Abstract
In anticipation of the massive burden of neurodegenerative disease within super-aged societies, great efforts have been made to utilize neural stem and progenitor cells for regenerative medicine. The capacity of intrinsic neural stem and progenitor cells to regenerate damaged brain tissue remains unclear, due in part to the lack of knowledge about how these newly born neurons integrate into functional circuitry. As sizable integration of adult-born neurons naturally occurs in the dentate gyrus region of the hippocampus, clarifying the mechanisms of this process could provide insights for applying neural stem and progenitor cells in clinical settings. There is convincing evidence of functional correlations between adult-born neurons and memory consolidation and sleep; therefore, we describe some new advances that were left untouched in our recent review.
Keywords: rapid eye movement sleep, sleep deprivation, optogenetics, real-time sleep analysis, hippocampus, fear memory, synaptic plasticity, memory processing
Dentate Gyrus Circuitry and Development
The principle cells in the dentate gyrus (DG) are densely packed granular neurons. These granular neurons receive input from superficial layers of the entorhinal cortex through the perforant pathway. Granular neurons mainly project to CA3 pyramidal cells through mossy fibers. Although homologous brain areas are found in birds and reptiles, the DG appears to have evolved recently and hence is unique to mammals. Ontogenetically, the DG matures relatively late during brain development and separately from the rest of the hippocampus, completing its major developmental process around postnatal day 15 in mice. At the border between the granular cell layer and the hilus, which is called the subgranular zone, neural stem and progenitor cells (NSPCs) mainly give rise to DG granular neurons. Intriguingly, these NSPCs continue to produce new neurons throughout the lifespan. Furthermore, across evolutionary lineages, adult neurogenesis has become increasingly restricted in terms of the number of neurogenic regions in the brain and the regenerative potential of these regions. Therefore, although evolutionary pressures appear to have favored relatively static brain circuity without the constant introduction of new neurons, the persistence of adult neurogenesis in specific regions of the mammalian brain, including the DG, suggests that these adult-born neurons serve certain adaptive functions that promote survival (Kempermann, 2012). We have performed a PubMed literature search of articles published in the period April 1995 on adult-neurogenesis in memory consolidation during sleep.
Mnemonic Function of the DG and Its Adult-Generated Neurons
A substantial body of evidence implicates the DG and its adult-generated neurons in learning and memory. In particular, recent advances in technology have enabled defined populations of neurons to be labeled with optogenes that can control neuronal activity in behaving animals upon light delivery through an implanted optical fiber. Using this optogenetic approach, it was shown that the reactivation of DG neurons that were active during earlier contextual fear conditioning produces a conditioned fear response in mice, indicating that activation of DG neurons integrated into a memory trace is sufficient to induce recall of the memory (Liu et al., 2012). Conversely, optogenetic silencing of the activity of adult-born DG neurons impairs the learning and retrieval of contextual fear memories (Gu et al., 2012; Danielson et al., 2016). Furthermore, using a transgene-mediated ablation approach, the specific removal of adult-born DG neurons after learning degrades contextual fear and spatial memories (Arruda-Carvalho et al., 2011). Therefore, DG neurons, including those generated during adulthood, play a critical role in forming hippocampal-dependent memory traces. At the same time, however, the incorporation of adult-born DG neurons into new memories may “overwrite” older memories and lead to forgetting, which might help animals adapt to changing environments by facilitating the learning of new contingencies (Akers et al., 2014).
One currently popular hypothesis is that adult-born DG neurons are incorporated into memory circuits during a specific time window in their development. In support of this hypothesis, optogenetic silencing of 4-week-old adult-born DG neurons impairs the retrieval of spatial and contextual fear memories, whereas silencing of 2- or 8-week-old adult-born neurons has no such effect (Gu et al., 2012). This reliance of memory networks on adult-born DG neurons during a narrow developmental period may be pinned to certain critical steps in their maturation, including the stabilization of dendritic branching and pruning, a period of hyper-excitability and enhanced long-term potentiation, and distinct patterns of connections within local circuitry that occurs around 4 weeks of age (Figure 1) (Gu et al., 2012; Toni and Schinder, 2015). One possibility is that during this transient hyper-plastic period, adult-born DG neurons are more likely to participate in the encoding of multiple representations at once, meaning that memories formed around the same point in time are encoded by overlapping neural networks containing the same adult-born neurons (Danielson et al., 2016). Thus, the incorporation of adult-born DG neurons into neural networks may serve as a memory “time-stamp” that could facilitate discrimination between similar representations of events (i.e., pattern separation) based on when those events occurred in the past.
