Keywords: nerve regeneration, folic acid, Schwann cell, cell functions, peripheral nerve injury, peripheral nerve repair, neurotrophic factor, tissue engineering, neural regeneration, biomaterial, neural regeneration
Abstract
After peripheral nerve injury, intraperitoneal injection of folic acid improves axon quantity, increases axon density and improves electromyography results. However, the mechanisms for this remain unclear. This study explored whether folic acid promotes peripheral nerve injury repair by affecting Schwann cell function. Primary Schwann cells were obtained from rats by in vitro separation and culture. Cell proliferation, assayed using the Cell Counting Kit-8 assay, was higher in cells cultured for 72 hours with 100 mg/L folic acid compared with the control group. Cell proliferation was also higher in the 50, 100, 150, and 200 mg/L folic acid groups compared with the control group after culture for 96 hours. Proliferation was markedly higher in the 100 mg/L folic acid group compared with the 50 mg/L folic acid group and the 40 ng/L nerve growth factor group. In Transwell assays, the number of migrated Schwann cells dramatically increased after culture with 100 and 150 mg/L folic acid compared with the control group. In nerve growth factor enzyme-linked immunosorbent assays, treatment of Schwann cell cultures with 50, 100, and 150 mg/L folic acid increased levels of nerve growth factor in the culture medium compared with the control group at 3 days. The nerve growth factor concentration of Schwann cell cultures treated with 100 mg/L folic acid group was remarkably higher than that in the 50 and 150 mg/L folic acid groups at 3 days. Nerve growth factor concentration in the 10, 50, and 100 mg/L folic acid groups was higher than that in the control group at 7 days. The nerve growth factor concentration in the 50 mg/L folic acid group was remarkably higher than that in the 10 and 100 mg/L folic acid groups at 7 days. In vivo, 80 μg/kg folic acid was intraperitoneally administrated for 7 consecutive days after sciatic nerve injury. Immunohistochemical staining showed that the number of Schwann cells in the folic acid group was greater than that in the control group. We suggest that folic acid may play a role in improving the repair of peripheral nerve injury by promoting the proliferation and migration of Schwann cells and the secretion of nerve growth factors.
Chinese Library Classification No1. R456; R338; R745
Introduction
Peripheral nerve injury is a serious clinical problem. In recent years progress has been made with various approaches involving autologous nerve grafts and nerve conduit combined with seed cells or neural growth factors (Pabari et al., 2011; Grinsell and Keating, 2014); however, functional recovery remains unsatisfactory. For peripheral nerve reconstruction, neurotrophic factors released from the target organ and the proximal stump that forms after Wallerian degeneration play critical roles in building an effective microenvironment (Lundborg et al., 1994; Cornejo et al., 2010; Wood et al., 2018). Because of the neurotrophic effects of neurotrophic factors, some studies have combined such factors with nerve conduit to improve repair after peripheral nerve injury (Chung et al., 2014; Kuihua et al., 2014; Choi et al., 2018). However, neurotrophic factors have limited application because of their short half-life and unstable characteristics. Thus, it is reasonable to search for another substances to overcome these drawbacks (Zeng et al., 2011, 2014). Folic acid shows great potential in repairing nervous system injury because of its neurotrophic effects (Balashova et al., 2018).
Folic acid, a derivative of water-soluble vitamins, plays a key role in the growth, differentiation and regeneration of the central nervous system (Iskandar et al., 2004, 2010). Folic acid supplements can prevent various diseases of the central nervous system, such as neural tube defects, development delays, and Alzheimer’s disease (Zammit et al., 2007; Ichi et al., 2012; Li et al., 2016). Moreover, folic acid is also good for peripheral nerve repair. In chronic peripheral nerve injury, such as carpal tunnel syndrome which is the most common type of peripheral entrapment neuropathy, folic acid combined with uridine monophosphate and vitamin B12 reduced pain score, intensity and characterization of pain and associated symptoms (Negrão et al., 2016). Yilmaz et al. (2016) demonstrated that folic acid can protect diabetic rats against diabetic peripheral neuropathy by reducing malondialdehyde levels and upregulating nerve growth factor (NGF) expression. In acute peripheral nerve injury, Harma et al. (2015) demonstrated that folic acid improved peripheral nerve healing and increased axon myelination in a rat sciatic nerve injury model. In conclusion, folic acid is important for peripheral nerve repair. However, the underlying mechanism of action remains unclear.
This study aimed to demonstrate whether folic acid affects the proliferation and migration of and secretion from Schwann cells, ultimately leading to promotion of peripheral nerve repair. Folic acid has great potential for use in the bioengineering of peripheral nerves because of its neurotrophic properties and stability and because it is easy to obtain. Our study examines a target cell-type affected by folic acid and the effects on its function. This contributes to development of local folic acid combined with nerve conduits for use in peripheral nerve repair.
Materials and Methods
Animals
Two specific-pathogen-free 3-day-old Sprague-Dawley (SD) male rats for primary culture of Schwann cells and nine specific-pathogen-free 3-month-old SD male rats for in vivo study (average weight 180–220 g) were purchased from Peking Weitonglihua Laboratory Animal Center (Beijing, China). Animal experiments were approved by the Animal Ethical Committee of the Neurosurgical Institute of Beijing, Capital Medical University, China (approval No. 201603001).
