Abstract
The impact of payment-for-performance (P4P) schemes in the health sector has been documented, but there has been little attention to the distributional effects of P4P across health facilities. We examined the distribution of P4P payouts over time and assessed whether increased service coverage due to P4P differed across facilities in Tanzania. We used two service outcomes that improved due to P4P [facility-based deliveries and provision of antimalarials during antenatal care (ANC)], to also assess whether incentive design matters for performance inequalities. We used data from 150 facilities from intervention and comparison areas in January 2012 and 13 months later. Our primary data were gathered through facility survey and household survey, while data on performance payouts were obtained from the programme administrator. Descriptive inequality measures were used to examine the distribution of payouts across facility subgroups. Difference-in-differences regression analyses were used to identify P4P differential effects on the two service coverage outcomes across facility subgroups. We found that performance payouts were initially higher among higher-level facilities (hospitals and health centres) compared with dispensaries, among facilities with more medical commodities and among facilities serving wealthier populations, but these inequalities declined over time. P4P had greater effects on coverage of institutional deliveries among facilities with low baseline performance, serving middle wealth populations and located in rural areas. P4P effects on antimalarials provision during ANC was similar across facilities. Performance inequalities were influenced by the design of incentives and a range of facility characteristics; however, the nature of the service being targeted is also likely to have affected provider response. Further research is needed to examine in more detail the effects of incentive design on outcomes and researchers should be encouraged to report on design aspects in their evaluations of P4P and systematically monitor and report subgroup effects across providers.
Keywords: Payment-for-performance, inequality, impact evaluation, incentive design, Tanzania
Key Messages
Inequality in payouts favoured better-off facilities, but declined over time.
Lower baseline performers improved most on institutional deliveries coverage.
Rural and middle wealth facilities improved most on deliveries coverage.
Performance on antimalarial provision was similar across facilities.
Introduction
Payment-for-Performance (P4P) programmes, involving financial incentives (payouts) to healthcare workers and healthcare facility for achievement of pre-defined performance outcomes, are aimed at improving the quality of care and, especially in low- and middle-income countries (LMICs) are aimed to increase service coverage and strengthen health systems more generally (Meessen et al., 2011; Witter et al., 2013). The measured effects of P4P on healthcare coverage and quality are mixed across programmes and settings (Gillam et al., 2012; Witter et al., 2012; Eijkenaar et al., 2013; Das et al., 2016; Renmans et al., 2016; Mendelson et al., 2017).
To date, most evaluations of P4P schemes have largely focused on average programme effects, and paid less attention to how this remuneration system affect the distribution of programme effects (Markovitz and Ryan, 2016; Sherry et al., 2017). The heterogeneity of P4P effects on service use among populations have been documented in the literature (Alshamsan et al., 2010; Renmans et al., 2016; Van de Poel et al., 2016; Binyaruka et al., 2018). However, from a theoretical point of view, it is not clear how P4P will affect the distribution of performance/performance inequalities across service providers. P4P could give the facilities that are lagging behind extra motivation to catch up and it may be easier to increase performance from a low level (Alshamsan et al., 2010; Meessen et al., 2011; Fritsche et al., 2014). But P4P could also increase performance inequalities by rewarding facilities that are better able to perform (e.g. better resourced facilities) (Ireland et al., 2011). The distributional effects of P4P will also depend, for example, on the exact design of the incentive scheme, and whether the reward depend linearly or non-linearly on performance score (Mehrotra et al., 2010; Van Herck et al., 2010; Levitt et al., 2012; Eijkenaar, 2013; Miller and Babiarz, 2013).
In this study, we measured how P4P in Tanzania affected service coverage and facility performance across facilities with different characteristics, and whether the design of performance incentives enhanced or mitigated inequalities in service provision across facilities. This assessment is important especially in LMICs given the substantial variation in health facility readiness to deliver services (MoHSW, 2013; O’Neill et al., 2013).
P4P intervention in Tanzania
The public sector dominates the Tanzanian health system, private for profit and the voluntary sector (faith-based) serve as important supplements (MoHSW, 2015). The public health system has a hierarchal administrative structure with three main facility levels of care: dispensaries, health centres and hospitals. Dispensaries and health centres provides primary healthcare services, and hospitals are referral facilities.
In 2011, the Ministry of Health and Social Welfare (MoHSW) in Tanzania, with support from the Government of Norway, introduced a P4P pilot scheme in all seven districts of Pwani region. Pwani region has >300 health facilities covering a population of just over a million (NBS, 2013). All facilities providing maternal and child health (MCH) services in Pwani were included in the scheme. The P4P scheme was introduced to reduce maternal, neonatal and child morbidity and mortality by improving the coverage and quality of MCH services. It also aimed to inform the national P4P roll out. P4P incentives were tied to coverage of services (e.g. facility-based/institutional delivery) and content of care targets [e.g. provision of Intermittent Preventive Treatment (IPT) doses for malaria during antenatal care (ANC)] (Borghi et al., 2013; Binyaruka et al., 2015). Since P4P aimed to increase service coverage, performance targets were set based on coverage rates. For example, facilities were rewarded with extra funding if facility-based deliveries surpassed a target percentage of all deliveries, and if the fraction of pregnant women that received at least two doses of IPT (IPT2) were above a target (Table 1).
Table 1.
