Abstract
Rats have been the primary model to study the process and underlying mechanisms of recovery after spinal cord injury. Two weeks after a severe spinal cord contusion, rats can regain weight-bearing abilities without therapeutic interventions, as assessed by the Basso, Beattie and Bresnahan locomotor scale. However, many human patients suffer from permanent loss of motor function following spinal cord injury. While rats are the most understood animal model, major differences in sensorimotor pathways between quadrupeds and bipeds need to be considered. Understanding the major differences between the sensorimotor pathways of rats, non-human primates, and humans is a start to improving targets for treatments of human spinal cord injury. This review will discuss the neuroplasticity of the brain and spinal cord after spinal cord injury in rats, non-human primates, and humans. A brief overview of emerging interventions to induce plasticity in humans with spinal cord injury will also be discussed.
Keywords: recovery, regeneration, spinal cord injury, plasticity, axons, animal studies, locomotor training, functional recovery
Introduction
Approximately 236 to 1009 per million people suffer from spinal cord injury (SCI) to varying degrees globally (Alam and He, 2014). Due to the lack of research and limited understanding of human SCI, there are currently no direct cures for the loss of motor function that results from an SCI. Historically, methods to enhance axonal regeneration were the dominant approach to spinal cord repair. However, recent studies using animal models do not show significant axonal regeneration caudal to the site of injury, suggesting an innate plastic ability of the central nervous system (CNS) to redirect signaling via alternate pathways. Taking into consideration the severe impacts SCIs have on quality of life, current lack of proven therapeutic interventions, and evidence of innate plasticity in other species, neuroscientists must further explore mechanisms by which damage from SCI occurs and the possibility of harnessing plasticity in the human CNS.
Animal models, especially rats, have provided immense insight into the biological processes within the CNS contributing to functional recovery after SCI (Figure 1D). However, it is recognized that motor recovery of rats after SCI greatly surpasses those observed in humans. Research has shown contused rats express non-significant corticospinal axonal regeneration caudal to the injury site, yet regain weight bearing function just one week after injury, as assessed by Basso, Beattie, and Bresnahan scores (Figure 1A–C). Such disparities in recovery arise from interspecies biological variations. Primarily, these include evolutionary changes in CNS structural dependence and post-injury neuroplastic adaptive recovery.
Figure 1.
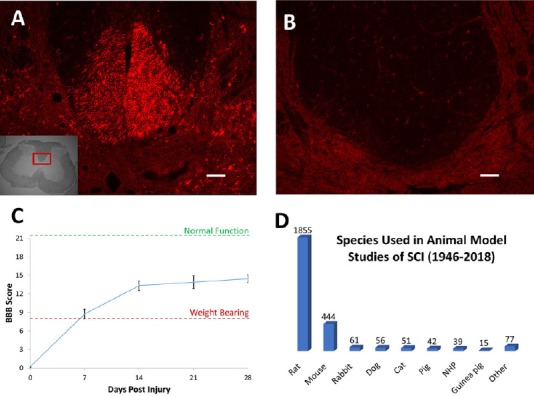
Animal models of SCI.
After an SCI, there are major differences in the extent of recovery that occurs in different animal models. Four weeks after a spinal contusion in rats, biotinylated dextran amine, an anterograde axon tracer, was injected into the motor cortex. In a transverse spinal cord section rostral to the injury, hundreds of axons are labeled in the dorsal corticospinal tract (A), however caudal to the injury there are no labeled axons (B). Even in the absence of the dorsal corticospinal tract, the rats regain the ability to bear weight and step seven days after injury, continuing to improve for at least a month post-SCI (C). Even though this recovery is much higher than what would be expected to occur in humans, rats are still the most widely used model in SCI experiments (D). Scale bars: 100 μm (A & B). Injections performed according to Hellenbrand et al. (2013). SCI: Spinal cord injury; BBB: Basso, Beattie, and Bresnahan scale.
This review discusses the differences between animal and human responses to SCI in order to better elucidate the causative factors in the discrepancy of post-SCI recovery rates. This includes establishing an understanding of the cellular and molecular mechanisms occurring in the spinal cord following injury amongst different species. Additionally, we discuss a comparison of the post-SCI intracerebral and intraspinal plastic reorganization observed across rats and mice, non-human primates, and humans. Finally, the discussion closes with an analysis of interventions being researched and implemented to induce neuroplasticity in humans with SCIs.
Literature Review
The articles used in this neuroplasticity review were retrieved by replicating the search terms of Sharif-Alhoseini et al. (2017). An electronic search of the Medline database for literature describing animal models of SCI from 1946 to 2018 was performed using the following conditions: SCI (MeSH Terms) AND (Models, Animal (MeSH Terms) OR Behavior, Animal/Physiology (MeSH Terms) OR Animal Experimentation (MeSH Terms). The results were further screened by title and abstract to only present rats, mice and non-human primates. Non-SCI experiments and review articles were also excluded.
In addition, an electronic search of the Medline database for methods of inducing human plasticity was completed. This included publications prior to March, 2018, with the following search criteria: spinal cord injury (SCI), inducing plasticity, human plasticity, and spinal cord plasticity. Subsequent searches were completed that were specifically relevant to each method discussed in the inducing plasticity with the following terms: locomotor training (LT), brain-machine interface (BMI), virtual reality (VR), and hypoxia. The articles that did not correspond to human models of SCI were excluded.
Pathophysiology of SCI
The CNS response following SCI establishes a unique inflammatory environment that has yet to be fully understood. This response is constituted by a multi-stage cascade of events at the gross, cellular, and molecular levels, each of which maintain drastic time-course variations, lasting from minutes to years after the injury (Silva et al., 2014). These phases consist of the primary, secondary, and tertiary inflammatory responses produced by the CNS, as well as an attempt at compensatory neural reorganization.
The primary stage occurs at gross anatomical level, arising from the injury itself; this phase is more the direct effect of the mechanical maceration, laceration, contusion, compression, or infarction affecting the spinal cord (Darian-Smith, 2009; Silva et al., 2014). Bleeding and the initiation of cell death are the two primary characteristics of damage associated with this phase (Darian-Smith, 2009). The presence of a secondary stage of damage was first proposed in 1911 by Dr. Alfred Allen (Allen, 1911); and, subsequent research over the last century has validated the existence of this secondary response phase.
The secondary stage consists of a series of vascular, biochemical, and cellular events occurring within minutes to weeks following the initial injury (Darian-Smith, 2009). This stage is the most complex, and, consequently, the least understood of the SCI phases. The vascular changes occurring during this timeframe result in severe complications including edema, ischemia, hypoxia, necrosis, inflammation, and a reduction in spinal perfusion (Tator, 1991; Tator and Fehlings, 1991; Bareyre and Schwab, 2003; Darian-Smith, 2009). The biochemical fluctuations are composed of events such as free radical and nitric oxide formation, lipid peroxidation, excitotoxic changes with specific regard to glutamate, protease release, and ionic imbalances of Na+, K+, and Ca2+ (Darian-Smith, 2009; Silva et al., 2014). These events result in a series of complications such as: oxidative death, reduced blood flow, edema, structural disruption of lipid layers, depolarization, metabolic enzyme impairment, loss of cellular function, tissue dissolution, and, most significantly, general neuron cell death (Sandler and Tator, 1976; Goodman et al., 1979; Hall and Braughler, 1982; Jamme et al., 1995; Farooque et al., 1996; Stys, 1998; Xu et al., 1998; Liu et al., 1999; McAdoo et al., 1999; Toborek et al., 1999; Darian-Smith, 2009). The cellular changes observed in the secondary stage consist of upregulation of macrophages and neutrophils, apoptosis, Wallerian degeneration, and the greatest level of inflammatory response (Beattie et al., 2000; Darian-Smith, 2009; Silva et al., 2014). The direct processes and subsequent effects of these changes are not well understood. It’s thought that apoptosis of oligodendrocytes via the activation of microglia, particularly, may contribute to demyelination (Crowe et al., 1997; Shuman et al., 1997). Microglia activation as well as upregulation of cytokine and reactive oxygen species by increased leukocyte extravasation contribute to further damage at the injury site (Means and Anderson, 1983; Popovich et al., 1997; Taoka et al., 1997; Mabon et al., 2000). The activation of microglia also contributes to the removal of tissue debris, often resulting in the formation of a fluid filled cyst at the injury site, which contributes to glial scar formation (Fawcett and Asher, 1999). However, it is important to note that the inflammatory response also provides various, time-sensitive tissue repair mechanisms that are not yet fully understood (Donnelly and Popovich, 2008; Silva et al., 2014).
During the course of the secondary stage, significant activity of molecules originating from the external environment also contributes to the inhibition of axonal regeneration. This inhibition arises from molecules such as the myelin-associated Nogo-A protein, the myelin-associated glycoprotein, and proteoglycans (Silva et al., 2014). Nogo-A and myelin-associated glycoprotein are both products of oligodendrocytes, which maintain inhibitory characteristics when bound to their receptors, such as neuron growth inhibition and growth cone collapse, and inhibition of white matter regeneration, respectively (Li et al., 1996; GrandPre et al., 2000; Prinjha et al., 2000; Fouad et al., 2001a; Domeniconi et al., 2002). It is thought these inhibitory molecules share a similar Nogo-receptor (NgR) based signaling pathway, which includes several inhibitory co-receptors and a connection with the neurite outgrowth blocking, growth cone retraction inducing Rho-associated kinase (ROCK) molecule (Bito et al., 2000; Liu et al., 2002; McKerracher and Winton, 2002; Wang et al., 2002; Mi et al., 2004; Shao et al., 2005; Venkatesh et al., 2005; Silva et al., 2014). Proteoglycans, specifically chondroitin sulphate proteoglycans, may also use this NgR signaling pathway (Dickendesher et al., 2012). Chondroitin sulphate proteoglycans are the most well understood of the four recognized proteoglycan classes, and when highly concentrated they establish a growth boundary for axons, contribute to neural pattern formation during development, and limit plasticity via synaptic stabilization in adults (Johnson-Green et al., 1991; Levine, 1994; McKeon et al., 1995; Geisert et al., 1996; Yamaguchi, 2000; Corvetti and Rossi, 2005). Finally, the proteoglycans are also known to play a key role in glial scar formation (Fawcett and Asher, 1999; Silver and Miller, 2004).
The glial scar arises as a result of astrocytic activity. It has been shown to maintain beneficial contributions to the healing process for up to two weeks post-injury, influencing blood-brain barrier repair, oligodendrocyte and neuronal death, and inflammatory-mediated cellular intrusion (Silva et al., 2014). However, later it becomes a physical barrier, impeding growth and preventing regeneration of spinal cord axons (Darian-Smith, 2009; Silva et al., 2014). The glial scar formation is considered to be the final step of the secondary phase and first step of the tertiary, chronic phase, following SCI.
The chronic phase of SCI can last days to years after the injury, leading to debilitating neurological impairments such as pain syndromes and mood disorders (Krause et al., 2000; Siddall et al., 2003). The pain syndromes stem from hyperexcitation of sensory neurons in the dorsal horns of the spinal cord causing chronic pain, and can contribute to mood disorders, such as depression, via the activation of the hypothalamic-pituitary-adrenal axis (Taylor et al., 1998; Blackburn-Munro and Blackburn-Munro, 2001; Drew et al., 2001; Hains et al., 2003a, b). Although the general progression of SCI pathophysiology is similar between species, there is variation in timing and neuroplastic capabilities resulting in varied abilities of functional recovery.
Animal Models of SCI
The majority of human SCIs are contusions, but many different injury types are studied in animal models (Figure 2A). Complete injuries in animal models involve a complete transection of the spinal cord, often done to observe axon growth through the injury site. This method prevents spared pathway reorganization from impacting functional recovery. Incomplete injuries include lateral and dorsal hemisections, contusions, compressions, exocytotic injuries, among others (Figure 2A). Contusion and compression models are thought to be the injury models that most closely resemble human SCIs (Sharif-Alhoseini et al., 2017). However, rat spinal cord contusions are less devastating than human spinal cord contusions. Furthermore, the injury type used is strategically determined by the focus of the study, with 37.3% observing for the effect of a drug or neurotrophic/growth factor, followed by 28.9% looking for pathophysiological changes (Figure 2B).
Figure 2.
