Keywords: nerve regeneration, facial nerve defects, olfactory ensheathing cells, nerve fibers, myelination, neurons, nerve muscle action potentials, facial nerve motor nucleus, neural regeneration
Abstract
Olfactory ensheathing cells from the olfactory bulb and olfactory mucosa have been found to increase axonal sprouting and pathfinding and promote the recovery of vibrissae motor performance in facial nerve transection injured rats. However, it is not yet clear whether olfactory ensheathing cells promote the reparation of facial nerve defects in rats. In this study, a collagen sponge and silicone tube neural conduit was implanted into the 6-mm defect of the buccal branch of the facial nerve in adult rats. Olfactory ensheathing cells isolated from the olfactory bulb of newborn Sprague-Dawley rats were injected into the neural conduits connecting the ends of the broken nerves, the morphology and function of the regenerated nerves were compared between the rats implanted with olfactory ensheathing cells with the rats injected with saline. Facial paralysis was assessed. Nerve electrography was used to measure facial nerve-induced action potentials. Visual inspection, anatomical microscopy and hematoxylin-eosin staining were used to assess the histomorphology around the transplanted neural conduit and the morphology of the regenerated nerve. Using fluorogold retrograde tracing, toluidine blue staining and lead uranyl acetate staining, we also measured the number of neurons in the anterior exterior lateral facial nerve motor nucleus, the number of myelinated nerve fibers, and nerve fiber diameter and myelin sheath thickness, respectively. After surgery, olfactory ensheathing cells decreased facial paralysis and the latency of the facial nerve-induced action potentials. There were no differences in the general morphology of the regenerating nerves between the rats implanted with olfactory ensheathing cells and the rats injected with saline. Between-group results showed that olfactory ensheathing cell treatment increased the number of regenerated neurons, improved nerve fiber morphology, and increased the number of myelinated nerve fibers, nerve fiber diameter, and myelin sheath thickness. In conclusion, implantation of olfactory ensheathing cells can promote regeneration and functional recovery after facial nerve damage in rats.
Chinese Library Classification No. R456; R364.3; R745
Introduction
Facial nerves are the seventh pair of cranial nerves, and regulate the muscles that control facial expression. Facial nerve injuries can be caused by trauma (Ghiasi and Banaei, 2016), surgery (Linder et al., 2017), inflammation (Basavaraj et al., 2014), or tumors (Gaudin et al., 2016), and can result in facial paralysis and dysphagia. The incidence of facial nerve injuries ranges from 20 to 30 cases per 100,000 people (Gaudin et al., 2016). Therapeutic approaches to facial nerve injuries include direct end-to-end coaptation (Siemionow et al., 2010), nerve grafts, artificial nerve conduits (Jiang et al., 2010), combinational administration of nerve conduits with nerve regeneration factors such as stem cells (Euler de Souza Lucena et al., 2014; Mohammadi et al., 2014; Ma et al., 2017) and neurotrophic factors (Ma et al., 2017), and low frequency electrical stimulation (Gaudin et al., 2016). As peripheral nerves, facial nerves more readily regenerate than central nerves; however, there are still many cases of facial nerve paralysis that do not fully recover due to the replacement of the enervated muscle tissues by adipose tissue and glial tissue (Choi and Dunn, 2001). Thus, more efforts to search for new approaches for achieving optimal recovery of facial nerve injuries are needed.
Olfactory ensheathing cells (OECs), also known as olfactory ensheathing glial cells or olfactory ensheathing glia, are the glial cells of the olfactory nervous system. OECs are unique glial cell types that migrate from the peripheral nervous system into the central nervous system; this characteristic distinguishes OECs from typical glial cells (Grosu-Bularda et al., 2015; Jiang et al., 2017). OECs can guide axonal outgrowth, enhance axon extension, and stimulate growth cone activity. In vitro studies have shown that OECs stimulate axonal regeneration and sprouting, increase remyelination, offer neuroprotection, enhance neovascularization, and replace lost cells (Wang et al., 2010; Radtke and Kocsis, 2014). The ability of OECs to create a favorable microenvironment (Radtke and Kocsis, 2012) and establish glial pathways (Raisman and Li, 2007; Zheng et al., 2013) for neurogenesis justifies their use in cellular transplantations for injuries of the central (Deumens et al., 2006; Zheng et al., 2013) and peripheral nervous systems, to achieve nerve recovery and increase axonal regrowth (Raisman and Li, 2007; Grosu-Bularda et al., 2015). Various nerve scaffolds and materials, including nanoparticles and poly(lactic-co-glycolic acid) conduit, have been used in combination with OECs for the repair of peripheral nerve defects, and have revealed promising results for repairing defects in facial and sciatic nerves (Tan et al., 2013; Radtke and Kocsis, 2014). These studies indicate that OECs have the potential to be used as stem cells for treatment of facial nerve damage.
OECs prepared from the olfactory bulb and the olfactory mucosa have been used to treat facial nerve transection injuries in rats (Guntinas-Lichius et al., 2001; Guntinas-Lichius et al., 2002); a resulting increase in axonal sprouting and pathfinding (Guntinas-Lichius et al., 2001), better recovery of vibrissae motor performance (Guntinas-Lichius et al., 2002), and an increased blinking rate of eyes (Ramer et al., 2004; Angelov et al., 2005) have been reported. However, the effects of transplantation of OECs on injured facial nerves still need further investigation. In this study, a 6-mm defect was surgically introduced into the upper buccal branch of the facial nerve in rats, and the therapeutic effects of transplantation of OECs on neural regeneration and functional recovery of the defected facial nerves were assessed.
