of diffusion metrics
ROI
A whole-brain WM mask was segmented using the FSL FAST toolbox on subject’s co-registered T1-weighted MPRAGE images in the diffusion space (see Pre-processing). This WM mask may or may not contain lesions, which will eventually be excluded by the lesion masks described below. For each subject, within the WM, three ROI types were selected. Lesion (red in Figure 2): T2-FLAIR hyperintense lesions were segmented using an in-house semi-automated approach (Wishart et al., 2010). All segmented WM lesions were used in this study while gray matter lesions were excluded. Perilesion (blue in Figure 2): Perilesions were WM voxels that surrounded the lesions with a thickness of one voxel. Similar to (Colasanti et al., 2014), the perilesion WM voxels were generated by intersecting dilated lesion masks and the WM mask. NAWM (green in Figure 2): NAWM were WM voxels surrounding the perilesions, but with a gap of at least one voxel from the perilesion. Similarly, the NAWM ROIs were also generated using the dilation approach. As demonstrated in Figure 2, a set of ROIs includes a lesion (red), a matched perilesion (blue), and NAWM (green); in this case, there were six sets identified for Patient no. 4. All ROIs were three dimensional and extended across several slices depending on their sizes.
Figure 2.
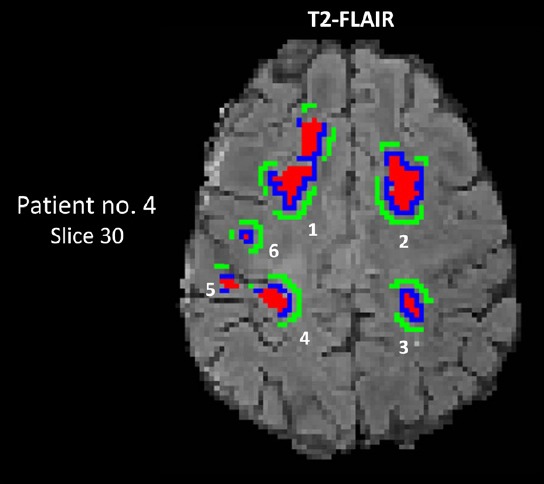
Segmentation of lesions, perilesions, and normal-appearing white matter (NAWM) on a T2-FLAIR map of patient No. 4.
A set of ROIs includes a lesion (red), a matched perilesion (blue) and NAWM (green); this patient (Expanded Disability Status Scale score was 3.5) had six sets of lesions (No. 4). The set number for this patient, sizes of corresponding ROIs, and anatomic locations in the white matter are listed in Table 4. The gray scale is 0–300 AU for the T2-FLAIR map. Numbers 1–6 denote ROI sets listed in Table 3. AU: Arbitrary unit; FLAIR: fluid attenuated inversion recovery; no: number; ROIs: regions of interest.
Statistical analyses
For each ROI, the means and standard deviations were computed for eight MRI measures: T2-FLAIR intensity and the seven diffusion metrics. Image data were assessed for outliers using boxplots and excluded if the values were outside the median ± 1.5 interquartile range (IQR). At the individual lesion level: For each set of ROIs, pairwise comparisons were performed between the lesion, matched perilesion, and NAWM, followed by multiple-comparison adjustment (across MRI measures and ROI sets). A false discovery rate (FDR) of less than 5% (i.e., q-value < 0.05) was considered significant. For each MRI measure and each comparison pair, the number of significant ROI sets was summarized as a percentage relative to the total number of sets detected in the participants. At the group level: Means of each ROI were collected according to the type of ROI (i.e., lesion, perilesion, and NAWM). The means of means and standard deviations of means were compared for each type of ROI by analysis of variance (ANOVA). Because T2-FLAIR intensity is not a quantitative measure, it was only included in the comparisons at the individual ROI level within individual subjects, and not in the group level analyses. Mauchly’s test of sphericity (Mauchly, 1940) was calculated for each ANOVA, and when the test indicated a violation of sphericity, the Greenhouse-Geisser adjustment was applied. Given the number of ANOVAs utilized in our analysis, we applied Bonferroni’s adjustment for multiple comparisons, yielding a critical (P) threshold of 0.007 (0.05/7 diffusion indices). For ANOVAs with a significant overall result, additional post hoc pairwise testing comparing between the three ROI types (i.e., lesion, perilesion, and NAWM) was conducted, with Bonferroni’s adjustment applied for three pairwise comparisons with a critical P = 0.017 (0.05/3 pairs). All analyses were performed using IBM SPSS Statistics, version 23 (IBM, Armonk, NY, USA).