Service indicators and performance targets for facilities implementing P4P in Tanzania
| P4P service indicators | Method | Baseline coverage (previous cycle) |
||||
|---|---|---|---|---|---|---|
| 0–20% | 21–40% | 41–70% | 71–85% | 85%+ | ||
| Coverage indicators | ||||||
| % of institutional/facility-based deliveries | Percentage point increase | 15% | 10% | 5% | 5% | Maintain |
| % of mothers attending a facility within 7 days of delivery. | Percentage point increase | 15% | 10% | 5% | 5% | Maintain |
| % of women using long term contraceptives | Percentage point increase | 20% | 15% | 10% | Maintain above 71% | Maintain |
| % children under 1 year received measles vaccine | Overall result | 50% | 65% | 75% | 80%+ | Maintain |
| % children under 1 year received Penta 3 vaccine | Overall result | 50% | 65% | 75% | 80%+ | Maintain |
| % of complete partographs | Overall result | 80% | 80% | 80% | 80%+ | Maintain above 80% |
| HMIS reports submitted to district managers on time and complete | Overall result | 100% | 100% | 100% | 100% | 100% |
| Content of care indicators | ||||||
| % ANC clients receiving two doses of IPT | Overall result | 80% | 80% | 80% | 80%+ | Maintain above 80% |
| % HIV+ ANC clients on ART | Overall result | 40% | 60% | 75% | 75%+ | Maintain |
| % of children receiving polio vaccine (OPV0) at birth | Overall result | 60% | 75% | 80% | 80%+ | Maintain |
Health managers were rewarded based on the overall performance of facilities in their district/region. Managers also had their own indicators that includes, maternal and newborn deaths audited properly and timely; reducing stock-out rates of essential drugs; timely reporting the facility data from district to regional level, and from regional to national level.
Source: The United Republic of Tanzania, Ministry of Health and Social Welfare. 2011. The Coast Region Pay for Performance (P4P) Pilot: Design Document.
85%+, 85% or more; 80%+, 80% or more; HMIS, Health Management Information System; ANC, antenatal care.
There were two methods of target setting (Table 1): a single threshold (absolute coverage target) and multiple thresholds based on baseline performance/previous cycle (relative change/overall result). For multiple thresholds, each group of facilities faced an absolute threshold based on baseline performance: Group 1 (0–20% coverage of said indicator), Group 2 (21–40%), Group 3 (41–70%), Group 4 (71–85%) and Group 5 (>85%). Group 5 was required to improve or maintain coverage for payment. District and regional managers were rewarded for the performance of facilities in their district or region.
Performance data were compiled by facilities and verified by the P4P implementing agency every 6 months (one cycle) before payments. The maximum payout per cycle differed by facility level of care: USD 820 per cycle for dispensaries; USD 3220 for health centres and USD 6790 for hospitals. From the total payout earned, the largest share (90% in hospitals and 75% in lower level facilities) was for staff bonuses, while the remainder was for facility improvement and to increase demand. P4P payments were additional to regular government funding for operational costs and salaries unrelated to performance. Full payment per indicator was made if 100% of a given target was achieved, 50% of payment was made for 75–99% achievement and no payment was made for lower levels of performance. Staff bonuses were almost equivalent to 10% of their monthly salary if all targets were fully attained. The maximum payout for district and regional managers was USD 3000 per cycle (Borghi et al., 2013).
An impact evaluation of the P4P programme in Tanzania showed a significant positive effect on two out of the eight incentivized service indicators: facility-based delivery rate and provision of antimalarials during ANC (Binyaruka et al., 2015). The programme also increased the availability of drugs and supplies, increased supportive supervision, reduced payment of user fees and resulted in greater provider kindness during delivery care (Binyaruka et al., 2015; Anselmi et al., 2017; Binyaruka and Borghi, 2017; Mayumana et al., 2017).
Conceptual framework
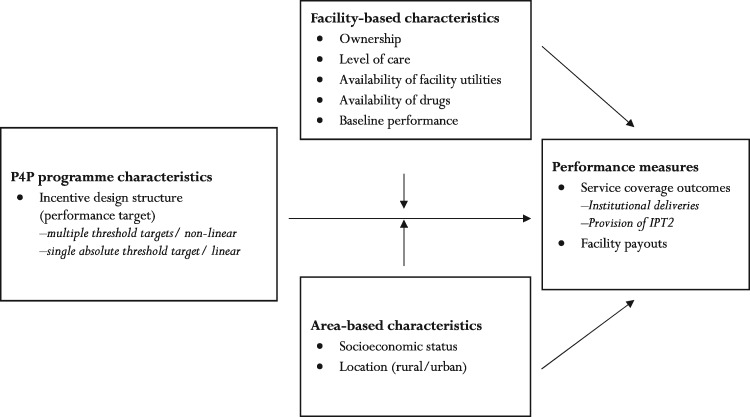
To conceptualise the pathways to distributional effects of P4P among health providers, we adapted the theoretical framework by Rittenhouse et al. (2010) and Markovitz and Ryan (2016) to the Tanzanian context (Figure 1).
Figure 1.
Conceptual framework for the determinants of performance in pay-for-performance programmes [we modified a conceptual framework which was initially developed by Rittenhouse et al. (2010) and Markovitz and Ryan (2016)]
In an incentive system like the one implemented in a P4P pilot in Tanzania, with a hierarchy of performance targets, two factors play a role for how incentives affect the distribution of performance across facilities; the distance from current performance to the target and how costly it is for the facility to reach the target level. The costs of increasing performance depend both on effort costs and on enabling factors.
Suppose performance in period t() is given by facility-level effort (), and a set of structural/enabling factors (): =p(, ). Performance is also assumed differentiable and weakly increasing in both arguments: ≥ 0, ≥0. We then consider two types of facilities: those with higher () and lower baseline performance (). At baseline we have: = – > 0, and after P4P is introduced we have = – . P4P incentive design structure and/or structural factors can affect performance across facilities over time, resulting in convergence in performance/positive distributional effects (); divergence in performance/negative distributional effects (); or similar performance across facilities (i.e. zero distributional effects) (). We discuss the extent to which the incentive design (P4P target setting) and structural factors (facility- and area-based characteristics) affect performance across facilities.