Materials and Methods
Isolation, culture and characterization of OECs
All experimental procedures with the animals complied with the animal ethics regulations and were approved by the Animal Ethics Committee of the Second Military Medical University, China. OECs were isolated according to the method of Dombrowski et al. (2006) with some modifications. Four newborn Sprague-Dawley rats supplied by the Experimental Animal Center of The Second Military Medical University of China (license No. SCXK (Hu) 2017-0002) were sacrificed using CO2. After removal of the frontal nasal bones through a sterile operation, the olfactory bulbs were separated from the surrounding tissues. After 1/3 of the olfactory bulbs at the tail ends had been cut off, the remaining 2/3 of the olfactory bulbs were put into D-Hanks solution in an ice bath. Under an anatomical microscope (Olympus, Tokyo, Japan), the capillaries and soft brain membrane were dissected from the olfactory bulb. After the white matter and the inner layer of the gray matter had been discarded, the olfactory nerve layer and the olfactory bulb granular layer were washed three times with D-Hanks solution, cut into pieces, and digested in 0.25% trypsin + 0.02% ethylenediaminetetraacetic acid at 37°C for 20 minutes. After the digestion was terminated by adding Dulbecco’s modified Eagle’s medium (DMEM)/F12 fetal calf serum medium (Gibco, Grand Island, New York, NY, USA), the cells were suspended through blowing several times with a Pasteur pipette sterilized in flames, and the cell masses were removed through filtration with a 200 nest. After centrifugation at 1000 r/min for 8 minutes, the supernatant was discarded; DMEM/F12-20% fetal calf serum medium was added to suspend the cells. After the cells were seeded into a T25 flask without gel coating for 36 hours, they were transferred to a second T25 bottle without gel coating. Thirty-six hours later, the suspended cells were seeded into poly-L-lysine (0.1 mg/mL) coated bottles, dishes, plates, or slides. The OECs culture media consisted of DMEM/Ham’s F-12, 15% fetal calf serum, 2 µM of forskolin, and 10 ng/mL of basic fibroblast growth factor. The media were replaced once every two days, and the cells were occasionally observed under a light microscope (Olympus).
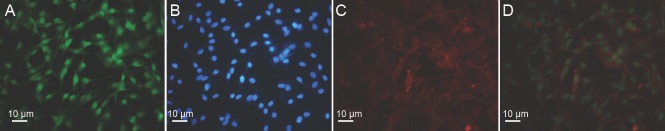
After 10–14 days, a bottle of OECs adhesive to the well was randomly selected and digested with 0.25% trypsin for characterization. The cells were fixed in 4% paraformaldehyde for 10 minutes, characterized with immunofluorescence using mouse IgG against glial fibrillary acidic protein (GFAP) (Chemicon, Tokyo, Japan) as a primary antibody and FITC-labeled donkey IgG (Jackson ImmunoResearch Laboratories, Inc., Baltimore, MD, USA) as a secondary antibody for GFAP (Figure 1A), rabbit IgG against S-100 (Boster Biological Technology, Pleasanton, CA, USA) as a primary antibody and TRITC-labeled donkey IgG (Jackson ImmunoResearch Laboratories, Inc.) as a secondary antibody for S-100 protein (Figure 1C), and stained with Hoechst 33342 (Sigma, Shanghai, China) (Figure 1B and D) to visualize nuclei. The Hoechst 33342-stained nuclei were blue, circular or oval, and varied in size (Figure 1B and D). GFAP-positive cells showed intact nuclei, faint green cytoplasm, and cytoplasm membrane (Figure 1A). S-100-positive cells showed intact nuclei, pink-yellow cytoplasm, and cytoplasm membrane (Figure 1C). The nuclei, S-100-positive cells, and GFAP-positive cells were counted in five high magnificent fields (200×) for each image using a ZEISS confocal laser scanning microscope (Leica, Wetzlar, Germany). The percentage of S-100- or GFAP-positive cells was calculated by dividing the number of the positive cells with the number of Hoechst-positive nuclei in the same section and multiplying by 100%. The mean values of the percentages of S-100- and GFAP-positive cells were used as the concentration of OECs, which was 70%. Trypan blue exclusion was used to detect viability of OECs. The viability of OECs was more than 90%.
Figure 1.
Characterization of the isolated OECs (immunofluorescence staining).
(A) OECs stained with GFAP (green); (B) OEC nuclei stained with Hoechst 33342 (blue) in the same section as A; (C) OECs stained with S-100 (red); (D) OEC nuclei stained with Hoechst 33342 in the same section as C. Scale bars: 10 µm. OECs: Olfactory ensheathing cells; GFAP: glial fibrillary acidic protein.
Preparation of the neural conduit
A silicone tube (Jinan Medical Silicon Rubber Product Ltd., Jinan, China) of 2.0 mm inner diameter and 2.1 mm outer diameter was cut into pieces of 10 mm in length, and washed and sterilized in ethylene oxide. Under sterile conditions, a collagen sponge was cut into cylinders of 6 mm in length and 2 mm in diameter, and inserted into the pieces of silicon tube.
Animal treatment
We used 32 eight-week-old healthy male and female Sprague-Dawley rats (the Experimental Animal Center of The Second Military Medical University of China) with body weights of 200–300 g. Mice received a surgically-induced 6-mm defect on the right side of the facial nerve trunk and were then randomly divided into two groups (n = 16 each). OEC-treated rats were implanted with a piece of neural tube containing OECs. Rats in the injury group were implanted with a piece of neural tube containing saline.