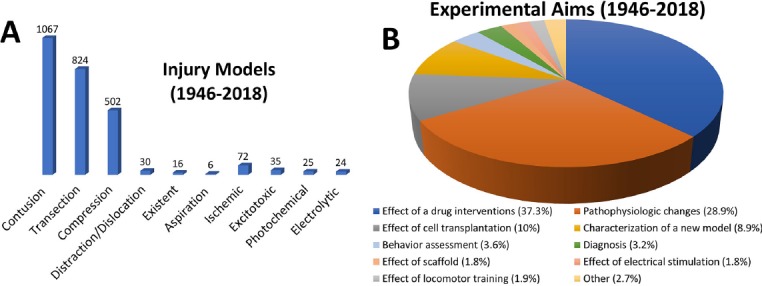
The goal of SCI research varies between studies.
Contusion injuries are the most commonly seen injury in humans and thus the most studied animal models (A). Although contusions and compressions are thought to best represent an injury seen in humans, other models may be helpful in understanding the cellular and molecular mechanisms post-SCI and the effects of treatments on SCI. An appropriate injury model is chosen depending on the aims of the study (B). Adapted from Sharif-Alhoseini et al. (2017). SCI: Spinal cord injury.
The creation of standardized SCI procedures has allowed for reproducible results, especially for rat spinal cord contusions and compressions (Zhang et al., 2014). The Basso, Beattie and Bresnahan locomotor scale (Basso et al., 1995), the horizontal ladder test (Lee et al., 2010), the catwalk (Van Meeteren et al., 2003), among other functional testing have allowed for direct comparison between rat SCI studies. This consistency has allowed for seemingly promising SCI treatments in rats, but human applications have yielded minimal positive outcomes (Tator, 2006). Anatomical and functional differences between rats and humans are large contributing factors to the disparity in functional recovery post-SCI. Using non-human primates as an intermediate model before translation to humans may help predict outcomes in humans (Nout et al., 2012).
Reorganization of Neuronal Circuitry in the Spinal Cord
The sensorimotor pathway is most extensively defined in the rat and mouse models, as they are the most commonly used for SCI research (Figure 1D). There are two general pathways for functional recovery: direct or indirect. Direct or monosynaptic connections between two cells arise with axonal regeneration. Indirect or multi-synaptic connections may help maintain input via one or more intermediate neurons, mediating communication between two cells. Spontaneous recovery of sensorimotor function following SCI most often results from the structural rewiring of spared axons and dendrites within the injury site, using both direct and indirect connections (Darian-Smith, 2009). Spared neurons after an incomplete SCI can develop axon collaterals and provide connections rostral or caudal to a glial scar, a process vital in the strengthening of existing connections and the creation of new ones across the injury site (Aoki et al., 1986; Li and Raisman, 1995; Galea and Darian-Smith, 1997b; Fouad et al., 2001b; Weidner et al., 2001; Bareyre et al., 2004; Ballermann and Fouad, 2006; Hagg, 2006; Fouad and Tse, 2008).
When descending corticospinal tract (CST) fibers are spared, combining pharmacotherapy, treadmill training and epidural stimulation can induce reorganization, thus improving functional recovery (van den Brand et al., 2012; Angeli et al., 2014). Without similar interventions, long-distance reorganization is minimal (Bradbury et al., 2002; Kim et al., 2004; Cafferty et al., 2010; Liu et al., 2010). However, the injured CST can undergo spontaneous short-distance sprouting after incomplete SCI with or without intervention (Thallmair et al., 1998; Cafferty and Strittmatter, 2006; Liu et al., 2010; Starkey et al., 2012; Geoffroy et al., 2015).
Rats and mice
In healthy, uninjured rats few axons from the dorsal CST cross the midline to innervate the ipsilateral spinal cord; however, post-incomplete injury, there is an increase of 6 to 10 fold of dorsal CST axons crossing the midline at the cervical and lumbar levels (Ghosh et al., 2009). Intact CST fibers contralateral to the lesion sprout across the midline into the denervated region, both caudal and rostral to the lesion (Thallmair et al., 1998). In addition, axons originating in the ipsilateral spinal cord spread across the transection, innervating spinal cord sections below the injury (Ghosh et al., 2009). This sprouting mediates the emergence of ipsilateral cortical control of ipsilateral hind paw function (Ghosh et al., 2010). It also suggests that compensatory growth by the CST develops indirect connections between the brain and spinal cord (Thallmair et al., 1998; Z’Graggen et al., 1998). However, the ability for the CST to sprout is largely impacted by the age of the animal. Perinatal mice (P7) CST axons may sprout across the midline after contralateral CST injury, but adult mice have a diminished ability to do so (Liu et al., 2010). Plasticity has been seen in minor CST components as well. The dorsolateral and ventral CST may remodel after dorsal CST lesioning, as shown by Bareyre et al. (2005) and Weidner et al. (2001).
Functional recovery after incomplete SCI can also be increased by spared descending motor pathways besides the CST. The rubrospinal tract and CST redundantly control many motor functions in quadrupeds. Zorner et al. (2014) demonstrated that the reticulospinal tract reorganizes after lateral hemisection in rats. Thus, the rubrospinal and reticulospinal tracts may aid in recovery of function when the CST is damaged (Kennedy and Humphrey, 1987; Kennedy, 1990; Kanagal and Muir, 2009; Lee and Lee, 2013; Williams et al., 2014; Siegel et al., 2015).
Propriospinal neurons (PSNs) also play an important role in functional recovery post-SCI (Jane et al., 1964; Basbaum, 1973; Stelzner and Cullen, 1991; Bareyre et al., 2004; Vavrek et al., 2006). Direct connections between the brain and locomotor circuits are not essential if transmission is mediated by de novo relay propriospinal circuits around the lesion (Courtine et al., 2008). Significant reorganization of descending tracts with PSNs has been observed post-partial descending tract lesions (Raineteau and Schwab, 2001; Bareyre et al., 2004; Jankowska and Edgley, 2006). After a dorsal hemisection injury, transected CST axons that normally innervate lumbar segments sprouted ipsilaterally into the cervical cord to innervate PSNs (Bareyre et al., 2004). These new CST-PSN connections likely serve as a bypass around the lesion, rerouting cortical stimuli to motor neurons caudal to the injury (Bareyre et al., 2004), as severing the CST at the medulla reverses the functional recovery seen (Bareyre et al., 2004). The CST-PSN connections formed post-SCI included transient connections (short PSNs, not bridging lesion) and long-term connections (long PSNs, bridging lesion) (Bareyre et al., 2004).
Non-human primates
Previous studies have shown that there is a significant loss of CST fibers post-unilateral pyramidotomy (Pernet and Hepp-Reymond, 1975), but the loss is minimal post-cervical lesioning (Wannier et al., 2005). Contradictory to Pernet and Hepp-Reymond (1975), Lassek (1946) and Bronson et al. (1978) demonstrated that CST survival was large, regardless of location of the injury. These spared axons can extend across the lesion site to contribute directly to the spontaneous recovery of dexterous manual movements (Schmidlin et al., 2004, Freund et al., 2006, 2009; Hoogewoud et al., 2013). In addition, collateral sprouting is observed in CST axons caudal to hemi-lesions at both the cervical and lumbar levels (Aoki et al., 1986; Galea and Darian-Smith, 1997a). However, Galea and Darian-Smith (1997a) observed a lack of reorganization of CST projections for over two years post-C3 hemisection, while Aoki et al. (1986) saw an increase in ipsilateral de novo CST projections for over 3 years post-hemisection. Moreover, extensive spontaneous sprouting of spared CST fibers occurred in adult rhesus monkeys after low cervical hemisections, resulting in coordinated muscle improvement and hand function and locomotion (Rosenzweig et al., 2010). It was observed that 60% of the CST axon density unilateral to the lesion was restored post-hemisection, likely due to contralateral sprouting of spared CST axons (Rosenzweig et al., 2009, 2010).
The spared CST fibers can also utilize interneurons to regain function. Indirect connections can be formed via CST sprouting into interneuron dense regions (Lacroix et al., 2004; Rosenzweig et al., 2009; Nakagawa et al., 2015). Nakagawa et al. (2015) observed preferential sprouting of spared CST fibers into spinal motor neuron regions, innervating motor neurons directly and forming button-like swellings. This preferential sprouting may allow for recovery of dexterous manual movements with limited CST fibers. Recovery can also be achieved with ipsilateral and re-crossing CST fibers (Rosenzweig et al., 2010; Martin, 2012).
Other descending motor tracts can also mediate functional recovery. Lawrence and Kuypers (1968a, b) deduced that the un-lesioned rubrospinal tract can partially take over the function of the lesioned CST. However, this partial recovery does not occur if there is damage to the red nucleus. Also, the reticulospinal tract can partially mediate functional recovery with a lesioned CST (Zaaimi et al., 2012).
Humans
The large majority of human SCIs are contusions, with significant amounts of surviving white and gray matter that can potentially be used for functional recovery (Kakulas, 1987). Axonal regeneration allowing for direct connection is very limited in humans, but indirect connections between cortical and spinal motor neurons may be used for functional recovery. Burns et al. (1997) demonstrated functional recovery was dependent on the amount of spared tissue in tetraplegic patients. The recovery of function may come from collateral sprouting of damaged or spared pathways, forming de novo neuronal circuitry (Bradbury and McMahon, 2006).
Electrophysiology studies have shown the human CST is capable of reorganization. Post-mortem spinal cord tissue shows anatomical changes in the CST, with some changes similar to animal models and others unique to humans (Oudega and Perez, 2012). Despite CST neuron atrophy, demyelination and retrograde degeneration at the injury site (Bronson et al., 1978; Fishman, 1987; Yamamoto et al., 1989; Buss et al., 2004; Wrigley et al., 2009a), normal numbers of CST axons were observed at a distance from the injury, suggesting collateral sprouting of surviving axons (Fishman, 1987). It is evident that the CST in humans is capable of reorganization post-SCI; however, the extent to which this impacts functional recovery is yet to be determined.
The reticulospinal tract in humans interacts with PSNs and other ascending and descending tracts. The reticulospinal tract works alongside the CST in humans, suggesting that if the CST is lesioned, the reticulospinal tract could contribute to functional recovery, especially to the upper limbs. When humans with complete and incomplete chronic cervical SCIs were required to complete tasks involving finger dexterity and grip, reticulospinal tract stimulation increased the speed of certain grip tasks, but not finger dexterity (Baker and Perez, 2017). Uninjured individuals performed all motor tasks quicker with reticulospinal tract stimulation, suggesting that the reticulospinal tract plays a role in recovery of gross hand movement, but not fine motor control post-SCI in humans (Baker and Perez, 2017).
Although the rubrospinal tract is well developed in rats and non-human primates, its function in humans is not well known. In humans, the rubrospinal tract only extends through the upper cervical segments, unlike in rats and non-human primates where it extends the entire spinal cord (Nathan and Smith, 1982). It is thought that the rubrospinal tract became rudimentary as the CST expanded in bipedal species (ten Donkelaar, 1988; Onodera and Hicks, 2009). Thus, the impact of the rubrospinal tract on functional recovery in humans remains unclear.
Differences in Spinal Cord Reorganization Post-SCI Between Animal Models
As the major descending motor pathway from the cerebral cortex to the spinal cord, the location, function, and size of the CST plays an important role in functional recovery post-SCI. Observed in Figure 3, the CST has a prominent dorsal region in rats, but is only present in the lateral and anterior regions in humans. The function of the CST thus differs between species. CST axons in rats must synapse onto interneurons to relay information to motor neurons, as no direct CST-motor neuron connections exist (Lemon and Griffiths, 2005). However, in primates, direct corticomotoneuronal projections omit the need for interneuron connections for forelimb movement (Nakajima et al., 2000). In addition to the evolution of direct corticospinal connections, an increase in the size and number of corticospinal fibers and excitatory postsynaptic potentials of the cortical neurons is observed in non-human primates and humans (Courtine et al., 2007).
Figure 3.
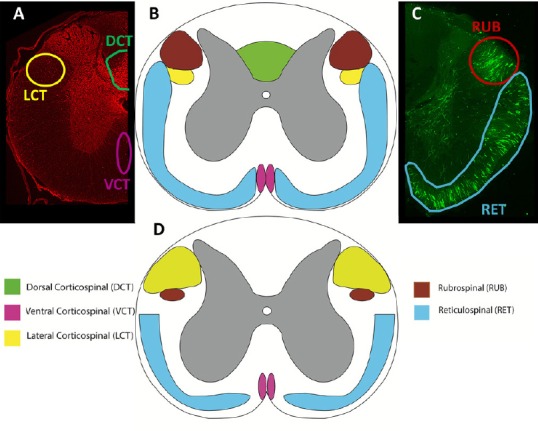
Comparison of spinal cord tracts.