Primary culture of Schwann cells
Two 3-day-old SD rats were euthanized in cold water and then immersed in 75% ethyl alcohol. Four sciatic nerves were dissected under sterile conditions and collected in buffered Dulbecco’s modified Eagle’s medium (DMEM, Corning, New York, NJ, USA). The nerves were cut into pieces after their epineurium was stripped. The tissues were digested in 3 mL of 0.25% trypsin (KeyGEN Biotech, Nanjing, China) and 1 mL of 0.2% collagenase type II (Sigma-Aldrich Corp., St. Louis, MO, USA) for 40 minutes at 37°C. The solution was then replaced with an equal volume of 0.2% collagenase type II after centrifugation at 180 × g for 5 minutes, and the cells were incubated for an additional 50 minutes at 37°C. Fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) (1 mL) was added to terminate the collagenase activity. The supernatant was discarded after centrifugation at 500 × g for 5 minutes at room temperature, and the cells were resuspended in DMEM (containing 20% FBS). The cells were seeded at a density of 1 × 105 in uncoated plastic Petri dishes (35 mm) and cultured at 37°C in 5% CO2. After 1.5 hours, the medium with unattached cells, mainly neurons, was removed, and 3 mL FBS-free DMEM was added. After 3 days of incubation, the cells were treated with cytosine arabinoside (10 µM, Macklin, Shanghai, China) for 48 hours in DMEM supplemented with 10% FBS, followed by forskolin (2 µM, Solarbio, Beijing, China) for 24 hours to induce Schwann cell formation (Mauritz et al., 2004). The medium was aspirated, and the cells were gently rinsed with cold phosphate buffered saline (PBS) at 4°C. Subsequently, the cultures were treated with a stream of cold DMEM at 4°C, which was gently applied by means of a 1 mL pipette tip and pipetted on and off 3–5 times (Jirsova et al., 1997). The suspension of floating cells was transferred to uncoated Petri dishes. After repeating the above procedure three times, the cells were seeded in a 24-well culture plate at 37°C in 5% CO2.
The purity of the cultures was determined by immunofluorescence staining for anti-S-100 (S-100β; Boster, Wuhan, China) as a Schwann cell marker. Cultured cells were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.2% Triton X-100 (KeyGEN Biotech) for 30 minutes. The cells were then incubated with anti-S-100 antibody at room temperature overnight. The cells were washed three times with PBS and then incubated with biotinylated goat anti-rabbit secondary antibody (Zhongshan Golden Bridge Biotechnology, Beijing, China) for 1 hour at room temperature. After a second rinsing step with PBS, the cells were incubated with streptavidin-conjugated fluorescein isothiocyanate (1:100; Zhongshan Golden Bridge Biotechnology) for 1 hour at room temperature. The nuclei of cells were visualized by staining with 4′,6-diamidino-2-phenylindole (DAPI) (KeyGEN Biotech). To identify the purity of Schwann cells, three random images were taken from each primary culture after immunofluorescence staining at 200× magnification using a fluorescence microscope (DM4000, Leica, Germany).
Schwann cell proliferation assay
Schwann cell proliferation assays were performed after culture of Schwann cells with folic acid for 24, 48, 72, and 96 hours (Zhou et al., 2017). Schwann cells were digested with 0.25% trypsin-ethylenediamine tetraacetic acid, and the suspended cells were collected and resuspended in DMEM. Cells at 1 × 104/well were seeded in 96-well culture plates and left to attach to the wells for 24 hours at 37°C. Cells were then exposed to different concentrations of folic acid (0, 10, 50, 100, 150, 200 mg/L; Macklin, Shanghai, China) or NGF (40 ng/L; Sigma-Aldrich Corp.). To evaluate the effects of folic acid on the proliferation of Schwann cells, a Cell Counting Kit-8 (CCK-8, KeyGEN Biotech) was used following the manufacturer’s protocol. After exposure to folic acid for 24, 48, 72, and 96 hours, 10 µL of CCK-8 solution was added to each well, and the plates were incubated for an additional 3 hours at 37°C. Cell proliferation was measured as the absorbance at 450 nm with a microplate reader (Beckman, Brea, CA, USA). The mean optical density value from eight wells for each treatment was used as an index of cell viability.
Schwann cell migration assay
Schwann cell migration was performed after incubation with folic acid for 12 hours. Schwann cells form tube-like structures called bands of Büngner that provide important guides along which axons can regenerate. The procedure depends on Schwann cell proliferation and migration. Transwells (Corning) with a pore size of 8.0 μm were used to demonstrate the tendency of Schwann cells to migrate toward folic acid. To count the number of migrated Schwann cells, six random images were taken at 400× magnification for each experiment. After treating with trypsin and rinsing with PBS, Schwann cells were suspended in 100 μL serum-free DMEM and seeded in the apical chamber of Transwell plates, and 500 μL DMEM containing 10% FBS and different concentrations of folic acid (0, 10, 50, 100, 150, or 200 mg/L) was added to the basolateral chamber of the Transwell plate. After incubation at 37°C for 12 hours, the apical chamber was washed with PBS, and all non-migrated cells were carefully scraped from the upper surface with a cotton swab. The cells on the lower side of the apical chamber were then fixed with 4% paraformaldehyde (Solarbio, Beijing, China) and stained with crystal violet (KeyGEN Biotech). Five random images were captured under an inverted microscope (Carl Zeiss microscopy GmbH37081, Gottingen, Germany). Schwann cell migration was determined by counting the number of stained cells with ImageJ software (National Institutes of Health, USA; http://rsbweb.nih.gov/ij; Open Source).
Enzyme-linked immunosorbent assay (ELISA) for NGF
An ELISA assay was performed to determine the concentration of any NGF released from the Schwann cells into the culture medium (Gomez and Schmidt, 2007) after administration of folic acid for 3 and 7 days. The concentration of NGF in the Schwann cell culture was detected on days 3 and 7 using an NGF-ELISA Kit (Cusabio Biotech, College Park, MD, USA) according to the manufacturer’s protocol. After treating with trypsin and rinsing with PBS, Schwann cells were suspended in 3 mL DMEM containing 10% FBS and different concentrations of folic acid (0, 10, 50, 100, 150, 200 mg/L), seeded on 6-well plates, and incubated for 3 or 7 days at 37°C. Five hundred microliters of medium were collected from each well on days 3 and 7. The supernatant was obtained after centrifugation for 15 minutes at 1000 × g. 96-well ELISA plates were coated with a primary goat anti-NGF antibody overnight at 4°C, blocked and then incubated with the samples and standards for 6 hours at room temperature. Plates were then incubated overnight with a second rat anti-NGF antibody (1:500; Gusabio Biotech, Colloge Park, MD, USA) followed by incubation with an anti-rat antibody conjugated to horseradish peroxidase for 2.5 hours. Developer was added, and the absorbance at 450 nm was recorded using a plate reader.