Rats were anesthetized abdominally with phenobarbital sodium. The right cheek was shaved, sterilized, and draped. A 1-cm incision was made 1 cm below the eyelid on the right cheek to expose the buccal branch of the facial nerve. A 6-mm defect was made by dissecting a 4-mm piece from the middle of the upper buccal branch of the facial nerve and retracting the broken nerves. Under an anatomical microscope (Olympus), 1.5 mm of the two ends of the broken facial nerve were inserted into a piece of neural tube and sutured to the silicon tube with 3 stiches of 9/0 absorbable thread at each end. For the rats treated with OECs, 0.1 mL of OECs suspension containing 1 × 105 cells was injected into the neural conduits, whereas 0.1 mL saline was injected into the neural conduits of the injury rats. The incision was sutured with 3/0 threads and sterilized with a 75% alcohol swab. After surgery, all rats were intramuscularly injected with 200,000.00 units of penicillin daily for 3 days. The rats were raised with standard food and drink for 12 weeks in the Experimental Animal Center of the Second Military Medical University.
Facial paralysis test
At 12 weeks after surgery, facial paralysis was evaluated with the sum of scores assigned to both blinking reflexes and vibrissa movement. For blinking reflexes, 2 mL of air was blown 3 cm away from the eye of the rats with an 18-gauge needle on a 5-mL syringe, and the blink speed and amplitude of the two eyes were compared. A score of 0 was assigned if the two eyes moved normally and symmetrically; a score of 1 was assigned if the movement of the right eye was delayed or weaker; and a score of 2 was assigned if no movement of the right eye was observed. For vibrissa movement, the swirling amplitudes of vibrissa on the two sides were compared; a score of 0 was assigned if the two vibrissae swirled normally and symmetrically, a score of 1 was assigned if the right vibrissa movement was weaker than the left, and a score of 2 was assigned if no right vibrissa movement was observed. The rats were identified as having no facial paralysis if the sum of both the blinking reflex score and vibrissa movement score was 0 or 1, partial facial paralysis if the sum was 2 or 3, and complete facial paralysis if the sum was 4 (Lal et al., 2008).
Nerve electrography
At 12 weeks after surgery, all rats were stimulated at the distal ends of the facial nerve defects with an electrical pulse of 20 mV, 1 Hz for 100–200 ms using a SEN-3201 electrical stimulator (Nihon Kohen, Tokyo, Japan). With a silver electrode in the trunks close to proximal ends of the nerve defects, the induced action potentials were detected, amplified with a preamplifier (5000 times, filtration 1000, and time factor 0.25 ms), stacked 64 times with an average. The latency and amplitude of the induced nerve action potentials in the facial nerves were recorded.
Morphological observation
At 12 weeks after surgery, the buccal branch of facial nerve was exposed by making an incision at the same site as that made during the original surgery. A morphological observation was made for inflammatory responses around the neural tube, adhesion of the tube to the surrounding tissues, out filtration, breaks on the wall of the tubes, breaks in the nerves, and the abnormality, color, diameter, and length of the regenerated nerves. These were measured using visual inspection and an anatomical microscope.
Fluorogold retrograde tracing
At 12 weeks after surgery, 6 rats from each group were randomly selected for a fluorogold retrograde tracing study of the regenerated nerves. After the rats were anesthetized abdominally with phenobarbital sodium, the facial nerves on the surgical sides were sectioned at the site 0.5 cm distal to fusion region of the ends of defected nerves, and the facial nerves on the healthy side were sectioned at the corresponding site. With a piece of photoplate under the section site, fluorogold particles (Fluorochrome, Englewood, CO, USA) were attached to a needle and transferred to the proximal end of the sectioned nerve. The proximal end was further inserted into a silicon tube containing 4% fluorogold. The two ends of the silicon tube were sealed with Vaseline to prevent fluorogold from leaking and being absorbed by other small nerves, and then the incision was sutured. After 72 hours, rats were first infused with saline through a catheter inserted into heart through aorta to wash out the blood, and then with 4% paraformaldehyde and 0.05% glutaraldehyde in 0.1 M phosphate buffered saline (PBS) (pH 7.4, 4°C) for 2–3 hours until the eluate became colorless. The brain stem was taken out and fixed in 4% paraformaldehyde at 4°C for 4–6 hours, and then transferred to 5% sucrose in PBS (pH 7.4) at 4°C for two days until the tissues settled to the bottom. Serial cry-sections of 35 µm were made, laid onto poly-lysine coated slides, dehydrated, cleared, and mounted in neutral resins. The slides were observed with a bright field microscope (Olympus) and the images were captured and analyzed using image analysis software (Shanghai Bio-Tech Co., Ltd., Shanghai, China). Ten slides from each rat were randomly selected, and five fields under a microscope with a magnification of 400 for each slide were counted for fluorogold-positive cells. The number of the positive cells for each slide was represented by the average of the numbers of the positive cells in the five fields. The number of fluorogold-positive cells for each rat was represented by the average of the number of the positive cells of the 10 slides.
Histological study
At 12 weeks after surgery, after the electrophysiological tests, 10 rats from each group were sacrificed using CO2. A 1-mm piece from the proximal end and a 1-mm piece from the distal end of the regenerated nerve were taken from each rat. The proximal pieces were fixed in 2.5% neutral glutaraldehyde for 24 hours and then 1% osmium tetroxide for 80 minutes, embedded in epoxy, and cut into semithin sections of 1 µm and ultrathin sections of 50 nm. The distal pieces were fixed in 4% neutral paraformaldehyde, embedded in paraffin, and cut into 4 µm cross-sections of the regenerated nerves and longitudinal sections across the fusion of the regenerated nerves with the distal ends of the nerve defects. The paraffin cross-sections and the paraffin longitudinal sections were stained with hematoxylin and eosin (Carriel et al., 2011). The semithin sections were stained with ammonium methylbenzene blue (Raimondo et al., 2009). The ultrathin sections were stained with lead uranyl acetate (Hirano, 2005). The images of the toluidine blue-stained semithin sections and the electronic microscopic (Leica, Germany) images of the lead uranyl acetate-stained ultrathin sections were analyzed using image analysis software (Shanghai Bio-Tech Co., Ltd.) for the number, diameter, and thickness of the myelin sheath of myelinated nerve fibers in the regenerated nerves. For each parameter tested, 10 slides were randomly selected for each rat, and five images were quantified. The average of the values from the five fields was calculated for each slide, and the final value for each rat was represented by the average of the 10 slides.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical analysis was performed using SAS.V9 software (SAS Institute, Cary, NC, USA). Between-group differences were analyzed using Student’s t-tests. P < 0.05 was considered as statistically significant.