Transverse sections of the rat (A-C) and human (D) demonstrating the approximate locations and sizes of the corticospinal, rubrospinal, and reticulospinal tracts. Rats were injected with an anterograde tracer, biotinylated dextran amine, into the motor cortex (A), reticular formation and red nucleus (B). Injections performed according to Hellenbrand et al. (2013). The corticospinal tract (CST) has larger lateral fibers in humans (yellow), while there is no dorsal CST in humans as compared to rats, who have a large dorsal CST (green). Both rats and humans have a ventral CST (pink). The rubrospinal tract (red) is prominent in rats, but largely reduced in humans, only passing through the upper cervical levels. All species express the reticulospinal tract (blue) prominently, with slight variations in location and size. High order non-human primates more closely resemble humans; however, tract size and exact location varies between non-human primate species. Adapted from Silva et al. (2013) and Watson et al. (2009).
In non-human primates, the CST has a strong influence on the activity of several clusters of motor neurons in the spinal cord, which then innervate distal muscles of the hands and feet (Courtine et al., 2007). For non-human primates, it is the most vital control pathway used for voluntary manual dexterity and is used for fine hand and finger movements (Darian-Smith, 2009). In rats, the CST plays a less important role in forepaw movement (Darian-Smith, 2009). The human CST is also responsible for gross and fine motor movement of the hands. The difference in location and function of the CST between species likely contributes to the varying degrees of functional recovery observed.
A prominent difference between humans, non-human primates, and rats is the presence of the rubrospinal tract. Across these species, the reticulospinal tract is thought to contribute to functional recovery in varying extents, but the rubrospinal tract likely has a limited contribution to functional recovery in humans. Bipedal species observe a reduction in the rubrospinal tract, likely due to the growth of the CST during the evolutionary transition from quadrupedal to bipedal (ten Donkelaar, 1988; Onodera and Hicks, 2009). Thus, quadrupeds like rats, and to an extent, non-human primates observe rubrospinal tract reorganization post-SCI while humans may not. It is possible for the rubrospinal tract to have an impact on functional recovery in humans, especially for cervical level injuries, but to the extent this occurs is not well known.
The pathophysiology of SCI also varies between species. The spinal shock phase lasts for several weeks or more in humans, but only lasts hours or days in animal models (Smith and Jeffery, 2005). Considering a significant amount of spontaneous functional recovery is seen within 2–6 months of injury (Bradbury and McMahon, 2006), the length of the spinal shock phase may affect spontaneous functional recovery, however, it is related to the rehabilitative treatment. In addition, the lesion area of chronic SCI in humans seems to contain less reactive astrocytes (Bunge et al., 1993; Puckett et al., 1997) than rodents (Murray et al., 1990). This reduced astrogliosis may impact long-term functional recovery.
Although axon sprouting may lead to functional recovery, it can also lead to aberrant connections with potentially detrimental consequences. Pain and autonomic dysreflexia have been attributed to increases in primary afferent sprouting in animal models (Romero et al., 2000, Weaver et al., 2006). Pain, autonomic dysreflexia, and spasticity are also common in humans with SCI and are likely increased with aberrant connections (Balazy, 1992; Burchiel and Hsu, 2001; Siddall et al., 2003; Adams and Hicks, 2005; Karlsson, 2006). In addition, the formation of cavities (Williams et al., 1981; Bunge, 1994; Fitch et al., 1999) and the slow spread of cell death from the injury site (Dusart and Schwab, 1994; Liu et al., 1997) impact recovery in humans and animal models alike.
Reorganization of Neuronal Circuitry in the Brain
The brain is continuously changing in response to learning, stimuli, and injury. Within minutes post-injury, continuing for several days, sensorimotor loss post-SCI induces reorganization within the brainstem cuneate nucleus and primary sensorimotor areas and their surrounding regions (Donoghue et al., 1990; Jain et al., 1997; Xu and Wall, 1999). Changes may include alterations in neuronal activity, synaptic efficacy (Dunlop, 2008), and astrocytic activity, in addition to changes in length and diameter of already established dendritic branches, or the growth of new ones (Bayona et al., 2005). This reorganization does not guarantee functional recovery; however, it does provide a good basis for motor recovery (Wolpaw, 2007).
Rats and mice
Early studies showed thalamocortical reorganization after lesions of the dorsal columns or of the gracilis nucleus (Wall and Egger, 1971). However, Jain et al. (1995) observed absence of any cortical reorganization up to 3 months after unilateral dorsal column section at T6–8, with neurons in the deafferented hindlimb cortex unresponsive to all input. This was similarly observed with complete thoracic spinal cord transection (Graziano et al., 2013). Cortical reorganization appeared to be confined to one week post-injury in thoracic bilateral dorsal sectioning (Ghosh et al., 2010) when using voltage-sensitive dye imaging (VSD). When thalamocortical reorganization occurs, it plays a critical role, observed by functional magnetic resonance imaging (fMRI) (Seminowicz et al., 2012) and altered subcortical processing (Moxon et al., 2014). However, it is especially involved in the development of neuropathic pain and disruption of thalamic processing (Hains et al., 2005, 2006; Masri et al., 2009; Whitt et al., 2013).
The motor and sensory cortices contribute to reorganization post-SCI. fMRI has shown increased blood-oxygen-level dependent signaling in the primary somatosensory cortex (S1) in response to stimulation after thoracic contusion (Hofstetter et al., 2003), thoracic transection (Endo et al., 2007), and thoracic bilateral dorsal section (Ghosh et al., 2010). Lateral hemisections have shown that the forelimb cortices responded to ipsilateral and contralateral forelimb stimulation (Ghosh et al., 2009; Bazley et al., 2014). After transection of the spinal cord, reorganization of the motor cortex (MC) is also seen (Raineteau and Schwab, 2001). The dense network of axons that exists in the MC connect different sub-regions with horizontal pathways regulated by gamma-aminobutyric acid (GABA). However, reorganization of the MC still needs to be better understood (Fouad et al., 2001b; Oza and Giszter, 2014).
Non-human primates
The sprouting of spared CST fibers to descending motor systems may induce the incorporation of new neurons into the existing architecture of newly cell-deficient regions of the brain. After complete high cervical dorsal column sectioning, portions of forelimb, trunk, and hindlimb representations in areas 3b and 1 of the somatosensory cortex become deactivated (Jain et al., 1997). In 6–8 months post-injury, afferents remaining from the arm activate neurons from the previously deafferented hand representations in areas 3b and 1 (Jain et al., 1997). In addition, afferents from the chin reactivated former hand representations and medial trunk and hindlimb representations (Jain et al., 1997). This reorganization is related to the sprouting within the trigeminal-dorsal column complex in the brainstem (Jain et al., 2000; Kambi et al., 2014) and is also observed at the thalamic level (Jain et al., 2008). Similar results were seen with incomplete dorsal column sections. Spared ascending axons from hand afferents contribute to the reactivation of somatosensory hand representations (Jain et al., 1997). This was dependent on the sprouting of preserved forelimb afferents in the cuneate nucleus. Reactivated cuneate neurons activated more neurons in the contralateral ventroposterior nucleus (Jain et al., 2001, 2008), which may have allowed for additional sprouting and spread of activation. Regions that were reactivated from spared hand or foot afferents were still responsive to input from the face, suggesting that these neurons became responsive to both face and hand (Jain et al., 1997, 2000).
However, axonal sprouting between borders, such as the hand and face, has been shown to produce phantom limb sensations rather than contribute to functional recovery (Kaas et al., 2008). Some functional recovery is observed with long-term reorganization in regards to finger dexterity (Schmidlin et al., 2004; Nishimura et al., 2007; Kambi et al., 2011), but near-normal recovery of cortical hand representation is correlated with higher functional recovery of the hand (Qi et al., 2014).
Impaired hand use after dorsal column sectioning may reflect motor cortex dysfunction from the disruption of sensory input from somatosensory cortical areas. The secondary somatosensory cortex (S2) and the parietal ventral area depend on areas 3b and 1 for activation. The output for S2 and parietal ventral area are to motor and pre-motor regions of the frontal lobe, in addition to posterior parietal regions that project to these motor regions (Kaas, 2004). Thus, it is plausible that the deactivation and reactivation of regions 3b and 1 impact all somatosensory cortical processing (Kaas et al., 2008; Tandon et al., 2009).
Humans
In humans, 30% of CST fibers arise from the primary MC (MC1 or Brodmann area 4), 30% from the premotor cortex (PM), and 40% from the somatosensory cortex. fMRI and positron emission tomography have shown increased activation of MC1, supplementary motor area (SMA) (Dettmers et al., 1995, 1996; Cramer et al., 2002), pre-motor cortex (PM) and the cerebellum (Dai et al., 2001) as force increases, which can occur in a few ways (Moxon et al., 2014).
First, the activation of MC1 can be shifted posteriorly (Green et al., 1998; Turner et al., 2003). Post-SCI, the increased loss of axons in MC1 may allow surviving S1 axons to contribute to the damaged CST. This is observed by an increase in activity in S1 (Green et al., 1999). This was observed in individuals with American Spinal Injury Association (ASIA) A–D, 4 to 6 weeks post-SCI. It is thought that a posterior shift may be a result of hyperactive S1 axons in neuropathic pain (Turner et al., 2003), or that the S1 axons are less susceptible to damage due to their location (Green et al., 1999). However, it is currently unknown which theory is true.
Second, the shift in activation can be in the direction of the deafferented representation within MC1, depending on the chronicity of the SCI. In some individuals with ASIA A–C, the displacement correlated to the time since SCI (Lotze et al., 2006). Within one year of the SCI, the displacement was minimal. From the same individuals who observed a posterior shift in Green et al. (1999), some observed an anterior shift as recovery progressed. It is possible that early after injury a posterior shift is observed, moving anterior towards the deafferented representation within MC1 as time goes on (Kokotilo et al., 2009).
The level of injury also plays a role, as those with paraplegia will have different reorganization as compared to those with tetraplegia (Bruehlmeier et al., 1998). A posterior shift in activation within M1 was seen in individuals with thoracic injury or higher. Transcranial magnetic stimulation has shown enlarged cortical sensorimotor areas of muscles preserved above the injury in tetraplegia (Levy et al., 1990), with medial-superior shifts in cortical activation with tongue movements (Mikulis et al., 2002). A complete injury can cause abnormal brain motor function during attempted or imagined movement (Cramer et al., 2005), but stable cortical topography can be established in incomplete injuries (Corbetta et al., 2002). Thoracic injuries, or lower, observe a shift in activation towards the deafferented region. An increased excitability and representation of muscles rostral to the injury is also observed with paraplegia (Topka et al., 1991; Curt et al., 2002a). It is known that afferent feedback is important for motor control, but the mechanism by which level and severity of the injury impact reorganization is not known. SCI has also been shown to affect the speed of cortical spontaneous electroencephalography activity in addition to affecting evoked sensorimotor activity (Tran et al., 2004; Boord et al., 2008; Wydenkeller et al., 2009).
Although cortical reorganization occurs post-SCI, it is associated with phantom sensations and neuropathic pain, in addition to functional recovery (Ness et al., 1998; Moore et al., 2000; Boord et al., 2008; Wrigley et al., 2009a, b, Wydenkeller et al., 2009; Gustin et al., 2010, 2012). Post-SCI, reduction of GABAergic inhibition in excitatory synapses is an important cause of short-term plasticity by contributing to the maintenance of projection neurons to the deafferented nuclei and cortices by keeping a state of increased excitation (Chen et al., 2002; Navarro et al., 2007). This increase in excitability of neurons in the sensory pathways contributes to subcortical and cortical reorganization, but also neuropathic pain (Navarro et al., 2007). The expanded activation of the somatosensory cortex is correlated with referred sensations, as shown with fMRI (Wall et al., 2002). In adolescents potentially, maladaptive plasticity after injury may account for long-term physical and emotional impairments. An increase in inhibition and a decrease of excitatory neurotransmission may affect long-term potentiation and long-term depression, which is associated with impaired plasticity and synaptogenesis in a developing brain (Li et al., 2014). Thus, age-specific treatments and injury prevention strategies are necessary.