Incentive design effect
We considered only target setting approach as potential incentive design element to affect performance (Figure 1). P4P schemes can reward using fee-for-service, geographical targeting, relative performance, single absolute threshold targets or multiple threshold targets (Rosenthal et al., 2005; Rosenthal and Dudley, 2007; Mehrotra et al., 2010; Eijkenaar, 2013; Fritsche et al., 2014). The distributional effects of P4P schemes will partly depend on how incentives, and especially targets, are designed. We specifically focus on absolute and multiple thresholds target since these were used in the Tanzanian P4P scheme.
Multiple threshold target designs can enhance convergence in performance (Rosenthal et al., 2005; Mehrotra et al., 2010; Eijkenaar, 2013) because they account for baseline performance and provide incentives for lower performers to catch up. However, absence of systematic convergence in performance with this design has been observed in the UK (Sutton et al., 2012). Absolute single threshold/linear targets can enhance divergence in performance if some providers are far above and below the target (Heath et al., 1999; Rosenthal and Dudley, 2007; Mehrotra et al., 2010; Mullen et al., 2010; Miller and Babiarz, 2013). Improvement is most likely for providers/facilities that are close to achieve the threshold target. Top performers have no incentive to improve, and those far below the target may perceive it as unattainable, a phenomenon referred to as ‘goal-gradient’ theory (Heath et al., 1999). A single target design fails to account for any variation in baseline performance (Rosenthal et al., 2005; Mehrotra et al., 2010; Mullen et al., 2010; Eijkenaar, 2013).
Structural effect
Variation in facility- and area-based factors that are potentially responsible for inequalities in baseline performance can also affect overall facility performance over time (Figure 1) (Markovitz and Ryan, 2016). This is given by ≥ 0. We further assume the change in effort devoted to affect performance is increasing in x, that is > 0. If facilities invest initial bonus payments in enabling factors, this may improve their future performance, but general predictions of effects based on variation in structural factors are difficult to make (Markovitz and Ryan, 2016). We hypothesise that public facilities in Tanzania are better able to respond to incentives than non-public providers, as they can offer free MCH services (under the fee exemption policy) and have more financial autonomy (Mayumana et al., 2017). However, it is also possible that P4P can level the playing field across providers of different ownership status (Meessen et al., 2011). We further hypothesise that facilities with greater resource availability (e.g. essential drugs) are better able to increase patient demand than their counterparts (Donabedian, 1988; Alderman and Lavy, 1996; WHO, 2004); and that dispensaries are less able to respond to incentives compared with health centres and hospitals since they are more resource constrained (MoHSW 2013).
Regarding area-based factors, facilities with wealthier catchment populations may respond better to incentives, as they can more readily increase service use and revenue through user fees (Castro-Leal et al., 2000; Victora et al., 2000; Doran et al., 2008; Chien et al., 2012). Facilities in rural areas may be less able to respond to incentives than their urban counterparts, because of human resource shortages, poor road infrastructure, and more scattered and disadvantaged populations (Munga and Maestad, 2009; Witter et al., 2013; Fritsche et al., 2014).
Apart from the above hypothesized pathways (incentive design and structural effect), provider response may also depend on the nature of the services targeted or incentivized. This is because performance improvement can be harder for some services compared with other services and this may confound the initial hypothesises of incentive design and structural effect. For instance, less efforts are needed by providers to influence clients’ continuation of care than initiation of care (Gertler and Vermeersch, 2013).
Materials and methods
Study design and data sources
This study was part of the large impact evaluation of the P4P scheme in Pwani region (Borghi et al., 2013; Binyaruka et al., 2015). The P4P evaluation study surveyed all seven districts in Pwani region (intervention arm), and four districts from Morogoro and Lindi regions (comparison arm). Comparison districts were selected to be comparable to intervention districts in terms of poverty and literacy rates, the rate of institutional deliveries, infant mortality, population per health facility and the number of children under 1 year of age per capita (Borghi et al., 2013).
Baseline data at facility and household-levels were collected in January 2012, with a follow-up round 13 months later. For each study arm, data on facility ownership (public or non-public facility), level of care (hospital, health centre or dispensary), availability of medical inputs (considered 37 essential drugs) and rural/urban location was obtained from 75 sampled facilities providing MCH services (6 hospitals, 16 health centres and 53 dispensaries). Data on socioeconomic status of the facility catchment populations and service coverage rates were obtained from households with women who had delivered in the 12 months prior to the baseline and endline surveys. We randomly sampled 20 eligible households from each facility’s catchment area, making a total of 1500 households in each arm per survey round. Facility payout data were obtained from the implementing agency for all incentivized indicators for the 75 intervention facilities in our sample over seven payment cycles (2011–14).
Performance outcomes
We considered two facility performance outcomes. First, for each facility in the intervention arm and for each of seven payment cycles, we generated a ‘payout score’. That score was constructed as the bonus payout received divided by the maximum potential payout (all targets had been met) and multiplied by 100. Payout score was used to capture for each level of care the relative facility performance. Second, we estimated facility-level average service coverage rates for households in the facility catchment area from both study arms. Our coverage rates were estimated using only two incentivized services which improved significantly on average due to P4P (Binyaruka et al., 2015); that is, the coverage of facility-based deliveries and provision of two doses of IPT for malaria during ANC (referred to as IPT2). We therefore considered only these two service outcomes to assess whether P4P effect differed across facilities.
Subgroups of facilities for distributional analyses
To examine whether incentive design and structural effects affected performance outcomes, we identified facility subgroups as shown in Table 2, pertaining to their baseline performance for the two incentivized indicators (above or below the median); facility characteristics (ownership, level of care, availability of utilities, rural-urban location); an un-weighted index of drug availability at baseline (Supplementary AppendixTable S1); and wealth status of the catchment population, based on mean wealth index scores across households in the facility-catchment area generated by principal component analysis (Vyas and Kumaranayake, 2006) (Supplementary AppendixTable S2).