Results
Typical maps of AD, RD, MD, FA, ODI, Vic, and P0 from a group of healthy volunteers (not included in this study) are shown in Additional Figure 1 (387.5KB, tif) . Similar maps for two patients from this study are shown in Figure 3. As expected from the parameter descriptions in Table 3, AD and RD have high WM contrast only in compact fiber tracts with known single fiber bundles, such as the anterior and posterior limbs of the internal capsule and corpus callosum. MD in the ventricles is very high, approximately 3 × 10–3 mm2/s. This value is close to the isotropic free diffusivity value in the cerebrospinal fluid as assumed in the NODDI calculations. FA maps show high intensity (> 0.5) in the WM, indicating high tissue coherence, and low intensity in grey matter (< 0.2) and cerebrospinal fluid, indicating more isotropic diffusion. Consistently, WM has a lower intensity (< 0.3) in the ODI maps, indicating lower dispersion (i.e., high coherence). Typical WM also has high intensity in the Vic map (> 0.7), which represents axonal density under the rigid stick assumption. The P0 map shows higher intensity in areas with restricted diffusion (e.g., WM and deep brain gray matter) and intensity > 0.8, as expected. Hyperintense lesions can be appreciated in the T2-FLAIR maps (Figure 3), whereas corresponding hypointense areas are observed in the FA, Vic, and P0 maps.
Figure 3.
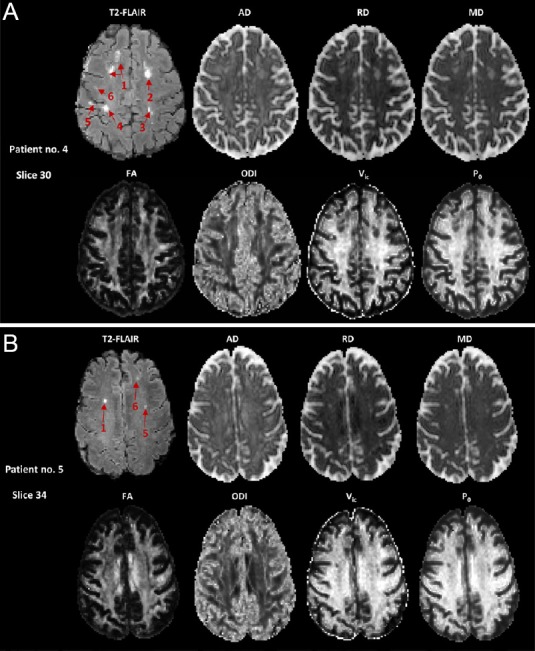
Co-registered MRI maps of two patients with multiple sclerosis (MS).
(A) Patient No. 4. Six hyperintense lesions in the T2-FLAIR image can be appreciated. Note that the two separated hyperintense regions of lesion 1 were connected in another slice (not shown). The lesion segmentation results in the same patient are shown in Figure 2. The corresponding areas in the diffusion maps appeared hyperintense in the AD, RD, and MD maps, and hypointense in the FA, Vic, and P0 maps. The gray scale is 0–300 AU for T2-FLAIR; 0–3 × 10–3 mm2/s for AD, RD, and MD; and 0–1 for FA, ODI, Vic, and P0. (B) Patient No. 5. Three hyperintense lesions in the T2-FLAIR image can be appreciated. AD: Axial diffusivity; AU: arbitrary unit; FA: fractional anisotropy; FLAIR: fluid attenuated inversion recovery; MD: mean diffusivity; ODI: orientation dispersion index; P0: q-space derived zero-displacement probability; RD: radial diffusivity; Vic: volume fraction for intra-axonal water.
Averaged AD, RD, MD, FA, ODI, Vic, and P0 maps from a group of healthy volunteers (not included in this study).
The gray scale for AD, RD, and MD ranges from 0 to 3.0 x10-3 mm2/s. The gray scale for FA is from 0.2 to 1; the larger the value, the higher the tissue coherence. The gray scale of the ODI map is from 0 to 1, while that of Vic is from 0.1 to 1. A smaller ODI indicates higher coherence and less dispersion, while a larger Vic indicates higher intra-cellular volume fraction. AD: Axial diffusivity; RD: radial diffusivity; MD: mean diffusivity; FA: fractional anisotropy; ODI: orientation dispersion index; Vic: volume fraction for intra-axonal water; P0: q-space derived zero-displacement probability.