Inducing Neuroplasticity in Humans
Beginning in the late 20th century, research regarding functional recovery, through means of physical rehabilitation and activity after SCI, began to play a new role in the post-injury treatment of patients. This was a shift from the near century old misguided belief that the CNS was incapable of repair and compensation-centered care (Behrman et al., 2006). Locomotor training (LT) therapies rely on activity-dependent, intrinsic plastic capabilities of the CNS. These methods centralize on activating the neuromuscular system below the injury level by stimulating the residual neural networks via familiar sensory input, thus inducing adaptive neural output and promoting modifications within the network (Harkema et al., 2012). Physical activity in animal models has been shown to have positive neuro-rehabilitative and neuroregenerative properties, which promote both macro and micro scale intrinsic recovery after SCI (Lovely et al., 1986; Skinner et al., 1996; de Leon et al., 1998; Rossignol et al., 1999; Beaumont et al., 2004; Engesser-Cesar et al., 2005; Cohen-Adad et al., 2014; Detloff et al., 2014; Theisen et al., 2017).
LT in humans has been shown to aid in the recovery of function in individuals with SCI and has accordingly been adopted into the clinical setting as a staple of SCI rehabilitation. Nevertheless, although LT has proven beneficial for recovery, further research is needed to determine which method provides the optimal conditions to afford the greatest level of relief (Morawietz and Moffat, 2013). LT can include the use of treadmills, such as in body weight-supported treadmill training, or robotic assistance to restore or improve functional ability in the lower extremities. These techniques, particularly when body-weight support assistance is provided, have been shown to enhance many factors of function on the gross scale including increased walking speed, ability to cover greater distances, improved balance and body weight-bearing, kinematics, and reduced spasticity (Harkema et al., 2012; Knikou, 2013; Manella and Field-Fote, 2013). Moreover, LT has also expressed beneficial impacts on somatosensory and corticospinal excitability, greater MC functional connectivity, normalization of the H-reflex phase-dependent modulation, electromyographic activity in leg muscles correspondent to movement, and reappearance and alterations of early and late flexor reflexes, respectively (Edgerton et al., 2006; Knikou, 2013; Smith et al., 2014; Chisholm et al., 2015).
Neuroplasticity in humans has also been induced in individuals with SCI after undergoing acute intermittent hypoxia (AIH) treatments. These treatments have been shown to activate chemoafferents in rodents, which in turn strengthen synaptic input and motor output via the release of episodic serotonin, brain-derived neurotrophic factor synthesis, and tyrosine kinase (TrkB) activation (Baker-Herman and Mitchell, 2002; Baker-Herman et al., 2004; Trumbower et al., 2012; Hayes et al., 2014). Regarding implementation in human studies, AIH is still a novel technique; though, initial results are encouraging. In 2012, a single AIH treatment consisting of alternating 9% O2 with 21% (environmental) O2 for a total of 15 episodes was, for the first time, shown to increase both motor output from the lumbosacral region via gastrocnemius electromyography and maximal voluntary ankle plantar flexion strength in individuals with SCI (Trumbower et al., 2012). A more recent study, completed in 2016, also found significantly enhanced ankle strength, up 30% from baseline, one hour after AIH, with an additional correlation observed between ankle strength and electromyography of the gastrocnemius and soleus muscles (Lynch et al., 2017). Determining AIH contributes to enhancing ankle strength presents a particularly important finding, as decreased ankle strength is a major mobility limiting characteristic of SCI, suggesting the opportunity for integration with the aforementioned rehabilitation techniques, possibly enhancing their results.
Building off the idea that AIH and LT would be mutually beneficial, Hayes et al. (2014) found daily AIH alone for five days increased walking speed and endurance, and even further enhancements in endurance were observed with daily AIH combined with LT in patients with chronic SCI. These findings are unique in that clinically significant changes in both endurance and speed were observed after just a five-day period in more than 70% and 30% of patients, respectively; whereas, traditional LT typically takes weeks to months to show clinically significant results (Hayes et al., 2014). Furthermore, Navarrete-Opazo et al. (2017) used an extended AIH protocol comprised of five treatments in the first week and three treatments in each of the subsequent three weeks, in combination with body weight-supported treadmill training. This demonstrated an increased walking speed and walking endurance after the first week, with walking endurance increased and speed maintained throughout the remaining three weeks. In several of the patients, these improvements persisted for up to five weeks post AIH (Navarrete-Opazo et al., 2017). The results of these studies, though limited in size, suggest that a combinatorial implementation of AIH and LT may be an effective, non-invasive, alternative therapy to induce plasticity and promote functional recovery following SCI.
Moreover, with newly developed technologies, SCI research has begun to wholly integrate modern machines into the therapies used to aid in recovery. One such integration is that of brain-machine-interface (BMI) training, with human test trials beginning in the early part of the 21st-century (Lebedev and Nicolelis, 2017). Unlike neurodegenerative diseases, SCI offers a relatively stable condition with a minimally affected MC, creating a most suitable environment for BMI integration (Curt et al., 2002b; Hotz-Boendermaker et al., 2008; Freund et al., 2011; Harkema et al., 2011; Collinger et al., 2013). These interfaces offer a bypass of the lower motor neurons, creating a direct link between the cortical motor neurons and peripheral nervous system or target muscles by means of functional electronic stimulation, prosthetics, or the spinal cord through epidural stimulation or intraspinal microstimulation (Chapin et al., 1999; Freund et al., 2011; Lebedev and Nicolelis, 2017). BMI integrations also show facilitation of neuroplasticity in monkeys through activity dependent neuromodulation and induced long-term potentiation (Fitzsimmons et al., 2009). With respect to animal models, these methods appear promising, though a large-scale translation to the human model will surely prove challenging.
BMI with functional electronic stimulation integration has shown promising results in several small-scale human trials and case studies for the recovery of upper limb movement after tetraplegia (Pfurtscheller et al., 2003, 2005; Bouton et al., 2016). These patients have been able to move prostheses, in the form of robotic arms and hands, to grasp and move simple objects through individualized training with BMI systems. Though no studies, to our knowledge, have been completed regarding the use of only functional electronic stimulation with BMI to attain lower limb functional recovery in humans, King et al. (2015) tested the feasibility of an functional electronic stimulation integrated BMI on a single paraplegic man in 2015, for the first time, demonstrating brain-controlled over ground locomotor function was feasible. Similarly, a 2014 study by To et al. (2014) explored the option of using functional neuromuscular stimulation for propulsion in combination with an exoskeleton for stability. The initial tests showed potential for use in rehabilitation of paraplegic individuals (To et al., 2014). Additionally, early data from non-human primate studies, have shown prospective for future use in humans (Lopez-Larraz et al., 2016; Lebedev and Nicolelis, 2017).
Furthermore, with regard to BMI control of prostheses, several human trials have shown applicability of this technique, but only one has, in combination with other techniques, demonstrated functional recovery. The BMI prostheses mechanism used in functional recovery therapy of paralysis of the lower limbs due to SCI is a variation of the type exoskeleton mentioned in the preceding section of this paper; and, like those therapeutic techniques, these BMI therapies can be considered a type of LT. Recent developments in BMI controlled lower limb exoskeletons have proposed user-interpreted electroencephalography recordings, such as steady-state visual evoked potentials, as a feasible alternative control system (Do et al., 2013; Kwak et al., 2015 Lopez-Larraz et al., 2016). Alternatively, the NeuroRex exoskeleton, developed in 2013, uses an electroencephalography recording to interpret and assist in the movement of impaired individuals (Contreras-Vidal and Grossman, 2013). The goal selection system, like the NeuroRex exoskeleton, provides a less fatiguing technique that is both faster and more accurate than the user-interpreted electroencephalography model (Royer et al., 2011). However, each of these studies was completed as a proof of concept to test the application of this technique rather than to determine the impact on recovery.
Another approach currently under development is the direct stimulation of the spinal cord below the injury through epidural stimulation or intraspinal microstimulation. Epidural stimulation requires direct electrode placement over the spinal cord (Lavrov et al., 2008; Hachmann et al., 2013). Using this method, paralyzed subjects in recent studies were able to elicit voluntary muscle control in the lower limbs for brief periods of time, upwards of twenty-five minutes, years after the initial injury (Harkema et al., 2011; Angeli et al., 2014; To et al., 2014). Intraspinal microstimulation, however, involves implantation of the stimulating electrodes within the spinal cord ventral grey matter (Bamford and Mushahwar, 2011). Application of intraspinal microstimulation in animal models has produced very promising results for SCI. Nevertheless, the requirement for implantation has raised several issues, including electrode-tissue biocompatibility and reversibility of the electrode-tissue interface charge capacity injection, among others, which have hindered implementation in human (Rosich et al., 2017).
Donati et al. (2016) described, what is considered to be, the first ever clinical study to show significant, partial recovery of neurological function across multiple individuals with chronic SCI, which is both consistent and reproducible. Through long-term training with virtual reality (VR) immersion, visual-tactile feedback, and two BMI-integrated exoskeletons, individuals were able to regain voluntary muscle control below the SCI and attain some level of improvement in somatic sensations. Specifically, half of the subjects were upgraded from complete to incomplete paraplegia and all saw improvements in neurologic sensation below the injury site (Donati et al., 2016). The results of this groundbreaking study suggest that combination therapy may be the best technique to induce plasticity in the human with SCI.
VR offers a unique strategy, serving as a platform to alter the sensory-perceptual experiences of the brain, forcing a compensatory response in the brain. This system is unique in that it provides immediate sensory feedback in a safe, customizable, immersive, and true-to-life environment, otherwise unattainable for patients with damage to the CNS (Rose et al., 1999; Kizony et al., 2005). The feasibility of virtual reality environments for neurological rehabilitation has been thoroughly tested, with results showing promising application with balance abilities, motor imagery facilitation tasks, control of BMI-exoskeletons, and electroencephalography-based BMI with functional electronic stimulation integration (Wang et al., 2012; King et al., 2013; Villiger et al., 2013; Roosink et al., 2016). Like many other techniques discussed in this section, the application and impact of VR rehabilitation on recovery of SCI has been minimally tested.
In 2013, Villiger et al. (2013) used an uncontrolled study of a virtual reality environments, in which the incompletely-injured patient could see virtual legs from a first-person point of view, combined with individual muscle training. Improvements were seen in lower limb function through balance, walking capacity, and strength, as well as through reduced neuropathic pain intensity and irritation. Two studies completed in 2016 offered further evidence for the positive impact of VR rehabilitation of SCI. The first was completed by Donati et al. (2016). The second, completed by Shokur et al. (2016), used a combination of VR and tactile feedback by means of sensory substitution and remapping. This combination elicited the incorporation of the virtual legs into the body schema, perception of the virtual legs’ position, and sensation of virtual floor textures. Lastly, Khurana et al. (2017) reported improvements in balance and functional performance in paraplegics after training with a VR-based game, greater than in paraplegics using task-specific, real-world, balance training.
Though it is clear these methods offer a beneficial approach to inducing plasticity in humans, further investigation is necessary to better understand the cellular and molecular actions taking place as a result of their implementation. With a more developed understanding of the intrinsic plastic capabilities of the CNS and of how exactly these techniques work to harness those properties, researchers will be able to better determine which methods can be combined to provide a more highly targeted and effective therapeutic path to recovery.
Conclusions
The evolutionary-based differences in the location and function of the descending motor tracts plays a large role in the functional recovery observed between species. The redundant, prominent motor tracts in rats allow for effective rerouting around the injury. The enlarged CST in primates, along with the reduced rubrospinal tract in humans may impact the efficacy of plasticity in humans. Continuing to evaluate the differences in tract reorganization between quadrupedal and bipedal species in conjunction with the use of locomotion therapy may contribute to future treatments.
Recent advances in technology have allowed for novel treatment methods to be tested in animal models and humans to improve functional recovery. The results are promising, giving hope to finding more effective therapies for humans. Although rats are a necessary starting point, we need to consider other models that better imitate the limitations of the human CST to better gauge the utility of therapeutic approaches.
Additional file: Open peer review reports 1 (116.8KB, pdf) and 2 (116.9KB, pdf) .
Footnotes
Conflicts of interest: The authors claim no existing conflicts of interest, financial or otherwise.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Xiangbing Wu, Indiana University School of Medicine, USA; Wei Wu, Indiana University School of Medicine-South Bend, USA.