Immunohistochemical staining
In the in vivo study, immunohistochemical staining was performed 7 days after the operation. The rats were divided randomly into three groups (Normal, Folic acid, Control group). Animals of the folic acid and control groups were anesthetized with chloral hydrate. The sciatic nerve was then exposed and cut 1 cm proximal to the sciatic/peroneal bifurcation using eye scissors. The nerve stumps were connected again by suturing the nerve epineurium with 8-0 polypropylene (Ethicon Endo Surgery, NJ, USA), then muscles and skin were closed (4-0 polyester, ARC Medical Supplies, China) (Hopkins et al., 2017). After the surgical procedure, 80 μg/kg of folic acid was administered to rats of folic acid group intraperitoneally for 7 days, while equal volume of physiological saline solution was administered to rats of control group. However, the rats under physiological condition served as normal group.
On day 7 after surgery, the distal sciatic nerve was transected 2 mm distal to the repair line, fixed with 4% paraformaldehyde for 2 hours and embedded in paraffin. This tissue was sectioned at 4 μm and then incubated with 0.6% hydrogen peroxide for 30 minutes after being dewaxed and rehydrated in PBS (pH 7.4). Sections were incubated in S100β antibody (mouse anti-rat antibody, 1:200 dilution; Boster, Wuhan, China) overnight at 4°C after non-specific immunoreactions were blocked by normal swine serum. After three washes with PBS, sections were incubated in biotinylated secondary antibody (Zhongshan Golden Bridge Biotechnology) for 1 hour at room temperature. Horseradish peroxidase-labeled secondary antibody was applied for 1 hour at room temperature. All sections were incubated with 3,3′-diaminobenzidine tetrahydrochloride chromogen substrate solution (DAKO, Carpinteria, CA, USA) for 10 minutes. The sections were then dehydrated, cleared, cover slipped, and examined under a light microscope (DM4000, Leica, Germany).
Statistical analysis
The data are expressed as the mean ± SEM and were analyzed by SPSS 21.0 software (IBM, Armonk, NY, USA). Normally distributed data (e.g., NGF-ELISA) were analyzed by one-way analysis of variance with the least significant difference post hoc method. Non-normal data (e.g., the number of migrated Schwann cells in Transwell assays) were analyzed by one-way analysis of variance with the Game-Howell post hoc method. The repeated measures data (e.g., CCK-8 cell proliferation assay) were analyzed with repeated measures analysis of variance followed by the least significant difference post hoc test. For the in vivo study, data were analyzed using independent unpaired t-tests. A value of P < 0.05 was considered statistically significant.
Results
Primary Schwann cell culture
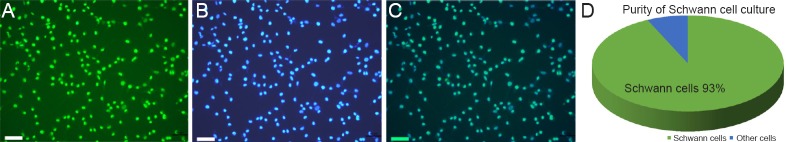
During the first 3 days of incubation, a small number of large fibroblasts did not grow in serum-free DMEM and formed a thin underlayer. The majority of cells other than fibroblasts had a bipolar or triangular shape, which is a characteristic of Schwann cells. Using the Schwann cell purification procedure described above, an S-100 antibody was used as a marker to identify the purity of Schwann cell culture. ImageJ software was used to count the number of cells stained with FITC and DAPI after images were captured using fluorescence microscopy. The purity of a Schwann cell culture was equal to the proportion of cells stained with FITC relative to all cell nuclei stained with DAPI. Schwann cell cultures of 93–98% purity were obtained (Figure 1).
Figure 1.
Purity of cultures determined by immunofluorescence staining for the Schwann cell marker S-100.
(A) Schwann cells were identified by S-100β immunofluorescence staining. (B) The nuclei of all cells were labeled with 4′,6-diamidino-2-phenylindole (DAPI). (C) Merged image of Schwann cells and nuclei of all cells. Scale bar: 100 μm. (D) Schwann cell culture purity was equal to the proportion of cells stained with fluorescein isothiocyanate (FITC) (green) among all cell nuclei stained with DAPI (blue). The purity of Schwann cells was 93–98%.
Effects of folic acid on Schwann cell migration
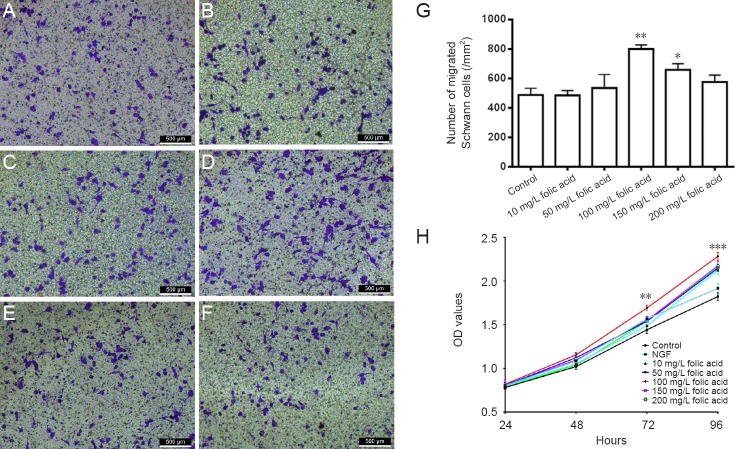
The influence of folic acid on Schwann cell migration was tested by the Transwell assay (Figure 2A–F). Figure 2G shows that a greater number of Schwann cells cultured with 100 mg/L folic acid migrated to the bottom of the insert compared with the control group (P < 0.01). A larger number of Schwann cells were observed to migrate to the bottom of the insert when cultured with 150 mg/L folic acid compared with other concentrations of folic acid (P < 0.05). These results demonstrate that 100 mg/L folic acid promotes Schwann cell migration.
Figure 2.
Influence of folic acid on Schwann cell migration and proliferation.
(A–F) Schwann cells cultured with 0, 10, 50, 100, 150, or 200 mg/L folic acid. Schwann cells that had migrated through the filter membrane of the Transwell were stained blue by crystal violet. Scale bars: 500 μm. (G) The number of migrated Schwann cells after culture with 100 mg/L (**P < 0.01) or 150 mg/L (*P < 0.05) folic acid were significantly higher than with other concentrations. Data are expressed as the mean ± SEM (n = 3; one-way analysis with the Game-Howell post hoc test). (H) Optical density (OD) value measured in the cell counting kit-8 assay reflects the proliferation of Schwann cells cultured with different concentrations of folic acid and nerve growth factor (NGF). The OD value of 100 mg/L folic acid was significantly higher than that of the control group at 72 (**P < 0.01) and 96 hours (***P < 0.001). Data are expressed as the mean ± SEM (n = 6; repeated measures analysis of variance followed by the least significant difference post hoc test).