Thirty-six lesions were segmented by an intensity-based semi-automated approach on T2-FLAIR images (Wishart et al., 2010). Thus, there were 36 sets of ROIs for lesions, perilesions, and NAWM. Table 4 lists the patient number, set number, ROI size, and their anatomic locations in the WM.
Table 4.
Classifications of region of interest (ROI)

At the individual lesion level
No outliers were evident in the image data. Results (q-values) of the pairwise comparisons between the lesion, matched perilesion, and NAWM are listed in Additional Table 1 (47.6KB, pdf) , whereas Table 5 summarizes the percentage of significant sets for each MRI measure and comparison pair. Most of the MRI measures demonstrated significant differences between lesions and the other two ROIs (i.e., perilesions and NAWM) in more than 80% of the ROI sets, except ODI, which did not differ between the three types of ROIs. For comparisons between perilesions and NAWM, only Vic and P0 were significantly different at least 89% of the ROI sets.
Table 5.
Pairwise comparisons of diffusion parameters between lesion-perilesion, lesion-NAWM, and perilesion-NAWM at the level of individual set of ROIs
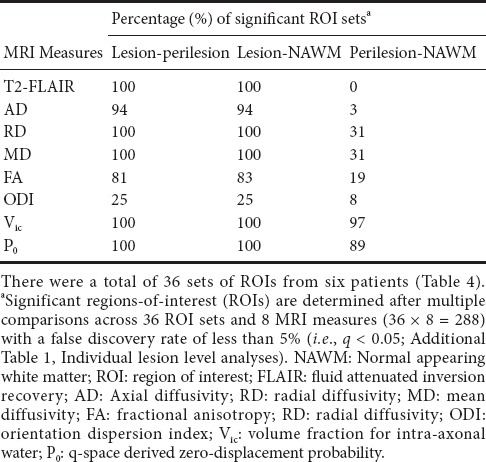
Results (q-values) of the pairwise comparisons between the lesion, matched perilesion, and NAWM
At the group level
The means and standard deviations for each MRI measure and ROI type are listed in Table 6. The table also lists the results of the ANOVAs and post-hoc pairwise t-tests. None of the DTI indices (AD, RD, MD, and FA) and NODDI-derived ODI differed significantly between ROIs. Vic and P0 differed among the three ROIs with a corrected P < 0.007. Post-hoc testing showed that Vic and P0 were significantly decreased (corrected P < 0.017) in lesions and perilesions compared with NAWM. The bar plots of the diffusion metrics in the three ROI types with significant findings are shown in Figure 4 with overhead arrows representing significant pairwise t-tests. T2-FLAIR intensity and DTI indices were similar between perilesions and NAWM, whereas Vic and P0 differed significantly in these two ROIs. Further, the extent of changes in the perilesions was intermediate between that in the lesions and NAWM.
Table 6.
Comparisons of diffusion parameters in the lesion, perilesion, and NAWM ROIs at the group level
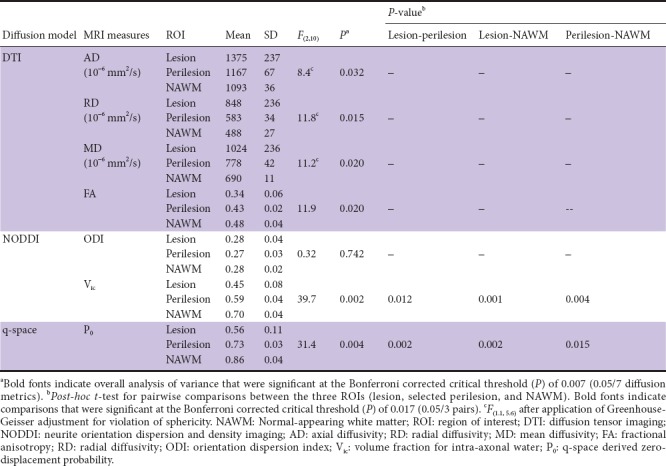
Figure 4.
Bar plots of T2-FLAIR and diffusion metrics in the three types of ROIs.