P-Reviewers: Wu X, Wu W; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577–586. doi: 10.1038/sj.sc.3101757. [DOI] [PubMed] [Google Scholar]
- 2.Alam M, He J. Lower-Limb Neuroprostheses: Restoring Walking after Spinal Cord Injury. In: Naik G, Guo Y, editors. Emerging Theory and Practice in Neuroprosthetics. Hershey, PA, USA: IGI Global; 2014. pp. 153–180. [Google Scholar]
- 3.Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. JAMA LVLII. 1911:878–880. [Google Scholar]
- 4.Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoki M, Fujito Y, Satomi H, Kurosawa Y, Kasaba T. The possible role of collateral sprouting in the functional restitution of corticospinal connections after spinal hemisection. Neurosci Res. 1986;3:617–627. doi: 10.1016/0168-0102(86)90058-1. [DOI] [PubMed] [Google Scholar]
- 6.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 7.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker SN, Perez MA. Reticulospinal contributions to gross hand function after human spinal cord injury. J Neurosci. 2017;37:9778–9784. doi: 10.1523/JNEUROSCI.3368-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balazy TE. Clinical management of chronic pain in spinal cord injury. Clin J Pain. 1992;8:102–110. doi: 10.1097/00002508-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci. 2006;23:1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- 11.Bamford JA, Mushahwar VK. Intraspinal microstimulation for the recovery of function following spinal cord injury. Prog Brain Res. 2011;194:227–239. doi: 10.1016/B978-0-444-53815-4.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 13.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 14.Bareyre FM, Schwab ME. Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci. 2003;26:555–563. doi: 10.1016/j.tins.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Basbaum AI. Conduction of the effects of noxious stimulation by short-fiber multisynaptic systems of the spinal cord in the rat. Exp Neurol. 1973;40:699–716. doi: 10.1016/0014-4886(73)90105-2. [DOI] [PubMed] [Google Scholar]
- 16.Basso DM, Beattie MS, Bresnahan JC. A Sensitive and Reliable Locomotor Rating-Scale for Open-Field Testing in Rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 17.Bayona NA, Bitensky J, Teasell R. Plasticity and reorganization of the uninjured brain. Top Stroke Rehabil. 2005;12:1–10. doi: 10.1310/A422-G91U-Q4HB-86XC. [DOI] [PubMed] [Google Scholar]
- 18.Bazley FA, Maybhate A, Tan CS, Thakor NV, Kerr C, All AH. Enhancement of bilateral cortical somatosensory evoked potentials to intact forelimb stimulation following thoracic contusion spinal cord injury in rats. IEEE Trans Neural Syst Rehabil Eng. 2014;22:953–964. doi: 10.1109/TNSRE.2014.2319313. [DOI] [PubMed] [Google Scholar]
- 19.Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- 20.Beaumont E, Houle JD, Peterson CA, Gardiner PF. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve. 2004;29:234–242. doi: 10.1002/mus.10539. [DOI] [PubMed] [Google Scholar]
- 21.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86:1406–1425. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- 22.Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron. 2000;26:431–441. doi: 10.1016/s0896-6273(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence. J Neuroendocrinol. 2001;13:1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 24.Boord P, Siddall PJ, Tran Y, Herbert D, Middleton J, Craig A. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord. 2008;46:118–123. doi: 10.1038/sj.sc.3102077. [DOI] [PubMed] [Google Scholar]
- 25.Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, Sharma G, Sederberg PB, Glenn BC, Mysiw WJ, Morgan AG, Deogaonkar M, Rezai AR. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533:247–250. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- 26.Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work. Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- 27.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 28.Bronson R, Gilles FH, Hall J, Hedley-Whyte ET. Long term post-traumatic retrograde corticospinal degeneration in man. Hum Pathol. 1978;9:602–607. doi: 10.1016/s0046-8177(78)80143-9. [DOI] [PubMed] [Google Scholar]
- 29.Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury. Eur J Neurosci. 1998;10:3918–3922. doi: 10.1046/j.1460-9568.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- 30.Bunge MB. Transplantation of purified populations of Schwann cells into lesioned adult rat spinal cord. J Neurol. 1994;242:S36–39. doi: 10.1007/BF00939240. [DOI] [PubMed] [Google Scholar]
- 31.Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- 32.Burchiel KJ, Hsu FP. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine. 2001;26:S146–160. doi: 10.1097/00007632-200112151-00024. [DOI] [PubMed] [Google Scholar]
- 33.Burns SP, Golding DG, Rolle WA, Jr, Graziani V, Ditunno JF., Jr Recovery of ambulation in motor-incomplete tetraplegia. Arch Phys Med Rehabil. 1997;78:1169–1172. doi: 10.1016/s0003-9993(97)90326-9. [DOI] [PubMed] [Google Scholar]
- 34.Buss A, Brook GA, Kakulas B, Martin D, Franzen R, Schoenen J, Noth J, Schmitt AB. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain. 2004;127:34–44. doi: 10.1093/brain/awh001. [DOI] [PubMed] [Google Scholar]
- 35.Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapin JK, Moxon KA, Markowitz RS, Nicolelis MA. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 1999;2:664–670. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- 38.Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 39.Chisholm AE, Peters S, Borich MR, Boyd LA, Lam T. Short-term cortical plasticity associated with feedback-error learning after locomotor training in a patient with incomplete spinal cord injury. Phys Ther. 2015;95:257–266. doi: 10.2522/ptj.20130522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen-Adad J, Martinez M, Delivet-Mongrain H, Rossignol S. Recovery of locomotion after partial spinal cord lesions in cats: assessment using behavioral, electrophysiological and imaging techniques. Acta Neurobiol Exp (Wars) 2014;74:142–157. doi: 10.55782/ane-2014-1981. [DOI] [PubMed] [Google Scholar]
- 41.Collinger JL, Boninger ML, Bruns TM, Curley K, Wang W, Weber DJ. Functional priorities, assistive technology, and brain-computer interfaces after spinal cord injury. J Rehabil Res Dev. 2013;50:145–160. doi: 10.1682/jrrd.2011.11.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Contreras-Vidal JL, Grossman RG. NeuroRex: a clinical neural interface roadmap for EEG-based brain machine interfaces to a lower body robotic exoskeleton. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:1579–1582. doi: 10.1109/EMBC.2013.6609816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corbetta M, Burton H, Sinclair RJ, Conturo TE, Akbudak E, McDonald JW. Functional reorganization and stability of somatosensory-motor cortical topography in a tetraplegic subject with late recovery. Proc Natl Acad Sci U S A. 2002;99:17066–17071. doi: 10.1073/pnas.262669099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corvetti L, Rossi F. Degradation of chondroitin sulfate proteoglycans induces sprouting of intact purkinje axons in the cerebellum of the adult rat. J Neurosci. 2005;25:7150–7158. doi: 10.1523/JNEUROSCI.0683-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME, Edgerton VR. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007;13:561–566. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cramer SC, Lastra L, Lacourse MG, Cohen MJ. Brain motor system function after chronic, complete spinal cord injury. Brain. 2005;128:2941–2950. doi: 10.1093/brain/awh648. [DOI] [PubMed] [Google Scholar]
- 48.Cramer SC, Weisskoff RM, Schaechter JD, Nelles G, Foley M, Finklestein SP, Rosen BR. Motor cortex activation is related to force of squeezing. Hum Brain Mapp. 2002;16:197–205. doi: 10.1002/hbm.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 50.Curt A, Alkadhi H, Crelier GR, Boendermaker SH, Hepp-Reymond MC, Kollias SS. Changes of non-affected upper limb cortical representation in paraplegic patients as assessed by fMRI. Brain. 2002a;125:2567–2578. doi: 10.1093/brain/awf250. [DOI] [PubMed] [Google Scholar]
- 51.Curt A, Bruehlmeier M, Leenders KL, Roelcke U, Dietz V. Differential effect of spinal cord injury and functional impairment on human brain activation. J Neurotrauma. 2002b;19:43–51. doi: 10.1089/089771502753460222. [DOI] [PubMed] [Google Scholar]
- 52.Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH. Relationship between muscle output and functional MRI-measured brain activation. Exp Brain Res. 2001;140:290–300. doi: 10.1007/s002210100815. [DOI] [PubMed] [Google Scholar]
- 53.Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist. 2009;15:149–165. doi: 10.1177/1073858408331372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- 55.Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houle JD. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp Neurol. 2014;255:38–48. doi: 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 1995;74:802–815. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- 57.Dettmers C, Ridding MC, Stephan KM, Lemon RN, Rothwell JC, Frackowiak RS. Comparison of regional cerebral blood flow with transcranial magnetic stimulation at different forces. J Appl Physiol (1985) 1996;81:596–603. doi: 10.1152/jappl.1996.81.2.596. [DOI] [PubMed] [Google Scholar]
- 58.Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Do AH, Wang PT, King CE, Chun SN, Nenadic Z. Brain-computer interface controlled robotic gait orthosis. J Neuroeng Rehabil. 2013;10:111. doi: 10.1186/1743-0003-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 61.Donati AR, Shokur S, Morya E, Campos DS, Moioli RC, Gitti CM, Augusto PB, Tripodi S, Pires CG, Pereira GA, Brasil FL, Gallo S, Lin AA, Takigami AK, Aratanha MA, Joshi S, Bleuler H, Cheng G, Rudolph A, Nicolelis MA. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci Rep. 2016;6:30383. doi: 10.1038/srep30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donoghue JP, Suner S, Sanes JN. Dynamic organization of primary motor cortex output to target muscles in adult rats. II. Rapid reorganization following motor nerve lesions. Exp Brain Res. 1990;79:492–503. doi: 10.1007/BF00229319. [DOI] [PubMed] [Google Scholar]
- 64.Drew GM, Siddall PJ, Duggan AW. Responses of spinal neurones to cutaneous and dorsal root stimuli in rats with mechanical allodynia after contusive spinal cord injury. Brain Res. 2001;893:59–69. doi: 10.1016/s0006-8993(00)03288-1. [DOI] [PubMed] [Google Scholar]
- 65.Dunlop SA. Activity-dependent plasticity: implications for recovery after spinal cord injury. Trends Neurosci. 2008;31:410–418. doi: 10.1016/j.tins.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6:712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 67.Edgerton VR, Kim SJ, Ichiyama RM, Gerasimenko YP, Roy RR. Rehabilitative therapies after spinal cord injury. J Neurotrauma. 2006;23:560–570. doi: 10.1089/neu.2006.23.560. [DOI] [PubMed] [Google Scholar]
- 68.Endo T, Spenger C, Tominaga T, Brene S, Olson L. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain. 2007;130:2951–2961. doi: 10.1093/brain/awm237. [DOI] [PubMed] [Google Scholar]
- 69.Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- 70.Farooque M, Hillered L, Holtz A, Olsson Y. Changes of extracellular levels of amino acids after graded compression trauma to the spinal cord: an experimental study in the rat using microdialysis. J Neurotrauma. 1996;13:537–548. doi: 10.1089/neu.1996.13.537. [DOI] [PubMed] [Google Scholar]
- 71.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 72.Fishman PS. Retrograde changes in the corticospinal tract of posttraumatic paraplegics. Arch Neurol. 1987;44:1082–1084. doi: 10.1001/archneur.1987.00520220078021. [DOI] [PubMed] [Google Scholar]
- 73.Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fitzsimmons NA, Lebedev MA, Peikon ID, Nicolelis MA. Extracting kinematic parameters for monkey bipedal walking from cortical neuronal ensemble activity. Front Integr Neurosci. 2009;3:3. doi: 10.3389/neuro.07.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fouad K, Dietz V, Schwab ME. Improving axonal growth and functional recovery after experimental spinal cord injury by neutralizing myelin associated inhibitors. Brain Res Brain Res Rev. 2001a;36:204–212. doi: 10.1016/s0165-0173(01)00096-0. [DOI] [PubMed] [Google Scholar]
