Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and prion disease are representative neurodegenerative diseases that share common sub-cellular features, the most obvious of which is a strong association with the accumulation of misfolded, aggregated, and insoluble forms of proteins in the brain, as evidenced in post-mortem brain tissues of patients.
Diagnosis of neurodegenerative diseases is often made by exclusion, and it is made when individuals are at the advanced stage of disease with a brain that is markedly damaged. Also, neurodegenerative diseases are not timely and effectively treated, and current treatments are limited to a small number of drugs that control some of the symptoms of early disease. This scenario emphasizes the need for alternative diagnostic and therapeutic approaches.
Profiles of altered microRNAs (miRNAs) (reflected by increase or decrease of miRNAs expression level with respect to the normal level) isolated from blood exosomes associated with neurodegenerative disorders suggest the possibility of discovering new potential candidate biomarkers for early diagnosis of neurodegenerative diseases (Van Giau and An, 2016). miRNAs, which are small non-coding RNAs that can post-transcriptionally regulate gene expression, are highly abundant in exosomes. In clinical setting, exosomes are frequently proposed as therapeutic drug carriers, but, since the release of exosomes and their molecular cargo are cell type specific, exosomes have been also proposed as potential biomarkers for disease diagnosis and monitoring (Lin et al., 2015).
Thus, exosome-based approaches aimed at targeting dysregulated miRNAs or identifying them in blood may be adopted as alternative strategies that might facilitate and anticipate the therapy and diagnosis, respectively, of neurodegenerative diseases. Figure 1 summarizes the potential fields of clinical application of blood exosomes and miRNA to the neurodegenerative disorders.
Figure 1.
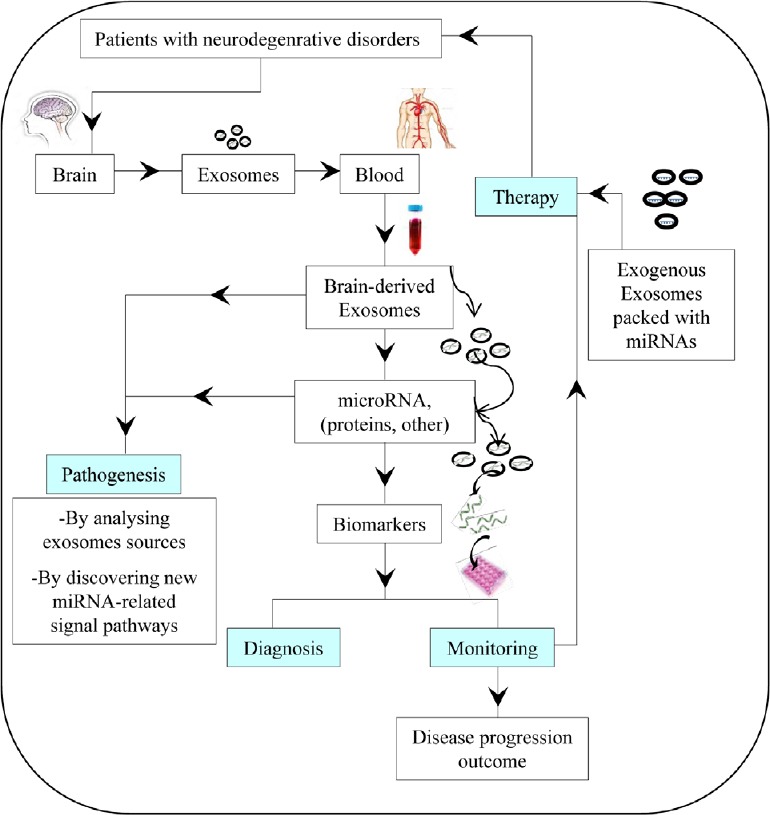
Potential fields of clinical application of exosomes and microRNA (miRNA) to the neurodegenerative diseases.
miRNAs as potential therapeutic agents for treating neurodegenerative diseases: Recently, together with a colleague, I explored the feasibility of the therapy of neurodegenerative diseases with miRNA through an extensive review of the literature concerning this specific topic (Ridolfi and Abdel-Haq, 2017). First, we performed a rapid overview of miRNAs involved in the pathogenesis of the above mentioned neurodegenerative diseases to underline the extent of this involvement and its crucial role. We found that multiple miRNAs are usually involved in the pathogenesis of neurodegeneration, and they often concern different signal pathways, but; sometimes they act on the same pathway modulating different down or upstream targets. On the other side, a single miRNA sequence can simultaneously affect multiple pathways related to a single pathology, such as miR-196a and miR-22 in Huntington’s disease [for details see Ridolfi and Abdel-Haq (2017)].