The lesion, perilesion, and NAWM ROIs are color-coded in red, blue, and green, respectively. Error bars denote the standard deviation across 36 ROI sets. The overhead arrows denote significant differences in measurements between pairs of ROIs at a Bonferroni-corrected P threshold of 0.017 (Table 6). Note that T2-FLAIR intensity is not a quantitative measure; thus, it was not tested for statistical significance at the group level. FLAIR: Fluid attenuated inversion recovery; NAWM: normal-appearing white matter; ROIs: regions of interest.
Discussion
In this study, a flexible dMRI technique, HYDI, was used to investigate changes in microstructural indices derived from different modeling approaches. The HYDI method is versatile and conducive to multiple strategies of diffusion data processing (Wu and Alexander, 2007; Wu et al., 2008). HYDI has at least 126 diffusion directions, which requires approximately 25 minutes of scan time with parallel imaging and less than 7 minutes when using simultaneous multislice (also called multi-band) techniques (Setsompop et al., 2012).
Using the HYDI dataset, we studied the differences in DTI indices (AD, RD, MD, and FA), NODDI indices (ODI and Vic), and P0 across lesion, perilesion, and NAWM ROIs in patients with MS. Compared with NAWM, MS lesions were characterized by decreased tissue restriction as described by P0, a model-free index from q-space analysis. Consistent with previous studies in an animal model of MS (Biton et al., 2006; Wu et al., 2011), decreased P0 suggests the deterioration of myelination and/or the absence of myelinated axons. The loss of myelinated axons is further implied by a decreased in Vic, a NODDI index of axonal density, consistent with an ex vivo MS spinal cord study (Grussu et al., 2017).
At both the lesion level and group level, Vic and P0 detected WM alterations between the perilesions and NAWM that were not detected in T2-FLAIR. Alterations of WM in these perilesion ROIs were significant, though more subtle than those in the lesions. Similarly, previous studies, though with different imaging modalities, demonstrated that MS perilesions are moderately associated with a decreased intensity of the magnetization transfer ratio, increased microglial density (Moll et al., 2011), and increased binding with a PET inflammation tracer, 18-kDa translocator protein (Colasanti et al., 2014). Our preliminary results suggest that dMRI metrics are sensitive to subtle changes in MS-affected WM that are not detectable using conventional imaging approaches. An advantage of model based diffusion metrics and q-space analysis is that they can provide insights into the underlying mechanisms.
In this study, the fiber dispersion inferred based on NODDI-derived ODI did not differ significantly among the three ROI types (i.e., lesions, perilesions, and NAWM). While not seen in the group level tests due to variations across ROI sets, decreased FA was observed at the individual lesion level and is consistent with a decrease in tissue coherence, as seen in previous reports of adult mild MS (Filippi et al., 2000), adult moderate MS (Filippi et al., 2001), and pediatric mild MS (Vishwas et al., 2013; Rocca et al., 2016). DTI diffusivities (i.e., AD, RD, and MD) have discrimination capabilities comparable to T2-FLAIR in separating the lesions from the rest of the WM. In this study they failed to distinguish perilesions from NAWM. The potential explanation may be the stringent multiple comparison adjustment filtered out those DTI metrics, which were otherwise significant.
This feasibility study has several limitations. First, although there were 36 ROIs, they were obtained from only six subjects, which could undermine the DTI results. In addition, the differences in subjects’ disease stage, ROI size, and anatomical locations of ROIs with distinct underlying microstructural compositions may contribute additional variations in the results. It is also possible that different anatomical locations of ROIs may have different degrees or types of neurodegeneration. Future studies may focus on the implication of anatomically varying diffusion metrics to underlying pathophysiological changes in MS.
Second, NODDI may not be the best model for characterizing MS lesions. While NODDI-derived Vic for axonal density is robust within WM, it tends to be overestimated in tissues with intermediate to low microscopic anisotropy, such as gray matter and lesions (Jelescu et al., 2016; Lampinen et al., 2017). The overestimation of NODDI could be caused by a violation of the NODDI assumptions of tortuosity and “rigid-stick” describing fixed axial diffusivities and close to zero radial diffusivity (compared with axial diffusivity). Further investigation is necessary to estimate intra-axonal diffusivities through other diffusion models, such as diffusion kurtosis imaging (Fieremans et al., 2011), diffusion basis spectrum imaging (Wang et al., 2011), restriction spectrum imaging (White et al., 2013), or the constrained diffusional variance decomposition method (Lampinen et al., 2017).