Results
Effect of OECs on facial paralysis scores of facial nerve defected rats
At 12 weeks after surgery, the facial paralysis scores were significantly lower in the OECs group compared with the injury group (35.0%, P < 0.01, n = 16; Figure 2).
Figure 2.
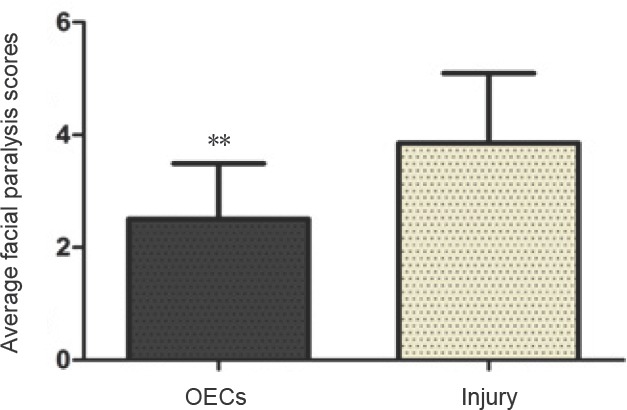
Effect of OECs on facial paralysis scores of facial nerve defected rats detected by the facial paralysis test.
Higher scores indicate a more severe facial nerve paralysis. Data are expressed as the mean ± SD (n = 16; Student’s t-test). **P < 0.01, vs. injury group. OECs: Olfactory ensheathing cells.
Effect of OECs on the nerve muscle action potential of facial nerve defected rats
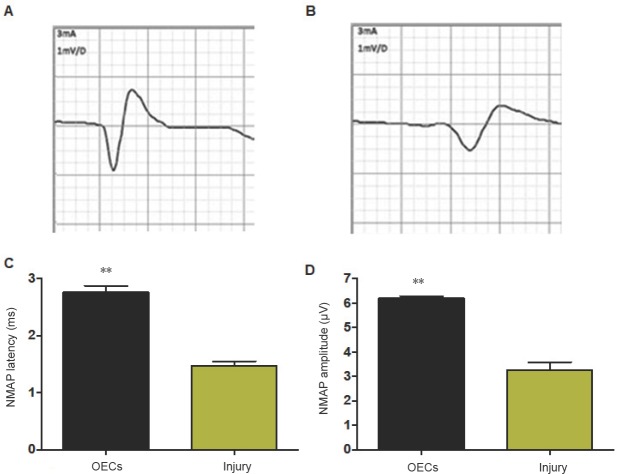
At 12 weeks after surgery, the regenerated facial nerves were stimulated with a current of threshold, and the action potentials were recorded (Figure 3A and B). The latency of the induced nerve muscle action potential was significantly shorter in the OECs group than in the injury group (n = 16, P < 0.01; Figure 3C). The amplitude of the induced nerve muscle action potential was significantly larger in the OECs group than in the injury group (P < 0.01; Figure 3D).
Figure 3.
Effect of OECs on nerve electrography of facial nerve defected rats detected by nerve electrography.
(A) NMAP in the OECs group; (B) NMAP in the injury group; (C) NMAP latency in the OEC-treated rats and untreated rats; (D) NMAP amplitude in the OEC-treated rats and untreated rats. Data are expressed as the mean ± SD (n = 16; Student’s t-test). **P < 0.01, vs. injury group. OECs: Olfactory ensheathing cells; NMAP: nerve muscle action potential.
Effect of OECs on the morphology of the regenerated nerves of facial nerve defected rats
At 12 weeks after surgery, all 32 rats survived. After anesthesia, the facial nerves of the rats were surgically exposed with incisions at the same sites as the original surgery. It was found that the neural silicone tubes were wrapped by connective tissue without local purulent exudate. After the connective tissue outside the tubes had been removed, it was found that the proximal nerves extended out of the defect, connected well with the distal ends and formed regenerated nerves in the semitransparent silicone tubes. The regenerated nerves were easily separable from the silicone tubes without adhesion to the tube walls. There was no purulent exudate inside the tubes. There were no differences between the OEC-treated rats and the injury rats with regard to the morphology of the nerves. The diameters of the regenerated nerves were similar to those of the nerve trunks both proximal and distal to the defects, without local swelling, atrophy or tumor formation. The regenerated nerves looked gray white with blood vessels around epineurium.
Effect of OECs on the number of neurons in the anterior exterior lateral facial nerve motor nucleus of facial nerve defected rats
At 12 weeks after surgery, cells in the anterior exterior lateral facial nerve motor nucleus region external to the anterior of the pons were fluorogold labeled. The labeled cells looked multi-angular or irregular, with a gold-yellow color unevenly distributed in the cytoplasm of the cell bodies and projections (Figure 4A and B). The number of fluorogold-labeled cells with intact shape and obvious projections on the surgical side was significantly higher in OECs group than in the injury group (n = 6, P < 0.01; Figure 4C).
Figure 4.