After this brief overview, we analyzed the experimental models performed till now using miRNAs (miRNA mimics, miRNA inhibitors or artificial miRNAs for miR-155, miR-146a, miR-124 or miR-7) to modulate the endogenous levels of individual miRNAs in Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease, thereby evaluating the pharmacologic feasibility of this approach for treating neurodegenerative diseases. Our analysis led to the identification of the inflammatory pathway as a potential target for the therapy of neurodegenerative diseases. In fact, the presence of an immune and inflammatory response modulated by key inflammatory miRNAs (for example, miRs 155, 146a and 124) has been observed in all the examined neurodegenerative diseases. Besides, this response was present throughout the disease but with a different extent and role during the different disease stages, suggesting the existence of a neuroinflammatory response modulated by distinct disease-stage miRNAs. The analysis also highlighted that, similarly to conventional therapeutics, miRNAs effect can be modulated by a structure-activity relationship, which could negatively influence the miRNA pharmacokinetic and pharmacodynamic profiles.
The most critical points that emerged during this analysis are mainly represented by the lack of adequate characterization of the secondary effects produced by miRNA-based therapies, and by the lack of basic data on the pharmacological properties of miRNA mimics and antagonists. Suitable pharmaco- and toxico-kinetics that focus on the tissue distribution and pharmacology safety are also needed to determine the superiority of miRNAs in terms of efficacy and safety relative to analogous conventional therapies.
A careful optimization and worldwide standardization of the miRNA detection methods and protocols would overcome many of the contradictions and inconsistencies observed in the literature data concerning the identification of dysregulated miRNAs related to neurodegenerations. Also, disease-stage studies for assessing miRNAs levels, as well as the availability and the choice of appropriate animal/cellular models for validating the efficacy of miRNA drugs, might appreciably facilitate the clinical application of miRNA to the therapy of neurodegenerative diseases.
Thus, there are challenges in the clinical application of miRNA-based therapy to neurodegenerative diseases. One more challenge is the availability of an effective drug delivery system to the brain that can guarantee high treatment efficacy and can increase patient compliance. Exosomes, which are small extracellular vesicles that have the intrinsic ability to traverse biological barriers and to transport functional small RNAs between cells naturally, are considered as an optimal delivery vehicle for miRNA. Efficient exosome-mediated delivery of miRNA and the feasibility of loading miRNA mimics, miRNA inhibitors, and shRNA expressing plasmids into exosomes are well-supported by proof-of-concept studies (El-Andaloussi et al., 2012; Lee et al., 2017).
Are exosomes a possible key step toward the clinical application of miRNAs to the therapy of neurodegenerative diseases? Exosomes are attractive for the delivery of miRNA for several reasons (Ridolfi and Abdel-Haq, 2017) but especially because they can prevent miRNAs degradation by RNase, thus allowing their stable circulation in the bloodstream and other fluids and tissues. Free miRNAs are degraded immediately and completely by RNase. Nevertheless, there are some challenges that can influence the loading efficiency of exosomes in miRNA, as well as the quantity of miRNA that reaches the brain (Ridolfi and Abdel-Haq, 2017).
Therefore, for an efficient treatment of neurodegenerative diseases, improved or innovative approaches/strategies are needed that enable a better implementation of exosomes and further increase and optimize their loading efficiency for efficient delivery of miRNAs to the central nervous system.