Finally, the definition of lesion, perilesion and NAWM ROIs may affect the results. In this study, lesion ROIs were defined based on a procedure optimized for detection of T2-FLAIR hyperintense regions. In addition, a small percentage (approximately 1.37 lesion count per brain (Wen et al., 2009)) of the lesions may be age-related white matter hyperintensities. It is also possible that perilesions have a partial volume effect from NAWM. Despite these factors, this study provides evidence and novel insights regarding the microstructural alterations from NAWM to intermediate perilesion areas to MS lesions.
Additional files:
Additional Figure 1 (387.5KB, tif) : Averaged AD, RD, MD, FA, ODI, Vic, and P0 maps from a group of healthy volunteers.
Additional Table 1 (47.6KB, pdf) : Results (q-values) of the pairwise comparisons between the lesion, matched perilesion, and NAWM.
Footnotes
Conflicts of interest: No competing financial interests exist.
Financial support: This work was supported by Indiana University-Purdue University Indianapolis Imaging Technology Development Program (IUPUI ITDP), National Institutes of Health (NIH) grant R21 NS075791, and R01 AG053993. Funders had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Institutional review board statement: All participants provided informed consent approved by the guidelines of the institutional review board of the Geisel School of Medicine at Dartmouth on October 17, 2013 (No. 11482) and this study was performed in accordance with the Declaration of Helsinki.
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understood that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity could not be guaranteed.
Reporting statement: This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by Jaroslaw Harezlak of Indiana University in USA
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: For data sharing, individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be shared. Individual participant data will not be available. However, the study protocol, statistical analysis plan, analytic code, and informed consent form without signatures will be made available beginning 3 months and ending 5 years following article publication to investigators whose proposed use of the data has been approved by an independent review committee identified to achieve aims in the approved proposal. Proposals should be directed to yucwu@iu.edu. To gain access, data requestors will need to sign a data access agreement and institutional IRB approval will be required.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by Indiana University-Purdue University Indianapolis Imaging Technology Development Program (IUPUI ITDP), National Institutes of Health (NIH) grant R21 NS075791, and R01 AG053993.
C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Liu XL
References
- 1.Assaf Y, Mayk A, Cohen Y. Displacement imaging of spinal cord using q-space diffusion-weighted MRI. Magn Reson Med. 2000;44:713–722. doi: 10.1002/1522-2594(200011)44:5<713::aid-mrm9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Assaf Y, Chapman J, Ben-Bashat D, Hendler T, Segev Y, Korczyn AD, Graif M, Cohen Y. White matter changes in multiple sclerosis: correlation of q-space diffusion MRI and 1H MRS. Magn Reson Imaging. 2005;23:703–710. doi: 10.1016/j.mri.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Assaf Y, Ben-Bashat D, Chapman J, Peled S, Biton IE, Kafri M, Segev Y, Hendler T, Korczyn AD, Graif M, Cohen Y. High b-value q-space analyzed diffusion-weighted MRI: application to multiple sclerosis. Magn Reson Med. 2002;47:115–126. doi: 10.1002/mrm.10040. [DOI] [PubMed] [Google Scholar]
- 4.Banaszek A, Bladowska J, Pokryszko-Dragan A, Podemski R, Sasiadek MJ. Evaluation of the degradation of the selected projectile, commissural and association white matter tracts within normal appearing white matter in patients with multiple sclerosis using diffusion tensor MR imaging - a preliminary study. Pol J Radiol. 2015;80:457–463. doi: 10.12659/PJR.894661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 7.Bester M, Lazar M, Petracca M, Babb JS, Herbert J, Grossman RI, Inglese M. Tract-specific white matter correlates of fatigue and cognitive impairment in benign multiple sclerosis. J Neurol Sci. 2013;330:61–66. doi: 10.1016/j.jns.