Effects of folic acid on Schwann cell proliferation
Consistent with the results of the CCK-8 assay, growth promotion was visible in Schwann cells administered 100 mg/L folic acid in comparison with the control group at 72 hours (P < 0.01) and 96 hours (P < 0.001). In contrast, there was no significant change in the proliferation of Schwann cells cultured with other concentrations of folic acid. In addition, exposure to 100 mg/L folic acid significantly improved the proliferation of Schwann cells compared to exposure to 40 ng/L NGF (P < 0.05). These results indicate that folic acid induced Schwann cell proliferation in a concentration-dependent manner, which peaked at 100 mg/L (Figure 2H).
Effects of folic acid on NGF release
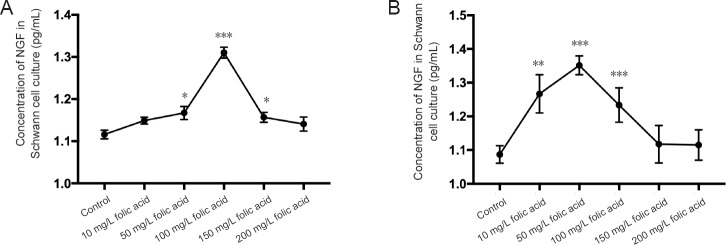
The amount of NGF released from Schwann cells into the culture medium was determined using ELISA. On day 3, folic acid at 50, 100, and 150 mg/L significantly promoted Schwann cells to secrete NGF compared to other concentrations of folic acid (P < 0.05), peaking at 100 mg/L (P < 0.001). However, 10, 50, and 100 mg/L folic acid also showed significant changes compared to the other concentrations on day 7 (P < 0.001), peaking at 50 mg/L (P < 0.001) (Figure 3A, B). These results indicated that 100 mg/L or 50 mg/L folic acid promotes NGF secretion by Schwann cells and is dependent on the culture time.
Figure 3.
Concentration of NGF in medium of Schwann cells cultured with folic acid for 3 (A) and 7 (B) days.
(A) NGF concentration in culture medium of Schwann cells cultured with 100 mg/L folic acid for 3 days was significantly higher than in other groups (***P < 0.001). The NGF concentrations in the 50 and 150 mg/L folic acid groups were significantly higher than in other concentrations (*P < 0.05). (B) NGF concentrations in culture medium of Schwann cells cultured with 10, 50 and 100 mg/L folic acid were significantly higher than in other groups after culture for 7 days (**P < 0.01); however, 50 mg/L folic acid was more effective than 10 mg/L (**P < 0.01) and 100 mg/L folic acid (***P < 0.001). Data are expressed as the mean ± SEM (n = 6; one-way analysis of variance with the least significant difference post hoc test).
Effects of folic acid on the number of Schwann cells in the distal sciatic nerve
To observe the effect of folic acid on Schwann cells after peripheral nerve injury, we performed immunohistochemistry at 7 days following peripheral nerve injury (Figure 4). In folic acid and control groups, normal nerve structures (Figure 4F) were observed to disappear. Low power views of folic acid and control groups were compared under light microscopy (Figure 4C). Some cavity, de-differentiated Schwann cells, and negative cells, possibly macrophages or fibroblasts, were observed (Figure 4B, D). However, the number of Schwann cells in the folic acid group was significantly higher than that in the control group (P < 0.05; Figure 4E).
Figure 4.
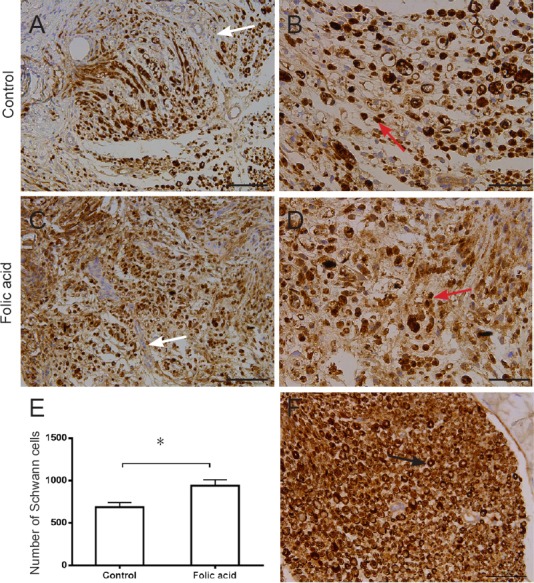
Immunohistochemistry of transected distal sciatic nerves, 2 mm distal to the repair site in rats administrated folic acid for 7 days.
Myelin sheath and Schwann cells were marked with S-100. Lower power views were observed in the control group (A) compared with that of the folic acid group (C). Some “cavity”, de-differentiated Schwann cells, and S-100-negative macrophages or fibroblasts were observed both in control (B) and folic acid groups (D) compared with the normal group (F). (E) The number of Schwann cells in the folic acid group was significantly higher than that in the control group (*P < 0.05). White arrows: negative cells; red arrows: Schwann cells; black arrow: normal structure. Data are expressed as the mean ± SEM (n = 3; independent unpaired unpaired t-test). Scale bars: 100 μm (A, C) and 50 μm (B, D, F).
Discussion
Folic acid, a stable water-soluble vitamin that is found in bacteria and plants, has been verified to play a critical role in cell metabolism (Henry et al., 2017). Folic acid affects neural stem cell differentiation, neuronal proliferation, and axonal elongation in the central nervous system (Iskandar et al., 2010; Kronenberg and Endres, 2010; Ichi et al., 2012; Liu et al., 2013; Li et al., 2016). Supplements of folic acid can prevent various clinical problems, such as neural tube defects, Alzheimer’s disease, and spinal cord regression (Ganesh et al., 2014; De-Regil et al., 2015; Li et al., 2016). Furthermore, intraperitoneal injection of folic acid can promote functional recovery after tibial nerve injury (Harma et al., 2015). Because of the great potential of folic acid to repair the nervous system, we used nerve conduits coated with folic acid to bridge sciatic nerve defects in preliminary experiments. The results indicated that folic acid improves the sciatic function index, nerve conduction velocity and cell proliferation. However, the target cells and functions that are affected by folic acid in the repair of peripheral nerve injury remains unclear.