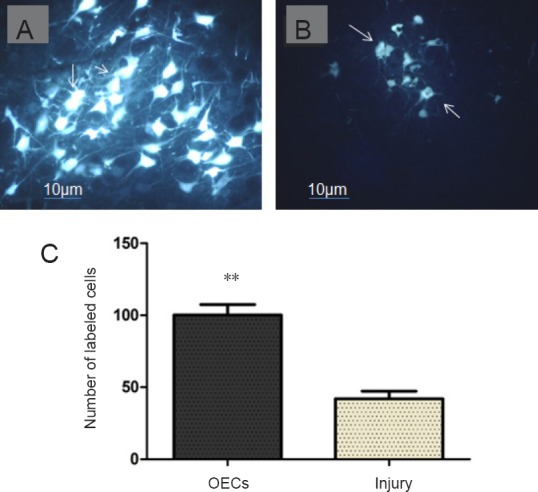
Effect of OECs on the number of neurons in the anterior exterior lateral facial nerve motor nucleus of facial nerve defected rats detected by fluorogold labeling.
(A, B) Fluorogold-labeled cells (arrows) in the facial nerve nucleus of the OECs group (A) and injury group (B) (×200). Scale bars: 10 µm. (C) Number of myelinated nerve fibers in the facial nerve nuclei in the OEC-treated rats and untreated rats. Arrows indicate fluorogold-positive cells. Data are expressed as the mean ± SD (n = 16; Student’s t-test). **P < 0.01, vs. injury group. OECs: Olfactory ensheathing cells.
Effect of OECs on the histological structures of the regenerated nerves of facial nerve defected rats
Hematoxylin-eosin staining
In the longitudinal section, many regenerated nerve fibers were tightly organized alongside and passed the fusion region to the distal end of the nerve in the OEC-treated rats. In the longitudinal section of the facial nerve of the injury rats, fewer nerve fibers were obviously twisted and there was comparatively more scar tissue 12 weeks after surgery (Figure 5).
Figure 5.
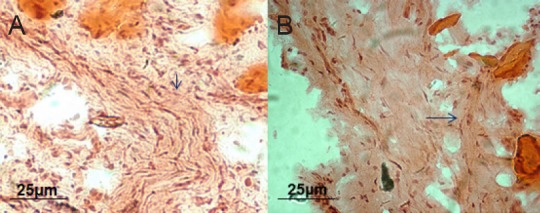
Effect of OECs on the structures of the regenerated nerves of facial nerve defected rats detected by hematoxylin-eosin staining.
(A) OECs group: The arrow points to the intact nerve fiber containing many newly generated axons and nuclei. (B) Injury group: Fewer axons and nuclei in the nerve fibers can be seen; the arrow points to scars extending into the nerve fibers and some vacuoles. Original magnification: 100×. Scale bars: 25 µm. OECs: Olfactory ensheathing cells.
Ammonium methylbenzene blue staining
At 12 weeks after surgery in ammonium methylbenzene blue-stained cross sections of the regenerated nerves, more regenerated nerve fibers were thickly myelinated and organized into bundles with little inter-bundle connective tissue in the OEC-treated rats. In the injury rats, there were fewer regenerated nerve fibers organized into bundles, and more inter-bundle connective tissue was observed compared with OEC-treated rats (Figure 6A and B). Quantitative analysis showed that the number of myelinated nerve fibers was significantly higher in OEC-treated rats than in injury rats (n = 10, P < 0.01; Figure 6C).
Figure 6.
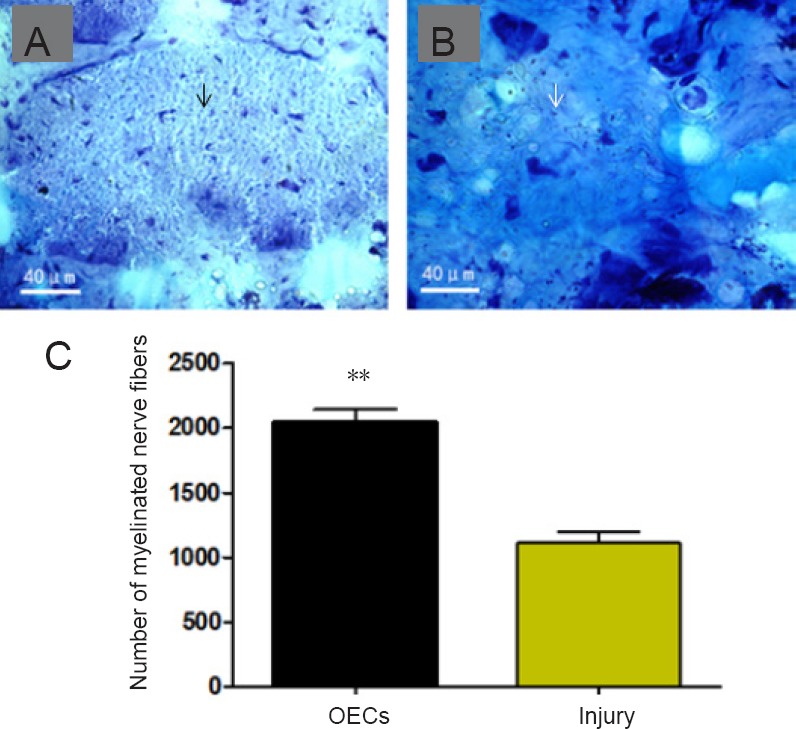
Effect of OECs on the structures of the regenerated nerves of facial nerve defected rats detected by ammonium methylbenzene blue staining.
(A, B) Regenerated nerves of the facial nerve in the OECs (A) and injury (B) groups. OECs obviously improved the morphological structure and increased the number of the regenerated nerve fibers. Each arrow indicates a nerve fiber. Scale bars: 40 µm. (C) Number of myelinated nerve fibers. Data are expressed as the mean ± SD (n = 16; Student’s t-test). **P < 0.01, vs. injury group. OECs: Olfactory ensheathing cells.