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billiet T, Vandenbulcke M, Madler B, Peeters R, Dhollander T, Zhang H, Deprez S, Van den Bergh BR, Sunaert S, Emsell L. Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiol Aging. 2015;36:2107–2121. doi: 10.1016/j.neurobiolaging.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Biton IE, Duncan ID, Cohen Y. High b-value q-space diffusion MRI in myelin-deficient rat spinal cords. Magn Reson Imaging. 2006;24:161–166. doi: 10.1016/j.mri.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Bodini B, Cercignani M, Khaleeli Z, Miller DH, Ron M, Penny S, Thompson AJ, Ciccarelli O. Corpus callosum damage predicts disability progression and cognitive dysfunction in primary-progressive MS after five years. Hum Brain Mapp. 2013;34:1163–1172. doi: 10.1002/hbm.21499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callaghan PT. Principles of nuclear magnetic resonance microscopy. Oxford: Clarendon Press; 1991. [Google Scholar]
- 12.Chiang CW, Wang Y, Sun P, Lin TH, Trinkaus K, Cross AH, Song SK. Quantifying white matter tract diffusion parameters in the presence of increased extra-fiber cellularity and vasogenic edema. Neuroimage. 2014;101:310–319. doi: 10.1016/j.neuroimage.2014.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark CA, Le Bihan D. Water diffusion compartmentation and anisotropy at high b values in the human brain. Magn Reson Med. 2000;44:852–859. doi: 10.1002/1522-2594(200012)44:6<852::aid-mrm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 14.Colasanti A, Guo Q, Muhlert N, Giannetti P, Onega M, Newbould RD, Ciccarelli O, Rison S, Thomas C, Nicholas R, Muraro PA, Malik O, Owen DR, Piccini P, Gunn RN, Rabiner EA, Matthews PM. In vivo assessment of brain white matter inflammation in multiple sclerosis with (18)F-PBR111 PET. J Nucl Med. 2014;55:1112–1118. doi: 10.2967/jnumed.113.135129. [DOI] [PubMed] [Google Scholar]
- 15.Colgan N, Siow B, O’Callaghan JM, Harrison IF, Wells JA, Holmes HE, Ismail O, Richardson S, Alexander DC, Collins EC, Fisher EM, Johnson R, Schwarz AJ, Ahmed Z, O’Neill MJ, Murray TK, Zhang H, Lythgoe MF. Application of neurite orientation dispersion and density imaging (NODDI) to a tau pathology model of Alzheimer’s disease. Neuroimage. 2015;125:739–744. doi: 10.1016/j.neuroimage.2015.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Santis S, Drakesmith M, Bells S, Assaf Y, Jones DK. Why diffusion tensor MRI does well only some of the time: Variance and covariance of white matter tissue microstructure attributes in the living human brain. Neuroimage. 2013;89:35–44. doi: 10.1016/j.neuroimage.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Descoteaux M, Deriche R, Le Bihan D, Mangin JF, Poupon C. Multiple q-shell diffusion propagator imaging. Med Image Anal. 2011;15:603–621. doi: 10.1016/j.media.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Fieremans E, Jensen JH, Helpern JA. White matter characterization with diffusional kurtosis imaging. Neuroimage. 2011;58:177–188. doi: 10.1016/j.neuroimage.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippi M, Rocca MA. Conventional MRI in multiple sclerosis. J Neuroimaging. 2007;17(Suppl 1):3S–9S. doi: 10.1111/j.1552-6569.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- 20.Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304–311. doi: 10.1212/wnl.56.3.304. [DOI] [PubMed] [Google Scholar]
- 21.Filippi M, Iannucci G, Cercignani M, Assunta Rocca M, Pratesi A, Comi G. A quantitative study of water diffusion in multiple sclerosis lesions and normal-appearing white matter using echo-planar imaging. Arch Neurol. 2000;57:1017–1021. doi: 10.1001/archneur.57.7.1017. [DOI] [PubMed] [Google Scholar]
- 22.Grussu F, Schneider T, Tur C, Yates RL, Tachrount M, Ianus A, Yiannakas MC, Newcombe J, Zhang H, Alexander DC, DeLuca GC Gandini Wheeler-Kingshott CAM. Neurite dispersion: a new marker of multiple sclerosis spinal cord pathology? Ann Clin Transl Neurol. 2017;4:663–679. doi: 10.1002/acn3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison DM, Shiee N, Bazin PL, Newsome SD, Ratchford JN, Pham D, Calabresi PA, Reich DS. Tract-specific quantitative MRI better correlates with disability than conventional MRI in multiple sclerosis. J Neurol. 2013;260:397–406. doi: 10.1007/s00415-012-6638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseinbor AP, Chung MK, Wu YC, Alexander AL. Bessel Fourier Orientation Reconstruction (BFOR): an analytical diffusion propagator reconstruction for hybrid diffusion imaging and computation of q-space indices. Neuroimage. 