This study reported the effect of folic acid on inducing the proliferation and migration of and secretion from Schwann cells. After peripheral nerve injury, Schwann cells dedifferentiate to a progenitor-like state and proliferate, forming bands of Büngner upon which axons can regrow (Napoli et al., 2012). Schwann cells cultured with 100 mg/L folic acid exhibited significantly promoted cell proliferation compared to cells cultured in other concentrations in our CCK-8 assay. To further observe the effect of folic acid on Schwann cell proliferation, nine rats were used. The rats of the folic acid group were intraperitoneally administered folic acid for 7 days after peripheral nerve injury. Immunohistochemistry showed that the number of Schwann cells in the folic acid group was higher than that in the control group. These findings indicate that folic acid acts as a neurotrophic factor to promote Schwann cell proliferation to improve formation of the bands of Büngner. Furthermore, Schwann cells administered 100 mg/L folic acid showed increased migration. As the primary type of glial cell, Schwann cells migrate to sites of axon injury to remyelinate the regenerating axons after peripheral nerve injury (Heermann and Schwab, 2013). Our results indicate that 100 mg/L folic acid remarkably promoted Schwann cell migration, which is favorable for remyelination. The concentration of NGF in Schwann cell cultures treated with 50 or 100 mg/L folic acid was dramatically higher than that in the other groups at different time points. This showed that folic acid not only acts as a neurotrophic factor but also promotes the secretion of neural growth factors from Schwann cells that support the survival of impaired neurons.
Harma et al. (2015) demonstrated that folic acid promotes the healing of peripheral nerve injury. In their study, increased axon quantity, density and myelination were observed in an in vivo sciatic nerve injury model. Folic acid combined with uridine and vitamin B12 reduced the pain and associated symptoms of carpal tunnel syndrome (Negrão et al., 2014, 2016) possibly due to regeneration/protection of the myelin sheath. However, the target cell affected by folic acid in peripheral nerve repair was not defined. In this study, we demonstrated that 100 mg/L folic acid markedly promoted the proliferation and migration of Schwann cells in vitro, which is important for axonal remyelination. Yilmaz et al. (2016) demonstrated that folic acid improved the compound muscle action potential amplitude related to increased axon density and increased expression of NGF, and decreased distal latency and malondialdehyde levels, to protect diabetic rats against diabetic peripheral neuropathy. Moreover, in our study, we demonstrated that 100 mg/L folic acid in vitro remarkably promoted NGF secretion by Schwann cells at 3 days and that 50 mg/L folic acid obviously promoted NGF secretion by Schwann cells at 7 days. These results indicate that folic acid may improve peripheral nerve repair by upregulating the expression of NGF.
Our results also indicated that folic acid plays a dose-dependent role in promoting functions of Schwann cells. It is established that locally delivered folic acid plays a dose-dependent role in inducing neuronal differentiation and increasing proliferation of neural stem cells (Li et al., 2013; Luo et al., 2013). Moreover, the effect of folate on axonal regeneration in the central nervous system was biphasic and dose-dependent, and associated with DNA methylation and expression of folate receptor Folr1 and de novo methyltransferases. However, a higher dose of folic acid induced relative hypomethylation associated with reduced de novo methyltransferase levels and decreased axonal regeneration (Iskandar et al., 2010). However, a folic acid dose higher than 100 mg/L showed no positive effect on promoting functions of Schwann cell for unclear reasons.
Biological agents such as NGF, glial cell-derived neurotrophic factor, or brain-derived neurotrophic factor are often directly incorporated in nerve guidance conduits alone or in combination to promote axonal outgrowth and neuronal survival after peripheral nerve injury (Barras et al., 2002; Hobson, 2002; Xu et al., 2003; Cui and Cui, 2007; Takagi et al., 2012; Chen et al., 2017). However, short biological half-lives, high initial burst release, and high cost, limit the use of these neurotrophic factors in peripheral nerve repair (Zhang et al., 2014; Zeng et al., 2014). Thus, there is a high demand for finding an inexpensive neurotrophic factor with a long half-life and gradual release. Folic acid, a stable, easily obtained, inexpensive vitamin, showed potential in promoting Schwann cell functions for peripheral nerve repair in our study. Further exploration is warranted to effectively locally utilize folic acid to repair peripheral nerve injury.
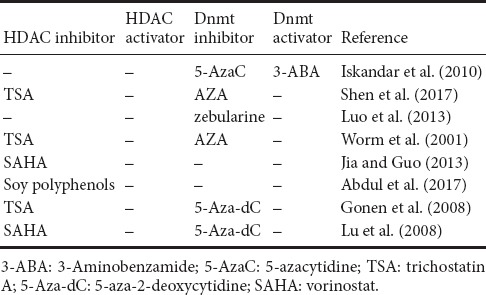
Folic acid participates in cell metabolism by affecting DNA methylation of genes such as Sox2, Lphn1, Dixdc1, Bhlhb9, and Ifrd1, thereby regulating central nervous system development, growth and repair (Worm et al., 2001; Gonen et al., 2008; Lu et al., 2008; Iskandar et al., 2010; Kronenberg and Endres, 2010; Crider et al., 2012; Jia and Guo, 2013; Luo et al., 2013; Qian et al., 2016; Abdul et al., 2017; Shen et al., 2017; Yang et al., 2017) (Related drugs altering DNA methylation are shown in Table 1). Folic acid has also been associated with the cyclic adenosine monophosphate signaling pathway (Sheean et al., 2014; Yu et al., 2014; Wang et al., 2015; Permpoonputtana et al., 2016), the Wnt signaling pathway, the ERK signaling pathway, and the PI3-Akt signaling pathway. However, the mechanism by which folic acid regulates DNA methylation of specific genes of a particular signaling pathway to promote the repair of peripheral nerve injury must be further explored.