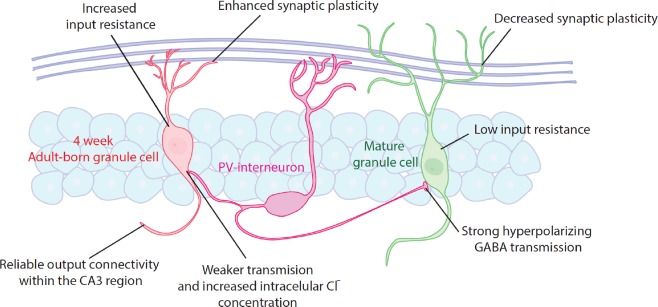
Figure 1.
Synaptic and intrinsic properties of 4-week-old adult-born granule cells vs. mature granule cells.
Behavioral data suggest a critical period for 4-week-old adult-born granule cells (ABGCs) in memory processing. During this period, ABGCs display increased amplitude and decreased induction threshold for long-term potentiation. Also, input-resistance is around two-fold higher than in mature granule cells, making them more efficient in transducing ionic currents into membrane depolarization. In addition, as the intracellular Cl- concentration is increased, γ-aminobutyric acid currents may produce shunting rather than hyperpolarizing inhibition of ABGCs. These properties afford ABGCs with a transient hyper-plastic period that may enhance their incorporation into memory circuits.
Contribution of Sleep to Memory Consolidation
During the time window when adult-born DG neurons incorporate into memory circuits, the sleep/wake cycle could dynamically change the mode of brain activity, thereby affecting adult-born DG neuron function and memory processing (Akers et al., 2010). In mammals, sleep mainly consists of rapid eye movement (REM) sleep and non-REM (NREM) sleep, which can be identified by brain-wide patterns of oscillatory neural activity. The most predominant rhythmic brain activities are theta waves and pont-geniculo-occipital waves during REM sleep and neocortical slow oscillations, thalamo-cortical spindles, and hippocampal sharp wave-ripples during NREM sleep. The theta waves occurring during REM sleep are observed throughout the hippocampus, whereas the hippocampal sharp wave-ripples occurring during NREM sleep arise from CA3 output to the CA1 (Diekelmann and Born, 2010; Girardeau and Zugaro, 2011; Boyce et al., 2016; Garner et al., 2017).
Although the functions of sleep are still not fully understood, several lines of evidence indicate that sleep promotes memory consolidation. For instance, early studies in both animals and humans show that learning increases the firing of hippocampal neurons, the duration of certain sleep stages, and the amount of oscillatory activity during subsequent sleep and that post-learning sleep enhances memory recall. Many studies have examined the consequences of lack of sleep on memory, reporting that sleep-deprived animals and humans show memory impairments in hippocampal-dependent but not hippocampal-independent tasks as well as deficits in hippocampal synaptic plasticity. However, sleep deprivation can induce significant stress and inflammatory responses, elicit arousal, and affect the function of non-hippocampal brain regions that are also important for cognition, making it difficult to disentangle the effects of sleep deprivation on hippocampal-dependent memory processing from other confounding effects (Ribeiro and Nicolelis, 2004; Diekelmann and Born, 2010).
Using more precise approaches to interfering with sleep, some studies show that disrupting certain types of oscillatory neural activity during sleep disrupts memory consolidation. For instance, rats in which hippocampal sharp wave-ripples are electrophysiologically suppressed across several days of training in spatial tasks show poorer task performance than control rats, suggesting that such ripple activity during sleep facilitates the consolidation of spatial memories (Girardeau and Zugaro, 2011). Also, optogenetically silencing upstream neurons to selectively reduce sleep-related hippocampal theta waves in mice after training impairs previously-learned hippocampal-dependent memories but not non-hippocampal-dependent memory, providing evidence of the critical contribution of hippocampal rhythmic activity during sleep to memory consolidation (Girardeau and Zugaro, 2011; Boyce et al., 2017).
Intriguingly, memories can be replayed in the hippocampus during sleep. Replayed memories can unfold in forward or backward directions in a temporally compressed manner, are usually observed during NREM sleep, and are thought to enable system-level consolidation from hippocampal-dependent to primarily neocortical-dependent networks. Moreover, the presentation of previously learned stimuli, known as “cueing”, during NREM sleep can induce the replay of memories and alter their consolidation. In humans, presenting a previously experienced olfactory stimulus during NREM sleep causes hippocampal reactivation of an odor-related object-place memory and enhances subsequent recall of that memory (Diekelmann and Born, 2010). Similarly, in mice, presenting a conditioned auditory stimulus during NREM sleep impairs the subsequent expression of auditory trace fear memory, although it is not yet fully understood why cueing during sleep sometimes enhances but other times impairs memory consolidation (Ribeiro and Nicolelis, 2004; Diekelmann and Born, 2010).