- 76.Fouad K, Pedersen V, Schwab ME, Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol. 2001b;11:1766–1770. doi: 10.1016/s0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- 77.Fouad K, Tse A. Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol Res. 2008;30:17–27. doi: 10.1179/016164107X251781. [DOI] [PubMed] [Google Scholar]
- 78.Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- 79.Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates--re-examination and extension of behavioral data. Eur J Neurosci. 2009;29:983–996. doi: 10.1111/j.1460-9568.2009.06642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, Craggs M, Friston K, Thompson AJ. Disability, atrophy and cortical reorganization following spinal cord injury. Brain. 2011;134:1610–1622. doi: 10.1093/brain/awr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galea MP, Darian-Smith I. Corticospinal projection patterns following unilateral section of the cervical spinal cord in the newborn and juvenile macaque monkey. J Comp Neurol. 1997a;381:282–306. [PubMed] [Google Scholar]
- 82.Galea MP, Darian-Smith I. Manual dexterity and corticospinal connectivity following unilateral section of the cervical spinal cord in the macaque monkey. J Comp Neurol. 1997b;381:307–319. [PubMed] [Google Scholar]
- 83.Geisert EE, Jr, Bidanset DJ, Del Mar N, Robson JA. Up-regulation of a keratan sulfate proteoglycan following cortical injury in neonatal rats. Int J Dev Neurosci. 1996;14:257–267. doi: 10.1016/0736-5748(96)00012-3. [DOI] [PubMed] [Google Scholar]
- 84.Geoffroy CG, Lorenzana AO, Kwan JP, Lin K, Ghassemi O, Ma A, Xu N, Creger D, Liu K, He Z, Zheng B. Effects of PTEN and Nogo codeletion on corticospinal axon sprouting and regeneration in mice. J Neurosci. 2015;35:6413–6428. doi: 10.1523/JNEUROSCI.4013-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghosh A, Haiss F, Sydekum E, Schneider R, Gullo M, Wyss MT, Mueggler T, Baltes C, Rudin M, Weber B, Schwab ME. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci. 2010;13:97–104. doi: 10.1038/nn.2448. [DOI] [PubMed] [Google Scholar]
- 86.Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zorner B, Schneider R, Baltes C, Rudin M, Weber B, Schwab ME. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci. 2009;29:12210–12219. doi: 10.1523/JNEUROSCI.1828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goodman JH, Bingham WG, Jr, Hunt WE. Platelet aggregation in experimental spinal cord injury. Ultrastructural observations. Arch Neurol. 1979;36:197–201. doi: 10.1001/archneur.1979.00500400051006. [DOI] [PubMed] [Google Scholar]
- 88.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 89.Graziano A, Foffani G, Knudsen EB, Shumsky J, Moxon KA. Passive exercise of the hind limbs after complete thoracic transection of the spinal cord promotes cortical reorganization. PLoS One. 2013;8:e54350. doi: 10.1371/journal.pone.0054350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical sensorimotor reorganization after spinal cord injury: an electroencephalographic study. Neurology. 1998;50:1115–1121. doi: 10.1212/wnl.50.4.1115. [DOI] [PubMed] [Google Scholar]
- 91.Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical motor reorganization after paraplegia: an EEG study. Neurology. 1999;53:736–743. doi: 10.1212/wnl.53.4.736. [DOI] [PubMed] [Google Scholar]
- 92.Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, Henderson LA. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization. J Neurosci. 2012;32:14874–14884. doi: 10.1523/JNEUROSCI.1733-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gustin SM, Wrigley PJ, Siddall PJ, Henderson LA. Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb Cortex. 2010;20:1409–1419. doi: 10.1093/cercor/bhp205. [DOI] [PubMed] [Google Scholar]
- 94.Hachmann JT, Jeong JH, Grahn PJ, Mallory GW, Evertz LQ, Bieber AJ, Lobel DA, Bennet KE, Lee KH, Lujan JL. Large animal model for development of functional restoration paradigms using epidural and intraspinal stimulation. PLoS One. 2013;8:e81443. doi: 10.1371/journal.pone.0081443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hagg T. Collateral sprouting as a target for improved function after spinal cord injury. J Neurotrauma. 2006;23:281–294. doi: 10.1089/neu.2006.23.281. [DOI] [PubMed] [Google Scholar]
- 96.Hains BC, Johnson KM, Eaton MJ, Willis WD, Hulsebosch CE. Serotonergic neural precursor cell grafts attenuate bilateral hyperexcitability of dorsal horn neurons after spinal hemisection in rat. Neuroscience. 2003a;116:1097–1110. doi: 10.1016/s0306-4522(02)00729-7. [DOI] [PubMed] [Google Scholar]
- 97.Hains BC, Saab CY, Waxman SG. Changes in electrophysiological properties and sodium channel Nav1. 3 expression in thalamic neurons after spinal cord injury. Brain. 2005;128:2359–2371. doi: 10.1093/brain/awh623. [DOI] [PubMed] [Google Scholar]
- 98.Hains BC, Saab CY, Waxman SG. Alterations in burst firing of thalamic VPL neurons and reversal by Na(v)1. 3 antisense after spinal cord injury. J Neurophysiol. 2006;95:3343–3352. doi: 10.1152/jn.01009.2005. [DOI] [PubMed] [Google Scholar]
- 99.Hains BC, Willis WD, Hulsebosch CE. Temporal plasticity of dorsal horn somatosensory neurons after acute and chronic spinal cord hemisection in rat. Brain Res. 2003b;970:238–241. doi: 10.1016/s0006-8993(03)02347-3. [DOI] [PubMed] [Google Scholar]
- 100.Hall ED, Braughler JM. Effects of intravenous methylprednisolone on spinal cord lipid peroxidation and Na+ + K+)-ATPase activity. Dose-response analysis during 1st hour after contusion injury in the cat. J Neurosurg. 1982;57:247–253. doi: 10.3171/jns.1982.57.2.0247. [DOI] [PubMed] [Google Scholar]
- 101.Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harkema SJ, Hillyer J, Schmidt-Read M, Ardolino E, Sisto SA, Behrman AL. Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch Phys Med Rehabil. 2012;93:1588–1597. doi: 10.1016/j.apmr.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 103.Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology. 2014;82:104–113. doi: 10.1212/01.WNL.0000437416.34298.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hellenbrand DJ, Kaeppler KE, Hwang E, Ehlers ME, Toigo RD, Giesler JD, Vassar-Olsen ER, Hanna A. Basic techniques for long distance axon tracing in the spinal cord. Microsc Res Tech. 2013;76:1240–1249. doi: 10.1002/jemt.22291. [DOI] [PubMed] [Google Scholar]
- 105.Hofstetter CP, Schweinhardt P, Klason T, Olson L, Spenger C. Numb rats walk - a behavioural and fMRI comparison of mild and moderate spinal cord injury. Eur J Neurosci. 2003;18:3061–3068. doi: 10.1111/j.1460-9568.2003.03062.x. [DOI] [PubMed] [Google Scholar]
- 106.Hoogewoud F, Hamadjida A, Wyss AF, Mir A, Schwab ME, Belhaj-Saif A, Rouiller EM. Comparison of functional recovery of manual dexterity after unilateral spinal cord lesion or motor cortex lesion in adult macaque monkeys. Front Neurol. 2013;4:101. doi: 10.3389/fneur.2013.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hotz-Boendermaker S, Funk M, Summers P, Brugger P, Hepp-Reymond MC, Curt A, Kollias SS. Preservation of motor programs in paraplegics as demonstrated by attempted and imagined foot movements. Neuroimage. 2008;39:383–394. doi: 10.1016/j.neuroimage.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 108.Jain N, Catania KC, Kaas JH. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature. 1997;386:495–498. doi: 10.1038/386495a0. [DOI] [PubMed] [Google Scholar]
- 109.Jain N, Florence SL, Kaas JH. Limits on plasticity in somatosensory cortex of adult rats: hindlimb cortex is not reactivated after dorsal column section. J Neurophysiol. 1995;73:1537–1546. doi: 10.1152/jn.1995.73.4.1537. [DOI] [PubMed] [Google Scholar]
- 110.Jain N, Florence SL, Qi HX, Kaas JH. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc Natl Acad Sci U S A. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jain N, Qi HX, Collins CE, Kaas JH. Large-scale reorganization in the somatosensory cortex and thalamus after sensory loss in macaque monkeys. J Neurosci. 2008;28:11042–11060. doi: 10.1523/JNEUROSCI.2334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jain N, Qi HX, Kaas JH. Long-term chronic multichannel recordings from sensorimotor cortex and thalamus of primates. Prog Brain Res. 2001;130:64–72. [PubMed] [Google Scholar]
- 113.Jamme I, Petit E, Divoux D, Gerbi A, Maixent JM, Nouvelot A. Modulation of mouse cerebral Na+, K(+)-ATPase activity by oxygen free radicals. Neuroreport. 1995;7:333–337. [PubMed] [Google Scholar]
- 114.Jane JA, Evans JP, Fisher LE. An investigation concerning the restitution of motor function following injury to the spinal cord. J Neurosurg. 1964;21:167–171. doi: 10.3171/jns.1964.21.3.0167. [DOI] [PubMed] [Google Scholar]
- 115.Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12:67–79. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnson-Green PC, Dow KE, Riopelle RJ. Characterization of glycosaminoglycans produced by primary astrocytes in vitro. Glia. 1991;4:314–321. doi: 10.1002/glia.440040309. [DOI] [PubMed] [Google Scholar]
- 117.Kaas JH. Evolution of somatosensory and motor cortex in primates. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1148–1156. doi: 10.1002/ar.a.20120. [DOI] [PubMed] [Google Scholar]
- 118.Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kakulas BA. The clinical neuropathology of spinal cord injury. A guide to the future. Paraplegia. 1987;25:212–216. doi: 10.1038/sc.1987.37. [DOI] [PubMed] [Google Scholar]
- 120.Kambi N, Halder P, Rajan R, Arora V, Chand P, Arora M, Jain N. Large-scale reorganization of the somatosensory cortex following spinal cord injuries is due to brainstem plasticity. Nat Commun. 2014;5:3602. doi: 10.1038/ncomms4602. [DOI] [PubMed] [Google Scholar]
- 121.Kambi N, Tandon S, Mohammed H, Lazar L, Jain N. Reorganization of the primary motor cortex of adult macaque monkeys after sensory loss resulting from partial spinal cord injuries. J Neurosci. 2011;31:3696–3707. doi: 10.1523/JNEUROSCI.5187-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kanagal SG, Muir GD. Task-dependent compensation after pyramidal tract and dorsolateral spinal lesions in rats. Exp Neurol. 2009;216:193–206. doi: 10.1016/j.expneurol.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 123.Karlsson AK. Autonomic dysfunction in spinal cord injury: clinical presentation of symptoms and signs. Prog Brain Res. 2006;152:1–8. doi: 10.1016/S0079-6123(05)52034-X. [DOI] [PubMed] [Google Scholar]
- 124.Kennedy PR. Corticospinal, rubrospinal and rubro-olivary projections: a unifying hypothesis. Trends Neurosci. 1990;13:474–479. doi: 10.1016/0166-2236(90)90079-p. [DOI] [PubMed] [Google Scholar]
- 125.Kennedy PR, Humphrey DR. The compensatory role of the parvocellular division of the red nucleus in operantly conditioned rats. Neurosci Res. 1987;5:39–62. doi: 10.1016/0168-0102(87)90022-8. [DOI] [PubMed] [Google Scholar]
- 126.Khurana M, Walia S, Noohu MM. Study on the effectiveness of virtual reality game-based training on balance and functional performance in individuals with paraplegia. Top Spinal Cord Inj Rehabil. 2017;23:263–270. doi: 10.1310/sci16-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 128.King CE, Wang PT, Chui LA, Do AH, Nenadic Z. Operation of a brain-computer interface walking simulator for individuals with spinal cord injury. J Neuroeng Rehabil. 2013;10:77. doi: 10.1186/1743-0003-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.King CE, Wang PT, McCrimmon CM, Chou CC, Do AH, Nenadic Z. The feasibility of a brain-computer interface functional electrical stimulation system for the restoration of overground walking after paraplegia. J Neuroeng Rehabil. 2015;12:80. doi: 10.1186/s12984-015-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kizony R, Raz L, Katz N, Weingarden H, Weiss PLT. Video-capture virtual reality system for patients with paraplegic spinal cord injury. J Rehabil Res Dev. 2005;42:595–608. doi: 10.1682/jrrd.2005.01.0023. [DOI] [PubMed] [Google Scholar]
- 131.Knikou M. Functional reorganization of soleus H-reflex modulation during stepping after robotic-assisted step training in people with complete and incomplete spinal cord injury. Exp Brain Res. 2013;228:279–296. doi: 10.1007/s00221-013-3560-y. [DOI] [PubMed] [Google Scholar]