Table 2.
Baseline facility and area-based characteristics by study arms
| Characteristics | Description | Intervention (n = 75) | Comparison (n = 75) | Difference (P-value) |
|---|---|---|---|---|
| Panel A: facility-based characteristics | ||||
| Facility ownership | =1 for public owned (%) | 84.0 | 82.7 | 1.3 (0.828) |
| Facility level of care | =1 for dispensary (%) | 70.7 | 70.7 | 0.0 (1.000) |
| Availability of facility utilities | =1 for electricity and water supply (%) | 54.7 | 52.0 | 2.7 (0.745) |
| Availability of drugs—index | Mean index (0–1) of 37 drugs [SD] | 0.61 [0.16] | 0.66 [0.12] | –0.05 (0.031) |
| Availability of drugs—subgroup | =1 for availability below the median (%) | 57.3 | 42.7 | 14.6 (0.073) |
| Baseline coverage level (deliveries) | =1 for facility below the median (%) | 53.3 | 46.7 | 6.6 (0.418) |
| Baseline coverage level (IPT2) | =1 for facility below the median (%) | 54.6 | 45.3 | 9.3 (0.256) |
| Panel B: area-based characteristics | ||||
| Wealth status index | Mean wealth index [SD] | –0.43 [1.8] | 0.32 [2.4] | –0.75 (0.028) |
| Wealth status—tercile 1 | =1 for poorest population (%) | 40.0 | 26.7 | 13.3 (0.084) |
| Wealth status—tercile 2 | =1 for middle wealth population (%) | 34.7 | 32.0 | 2.7 (0.731) |
| Wealth status—tercile 3 | =1 for least poor population (%) | 25.3 | 41.3 | –16.0 (0.038) |
| Facility location | =1 for facility in rural district (%) | 78.7 | 84.0 | –5.3 (0.405) |
Three quantiles (terciles) were used for wealth status of the facility’s catchment population; Availability of drugs include 37 drugs and analysis used a dummy variable classified based on baseline availability distribution (=1 for availability below the median/bottom half and 0, otherwise); SD, standard deviation; reference category in brackets: public (vs non-public), dispensary (vs health centre and hospital), with electricity and water supply at baseline (vs none), baseline availability of drugs below the median/in bottom half (vs top half), baseline lower performer/below the median (vs higher performer), rural (vs urban district); for distributional analyses, wealth index and drugs availability index were re-classified on each arm separately and equally to avoid the imbalance across arms at baseline.
Analysis
We first compared the sample means at baseline for each of the facility subgroups across study arms, and examined eventual differences between study arms using the t-test.
Distribution of bonus payouts
To assess how bonus payouts were distributed across intervention facilities, we used three measures of inequality: an absolute measure (the gap) and two relative measures [the ratio and the concentration index (CI)] (O'Donnell et al., 2008; WHO, 2013). The gap was measured as the difference in payout scores between facility subgroups. The ratio was measured as the ratio of payout scores between subgroups. In relation to wealth subgroups, a positive (negative) gap and a ratio greater (less) than one defines a pro-rich (pro-poor) distribution, respectively. A gap of zero and a ratio of one defines an equal distribution. We tested whether the gaps were significantly different from zero by using t-tests.
The CI was computed on a ranking variable of area-based wealth status to examine wealth-related inequality in the distribution of payouts (Kakwani et al., 1997; O'Donnell et al., 2008). The CI ranges between [−1 and +1], whereby zero indicate equality between wealth subgroups, while negative and positive values indicate that payouts are pro-poor and pro-rich, respectively. We tested whether the CIs were significantly different from zero.
Heterogeneity in service coverage outcomes
We measured the difference in mean baseline coverage of the two incentivized services between facility subgroups (the coverage gap; WHO, 2013) and tested for significant differences between subgroups.
Based on the two incentivized services that were improved by P4P (i.e. facility-based deliveries by 8.2% points, and provision of IPT2 by 10.3% points) (Binyaruka et al., 2015), we assessed whether the effects differed by facility subgroup. We used a linear difference-in-differences regression model with a three-way interaction term between the average treatment effect () and facility subgrouping variable . The associated two-order interaction terms were also included in the model as shown in Equation (1).
| (1) |
where is the service coverage outcome of facility i at time t. is a dummy variable, taking the value 1 if a facility is exposed to P4P and zero otherwise. We controlled for unobserved time-invariant facility-level characteristics with facility fixed-effects estimation, and included for year fixed-effects. We also controlled for time-varying facility-level covariates (availability of electricity and water supply, and the mean wealth index for households sampled in the catchment area of the facility) as potential confounding factors. The error term is . Our statistical inference for regression was based on standard errors clustered at the facility level to account for serial correlation of at the facility level. The coefficient of interest for the differential effect across facility subgroups is .
Causal inference using the difference-in-differences approach relies on the key identifying assumption that the trends in outcomes would have been parallel across study arms in the absence of the intervention (Khandker et al., 2010). While this cannot be formally tested, we justified the assumption by verifying that the pre-intervention trends were parallel in Tanzania (Binyaruka et al., 2015; Anselmi et al., 2017). This was verified in women who had delivered in the past 12 months at baseline for the following outcomes for which we had monthly data: share of institutional deliveries, caesarean section deliveries, women who breastfeed within 1 h of birth, and women who paid for delivery care. We also verified pre-intervention trends to be parallel in facility service utilization levels based on patient registers.