In this respect, System Biosciences (SBI) has developed a new exosome-loading system in which a specific RNA sequence tag “XMotif” has been fused to a miRNA (XMIRs) or anti-miRNA (AXMIRs) sequence by using a designed oligo (SBI: http://www.systembio.com). After transfection into cells, the XMotif sequence drives the selective encapsulation of the small RNA into exosome. This latter can be delivered to target cells after isolation. Such an approach would significantly increase the amount of miRNA incorporated and delivered to the site of action.
miRNA stability and, consequently, duration of its activity and efficacy can be increased by binding of miRNA to specific miRNA-binding proteins [such as nucleophosmin 1 (NPM1)] at the level of the adenylate/uridylate-rich elements (AREs) structure; this protects the miRNA from degradation before loading into exosomes. Empty synthetic exosomes or engineered exosomes packaged with miRNA-binding protein complex can be delivered to target cells. This approach would significantly improve the quantity and quality of miRNA reaching the site of action due to the double protection offered by the miRNA-binding protein and the exosomes themselves.
Delivering miRNA-containing exosomes secreted by differentiated stem cells is another powerful approach that would increase and improve the delivery of miRNA to target cells. After transfection of stem cells with the therapeutic miRNA and differentiation into the desired precursor cells, exosomes secreted by miRNA-overexpressing precursor cells can be delivered to target cells where they transfer their content, including the therapeutic miRNA. This approach has several advantages over transplantation of stem cells and can mitigate or even eliminate many of the safety concerns and limitations associated with transplantation. However, for technical, ethical, and legal issues, this promising approach will not be immediate and might advance to clinical applications within the next few years.
miRNA-based therapy should be optimized to achieve the highest efficacy but avoiding many of the side effects caused by both primary pharmacodynamics (action on the pharmacologic target) and secondary pharmacodynamics (off-target effects), where these latter represent the main limitation of miRNA-based therapy. Such an optimization could be performed by monitoring treatment efficacy and disease progression. The possibility to monitor treatment efficacy offers the opportunity to adjust treatment protocol or change therapeutic strategy as early as possible, when necessary.
Thus, as a next step or in parallel to miRNA-based therapy, efforts should focus on providing a valid means/tool for monitoring response to treatment/disease progression. Exosomes are the elective candidates for this purpose since they are now largely known as an enriched source of functional biomarkers for several diseases, including brain disorders and neurodegenerative diseases (Lin et al., 2015).
Future prospects: blood exosomes as biomarkers for monitoring treatment outcome and disease progression: Monitoring the response to therapy for brain disorders is hampered by the fact that the core pathology lies in the brain, hidden from a direct study in living patients. Thus, monitoring therapy outcome by using brain-derived exosomes from tissues or biofluids outside the brain would be a great advance for the therapy of brain disorders.
Exosomes are secreted by most cell types, including neuronal cells in vivo and in vitro and have been found in various biofluids including blood, urine, amniotic fluid, breast milk, malignant ascites fluid, and cerebrospinal fluid (Lin et al., 2015). Exosomes contain distinct subsets of RNAs and proteins depending upon the cell type from which they are secreted, making them useful as an enriched source of molecules for biomarker discovery and profiling. Furthermore, since a drug target is part of a signal pathway and, in many cases, it is also related to the biomarker of the disease, exosomes also represent a valuable means for monitoring therapy efficacy and disease progression.
Additionally, toxic forms of α-synuclein, amyloid beta, and prion protein, which are hallmarks of Parkinson’s disease, Alzheimer’s disease, and prion diseases, respectively, have been shown to be effectively packaged into exosomes that had been isolated from the respective disease-associated material (Quek and Hill, 2017).
Using biofluids as a source of brain-derived exosomes for monitoring the response to treatment for neurodegenerative diseases is preferred, and the cerebrospinal fluid is the best biofluid for this purpose due to its proximity to the brain. However, cerebrospinal fluid is not an ideal specimen for routine monitoring due to the invasive nature of its collection by lumbar puncture, and therefore blood is preferred for such purposes.
Blood, besides being routinely available and much less invasive than cerebrospinal fluid, is similarly rich in brain-derived exosomes. Additionally, the feasibility and reliability of using blood exosomes as a source of brain-derived biomarkers for Alzheimer’s and other neurodegenerative diseases have already been assessed by several proof-of-concept studies (Fiandaca et al., 2015; Kapogiannis et al., 2015; Winston et al., 2016; Mustapic et al., 2017).
Using blood, exosomes can be collected noninvasively over a long period, allowing for continuous monitoring of disease progression and response to therapy. Besides, blood exosomes are abundant and can be preserved through several freeze-and-thaw cycles.