- 132.Kokotilo KJ, Eng JJ, Curt A. Reorganization and preservation of motor control of the brain in spinal cord injury: a systematic review. J Neurotrauma. 2009;26:2113–2126. doi: 10.1089/neu.2008.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krause JS, Kemp B, Coker J. Depression after spinal cord injury: relation to gender, ethnicity, aging, and socioeconomic indicators. Arch Phys Med Rehabil. 2000;81:1099–1109. doi: 10.1053/apmr.2000.7167. [DOI] [PubMed] [Google Scholar]
- 134.Kwak NS, Muller KR, Lee SW. A lower limb exoskeleton control system based on steady state visual evoked potentials. J Neural Eng. 2015;12:056009. doi: 10.1088/1741-2560/12/5/056009. [DOI] [PubMed] [Google Scholar]
- 135.Lacroix S, Havton LA, McKay H, Yang H, Brant A, Roberts J, Tuszynski MH. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J Comp Neurol. 2004;473:147–161. doi: 10.1002/cne.20051. [DOI] [PubMed] [Google Scholar]
- 136.Lassek AM. The pyramidal tract; speed of degeneration in axons following ablation of cells of origin in the monkey. J Comp Neurol. 1946;85:45–51. doi: 10.1002/cne.900850105. [DOI] [PubMed] [Google Scholar]
- 137.Lavrov I, Courtine G, Dy CJ, van den Brand R, Fong AJ, Gerasimenko Y, Zhong H, Roy RR, Edgerton VR. Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input. J Neurosci. 2008;28:7774–7780. doi: 10.1523/JNEUROSCI.1069-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968a;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 139.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968b;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- 140.Lebedev MA, Nicolelis MA. Brain-machine interfaces: from basic science to neuroprostheses and neurorehabilitation. Physiol Rev. 2017;97:767–837. doi: 10.1152/physrev.00027.2016. [DOI] [PubMed] [Google Scholar]
- 141.Lee DH, Lee JK. Animal models of axon regeneration after spinal cord injury. Neurosci Bull. 2013;29:436–444. doi: 10.1007/s12264-013-1365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee JH, Tigchelaar S, Liu J, Stammers AM, Streijger F, Tetzlaff W, Kwon BK. Lack of neuroprotective effects of simvastatin and minocycline in a model of cervical spinal cord injury. Exp Neurol. 2010;225:219–230. doi: 10.1016/j.expneurol.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 143.Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization. Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- 144.Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levy WJ, Jr, Amassian VE, Traad M, Cadwell J. Focal magnetic coil stimulation reveals motor cortical system reorganized in humans after traumatic quadriplegia. Brain Res. 1990;510:130–134. doi: 10.1016/0006-8993(90)90738-w. [DOI] [PubMed] [Google Scholar]
- 146.Li M, Shibata A, Li C, Braun PE, McKerracher L, Roder J, Kater SB, David S. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. J Neurosci Res. 1996;46:404–414. doi: 10.1002/(SICI)1097-4547(19961115)46:4<404::AID-JNR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 147.Li N, Yang Y, Glover DP, Zhang J, Saraswati M, Robertson C, Pelled G. Evidence for impaired plasticity after traumatic brain injury in the developing brain. J Neurotrauma. 2014;31:395–403. doi: 10.1089/neu.2013.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li Y, Raisman G. Sprouts from cut corticospinal axons persist in the presence of astrocytic scarring in long-term lesions of the adult rat spinal cord. Exp Neurol. 1995;134:102–111. doi: 10.1006/exnr.1995.1041. [DOI] [PubMed] [Google Scholar]
- 149.Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 150.Liu D, Xu GY, Pan E, McAdoo DJ. Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience. 1999;93:1383–1389. doi: 10.1016/s0306-4522(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 151.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lopez-Larraz E, Trincado-Alonso F, Rajasekaran V, Perez-Nombela S, Del-Ama AJ, Aranda J, Minguez J, Gil-Agudo A, Montesano L. Control of an ambulatory exoskeleton with a brain-machine interface for spinal cord injury gait rehabilitation. Front Neurosci. 2016;10:359. doi: 10.3389/fnins.2016.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lotze M, Laubis-Herrmann U, Topka H. Combination of TMS and fMRI reveals a specific pattern of reorganization in M1 in patients after complete spinal cord injury. Restor Neurol Neurosci. 2006;24:97–107. [PubMed] [Google Scholar]
- 155.Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- 156.Lynch M, Duffell L, Sandhu M, Srivatsan S, Deatsch K, Kessler A, Mitchell GS, Jayaraman A, Rymer WZ. Effect of acute intermittent hypoxia on motor function in individuals with chronic spinal cord injury following ibuprofen pretreatment: A pilot study. J Spinal Cord Med. 2017;40:295–303. doi: 10.1080/10790268.2016.1142137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mabon PJ, Weaver LC, Dekaban GA. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the integrin alphaD: a potential new anti-inflammatory treatment. Exp Neurol. 2000;166:52–64. doi: 10.1006/exnr.2000.7488. [DOI] [PubMed] [Google Scholar]
- 158.Manella KJ, Field-Fote EC. Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci. 2013;31:633–646. doi: 10.3233/RNN-120255. [DOI] [PubMed] [Google Scholar]
- 159.Martin JH. Systems neurobiology of restorative neurology and future directions for repair of the damaged motor systems. Clin Neurol Neurosurg. 2012;114:515–523. doi: 10.1016/j.clineuro.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 160.Masri R, Quiton RL, Lucas JM, Murray PD, Thompson SM, Keller A. Zona incerta: a role in central pain. J Neurophysiol. 2009;102:181–191. doi: 10.1152/jn.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McAdoo DJ, Xu GY, Robak G, Hughes MG. Changes in amino acid concentrations over time and space around an impact injury and their diffusion through the rat spinal cord. Exp Neurol. 1999;159:538–544. doi: 10.1006/exnr.1999.7166. [DOI] [PubMed] [Google Scholar]
- 162.McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 163.McKerracher L, Winton MJ. Nogo on the go. Neuron. 2002;36:345–348. doi: 10.1016/s0896-6273(02)01018-8. [DOI] [PubMed] [Google Scholar]
- 164.Means ED, Anderson DK. Neuronophagia by leukocytes in experimental spinal cord injury. J Neuropathol Exp Neurol. 1983;42:707–719. doi: 10.1097/00005072-198311000-00009. [DOI] [PubMed] [Google Scholar]
- 165.Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 166.Mikulis DJ, Jurkiewicz MT, McIlroy WE, Staines WR, Rickards L, Kalsi-Ryan S, Crawley AP, Fehlings MG, Verrier MC. Adaptation in the motor cortex following cervical spinal cord injury. Neurology. 2002;58:794–801. doi: 10.1212/wnl.58.5.794. [DOI] [PubMed] [Google Scholar]
- 167.Moore CI, Stern CE, Dunbar C, Kostyk SK, Gehi A, Corkin S. Referred phantom sensations and cortical reorganization after spinal cord injury in humans. Proc Natl Acad Sci U S A. 2000;97:14703–14708. doi: 10.1073/pnas.250348997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil. 2013;94:2297–2308. doi: 10.1016/j.apmr.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 169.Moxon KA, Oliviero A, Aguilar J, Foffani G. Cortical reorganization after spinal cord injury: always for good. Neuroscience. 2014;283:78–94. doi: 10.1016/j.neuroscience.2014.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Murray M, Wang SD, Goldberger ME, Levitt P. Modification of astrocytes in the spinal cord following dorsal root or peripheral nerve lesions. Exp Neurol. 1990;110:248–257. doi: 10.1016/0014-4886(90)90036-r. [DOI] [PubMed] [Google Scholar]
- 171.Nakagawa H, Ninomiya T, Yamashita T, Takada M. Reorganization of corticospinal tract fibers after spinal cord injury in adult macaques. Sci Rep. 2015;5:11986. doi: 10.1038/srep11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nakajima K, Maier MA, Kirkwood PA, Lemon RN. Striking differences in transmission of corticospinal excitation to upper limb motoneurons in two primate species. J Neurophysiol. 2000;84:698–709. doi: 10.1152/jn.2000.84.2.698. [DOI] [PubMed] [Google Scholar]
- 173.Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain. 1982;105:223–269. doi: 10.1093/brain/105.2.223. [DOI] [PubMed] [Google Scholar]
- 174.Navarrete-Opazo A, Alcayaga J, Sepulveda O, Rojas E, Astudillo C. Repetitive intermittent hypoxia and locomotor training enhances walking function in incomplete spinal cord injury subjects: a randomized, triple-blind, placebo-controlled clinical trial. J Neurotrauma. 2017;34:1803–1812. doi: 10.1089/neu.2016.4478. [DOI] [PubMed] [Google Scholar]
- 175.Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 176.Ness TJ, San Pedro EC, Richards JS, Kezar L, Liu HG, Mountz JM. A case of spinal cord injury-related pain with baseline rCBF brain SPECT imaging and beneficial response to gabapentin. Pain. 1998;78:139–143. doi: 10.1016/S0304-3959(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 177.Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- 178.Nout YS, Rosenzweig ES, Brock JH, Strand SC, Moseanko R, Hawbecker S, Zdunowski S, Nielson JL, Roy RR, Courtine G, Ferguson AR, Edgerton VR, Beattie MS, Bresnahan JC, Tuszynski MH. Animal models of neurologic disorders: a nonhuman primate model of spinal cord injury. Neurotherapeutics. 2012;9:380–392. doi: 10.1007/s13311-012-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Onodera S, Hicks TP. A comparative neuroanatomical study of the red nucleus of the cat, macaque and human. PLoS One. 2009;4:e6623. doi: 10.1371/journal.pone.0006623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol. 2012;590:3647–3663. doi: 10.1113/jphysiol.2012.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oza CS, Giszter SF. Plasticity and alterations of trunk motor cortex following spinal cord injury and non-stepping robot and treadmill training. Exp Neurol. 2014;256:57–69. doi: 10.1016/j.expneurol.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pernet U, Hepp-Reymond MC. Retrograde degeneration of the pyramidal cells in the motor cortex of apes (Macaca fascicularis) Acta Anat (Basel) 1975;91:552–561. [PubMed] [Google Scholar]
- 183.Pfurtscheller G, Muller GR, Pfurtscheller J, Gerner HJ, Rupp R. ‘Thought’ - control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett. 2003;351:33–36. doi: 10.1016/s0304-3940(03)00947-9. [DOI] [PubMed] [Google Scholar]
- 184.Pfurtscheller J, Rupp R, Muller GR, Fabsits E, Korisek G, Gerner HJ, Pfurtscheller G. Functional electrical stimulation instead of surgery. Improvement of grasping function with FES in a patient with C5 tetraplegia? Unfallchirurg. 2005;108:587–590. doi: 10.1007/s00113-004-0876-x. [DOI] [PubMed] [Google Scholar]
- 185.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 186.Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 187.Puckett WR, Hiester ED, Norenberg MD, Marcillo AE, Bunge RP. The astroglial response to Wallerian degeneration after spinal cord injury in humans. Exp Neurol. 1997;148:424–432. doi: 10.1006/exnr.1997.6692. [DOI] [PubMed] [Google Scholar]
- 188.Qi HX, Reed JL, Gharbawie OA, Burish MJ, Kaas JH. Cortical neuron response properties are related to lesion extent and behavioral recovery after sensory loss from spinal cord injury in monkeys. J Neurosci. 2014;34:4345–4363. doi: 10.1523/JNEUROSCI.4954-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 190.Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J Neurosci. 2000;20:4435–4445. doi: 10.1523/JNEUROSCI.20-12-04435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Roosink M, Robitaille N, Jackson PL, Bouyer LJ, Mercier C. Interactive virtual feedback improves gait motor imagery after spinal cord injury: An exploratory study. Restor Neurol Neurosci. 2016;34:227–235. doi: 10.3233/RNN-150563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rose FD, Brooks BM, Attree EA, Parslow DM, Leadbetter AG, McNeil JE, Jayawardena S, Greenwood R, Potter J. A preliminary investigation into the use of virtual environments in memory retraining after vascular brain injury: indications for future strategy. Disabil Rehabil. 1999;21:548–554. doi: 10.1080/096382899297206. [DOI] [PubMed] [Google Scholar]
- 193.Rosenzweig ES, Brock JH, Culbertson MD, Lu P, Moseanko R, Edgerton VR, Havton LA, Tuszynski MH. Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys. J Comp Neurol. 2009;513:151–163. doi: 10.1002/cne.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS, Havton LA, Bresnahan JC, Edgerton VR, Tuszynski MH. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rosich K, Hanna B, Ibrahim RK, Hellenbrand DJ, Hanna A. The effects of glial cell line-derived neurotrophic factor after spinal cord injury. J Neurotrauma. 2017;34:3311–3325. doi: 10.1089/neu.2017.5175. [DOI] [PubMed] [Google Scholar]