We performed some robustness checks. First, we re-estimated the model for facility-based deliveries excluding hospitals (8% of facilities per arm), as hospitals have less clearly defined catchment populations. Second, we clustered the standard errors at the district level and used a bootstrapping method to adjust the small number of district-clusters (Cameron and Miller, 2015). Third, we reclassified the mean wealth scores into two quantiles (below or above the median) to check whether the wealth effect was sensitive to classification of the wealth groupings. Lastly, apart from using a conventional parametric test (a t-test) to assess whether differences in payouts between subgroups were significant, a non-parametric test (Wilcoxon rank-sum test) was also used (Kitchen, 2009). All the analyses were performed using STATA version 13.
Results
Facility and area-based characteristics were generally similar in the intervention and comparison arms at baseline (Table 2), although intervention facilities served poorer populations, and had marginally lower availability of drugs than comparison facilities.
Distribution of bonus payouts
There was an increase in average payout scores between payment cycle 1 (50.1% of total potential payout) and cycle 7 (77.7%) (Table 3), and the payouts were highest for facilities with least poor catchment populations. This pro-rich effect was confirmed by positive equity gaps and concentration indices, and an equity ratio that was greater than one across all payment cycles (Table 3, Columns 5–7). The inequalities were generally stronger in early compared with later cycles (Table 3).
Table 3.
Distribution of facility payout scores by wealth status of the catchment populations
| Payment cycle | All | Area-based wealth status (terciles) |
Equity |
CI (P-value) | |||
|---|---|---|---|---|---|---|---|
| Mean [SD] | Least poor | Middle | Poorest | Gap (P-value) | Ratio | ||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
| CYCLE 1 (%) | 50.1 [19.4] | 54.7 | 52.3 | 43.1 | 11.6 (0.027) | 1.27 | 0.042 (0.099) |
| CYCLE 2 (%) | 50.3 [19.1] | 58.4 | 49.7 | 42.4 | 16.0 (0.002) | 1.38 | 0.088 (0.000) |
| CYCLE 3 (%) | 64.6 [18.8] | 69.2 | 65.1 | 59.6 | 9.6 (0.062) | 1.16 | 0.036 (0.054) |
| CYCLE 4 (%) | 67.5 [19.5] | 67.8 | 69.6 | 65.1 | 2.7 (0.623) | 1.04 | 0.007 (0.699) |
| CYCLE 5 (%) | 74.5 [18.5] | 75.3 | 74.9 | 73.4 | 1.9 (0.707) | 1.03 | 0.007 (0.669) |
| CYCLE 6 (%) | 69.6 [20.1] | 72.0 | 75.3 | 61.3 | 10.7 (0.046) | 1.17 | 0.035 (0.058) |
| CYCLE 7 (%) | 77.7 [16.3] | 79.2 | 76.9 | 76.9 | 2.3 (0.619) | 1.03 | 0.006 (0.672) |
| Pooled—all cycles (1–7) (%) | 64.7 [11.7] | 68.1 | 66.3 | 60.5 | 7.6 (0.015) | 1.13 | 0.027 (0.022) |
Analysis restricted to intervention facilities only (n = 75); p-values in Column (5) were from t-test of the null hypothesis that the gap [Columns (2)–(4)] is equal to zero; p-values in Column (7) were for testing the null hypothesis of zero CI; SD, standard deviation; terciles for wealth status were generated with equal-size from intervention arm separately; Gap, least poor—poorest; ratio, least poor/poorest; the results were generally similar in Column (5) when non-parametric test (Wilcoxon rank-sum) is used (Supplementary Table S6).
Facilities with greater availability of drugs at baseline, hospitals and health centres had significantly higher payout scores than facilities with more limited drug availability and dispensaries (Table 4). The equity ratios were ∼1, near equality, between most subgroups (Table 4).
Table 4.
Distribution of facility payout scores by other subgroups of facilities
| Facility subgroups | By payment cycle |
Pooled average cycles | ||||||
|---|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 6 | Cycle 7 | Cycles 1–7 | |
| Facility location | ||||||||
| Rural (%) | 52.2 | 48.5 | 66.3 | 69.5 | 76.4 | 71.3 | 80.0 | 66.4 |
| Urban (%) | 42.3 | 56.7 | 58.3 | 60.1 | 68.1 | 63.2 | 68.9 | 59.7 |
| Gap (%) | 9.9** | –8.2* | 8.0 | 9.4* | 8.3 | 8.1 | 11.1** | 6.7* |
| Ratio | 1.2 | 0.9 | 1.1 | 1.2 | 1.1 | 1.1 | 1.2 | 1.1 |
| Ownership status | ||||||||
| Public owned (%) | 49.9 | 49.5 | 66.0 | 68.8 | 75.8 | 70.0 | 78.4 | 65.6 |
| Non-public (%) | 50.9 | 54.4 | 56.9 | 60.6 | 66.7 | 67.1 | 73.6 | 61.5 |
| Gap (%) | –1.0 | –4.9 | 9.1 | 8.2 | 9.1 | 2.9 | 4.8 | 4.1 |
| Ratio | 1.0 | 0.9 | 1.2 | 1.1 | 1.1 | 1.0 | 1.1 | 1.1 |
| Level of care | ||||||||
| Dispensary (%) | 47.7 | 46.9 | 60.2 | 63.5 | 71.5 | 66.9 | 75.4 | 61.9 |
| HC and hospital (%) | 55.8 | 58.3 | 75.3 | 77.0 | 81.7 | 75.8 | 82.9 | 72.4 |
| Gap (%) | −8.1* | −11.4** | −15.1*** | −13.5*** | −10.2*** | −8.9** | −7.5** | −10.5*** |
| Ratio | 0.9 | 0.8 | 0.8 | 0.8 | 0.9 | 0.9 | 0.9 | 0.9 |
| Electricity and water supply | ||||||||
| Available (%) | 53.6 | 51.9 | 66.7 | 69.1 | 76.8 | 71.3 | 81.1 | 67.2 |
| None (%) | 45.9 | 48.3 | 62.1 | 65.5 | 71.7 | 67.5 | 73.5 | 62.2 |
| Gap (%) | 7.7* | 3.6 | 4.6 | 3.6 | 5.1 | 3.8 | 7.6** | 5.0* |
| Ratio | 1.2 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| Availability of drugs | ||||||||
| Above the median (%) | 50.6 | 58.6 | 68.3 | 72.2 | 76.0 | 74.6 | 79.3 | 68.5 |
| Below the median (%) | 49.7 | 41.8 | 61.0 | 62.9 | 73.2 | 64.6 | 76.0 | 61.5 |
| Gap (%) | 0.9 | 16.8*** | 7.3* | 9.3** | 2.8 | 10.0** | 3.3 | 7.0*** |
| Ratio | 1.0 | 1.4 | 1.1 | 1.1 | 1.0 | 1.2 | 1.0 | 1.1 |
Analysis restricted to intervention facilities only (n = 75); Gap is the difference in payout score between two subgroups of facilities; ratio is the ratio of payout scores for two subgroups; the significance test was by t-test for the null hypothesis of gap equals zero; the results were generally similar when non-parametric test (Wilcoxon rank-sum) was used to test the significant of the gap (results not shown).