Possibly in conjunction with memory replay, another way in which sleep could promote memory consolidation is by downscaling synaptic strength to reset the increase in synaptic strength that occurs during wakefulness. According to the synaptic homeostasis hypothesis, a generalized down-regulation of synaptic strength during sleep eliminates weak connections while sparing strong connections, which increases the signal-to-noise ratio by allowing the removal of less meaningful information without jeopardizing the consolidation of more meaningful information. However, some recent studies report the upscaling of synaptic strength during sleep, casting doubt upon the synaptic homeostasis hypothesis. For instance, rats that were exposed to novel objects during prior wakefulness but not non-exposed rats show up-regulation of plasticity-related genes in the hippocampus during REM sleep. Such findings provide support for the competing synaptic embossing hypothesis, which proposes that specific increases in synaptic strength occur against the backdrop of a generalized decrease in synaptic strength during sleep, which “embosses” certain memory traces in the brain. This active restructuring of patterns of synaptic strength during sleep might also serve to explain how cueing during sleep alters memory processing (Ribeiro and Nicolelis, 2004; Tononi and Cirelli, 2006).
Sleep and Adult DG Neurogenesis
Given the role of the hippocampus in memory consolidation during sleep, research has begun to investigate the contribution of adult-generated DG neurons to this process. So far, in addition to evidence linking alterations in circadian clock genes with different neurogenic phenotypes, several studies report a dose-response relationship between sleep deprivation and adult DG neurogenesis in rodents, with longer periods of sleep deprivation causing greater impairments in neurogenesis. Also, it has been recently reported that depriving pregnant rats of sleep suppresses DG neurogenesis, impairs hippocampal-dependent learning and memory, decreases hippocampal synaptic plasticity, and increases depressive and anxiety-like behavior in adult offspring, suggesting an epigenetic link between sleep deprivation and adult DG neurogenesis. Again, however, because sleep deprivation usually induces a stress response, it is possible that these outcomes are primarily due to an increased exposure to stress hormones. To more clearly determine whether adult-born DG neurons make a special contribution to memory consolidation during sleep, future studies could optogenetically manipulate the activity of these neurons during specific stages of sleep without changing overall sleep architecture (López-Armas et al., 2016; Akers et al., 2018; Murata et al., 2018).
Conclusions
The studies covered in this review hint at a complex interplay among adult DG neurogenesis, sleep, and mnemonic function, indicating the potential value of future studies directly aimed at identifying and unraveling the interconnections among these factors. In particular, it is imperative to develop a fuller understanding of how adult-born DG neurons integrate into the existing neural circuitry (Figure 2), which may soon be realized through exciting advances in technology and computing. By ensuring the optimal functional integration of adult-generated DG neurons, hippocampal NSPCs could be clinically applied to treat patients with age-related cognitive decline, dementia, depression and anxiety, phobias, or posttraumatic stress disorder.
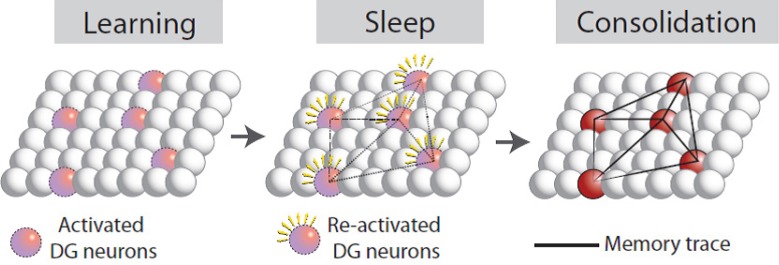
Figure 2.
A hypothetical process of dentate gyrus (DG) neuron involvement in memory consolidation during sleep.
Memory encoded by DG neurons during learning is unstable and susceptible to interference. Selective re-activation of DG neurons encoding a memory during sleep may strengthen their connections, thereby consolidating the memory trace.
Additional file: Open peer review report 1 (118.4KB, pdf) .