Table 1.
Drugs used to alter DNA methylation
In our study, we explored the target cell affected by folic acid in peripheral nerve repair. These results indicated that folic acid improved the recovery of peripheral nerve injury, which was probably associated with increased Schwann cell proliferation, migration and secretion of NGF. Moreover, we demonstrated that folic acid plays a dose-dependent role in promoting functions of Schwann cells, peaking at 100 mg/L. However, we only observed the phenotype of Schwann cells administrated folic acid and the underlying mechanism needs further exploration. The critical molecular responses of Schwann cells to folic acid need to be determined. It would be interesting to find out which specific genes are regulated at the DNA methylation level by folic acid. Axon elongation after peripheral nerve injury is important for peripheral nerve regeneration; however, we only observed the effect of folic acid on Schwann cells. To explain the neurotrophic effect of folic acid on peripheral nerve regeneration, the effect of folic acid on neurons needs further investigation.
In summary, folic acid can act as an inexpensive, stable neurotrophic factor that promotes proliferation and migration of and secretion from Schwann cells. These findings will assist in understanding the neurotrophic effects produced by folic acid to help develop locally released folic acid as a new therapeutic strategy for peripheral nerve injury repair.
Additional file: Open peer review report 1 (42.3KB, pdf) .
Acknowledgments:
We are very grateful to Yang Jian and Gloria Kim from Department of Biomedical Engineering, Materials Research Institute, The Huck Institutes of The Life Sciences, The Pennsylvania State University, for providing nerve conduits and the theory supporting.
Footnotes
Conflicts of interest: The authors declare that there is no duality of interest associated with this manuscript.
Financial support: This study was supported by the High Levels of Health Technical Personnel in Beijing City Health System of China, No. 2013-3-050 (to JZY). The funding source had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All experimental procedures and protocols were approved by the Animal Ethics Committee of the Neurosurgical Institute of Beijing, Capital Medical University, China (approval No. 201603001). All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Filip Petkovic, Universitat Autonoma de Barcelona, Spain.
Funding: This study was supported by the High Levels of Health Technical Personnel in Beijing City Health System of China, No. 2013-3-050 (to JZY).
P-Reviewer: Petkovic F; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Abdul QA, Yu BP, Chung HY, Jung HA, Choi JS. Epigenetic modifications of gene expression by lifestyle and environment. Arch Pharmacal Res. 2017;40:1219–1237. doi: 10.1007/s12272-017-0973-3. [DOI] [PubMed] [Google Scholar]
- 2.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Balashova OA, Visina O, Borodinsky LN. Folate action in nervous system development and disease. Dev Neurobiol. 2018 doi: 10.1002/dneu.22579. DOI:10.1002/dneu.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barras FM, Pasche P, Bouche N, Aebischer P, Zurn AD. Glial cell line-derived neurotrophic factor released by synthetic guidance channels promotes facial nerve regeneration in the rat. J Neurosci Res. 2002;70:746–755. doi: 10.1002/jnr.10434. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Deng ZL, Chen SM, Wong Z, Huang F. Expression of adenovirus-mediated nerve growth factor and myelin-associated glycoprotein double-gene in sciatic nerve injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:3183–3189. [Google Scholar]
- 6.Choi J, Kim JH, Jang JW, Kim HJ, Choi SH, Kwon SW. Decellularized sciatic nerve matrix as a biodegradable conduit for peripheral nerve regeneration. Neural Regen Res. 2018;13:1796–1803. doi: 10.4103/1673-5374.237126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung TW, Lai DM, Chen SD, Lin YI. Poly (epsilon-caprolactone) scaffolds functionalized by grafting NGF and GRGD promote growth and differentiation oxf PC12 cells. J Biomed Mater Res A. 2014;102:315–323. doi: 10.1002/jbm.a.34693. [DOI] [PubMed] [Google Scholar]
- 8.Cornejo M, Nambi D, Walheim C, Somerville M, Walker J, Kim L, Ollison L, Diamante G, Vyawahare S, de Bellard ME. Effect of NRG1, GDNF, EGF and NGF in the migration of a Schwann cell precursor line. Neurochem Res. 2010;35:1643–1651. doi: 10.1007/s11064-010-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3:21–38. doi: 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui YZ, Cui BB. Promotion of brain-derived neurotrophic factor to the repair and regeneration of injured peripheral nerve. Zhongguo Zuzhi Gongcheng Yanjiu. 2007;11:3800–3801. [Google Scholar]
- 11.Dalamagkas K, Tsintou M, Seifalian A. Advances in peripheral nervous system regenerative therapeutic strategies: a biomaterials approach. Mater Sci Eng C Mater Biol Appl. 2016;65:425–432. doi: 10.1016/j.msec.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 12.De-Regil LM, Pena-Rosas JP, Fernandez-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD007950.pub3. DOI: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganesh D, Sagayaraj BM, Barua RK, Sharma N, Ranga U. Arnold Chiari malformation with spina bifida: a lost opportunity of folic Acid supplementation. J Clin Diag Res. 2014;8:Od01–Od03. doi: 10.7860/JCDR/2014/11242.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: bioactive electrically conducting polymer for enhanced neurite extension. J Biomed Mater Res A. 2007;81:135–149. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonen N, Bram EE, Assaraf YG. PCFT/SLC46A1 promoter methylation and restoration of gene expression in human leukemia cells. Biochem Biophys Res Commun. 2008;376:787–792. doi: 10.1016/j.bbrc.2008.09.074. [DOI] [PubMed] [Google Scholar]