Significance at 10% level.
Significance at 5% level.
Significance at 1% level.
Heterogeneity in service coverage outcomes
Baseline facility-based delivery rates and coverage of IPT2 during ANC were similar between most facility subgroups (Table 5). Exceptions were higher facility-based delivery rates in facilities with the least poor catchment populations, and higher coverage of IPT2 among the poorest catchment populations. Coverage of IPT2 was higher among dispensaries than health centres and hospitals, but there were lower levels of coverage in both outcomes in the comparison arm at baseline (Table 5).
Table 5.
Baseline coverage levels by facility subgroups across study arms
| Outcome variable/subgrouping variable | Intervention arm (n = 75) |
Comparison arm (n = 75) |
||||
|---|---|---|---|---|---|---|
| Yes | No | Gap | Yes | No | Gap | |
| (1) | (2) | (3) | (4) | (5) | (6) | |
| OUTCOME 1: institutional/facility-based deliveries | ||||||
| Public facility (%) | 84.6 | 84.7 | –0.1 | 86.4 | 89.0 | –2.6 |
| Dispensary facility (%) | 82.5 | 89.5 | –7.0 | 85.3 | 90.7 | –5.4** |
| Facility with utilities (electricity and water supply) (%) | 86.9 | 81.7 | 5.2 | 88.3 | 85.4 | 2.9 |
| Facility with drugs availability below the median (%) | 83.9 | 85.2 | –1.3 | 88.6 | 85.1 | 3.5 |
| Facility with poorest catchment population (%) | 84.6 | 89.7 | –5.1* | 81.5 | 92.7 | –11.2*** |
| Facility with middle wealth catchment population (%) | 79.5 | 89.7 | –10.2** | 86.3 | 92.7 | –6.4** |
| Facility in rural district (%) | 83.9 | 87.1 | –3.2 | 85.9 | 92.0 | –6.1* |
| Lower performer (below the median) (%) | 73.9 | 95.6 | –21.7*** | 80.4 | 95.9 | –15.5*** |
| OUTCOME 2: provision of IPT2 to ANC clients | ||||||
| Public facility (%) | 50.2 | 50.6 | –0.4 | 57.0 | 51.3 | 5.7 |
| Dispensary facility (%) | 53.8 | 41.7 | 12.1*** | 54.1 | 60.5 | –6.4* |
| Facility with utilities (electricity and water supply) (%) | 47.7 | 53.2 | –5.5 | 57.8 | 54.1 | 3.7 |
| Facility with drugs availability below the median (%) | 53.6 | 46.7 | 6.9* | 57.2 | 54.7 | 2.5 |
| Facility with poorest catchment population (%) | 49.5 | 45.7 | 3.8 | 61.6 | 52.5 | 9.1** |
| Facility with middle wealth catchment population (%) | 55.5 | 45.7 | 9.8** | 53.8 | 52.5 | 1.3 |
| Facility in rural district (%) | 50.8 | 47.9 | 2.9 | 56.1 | 55.3 | 0.8 |
| Lower performer (below the median) (%) | 37.3 | 63.5 | –26.2*** | 44.0 | 68.9 | –24.9*** |
We used a t-test to test the null hypothesis of a gap (Columns 3 and 6) equals to zero; Terciles classified in each arm separately were used for wealth status of the facility’s catchment population; availability of drugs included 37 essential drugs and analysis used a dummy variable classified in each arm separately based on baseline availability distribution (=1 for availability below the median/bottom half and 0, otherwise); reference category for ‘NO’ column in brackets: public (vs non-public), dispensary (vs health centre and hospital), with electricity and water supply at baseline (vs none), baseline availability of drugs below the median/in bottom half (vs top half), baseline lower performer/below the median (vs higher performer); similar pattern of results when hospitals are excluded for facility-based delivery outcome; overall baseline coverage in facility-based deliveries was (84.7 and 86.8%) and IPT2 coverage was (49.5 and 56.7%) for intervention and control arm, respectively (Binyaruka et al., 2015).
Significance at 10% level.
Significance 5% level.
Significance at 1% level.
P4P resulted in a greater increase in facility-based deliveries among facilities with lower baseline coverage levels than those with higher baseline coverage levels (by 13.0% points, P = 0.006) (Table 6), and among facilities serving middle wealth populations than those serving least poor populations (by 14.3% points, P = 0.004) (Table 6). P4P also resulted in a greater increase in facility-based deliveries among facilities in rural compared with urban districts (by 10.0% points, P = 0.030). The effect of P4P on coverage of IPT2 increased over time and was similar across all facility subgroups (Table 6).