2013;64:650–670. doi: 10.1016/j.neuroimage.2012.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jelescu IO, Veraart J, Fieremans E, Novikov DS. Degeneracy in model parameter estimation for multi-compartmental diffusion in neuronal tissue. NMR Biomed. 2016;29:33–47. doi: 10.1002/nbm.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 27.Jespersen SN, Bjarkam CR, Nyengaard JR, Chakravarty MM, Hansen B, Vosegaard T, Ostergaard L, Yablonskiy D, Nielsen NC, Vestergaard-Poulsen P. Neurite density from magnetic resonance diffusion measurements at ultrahigh field: comparison with light microscopy and electron microscopy. Neuroimage. 2010;49:205–216. doi: 10.1016/j.neuroimage.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kodiweera C, Alexander AL, Harezlak J, McAllister TW, Wu YC. Age effects and sex differences in human brain white matter of young to middle-aged adults: A DTI, NODDI, and q-space study. Neuroimage. 2016;128:180–192. doi: 10.1016/j.neuroimage.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunz N, Zhang H, Vasung L, O’Brien KR, Assaf Y, Lazeyras F, Alexander DC, Huppi PS. Assessing white matter microstructure of the newborn with multi-shell diffusion MRI and biophysical compartment models. Neuroimage. 2014;96:288–299. doi: 10.1016/j.neuroimage.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 30.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 31.Lally P, Zhang H, Pauliah S, Price D, Bainbridge A, Balraj G, Cady E, Shankaran S, Thayyil S. Microstructural changes in neonatal encephalopathy revealed with the neurite orientation dispersion and density imaging (NODDI) model. Arch Dis Child Fetal Neonatal Ed. 2014;99(Suppl 1):A14. [Google Scholar]
- 32.Lampinen B, Szczepankiewicz F, Martensson J, van Westen D, Sundgren PC, Nilsson M. Neurite density imaging versus imaging of microscopic anisotropy in diffusion MRI: A model comparison using spherical tensor encoding. Neuroimage. 2017;147:517–531. doi: 10.1016/j.neuroimage.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Mitchell PJ, Kilpatrick TJ, Stein MS, Harrison LC, Baker J, Ditchfield M, Li K, Egan GF, Butzkueven H, Kolbe SC. Diffusion tensor imaging of acute inflammatory lesion evolution in multiple sclerosis. J Clin Neurosci. 2012;19:1689–1694. doi: 10.1016/j.jocn.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Manjon JV, Coupe P, Concha L, Buades A, Collins DL, Robles M. Diffusion weighted image denoising using overcomplete local PCA. PLoS One. 2013;8:e73021. doi: 10.1371/journal.pone.0073021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauchly JW. Significance test for sphericity of a normal n-variate distribution. Ann Math Statist. 1940;11:204–209. [Google Scholar]
- 36.Moll NM, Rietsch AM, Thomas S, Ransohoff AJ, Lee JC, Fox R, Chang A, Ransohoff RM, Fisher E. Multiple sclerosis normal-appearing white matter: pathology-imaging correlations. Ann Neurol. 2011;70:764–773. doi: 10.1002/ana.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mustafi SM, Kodiweera C, Flashman LA, McAllister TW, Wu YC. International Society of Magnetic Resonance in Medicine (ISMRM) 24th Annual Meeting. Singapore: 2016. Hybrid diffusion imaging to detect acute white matter injury after mild TBI. [Google Scholar]
- 38.Nazeri A, Chakravarty MM, Rotenberg DJ, Rajji TK, Rathi Y, Michailovich OV, Voineskos AN. Functional consequences of neurite orientation dispersion and density in humans across the adult lifespan. J Neurosci. 2015;35:1753–1762. doi: 10.1523/JNEUROSCI.3979-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozarslan E, Koay CG, Shepherd TM, Komlosh ME, Irfanoglu MO, Pierpaoli C, Basser PJ. Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage. 2013;78:16–32. doi: 10.1016/j.neuroimage.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62:717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- 41.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocca MA, Sonkin M, Copetti M, Pagani E, Arnold DL, Narayanan S, Sled JG, Banwell B, Filippi M. Diffusion tensor magnetic resonance imaging in very early onset pediatric multiple sclerosis. Mult Scler. 2016;22:620–627. doi: 10.1177/1352458515596600. [DOI] [PubMed] [Google Scholar]