Double oxygen acetic uranium and citrate lead staining
At 12 weeks after surgery, the ultra-sections of the regenerated nerves were stained with double oxygen acetic uranium and citrate lead. Electron microscopy showed that in the OEC-treated rats, the regenerated nerve fibers were mainly myelinated and regular in shape, the myelin sheath was clearly composed of intermittently stacked bright layers and dark layers, and there were plenty of organelles in the cytosol of the nerve fibers (Figure 7A). In the injury rats, the regenerated nerve fibers were poorly myelinated and irregular, contained few organelles in the cytosol of the axons, and some degenerated nerve fibers were still visible (Figure 7B). Quantitative analysis showed that the diameters of the nerve fibers were significantly larger in OEC-treated rats than in the injury rats (P < 0.01; Figure 7C). The myelin sheath of nerve fibers was significantly thicker in the OEC-treated rats than in the untreated rats (P < 0.01; Figure 7D).
Figure 7.
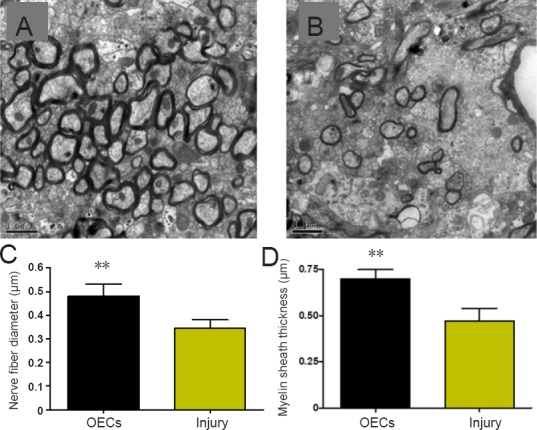
Effect of OECs on the structures of the regenerated nerves of facial nerve defected rats detected by double oxygen acetic uranium and citrate lead staining.
The facial nerve fibers of the regenerated facial nerves had larger diameters and thicker myelin sheaths in the OECs group (A) compared with the injury group (B). Scale bars: 1 µm. (C) Diameters of the facial nerve fibers. (D) Myelin sheath thickness of the facial nerve fibers. Data are expressed as the mean ± SD (n = 16; Student’s t-test). **P < 0.01, vs. injury group. OECs: Olfactory ensheathing cells.
Discussion
In this study, we implanted OECs into the defects in the facial nerve trunks of rats to investigate the effects of OECs on the repair of facial nerve damage. OEC treatment increased the thickness of myelin sheath, the diameter and the number of myelinated facial nerve fibers, and the number of intact neurons in the anterior exterior lateral facial nerve motor nucleus. OECs also improved the organization of nerve fibers and the nerve pulse conductivity of the regenerated nerves, increased the amplitude of the induced action potentials, and relieved facial paralysis. Overall, OECs promoted the nerve regeneration and functional recovery in facial nerve defected rats.
The implanted OEC-mediated increase in the number of nerve fibers in the regenerated nerves is in agreement with observation that OECs enhance axonal sprouting in facial nerve injured rats (Guntinas-Lichius et al., 2001). The finding is also consistent with observation that OECs increase the numbers of regenerated myelinated fibers in vague nerve defected rats (Paviot et al., 2011). The increase in myelin sheath thickness in OEC-treated rats supports findings that transplantation of OECs promoted myelination of the regenerated nerve fibers (Angelov et al., 2005). Since no cell tracking test was performed in this study, it was not possible to assess whether the implanted OEC-mediated increase in myelination was due to the potential of OECs to form the myelin sheath themselves (Angelov et al., 2005; Radtke et al., 2011) or the OEC-mediated increase in the capacity of the host Schwann cells to myelinate the nerve fibers (Boyd et al., 2005). The increase in nerve fiber diameter and the number of organelles in the cytosol of the nerve fibers suggests that implanted OECs may improve the subcellular structures of the regenerated axons and their functions to transport neurotransmitters. The improvement in the organization of nerve fibers into bundles indicates that OECs may create a three-dimensional matrix that provides a permissive microenvironment for axons to directionally extend (Guntinas-Lichius et al., 2002; Boyd et al., 2005). For the first time, we found an OEC-mediated increase in the number of cells in the anterior exterior lateral facial nerve motor nucleus, which suggests that OECs may migrate into the brainstem and differentiate into the neurons in the nucleus (Sandvig et al., 2012; Radtke and Kocsis, 2014). It might also be due to an OEC-mediated increase in proliferation and decrease in apoptosis of the host neurons in the nucleus. A cell tracking test would be required to differentiate between the two mechanisms. Altogether, these results demonstrate that OECs promote the physical regeneration of the defected facial nerves in rats.
In the present study, OECs also decreased the latency and increased the amplitude of the induced action potentials in the regenerated facial nerves. These results indicate that the increase in myelin sheath thickness, nerve fiber diameter, the number of organelles in the cytosol of the nerve fibers, and the number of well-organized nerve fiber bundles in the regenerated facial nerves improved action potential generation and nerve pulse conductivity, which is consistent with previous studies (Schröder, 1972; Ikeda and Oka, 2012; Arancibia-Carcamo et al., 2017). OEC treatment also relieved facial paralysis, which is in agreement with previous findings that OECs promoted recovery of vibrissae motor performance (Guntinas-Lichius et al., 2002) and increased the rate of eye closure (Ramer et al., 2004; Angelov et al., 2005) in facial nerve injured rats. Thus, these results demonstrate that OECs improved the physiological function of the regenerated facial nerves in rats.
This study is limited by the lack of a sham group. This made it difficult to determine whether the surgical procedures other than dissecting facial nerves influenced the morphology and function of facial nerves. It was also impossible to assess if the OEC implantation repaired the damaged facial nerves to a morphological and functional state comparable to that of facial nerves in sham group rats.