Table 6.
Heterogeneity in the effect of P4P on service coverage outcomes
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
|---|---|---|---|---|---|---|---|
| Outcome 1: facility-based delivery | |||||||
| P4P effect | 4.0 | 5.7 | 9.2*** | 4.4 | 1.0 | 1.4 | –0.8 |
| P4P effect×public facility | 4.4 | ||||||
| P4P effect×dispensary facility | 2.7 | ||||||
| P4P effect×with available utilities | –2.9 | ||||||
| P4P effect×low availability of drugs | 6.3 | ||||||
| P4P effect×lower baseline performer | 13.0*** | ||||||
| P4P effect×poorest population | 4.0 | ||||||
| P4P effect×middle wealth population | 14.3*** | ||||||
| P4P effect×rural facilities | 10.0** | ||||||
| Control mean at baseline | 86.8 | 86.6 | 86.8 | 86.4 | 86.5 | 86.5 | 86.8 |
| Observation (n) | 300 | 300 | 300 | 300 | 300 | 300 | 300 |
| Outcome 2: IPT2 coverage | |||||||
| P4P effect | 5.4 | 15.9*** | 9.4* | 10.2** | 5.8* | 9.2* | 4.8 |
| P4P effect×public facility | 4.5 | ||||||
| P4P effect×dispensary facility | –9.6 | ||||||
| P4P effect×with available utilities | –0.2 | ||||||
| P4P effect×low availability of drugs | –1.8 | ||||||
| P4P effect×lower baseline performer | 7.5 | ||||||
| P4P effect×poorest population | 6.4 | ||||||
| P4P effect×middle wealth population | –6.4 | ||||||
| P4P effect×rural facilities | 5.2 | ||||||
| Control mean at baseline | 51.4 | 51.2 | 51.6 | 51.6 | 51.4 | 51.9 | 51.7 |
| Observation (n) | 300 | 300 | 300 | 300 | 300 | 300 | 300 |
All regressions are ordinary least square (OLS). All specifications leads to an estimated Beta showing percentage point after controlling for a year dummy, facility-fixed effects and facility-level covariates (availability of utilities and wealth status of the catchment population); availability of drugs include 37 drugs and analysis used a dummy variable classified in each arm separately based on baseline availability distribution (=1 for availability below the median/bottom half and 0, otherwise); reference category in brackets: public (vs non-public), dispensary (vs health centre and hospital), with electricity and water supply at baseline (vs none), baseline availability of drugs below the median/in bottom half (vs top half), baseline lower performer/below the median (vs higher performer), rural (vs urban district), poorest/middle wealth (vs least poor).
Significance at 10% level.
Significance 5% level.
Significance at 1% level.
The results on facility-based deliveries were similar when we restricted the analysis to primary care facilities, except for the difference between rural/urban locations that became insignificant (Supplementary Table S3). The results were generally robust to clustering at the district level, except that there was no longer a differential effect on deliveries by wealth subgroups (Supplementary Table S4). When two quantiles of wealth scores (lower and higher) were used, the differential effect for facility-based deliveries became insignificant (Supplementary Table S5). The use of non-parametric tests of differences between payouts across facilities revealed similar results to those using parametric tests (Supplementary Table S6).
Discussion
We examined the distribution of P4P payouts over time and assessed how P4P effects on service coverage differed across facility subgroups in Tanzania. We then assessed whether facility performance was shaped by the incentive design and/or facility and area-based characteristics. This study is one of the few that examine how P4P payouts are distributed and that examine broadly whether there was supply-side heterogeneous P4P effects due to incentive design or structural factors in a LMIC. We found some evidence of both incentive design effects, and effects from structural differences at baseline on performance inequalities. However, the inequalities in payouts distribution declined over time.
Our finding of reduced inequalities in payouts distribution (convergence in performance) by population wealth status over time is partly consistent with the ‘inverse equity hypothesis’ (Victora et al., 2000). The hypothesis suggests that better-off groups will initially benefit from a new intervention, widening inequalities, but over time the worse-off will catch up especially when the better-off have extracted maximum benefit. This convergence in payouts over time is also consistent with US evidence that wealthier hospitals initially received higher payouts than their counterparts, but the distribution of payouts levelled over time (Ryan et al., 2012). The reduced payout inequalities in the US was partly due a change in the incentive design from only rewarding top performers to rewarding any improvement where all providers were likely to receive a payout (Ryan et al., 2012).
The finding that P4P had greatest effect on facility-based deliveries (with multiple threshold targets) among baseline lower performers indicates convergence in performance and is consistent with evidence on quality improvements from the UK (Doran et al., 2008), Canada (Li et al., 2014) and the US (Rosenthal et al., 2005; Lindenauer et al., 2007; Blustein et al., 2010; Chen et al., 2010; Jha et al., 2012). In Rwanda, however, a P4P programme rewards on a fee-for-service system and several rewarded services improved most among facilities with middle baseline quality scores (Sherry et al., 2017). The convergence in performance in HICs was partly linked to a design with multiple threshold targets in the UK (Doran et al., 2008) and Canada (Li et al., 2014) and to a US design system (relative incentive design) that rewarded the highest performers and penalized the lowest performers (Rosenthal et al., 2005; Lindenauer et al., 2007). However, another study in the UK of a hospital incentive scheme with multiple thresholds found evidence of divergence in performance in relation to mortality outcomes linked to pneumonia but not for other conditions (Sutton et al., 2012).