- 43.Rovaris M, Filippi M. Diffusion tensor MRI in multiple sclerosis. J Neuroimaging. 2007;17(Suppl 1):27S–30S. doi: 10.1111/j.1552-6569.2007.00133.x. [DOI] [PubMed] [Google Scholar]
- 44.Sbardella E, Tona F, Petsas N, Pantano P. DTI measurements in multiple sclerosis: Evaluation of brain damage and clinical implications. Mult Scler Int 2013. 2013:671730. doi: 10.1155/2013/671730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider T, Brownlee W, Zhang H, Ciccarelli O, Miller DH, Wheeler-Kingshott CG. Sensitivity of multi-shell NODDI to multiple sclerosis white matter changes: a pilot study. Funct Neurol. 2017;32:97–101. doi: 10.11138/FNeur/2017.32.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sepehrband F, Clark KA, Ullmann JF, Kurniawan ND, Leanage G, Reutens DC, Yang Z. Brain tissue compartment density estimated using diffusion-weighted MRI yields tissue parameters consistent with histology. Hum Brain Mapp. 2015;36:3687–3702. doi: 10.1002/hbm.22872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67:1210–1224. doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Timmers I, Zhang H, Bastiani M, Jansma BM, Roebroeck A, Rubio-Gozalbo ME. White matter microstructure pathology in classic galactosemia revealed by neurite orientation dispersion and density imaging. J Inherit Metab Dis. 2015;38:295–304. doi: 10.1007/s10545-014-9780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vishwas MS, Healy BC, Pienaar R, Gorman MP, Grant PE, Chitnis T. Diffusion tensor analysis of pediatric multiple sclerosis and clinically isolated syndromes. AJNR Am J Neuroradiol. 2013;34:417–423. doi: 10.3174/ajnr.A3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Wang Q, Haldar JP, Yeh FC, Xie M, Sun P, Tu TW, Trinkaus K, Klein RS, Cross AH, Song SK. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134:3590–3601. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warlop NP, Achten E, Fieremans E, Debruyne J, Vingerhoets G. Transverse diffusivity of cerebral parenchyma predicts visual tracking performance in relapsing-remitting multiple sclerosis. Brain Cogn. 2009;71:410–415. doi: 10.1016/j.bandc.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Wen W, Sachdev PS, Li JJ, Chen X, Anstey KJ. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44-48. Hum Brain Mapp. 2009;30:1155–1167. doi: 10.1002/hbm.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 54.White NS, Leergaard TB, D’Arceuil H, Bjaalie JG, Dale AM. Probing tissue microstructure with restriction spectrum imaging: Histological and theoretical validation. Hum Brain Mapp. 2013;34:327–346. doi: 10.1002/hbm.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wishart HA, Kee ER, Ford JF, MacDonald JW, Aney S, J. LZ. Acapulco, Mexico: Annual Meeting of the International Neuropsychological Society; 2010. A novel approach for semi-automated segmentation of MS lesions on FLAIR imaging: Reliability and clinical correlates. [Google Scholar]
- 56.Wu YC, Alexander AL. Hybrid diffusion imaging. Neuroimage. 2007;36:617–629. doi: 10.1016/j.neuroimage.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu YC, Field AS, Alexander AL. Computation of diffusion function measures in q-space using magnetic resonance hybrid diffusion imaging. IEEE Trans Med Imaging. 2008;27:858–865. doi: 10.1109/TMI.2008.922696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu YC, Field AS, Duncan ID, Samsonov AA, Kondo Y, Tudorascu D, Alexander AL. High b-value and diffusion tensor imaging in a canine model of dysmyelination and brain maturation. Neuroimage. 2011;58:829–837. doi: 10.1016/j.neuroimage.2011.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Averaged AD, RD, MD, FA, ODI, Vic, and P0 maps from a group of healthy volunteers (not included in this study).
The gray scale for AD, RD, and MD ranges from 0 to 3.0 x10-3 mm2/s. The gray scale for FA is from 0.2 to 1; the larger the value, the higher the tissue coherence. The gray scale of the ODI map is from 0 to 1, while that of Vic is from 0.1 to 1. A smaller ODI indicates higher coherence and less dispersion, while a larger Vic indicates higher intra-cellular volume fraction. AD: Axial diffusivity; RD: radial diffusivity; MD: mean diffusivity; FA: fractional anisotropy; ODI: orientation dispersion index; Vic: volume fraction for intra-axonal water; P0: q-space derived zero-displacement probability.
Results (q-values) of the pairwise comparisons between the lesion, matched perilesion, and NAWM