- 16.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int 2014. 2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harma A, Sahin MS, Zorludemir S. Effects of intraperitoneally administered folic acid on the healing of repaired tibial nerves in rats. J Reconstr Microsurg. 2015;31:191–197. doi: 10.1055/s-0034-1395414. [DOI] [PubMed] [Google Scholar]
- 18.Heermann S, Schwab MH. Molecular control of Schwann cell migration along peripheral axons: keep moving. Cell Adhesion Migration. 2013;7:18–22. doi: 10.4161/cam.22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry CJ, Nemkov T, Casas-Selves M, Bilousova G, Zaberezhnyy V, Higa KC, Serkova NJ, Hansen KC, D’Alessandro A, De Gregori J. Folate dietary insufficiency and folic acid supplementation similarly impair metabolism and compromise hematopoiesis. Haematologica. 2017 doi: 10.3324/haematol.2017.171074. DOI: 10.3324/haematol.2017.171074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobson MI. Increased vascularisation enhances axonal regeneration within an acellular nerve conduit. Ann R Coll Surg Engl. 2002;84:47–53. [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins TM, Little KJ, Vennemeyer JJ, Triozzi JL, Turgeon MK, Heilman AM, Minteer D, Marra K, Hom DB, Pixley SK. Short and long gap peripheral nerve repair with magnesium metal filaments. J Biomed Mater Res A. 2017;105:3148–3158. doi: 10.1002/jbm.a.36176. [DOI] [PubMed] [Google Scholar]
- 22.Ichi S, Nakazaki H, Boshnjaku V, Singh RM, Mania-Farnell B, Xi G, McLone DG, Tomita T, Mayanil CS. Fetal neural tube stem cells from Pax3 mutant mice proliferate, differentiate, and form synaptic connections when stimulated with folic acid. Stem Cells Dev. 2012;21:321–330. doi: 10.1089/scd.2011.0100. [DOI] [PubMed] [Google Scholar]
- 23.Iskandar BJ, Nelson A, Resnick D, Skene JH, Gao P, Johnson C, Cook TD, Hariharan N. Folic acid supplementation enhances repair of the adult central nervous system. Ann Neurol. 2004;56:221–227. doi: 10.1002/ana.20174. [DOI] [PubMed] [Google Scholar]
- 24.Iskandar BJ, Rizk E, Meier B, Hariharan N, Bottiglieri T, Finnell RH, Jarrard DF, Banerjee RV, Skene JH, Nelson A, Patel N, Gherasim C, Simon K, Cook TD, Hogan KJ. Folate regulation of axonal regeneration in the rodent central nervous system through DNA methylation. J Clin Invest. 2010;120:1603–1616. doi: 10.1172/JCI40000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia Y, Guo M. Epigenetic changes in colorectal cancer. Ai Zheng. 2013;32:21–30. doi: 10.5732/cjc.011.10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jirsova K, Sodaar P, Mandys V, Bar PR. Cold jet: a method to obtain pure Schwann cell cultures without the need for cytotoxic, apoptosis-inducing drug treatment. J Neurosci Methods. 1997;78:133–137. doi: 10.1016/s0165-0270(97)00146-5. [DOI] [PubMed] [Google Scholar]
- 27.Kronenberg G, Endres M. Neuronal injury: folate to the rescue. J Clin Invest. 2010;120:1383–1386. doi: 10.1172/JCI40764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuihua Z, Chunyang W, Cunyi F, Xiumei M. Aligned SF/P(LLA-CL)-blended nanofibers encapsulating nerve growth factor for peripheral nerve regeneration. J Biomed Mater Res A. 2014;102:2680–2691. doi: 10.1002/jbm.a.34922. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Yu M, Luo S, Liu H, Gao Y, Wilson JX, Huang G. DNA methyltransferase mediates dose-dependent stimulation of neural stem cell proliferation by folate. J Nutrit Biochem. 2013;24:1295–1301. doi: 10.1016/j.jnutbio.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Liu H, Yu M, Zhang X, Zhang Y, Liu H, Wilson JX, Huang G. Folic acid alters methylation profile of JAK-STAT and long-term depression signaling pathways in Alzheimer’s disease models. Mol Neurobiol. 2016;53:6548–6556. doi: 10.1007/s12035-015-9556-9. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Cao J, Zhang H, Qin S, Yu M, Zhang X, Wang X, Gao Y, Wilson JX, Huang G. Folic acid stimulates proliferation of transplanted neural stem cells after focal cerebral ischemia in rats. J Nutr Biochem. 2013;24:1817–1822. doi: 10.1016/j.jnutbio.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Lu X, Freund JN, Muller M, Ravey J, Nicolas JP, Gueant JL, Namour F. Differential regulation of CDX1 and CDX2 gene expression by deficiency in methyl group donors. Biochimie. 2008;90:697–704. doi: 10.1016/j.biochi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Lundborg G, Dahlin L, Danielsen N, Zhao Q. Trophism, tropism, and specificity in nerve regeneration. J Reconstruc Microsurg. 1994;10:345–354. doi: 10.1055/s-2007-1006604. [DOI] [PubMed] [Google Scholar]
- 34.Luo S, Zhang X, Yu M, Yan H, Liu H, Wilson JX, Huang G. Folic acid acts through DNA methyltransferases to induce the differentiation of neural stem cells into neurons. Cell Biochem Biophys. 2013;66:559–566. doi: 10.1007/s12013-012-9503-6. [DOI] [PubMed] [Google Scholar]
- 35.Mauritz C, Grothe C, Haastert K. Comparative study of cell culture and purification methods to obtain highly enriched cultures of proliferating adult rat Schwann cells. J Neurosci Res. 2004;77:453–461. doi: 10.1002/jnr.20166. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadi R, Hirsaee MA, Amini K. Improvement of functional recovery of transected peripheral nerve by means of artery grafts filled with diclofenac. Int J Surg. 2013;11:259–264. doi: 10.1016/j.ijsu.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 38.Negrão L, Nunes P Portuguese Group for the Study of Peripheral Neuropathy. Uridine monophosphate, folic acid and vitamin B12 in patients with symptomatic peripheral entrapment neuropathies. Pain Manage. 2016;6:25–29. doi: 10.2217/pmt.15.60. [DOI] [PubMed] [Google Scholar]
- 39.Negrão L, Almeida P, Alcino S, Duro H, Liborio T, Melo Silva U, Figueira R, Goncalves S, Neto Parra L. Effect of the combination of uridine nucleotides, folic acid and vitamin B12 on the clinical expression of peripheral neuropathies. Pain Manag. 2014;4:191–196. doi: 10.2217/pmt.14.10. [DOI] [PubMed] [Google Scholar]