- 196.Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. Prog Brain Res. 1999;123:349–365. doi: 10.1016/s0079-6123(08)62870-8. [DOI] [PubMed] [Google Scholar]
- 197.Royer AS, Rose ML, He B. Goal selection versus process control while learning to use a brain-computer interface. J Neural Eng. 2011;8:036012. doi: 10.1088/1741-2560/8/3/036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sandler AN, Tator CH. Review of the effect of spinal cord trama on the vessels and blood flow in the spinal cord. J Neurosurg. 1976;45:638–646. doi: 10.3171/jns.1976.45.6.0638. [DOI] [PubMed] [Google Scholar]
- 199.Schmidlin E, Wannier T, Bloch J, Rouiller EM. Progressive plastic changes in the hand representation of the primary motor cortex parallel incomplete recovery from a unilateral section of the corticospinal tract at cervical level in monkeys. Brain Res. 2004;1017:172–183. doi: 10.1016/j.brainres.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 200.Seminowicz DA, Jiang L, Ji Y, Xu S, Gullapalli RP, Masri R. Thalamocortical asynchrony in conditions of spinal cord injury pain in rats. J Neurosci. 2012;32:15843–15848. doi: 10.1523/JNEUROSCI.2927-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, Murray B, Jung V, Pepinsky RB, Mi S. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 202.Sharif-Alhoseini M, Khormali M, Rezaei M, Safdarian M, Hajighadery A, Khalatbari MM, Safdarian M, Meknatkhah S, Rezvan M, Chalangari M, Derakhshan P, Rahimi-Movaghar V. Animal models of spinal cord injury: a systematic review. Spinal Cord. 2017;55:714–721. doi: 10.1038/sc.2016.187. [DOI] [PubMed] [Google Scholar]
- 203.Shokur S, Gallo S, Moioli RC, Donati AR, Morya E, Bleuler H, Nicolelis MA. Assimilation of virtual legs and perception of floor texture by complete paraplegic patients receiving artificial tactile feedback. Sci Rep. 2016;6:32293. doi: 10.1038/srep32293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50:798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 205.Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 206.Siegel CS, Fink KL, Strittmatter SM, Cafferty WB. Plasticity of intact rubral projections mediates spontaneous recovery of function after corticospinal tract injury. J Neurosci. 2015;35:1443–1457. doi: 10.1523/JNEUROSCI.3713-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 208.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 209.Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996;729:127–131. [PubMed] [Google Scholar]
- 210.Smith AC, Mummidisetty CK, Rymer WZ, Knikou M. Locomotor training alters the behavior of flexor reflexes during walking in human spinal cord injury. J Neurophysiol. 2014;112:2164–2175. doi: 10.1152/jn.00308.2014. [DOI] [PubMed] [Google Scholar]
- 211.Smith PM, Jeffery ND. Spinal shock--comparative aspects and clinical relevance. J Vet Intern Med. 2005;19:788–793. doi: 10.1892/0891-6640(2005)19[788:ssaacr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 212.Starkey ML, Bartus K, Barritt AW, Bradbury EJ. Chondroitinase ABC promotes compensatory sprouting of the intact corticospinal tract and recovery of forelimb function following unilateral pyramidotomy in adult mice. Eur J Neurosci. 2012;36:3665–3678. doi: 10.1111/ejn.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Stelzner DJ, Cullen JM. Do propriospinal projections contribute to hindlimb recovery when all long tracts are cut in neonatal or weanling rats. Exp Neurol. 1991;114:193–205. doi: 10.1016/0014-4886(91)90036-c. [DOI] [PubMed] [Google Scholar]
- 214.Stys PK. Anoxic and ischemic injury of myelinated axons in CNS white matter: from mechanistic concepts to therapeutics. J Cereb Blood Flow Metab. 1998;18:2–25. doi: 10.1097/00004647-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 215.Tandon S, Kambi N, Lazar L, Mohammed H, Jain N. Large-scale expansion of the face representation in somatosensory areas of the lateral sulcus after spinal cord injuries in monkeys. J Neurosci. 2009;29:12009–12019. doi: 10.1523/JNEUROSCI.2118-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H, Takatsuki K. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 217.Tator CH. Review of experimental spinal cord injury with emphasis on the local and systemic circulatory effects. Neurochirurgie. 1991;37:291–302. [PubMed] [Google Scholar]
- 218.Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. discussion 982-987. [DOI] [PubMed] [Google Scholar]
- 219.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 220.Taylor BK, Akana SF, Peterson MA, Dallman MF, Basbaum AI. Pituitary-adrenocortical responses to persistent noxious stimuli in the awake rat: endogenous corticosterone does not reduce nociception in the formalin test. Endocrinology. 1998;139:2407–2413. doi: 10.1210/endo.139.5.5993. [DOI] [PubMed] [Google Scholar]
- 221.ten Donkelaar HJ. Evolution of the red nucleus and rubrospinal tract. Behav Brain Res. 1988;28:9–20. doi: 10.1016/0166-4328(88)90072-1. [DOI] [PubMed] [Google Scholar]
- 222.Thallmair M, Metz GA, Z’Graggen WJ, Raineteau O, Kartje GL, Schwab ME. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- 223.Theisen CC, Sachdeva R, Austin S, Kulich D, Kranz V, Houle JD. Exercise and peripheral nerve grafts as a strategy to promote regeneration after acute or chronic spinal cord injury. J Neurotrauma. 2017;34:1909–1914. doi: 10.1089/neu.2016.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.To CS, Kobetic R, Bulea TC, Audu ML, Schnellenberger JR, Pinault G, Triolo RJ. Sensor-based hip control with hybrid neuroprosthesis for walking in paraplegia. J Rehabil Res Dev. 2014;51:229–244. doi: 10.1682/JRRD.2012.10.0190. [DOI] [PubMed] [Google Scholar]
- 225.Toborek M, Malecki A, Garrido R, Mattson MP, Hennig B, Young B. Arachidonic acid-induced oxidative injury to cultured spinal cord neurons. J Neurochem. 1999;73:684–692. doi: 10.1046/j.1471-4159.1999.0730684.x. [DOI] [PubMed] [Google Scholar]
- 226.Topka H, Cohen LG, Cole RA, Hallett M. Reorganization of corticospinal pathways following spinal cord injury. Neurology. 1991;41:1276–1283. doi: 10.1212/wnl.41.8.1276. [DOI] [PubMed] [Google Scholar]
- 227.Tran Y, Boord P, Middleton J, Craig A. Levels of brain wave activity (8-13 Hz) in persons with spinal cord injury. Spinal Cord. 2004;42:73–79. doi: 10.1038/sj.sc.3101543. [DOI] [PubMed] [Google Scholar]
- 228.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26:163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- 229.Turner JA, Lee JS, Schandler SL, Cohen MJ. An fMRI investigation of hand representation in paraplegic humans. Neurorehabil Neural Repair. 2003;17:37–47. doi: 10.1177/0888439002250443. [DOI] [PubMed] [Google Scholar]
- 230.van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 231.Van Meeteren NL, Eggers R, Lankhorst AJ, Gispen WH, Hamers FP. Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J Neurotrauma. 2003;20:1029–1037. doi: 10.1089/089771503770195876. [DOI] [PubMed] [Google Scholar]
- 232.Vavrek R, Girgis J, Tetzlaff W, Hiebert GW, Fouad K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–1545. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- 233.Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Villiger M, Bohli D, Kiper D, Pyk P, Spillmann J, Meilick B, Curt A, Hepp-Reymond MC, Hotz-Boendermaker S, Eng K. Virtual reality-augmented neurorehabilitation improves motor function and reduces neuropathic pain in patients with incomplete spinal cord injury. Neurorehabil Neural Repair. 2013;27:675–683. doi: 10.1177/1545968313490999. [DOI] [PubMed] [Google Scholar]
- 235.Wall JT, Xu J, Wang X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Brain Res Rev. 2002;39:181–215. doi: 10.1016/s0165-0173(02)00192-3. [DOI] [PubMed] [Google Scholar]
- 236.Wall PD, Egger MD. Formation of new connexions in adult rat brains after partial deafferentation. Nature. 1971;232:542–545. doi: 10.1038/232542a0. [DOI] [PubMed] [Google Scholar]
- 237.Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- 238.Wang PT, King CE, Chui LA, Do AH, Nenadic Z. Self-paced brain-computer interface control of ambulation in a virtual reality environment. J Neural Eng. 2012;9:056016. doi: 10.1088/1741-2560/9/5/056016. [DOI] [PubMed] [Google Scholar]
- 239.Wannier T, Schmidlin E, Bloch J, Rouiller EM. A unilateral section of the corticospinal tract at cervical level in primate does not lead to measurable cell loss in motor cortex. J Neurotrauma. 2005;22:703–717. doi: 10.1089/neu.2005.22.703. [DOI] [PubMed] [Google Scholar]
- 240.Watson C, Paxinos G, Kayalioglu G, Heise C. The Spinal Cord. San Diego, CA, USA: Elsevier Associated Press; 2009. Atlas of the rat spinal cord; pp. 238–307. [Google Scholar]
- 241.Weaver LC, Marsh DR, Gris D, Brown A, Dekaban GA. Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention. Prog Brain Res. 2006;152:245–263. doi: 10.1016/S0079-6123(05)52016-8. [DOI] [PubMed] [Google Scholar]
- 242.Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Whitt JL, Masri R, Pulimood NS, Keller A. Pathological activity in mediodorsal thalamus of rats with spinal cord injury pain. J Neurosci. 2013;33:3915–3926. doi: 10.1523/JNEUROSCI.2639-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Williams B, Terry AF, Jones F, McSweeney T. Syringomyelia as a sequel to traumatic paraplegia. Paraplegia. 1981;19:67–80. doi: 10.1038/sc.1981.18. [DOI] [PubMed] [Google Scholar]
- 245.Williams PT, Kim S, Martin JH. Postnatal maturation of the red nucleus motor map depends on rubrospinal connections with forelimb motor pools. J Neurosci. 2014;34:4432–4441. doi: 10.1523/JNEUROSCI.5332-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wolpaw JR. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol (Oxf) 2007;189:155–169. doi: 10.1111/j.1748-1716.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- 247.Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, Siddall PJ, Henderson LA. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009a;19:224–232. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- 248.Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009b;141:52–59. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 249.Wydenkeller S, Maurizio S, Dietz V, Halder P. Neuropathic pain in spinal cord injury: significance of clinical and electrophysiological measures. Eur J Neurosci. 2009;30:91–99. doi: 10.1111/j.1460-9568.2009.06801.x. [DOI] [PubMed] [Google Scholar]
- 250.Xu GY, McAdoo DJ, Hughes MG, Robak G, de Castro R., Jr Considerations in the determination by microdialysis of resting extracellular amino acid concentrations and release upon spinal cord injury. Neuroscience. 1998;86:1011–1021. doi: 10.1016/s0306-4522(98)00063-3. [DOI] [PubMed] [Google Scholar]
- 251.Xu J, Wall JT. Functional organization of tactile inputs from the hand in the cuneate nucleus and its relationship to organization in the somatosensory cortex. J Comp Neurol. 1999;411:369–389. [PubMed] [Google Scholar]
- 252.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yamamoto T, Yamasaki M, Imai T. Retrograde pyramidal tract degeneration in a patient with cervical haematomyelia. J Neurol Neurosurg Psychiatry. 1989;52:382–386. doi: 10.1136/jnnp.52.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135:2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Z’Graggen WJ, Metz GA, Kartje GL, Thallmair M, Schwab ME. Functional recovery and enhanced corticofugal plasticity after unilateral pyramidal tract lesion and blockade of myelin-associated neurite growth inhibitors in adult rats. J Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang N, Fang M, Chen H, Gou F, Ding M. Evaluation of spinal cord injury animal models. Neural Regen Res. 2014;9:2008–2012. doi: 10.4103/1673-5374.143436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zorner B, Bachmann LC, Filli L, Kapitza S, Gullo M, Bolliger M, Starkey ML, Rothlisberger M, Gonzenbach RR, Schwab ME. Chasing central nervous system plasticity: the brainstem’s contribution to locomotor recovery in rats with spinal cord injury. Brain. 2014;137:1716–1732. doi: 10.1093/brain/awu078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