Though it is not known if the damaged facial nerves have been fully repaired or not by implantation of OECs, our comparative study between the facial nerve damaged rats treated with OECs and the facial nerve damaged rats without OEC treatment is sufficient to uncover the therapeutic effects of OECs on damaged facial nerves in rats. To our knowledge, this is the first study to demonstrate that OECs promote the physical regeneration and the physiological function recovery of the defected facial nerves in rats. This finding suggests that OEC implantation has the potential to be clinically applied for the treatment of facial nerve injuries in humans. Studies to achieve nerve generation and functional recovery in bigger animals, such as dogs with facial nerve defects, by implantation of OECs are warranted before implantation of OECs can be clinically tested for treatment of human facial nerve injuries.
In conclusion, OECs promote the physical regeneration and improve the physiological function of regenerated facial nerves in rats. Implantation of OECs has the potential clinical application for the treatment of facial nerve injuries in humans.
Additional file: Open peer review report 1 (6.1KB, pdf) .
Footnotes
Conflicts of interest: All the authors declare that they have no conflicts of interest.
Financial support: This study was supported by the Foundation for Military Medicine, China, No. BWS11J035 (to JPF); the Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai of China, No. PWZxq2017-09 (to XPC and JPF). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by Animal Ethic Committee of the Second Military Medical University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Shan-hui Hsu, National Taiwan University, China.
Funding: This study was supported by the Foundation for Military Medicine, China, No. BWS11J035 (to JPF); the Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai of China, No. PWZxq2017-09 (to XPC and JPF).
P-Reviewer: Hsu S; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Cason N, Stow A, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Angelov DN, Guntinas-Lichius O, Wewetzer K, Neiss WF, Streppel M. Axonal branching and recovery of coordinated muscle activity after transection of the facial nerve in adult rats. Adv Anat Embryol Cell Biol. 2005;180:1–130. [PubMed] [Google Scholar]
- 2.Arancibia-Carcamo IL, Ford MC, Cossell L, Ishida K, Tohyama K, Attwell D. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. Elife 6. 2017 doi: 10.7554/eLife.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basavaraj S, Prakash BG, Shetty TS, Sandhya D, Kallada S. Delayed facial nerve weakness after intact canal wall tympanomastoidectomy. Otol Neurotol. 2014;35:1003–1006. doi: 10.1097/MAO.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 4.Boyd JG, Doucette R, Kawaja MD. Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. FASEB J. 2005;19:694–703. doi: 10.1096/fj.04-2833rev. [DOI] [PubMed] [Google Scholar]
- 5.Carriel V, Garzón I, Alaminos M, Campos A. Evaluation of myelin sheath and collagen reorganization pattern in a model of peripheral nerve regeneration using an integrated histochemical approach. Histochem Cell Biol. 2011;136:709–717. doi: 10.1007/s00418-011-0874-3. [DOI] [PubMed] [Google Scholar]
- 6.Choi D, Dunn LT. Facial nerve repair and regeneration: an overview of basic principles for neurosurgeons. Acta Neurochir (Wien) 2001;143:107–114. doi: 10.1007/s007010170114. [DOI] [PubMed] [Google Scholar]
- 7.Deumens R, Koopmans GC, Lemmens M, Mollers S, Honig WM, Steinbusch HW, Brook G, Joosten EA. Neurite outgrowth promoting effects of enriched and mixed OEC/ONF cultures. Neurosci Lett. 2006;397:20–24. doi: 10.1016/j.neulet.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 8.Dombrowski MA, Sasaki M, Lankford KL, Kocsis JD, Radtke C. Myelination and nodal formation of regenerated peripheral nerve fibers following transplantation of acutely prepared olfactory ensheathing cells. Brain Res. 2006;1125:1–8. doi: 10.1016/j.brainres.2006.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Euler de Souza Lucena E, Guzen FP, Lopes de Paiva Cavalcanti JR, Galvao Barboza CA, Silva do Nascimento Junior E, Cavalcante Jde S. Experimental considerations concerning the use of stem cells and tissue engineering for facial nerve regeneration: a systematic review. J Oral Maxillofac Surg. 2014;72:1001–1012. doi: 10.1016/j.joms.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Gaudin R, Knipfer C, Henningsen A. Approaches to Peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Res Int 2016. 2016:3856262. doi: 10.1155/2016/3856262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghiasi S, Banaei M. Bilateral facial paralysis caused by temporal bone fracture: a case report. Arch Trauma Res. 2016;5:e26892. doi: 10.5812/atr.26892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosu-Bularda A, Manea C, Lascar I. The role of olfactory ensheating cells in regenerative medicine: review of the literature. Rom J Rhinol. 2015;5:75. [Google Scholar]
- 13.Guntinas-Lichius O, Angelov DN, Tomov TL, Dramiga J, Neiss WF, Wewetzer K. Transplantation of olfactory ensheathing cells stimulates the collateral sprouting from axotomized adult rat facial motoneurons. Exp Neurol. 2001;172:70–80. doi: 10.1006/exnr.2001.7774. [DOI] [PubMed] [Google Scholar]