- 40.Niu Y, Li L, Chen KC, Chen F, Liu X, Ye J, Li W, Xu K. Scaffolds from alternating block polyurethanes of poly (varepsilon-caprolactone) and poly (ethylene glycol) with stimulation and guidance of nerve growth and better nerve repair than autograft. J Biomed Mater Res A. 2015;103:2355–2364. doi: 10.1002/jbm.a.35372. [DOI] [PubMed] [Google Scholar]
- 41.Pabari A, Yang SY, Mosahebi A, Seifalian AM. Recent advances in artificial nerve conduit design: strategies for the delivery of luminal fillers. J Control Release. 2011;156:2–10. doi: 10.1016/j.jconrel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Permpoonputtana K, Porter JE, Govitrapong P. Calcitonin gene-related peptide mediates an inflammatory response in Schwann cells via cAMP-dependent ERK signaling cascade. Life Sci. 2016;144:19–25. doi: 10.1016/j.lfs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Qian YY, Huang XL, Liang H, Zhang ZF, Xu JH, Chen JP, Yuan W, He L, Wang L, Miao MH, Du J, Li DK. Effects of maternal folic acid supplementation on gene methylation and being small for gestational age. J Hum Nutr Diet. 2016;29:643–651. doi: 10.1111/jhn.12369. [DOI] [PubMed] [Google Scholar]
- 44.Sheean ME, McShane E, Cheret C, Walcher J, Muller T, Wulf-Goldenberg A, Hoelper S, Garratt AN, Kruger M, Rajewsky K, Meijer D, Birchmeier W, Lewin GR, Selbach M, Birchmeier C. Activation of MAPK overrides the termination of myelin growth and replaces Nrg1/ErbB3 signals during Schwann cell development and myelination. Genes Dev. 2014;28:290–303. doi: 10.1101/gad.230045.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen J, Wu S, Guo W, Liang S, Li X, Yang X. Epigenetic regulation of pro-inflammatory cytokine genes in lipopolysaccharide -stimulated peripheral blood mononuclear cells from broilers. Immunobiology. 2017;222:308–315. doi: 10.1016/j.imbio.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Takagi T, Kimura Y, Shibata S, Saito H, Ishii K, Okano HJ, Toyama Y, Okano H, Tabata Y, Nakamura M. Sustained bFGF-release tubes for peripheral nerve regeneration: comparison with autograft. Plast Reconstr Surg. 2012;130:866–876. doi: 10.1097/PRS.0b013e318262f36e. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Yuan D, Zhang D, Zhang W, Liu C, Cheng H, Song Y, Tan Q. Ginsenoside Re promotes nerve regeneration by facilitating the proliferation, differentiation and migration of Schwann cells via the ERK- and JNK-dependent pathway in rat model of sciatic nerve crush injury. Cell Mol Neurobiol. 2015;35:827–840. doi: 10.1007/s10571-015-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood RL, Karlinsey KS, Thompson AD, Rigby MN, Boatright GD, Pitt WG, Roeder BL, Steffensen SC, Cook AD. Baseline effects of lysophosphatidylcholine and nerve growth factor in a rat model of sciatic nerve regeneration after crush injury. Neural Regen Res. 2018;13:846–853. doi: 10.4103/1673-5374.232479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Worm J, Kirkin AF, Dzhandzhugazyan KN, Guldberg P. Methylation-dependent silencing of the reduced folate carrier gene in inherently methotrexate-resistant human breast cancer cells. J Biol Chem. 2001;276:39990–40000. doi: 10.1074/jbc.M103181200. [DOI] [PubMed] [Google Scholar]
- 50.Xu F, Zhang K, Lv P, Lu R, Zheng L, Zhao J. NECL1 coated PLGA as favorable conduits for repair of injured peripheral nerve. Mater Sci Eng C Mater Biol Appl. 2017;70:1132–1140. doi: 10.1016/j.msec.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 51.Xu X, Yee WC, Hwang PYK, Yu H, Wan ACA, Gao S, Boon KL, Mao HQ, Leong KW, Wang S. Peripheral nerve regeneration with sustained release of poly (phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials. 2003;24:2405–2412. doi: 10.1016/s0142-9612(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, Huang Y, Sun C, Li J. Maternal prenatal folic acid supplementation programs offspring lipid metabolism by aberrant DNA methylation in hepatic ATGL and adipose LPL in rats. Nutrients. 2017;9:E935. doi: 10.3390/nu9090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yilmaz M, Aktug H, Oltulu F, Erbas O. Neuroprotective effects of folic acid on experimental diabetic peripheral neuropathy. Toxicol Indust Health. 2016;32:832–840. doi: 10.1177/0748233713511513. [DOI] [PubMed] [Google Scholar]
- 54.Yu M, Li W, Luo S, Zhang Y, Liu H, Gao Y, Wang X, Wilson JX, Huang G. Folic acid stimulation of neural stem cell proliferation is associated with altered methylation profile of PI3K/Akt/CREB. J Nutr Biochem. 2014;25:496–502. doi: 10.1016/j.jnutbio.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Zammit S, Lewis S, Gunnell D, Smith GD. Schizophrenia and neural tube defects: comparisons from an epidemiological perspective. Schizophr Bull. 2007;33:853–858. doi: 10.1093/schbul/sbl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng W, Huang J, Hu X, Xiao W, Rong M, Yuan Z, Luo Z. Ionically cross-linked chitosan microspheres for controlled release of bioactive nerve growth factor. Int J Pharm. 2011;421:283–290. doi: 10.1016/j.ijpharm.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Zeng W, Rong M, Hu X, Xiao W, Qi F, Huang J, Luo Z. Incorporation of chitosan microspheres into collagen-chitosan scaffolds for the controlled release of nerve growth factor. PLoS One. 2014;9:e101300. doi: 10.1371/journal.pone.0101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Zhou Y, Li G, Zhao Y, Gu X, Yang Y. Nanoparticle mediated controlled delivery of dual growth factors. Sci China Life Sci. 2014;57:256–262. doi: 10.1007/s11427-014-4606-5. [DOI] [PubMed] [Google Scholar]
- 59.Zhou XH, Lin W, Ren YM, Liu S, Fan BY, Wei ZJ, Shi GD, Cheng X, Hao Y, Feng SQ. Comparison of DNA methylation in Schwann cells before and after peripheral nerve injury in rats. Biomed Res Int. 2017;2017:5393268. doi: 10.1155/2017/5393268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.