Growing evidence indicates that the content of exosomes secreted by neuronal cells in blood can reflect the health-state of their originating cells/tissues. Indeed, several Alzheimer’s disease-related proteins such as amyloid beta, tau and phosphorylated tau, and amyloid precursor protein and its cleavage products have been shown to integrate and secrete within blood exosomes from neuronal cells (Fiandaca et al., 2015; Quek and Hill, 2017). Additionally, it has been found that blood exosomes contain proteins such as cathepsin D, lysosome-associated membrane protein 1, ubiquitinylated proteins, and heat shock protein 70, which levels are altered in preclinical Alzheimer’s disease years before disease onset (Goetzl et al., 2015). Interestingly, Kapogiannis et al. (2015) found that the ratio of phospho (P)-serine-type 1 insulin receptor substrate (P-serine312-IRS-1) to P-pan-tyrosine-IRS-1 in neural-derived blood exosomes could predict the development of Alzheimer’s disease (up to 10 years before Alzheimer’s disease onset) in type 2 diabetic patients. While, plasma exosomes from Parkinson’s disease patients contained alpha-synuclein (Shi et al., 2014).
Monitoring action can be performed by assessing the extent of the level of restoration of distinct molecules after treatment. For example, it is possible to monitor the levels of the dysregulated miRNA since it has been reported that transferred miRNA might be encoded in the cargo of the exosomes of the recipient (target) cells. Otherwise, it is possible to monitor the levels of molecules regulated by the dysregulated miRNA or molecules closely correlated with the pathological mechanism (hallmark biomarkers).
The fact that exosomes express markers that allow their tracking to the cell of origin makes the use of exosomes for diagnosis and monitoring purposes particularly appealing.
Conclusions: Optimization of miRNA-based therapy for neurodegenerative diseases by using blood exosomes as a tool for monitoring both clinical and treatment outcomes might represent a considerable challenge but offers opportunities for a wide range of brain diseases (e.g., neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and prions) to be timely and effectively treated with the most suitable treatment and approach. This could represent a key step toward a correct and efficient clinical application of miRNAs.
Furthermore, this approach would reduce the global burden and the costs of care for these diseases. Moreover, this approach might allow for studying the underlying mechanisms involved, but not previously associated with neurodegenerative diseases. Finally, neural-derived blood exosomes would make brain content (of limited accessibility) available within the readily accessible blood.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJ. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc. 2012;7:2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 2.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015;11:600–607.e1. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Kapogiannis D. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85:40–47. doi: 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015;29:589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee ST, Im W, Ban JJ, Lee M, Jung KH, Lee SK, Chu K, Kim M. Exosome-based delivery of miR-124 in a Huntington’s disease model. J Mov Disord. 2017;10:45–52. doi: 10.14802/jmd.16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J, Li J, Huang B, Liu J, Chen X, Chen XM, Xu YM, Huang LF, Wang XZ. Exosomes: novel biomarkers for clinical diagnosis. Scientific World Journal 2015. 2015:657086. doi: 10.1155/2015/657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustapic M, Eitan E, Werner JK, Jr, Berkowitz ST, Lazaropoulos MP, Tran J, Goetzl EJ, Kapogiannis D. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci. 2017;11:278. doi: 10.3389/fnins.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quek C, Hill AF. The role of extracellular vesicles in neurodegenerative diseases. Biochem Biophys Res Commun. 2017;483:1178–1186. doi: 10.1016/j.bbrc.2016.09.090. [DOI] [PubMed] [Google Scholar]
- 9.Ridolfi B, Abdel-Haq H. Neurodegenerative disorders treatment: the microRNA role. Curr Gene Ther. 2017;17:327–363. doi: 10.2174/1566523218666180119120726. [DOI] [PubMed] [Google Scholar]
- 10.Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Banks WA, Zhang J. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014;128:639–650. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Giau V, An SS. Emergence of exosomal miRNAs as a diagnostic biomarker for Alzheimer’s disease. J Neurol Sci. 2016;360:141–152. doi: 10.1016/j.jns.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, Masliah E, Rissman RA. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 2016;3:63–72. doi: 10.1016/j.dadm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