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: This work was partially supported by the MEXT World Premier International Research Center Initiative, CREST JST, MEXT KAKENHI for Scientific Research on Innovative Areas “Microendophenotype” (25116530) and “Memory Dynamism” (26115502), JSPS KAKENHI Grants (16K18359, 15F15408), Research Foundation for Opto-Science and Technology, Kato Memorial Bioscience Foundation, Japan Foundation for Applied Enzymology, Uehara Memorial Foundation, 2016 Inamori Research Grants Program, Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care, Life Science Foundation of Japan, Kowa Life Science Foundation Research Grant, GSK Japan Research Grant, and KANAE Foundation for the Promotion of Medical Science, Shimadzu Foundation for the Promotion of Science and Technology, Takeda Science Foundation to MS and The Tokyo Biochemical Research Foundation to SS.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Jia-Xu Chen, Beijing University of Chinese Medicine, China.
Funding: This work was partially supported by the MEXT World Premier International Research Center Initiative, CREST JST, MEXT KAKENHI for Scientific Research on Innovative Areas “Microendophenotype” (25116530) and “Memory Dynamism” (26115502), JSPS KAKENHI Grants (16K18359, 15F15408), Research Foundation for Opto-Science and Technology, Kato Memorial Bioscience Foundation, Japan Foundation for Applied Enzymology, Uehara Memorial Foundation, 2016 Inamori Research Grants Program, Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care, Life Science Foundation of Japan, Kowa Life Science Foundation Research Grant, GSK Japan Research Grant, and KANAE Foundation for the Promotion of Medical Science, Shimadzu Foundation for the Promotion of Science and Technology, Takeda Science Foundation to MS and The Tokyo Biochemical Research Foundation to SS.
P-Reviewer: Chen JX; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Akers KG, Sakaguchi M, Arruda-Carvalho M. Functional contribution of adult-generated olfactory bulb interneurons: odor discrimination versus odor memory. J Neurosci. 2010;30:4523–4525. doi: 10.1523/JNEUROSCI.0443-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akers KG, Cherasse Y, Fujita Y, Srinivasan S, Sakurai T, Sakaguchi M. Concise review: Regulatory influence of sleep and epigenetics on adult hippocampal neurogenesis and cognitive and emotional function. Stem Cells. 2018 doi: 10.1002/stem.2815. doi: 10.1002/stem.2815. [DOI] [PubMed] [Google Scholar]
- 3.Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- 4.Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31:15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce R, Williams S, Adamantidis A. REM sleep and memory. Curr Opin Neurobiol. 2017;44:167–177. doi: 10.1016/j.conb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 2016;352:812–816. doi: 10.1126/science.aad5252. [DOI] [PubMed] [Google Scholar]
- 7.Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, Balough EM, Goldberg AR, Drew LJ, Hen R, Losonczy A, Kheirbek MA. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron. 2016;90:101–112. doi: 10.1016/j.neuron.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 9.Garner JM, Chambers J, Barnes AK, Datta S. Changes in brain-derived neurotrophic factor expression influence sleep-wake activity and homeostatic regulation of rapid eye movement sleep. Sleep. 2017 doi: 10.1093/sleep/zsx194. doi: 10.1093/sleep/zsx194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girardeau G, Zugaro M. Hippocampal ripples and memory consolidation. Curr Opin Neurobiol. 2011;21:452–459. doi: 10.1016/j.conb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci. 2012;15:1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempermann G. New neurons for ‘survival of the fittest’. Nat Rev Neurosci. 2012;13:727–736. doi: 10.1038/nrn3319. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Armas G, Flores-Soto ME, Chaparro-Huerta V, Jave-Suarez LF, Soto-Rodríguez S, Rusanova I, Acuña-Castroviejo D, González-Perez O, González-Castañeda RE. Prophylactic role of oral melatonin administration on neurogenesis in adult Balb/C mice during REM sleep deprivation. Oxid Med Cell Longev. 2016;2016:2136902. doi: 10.1155/2016/2136902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murata Y, Oka A, Iseki A, Mori M, Ohe K, Mine K, Enjoji M. Prolonged sleep deprivation decreases cell proliferation and immature newborn neurons in both dorsal and ventral hippocampus of male rats. Neurosci Res. 2018;131:45–51. doi: 10.1016/j.neures.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro S, Nicolelis MA. Reverberation, storage, and postsynaptic propagation of memories during sleep. Learn Mem. 2004;11:686–696. doi: 10.1101/lm.75604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toni N, Schinder AF. Maturation and functional integration of new granule cells into the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;8:a018903. doi: 10.1101/cshperspect.a018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.