- 14.Guntinas-Lichius O, Wewetzer K, Tomov TL, Azzolin N, Kazemi S, Streppel M, Neiss WF, Angelov DN. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J Neurosci. 2002;22:7121–7131. doi: 10.1523/JNEUROSCI.22-16-07121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano A. The role of electron microscopy in neuropathology. Acta Neuropathol. 2005;109:115–123. doi: 10.1007/s00401-004-0960-x. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda M, Oka Y. The relationship between nerve conduction velocity and fiber morphology during peripheral nerve regeneration. Brain Behav. 2012;2:382–390. doi: 10.1002/brb3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X, Lim SH, Mao HQ, Chew SY. Current applications and future perspectives of artificial nerve conduits. Exp Neurol. 2010;223:86–101. doi: 10.1016/j.expneurol.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Chi XF, Zou XJ, Ci Y, Yao Q, Yang MW. Combination of olfactory ensheathing cell transplantation and repetitive transcranial magnetic stimulation for the treatment of spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:98–102. [Google Scholar]
- 19.Lal D, Hetzler LT, Sharma N, Wurster RD, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation facilitates rat facial nerve recovery from a crush injury. Otolaryngol Head Neck Surg. 2008;139:68–73. doi: 10.1016/j.otohns.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Linder T, Mulazimoglu S, El Hadi T, Darrouzet V, Ayache D, Somers T, Schmerber S, Vincent C, Mondain M, Lescanne E, Bonnard D. Iatrogenic facial nerve injuries during chronic otitis media surgery: a multicentre retrospective study. Clin Otolaryngol. 2017;42:521–527. doi: 10.1111/coa.12755. [DOI] [PubMed] [Google Scholar]
- 21.Ma F, Zhu T, Xu F, Wang Z, Zheng Y, Tang Q, Chen L, Shen Y, Zhu J. Neural stem/progenitor cells on collagen with anchored basic fibroblast growth factor as potential natural nerve conduits for facial nerve regeneration. Acta Biomater. 2017;50:188–197. doi: 10.1016/j.actbio.2016.11.064. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi R, Vahabzadeh B, Amini K. Sciatic nerve regeneration induced by transplantation of in vitro bone marrow stromal cells into an inside-out artery graft in rat. J Craniomaxillofac Surg. 2014;42:1389–1396. doi: 10.1016/j.jcms.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Paviot A, Guérout N, Bon-Mardion N, Duclos C, Jean L, Boyer O, Marie JP. Efficiency of laryngeal motor nerve repair is greater with bulbar than with mucosal olfactory ensheathing cells. Neurobiol Dis. 2011;41:688–694. doi: 10.1016/j.nbd.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Radtke C, Kocsis JD. Peripheral nerve injuries and transplantation of olfactory ensheathing cells for axonal regeneration and remyelination: fact or fiction? Int J Mol Sci. 2012;13:12911–12924. doi: 10.3390/ijms131012911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radtke C, Kocsis JD. Olfactory-ensheathing cell transplantation for peripheral nerve repair: update on recent developments. Cells Tissues Organs. 2014;200:48–58. doi: 10.1159/000369006. [DOI] [PubMed] [Google Scholar]
- 26.Radtke C, Sasaki M, Lankford KL, Gallo V, Kocsis JD. CNPase expression in olfactory ensheathing cells. J Biomed Biotechnol 2011. 2011:608496. doi: 10.1155/2011/608496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raimondo S, Fornaro M, Di Scipio F, Ronchi G, Giacobini-Robecchi MG, Geuna S. Chapter 5: Methods and protocols in peripheral nerve regeneration experimental research: part II-morphological techniques. Int Rev Neurobiol. 2009;87:81–103. doi: 10.1016/S0074-7742(09)87005-0. [DOI] [PubMed] [Google Scholar]
- 28.Raisman G, Li Y. Repair of neural pathways by olfactory ensheathing cells. Nat Rev Neurosci. 2007;8:312–319. doi: 10.1038/nrn2099. [DOI] [PubMed] [Google Scholar]
- 29.Ramer LM, Richter MW, Roskams AJ, Tetzlaff W, Ramer MS. Peripherally-derived olfactory ensheathing cells do not promote primary afferent regeneration following dorsal root injury. Glia. 2004;47:189–206. doi: 10.1002/glia.20054. [DOI] [PubMed] [Google Scholar]
- 30.Sandvig I, Hoang L, Sardella TC, Barnett SC, Brekken C, Tvedt K, Berry M, Haraldseth O, Sandvig A, Thuen M. Labelling of olfactory ensheathing cells with micron-sized particles of iron oxide and detection by MRI. Contrast Media Mol Imaging. 2012;7:403–410. doi: 10.1002/cmmi.1465. [DOI] [PubMed] [Google Scholar]
- 31.Schröder JM. Altered ratio between axon diameter and myelin sheath thickness in regenerated nerve fibers. Brain Res. 1972;45:49–65. doi: 10.1016/0006-8993(72)90215-6. [DOI] [PubMed] [Google Scholar]
- 32.Siemionow M, Bozkurt M, Zor F. Regeneration and repair of peripheral nerves with different biomaterials: review. Microsurgery. 2010;30:574–588. doi: 10.1002/micr.20799. [DOI] [PubMed] [Google Scholar]
- 33.Tan CW, Ng MH, Ohnmar H, Lokanathan Y, Nur-Hidayah H, Roohi SA, Ruszymah B, Nor-Hazla MH, Shalimar A, Naicker AS. Sciatic nerve repair with tissue engineered nerve: Olfactory ensheathing cells seeded poly(lactic-co-glygolic acid) conduit in an animal model. Indian J Orthop. 2013;47:547–552. doi: 10.4103/0019-5413.121572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang XY, Sheng BY, Huang ZY, Zhang XM, Wei CJ. Proliferation and differentiation from neural stem cells into neurons following coculture with olfactory ensheathing cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2010;14:7501–7504. [Google Scholar]
- 35.Zheng Z, Liu G, Chen Y, Wei S. Olfactory ensheathing cell transplantation improves sympathetic skin responses in chronic spinal cord injury. Neural Regen Res. 2013;8:2849–2855. doi: 10.3969/j.issn.1673-5374.2013.30.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.