Our finding that the effects of P4P on facility-based deliveries differed according to the wealth status of facility catchment populations is somewhat different to that reported in the UK and the US with respect to quality of care improvements (Doran et al., 2008; Gravelle et al., 2008; Alshamsan et al., 2010; Blustein et al., 2010; Chien et al., 2012; Kontopantelis et al., 2013). While these studies found that providers serving low-income populations performed initially less well but improved most over time, we found that facilities serving middle wealth populations with initial low coverage improved more over time than those with least poor populations. Moreover, while we found that the effect of P4P on coverage of institutional deliveries was greater for rural facilities in Tanzania, a US study found no association between performance on quality and rural/urban location (Ryan and Blustein, 2011); and studies in the UK showed that P4P had less effect in rural than in urban areas (Gravelle et al., 2008; Kontopantelis et al., 2013).
We found similar improvements on IPT2 coverage across facilities (no differential effect of P4P), which is in contrast to literature that suggests a design with a single threshold target, as used for IPT2, fails to account for baseline performance and can enhance divergence in performance (Heath et al., 1999; Rosenthal et al., 2005; Rosenthal and Dudley, 2007; Mehrotra et al., 2010; Mullen et al., 2010; Eijkenaar, 2013). Our finding might be explained by the almost universal coverage of one ANC visit in Tanzania (Binyaruka et al., 2015; TDHS, 2016), and the nature of the targeted service (content of care, rather than service use) may have meant that minimal effort was needed for providers to achieve the target for IPT2.
Our results lend support to the notion that the incentive design, facility characteristics and the nature of services being targeted themselves, will determine how providers respond to P4P, their ability to achieve targets and receive P4P payouts, and the extent to which P4P leads to convergence in performance across providers. Although P4P is typically talked about as a single or uniform intervention, there is in fact substantial variation in incentive structures and scheme designs (Eijkenaar, 2013; Miller and Babiarz, 2013). Our study suggests that design details may be important for determining the distributional effects of P4P across providers, and whether P4P will enhance or reduce existing performance inequalities (Rosenthal et al., 2005; Rosenthal and Frank, 2006; Rosenthal and Dudley, 2007; Ryan et al., 2012). Further research is needed to examine the effects of incentive design on outcomes, and researchers should be encouraged to report on programme design aspects in their evaluations of P4P and systematically monitor and report subgroup effects across providers.
In addition to consideration of incentive design, a number of policies could be introduced to tackle structural factors to increase the likelihood of reducing performance inequalities with the introduction of P4P. ‘Equity bonuses’ have been suggested as a means to enhance performance among disadvantaged facilities so they benefit from payouts from the start (Rosenthal and Dudley, 2007; Meessen et al., 2011; Fritsche et al., 2014). Facility readiness assessment studies and potential quality boosting investments are also important to harmonise the capacity to deliver services prior to P4P. These are standard practices for most P4P programmes funded by the World Bank in LMICs, and the national P4P rollout programme in Tanzania has similarly incorporated these practices.
This study has a number of limitations. First, the administrative data on payouts did not allow for a disaggregation of payouts by service indicator, and thus we used the total payout per cycle which reflects performance across all P4P indicators. Second, since information about payout distribution was limited to intervention facilities, our results represent associations rather than causal effects. Third, we used household data from a random sample of 20 households per facility to proxy service coverage at facility level and wealth status of the facility’s catchment population, and these may have not been representative of the entire catchment populations surrounding facilities. Furthermore, our analysis assumed that households in a facility’s catchment population would have used the facility for care seeking, whereas it is possible that households bypassed their nearest provider to seek care at higher level or more distant facilities. Fourth, the finding of the convergence in coverage of institutional deliveries over 13 months may reflect a regression to the mean principle (a random fluctuation rather than a true causal effect) due to a ‘shorter term’ assessment (Barnett et al., 2005), although the distribution in terms of payouts over the ‘longer term’ of seven payment cycles showed a consistent pattern on convergence. Fifth, as our two service coverage outcomes differed both in terms of incentive design as well as the nature of the service being targeted, it was not possible to determine the extent to which the difference in provider performance response was due to the former or the latter. Finally, because of sample size constraints, we examined differential effects across facility subgroups using a three-way interaction term, and were unable to run separate models for each subgroup (subgroup effects) and compare their effects for better understanding of programme effect. We also classified baseline performance into two subgroups rather than five subgroups as used in the design, due to insufficient sample size. As a result, it was not possible to determine what effect the ‘maintain coverage’ target had on performance relative to the ‘improve coverage’ target.
Conclusion
In this study, better-off facilities (hospitals, health centres, facilities with more medical commodities and serving wealthier populations) benefited more from P4P payouts than worse-off facilities in the short term; but these inequalities declined over time as worse-off facilities caught up. The increased coverage of facility-based deliveries was greater among facilities with lower levels of baseline coverage, with middle wealth catchment populations, and located in rural areas; whereas the increased IPT2 coverage was similar across facility subgroups. The design of incentives and a range of facility characteristics seem to have influenced performance inequalities; however, the nature of the service being targeted is also likely to have affected provider response. While P4P can help to improve service coverage and quality, and to reduce performance inequalities, care must be taken to ensure that P4P design does not disproportionally benefit those who are already better-off.
Ethical approval
The evaluation study received ethical approval from the Ifakara Health Institute institutional review board (approval number: 1BI1IRB/38) and the ethics committee of the London School of Hygiene & Tropical Medicine. Study participants provided written consent to participate in this study, requiring them to sign a written consent form that was read out to them by the interviewers. This consent form was reviewed and approved by the ethics committees prior to the start of the research.
Supplementary Material
Acknowledgements
We would like to thank Kara Hanson and Ottar Mæstad for reviewing the paper and for valuable comments and suggestions. We also thank the whole P4P evaluation research team. We also acknowledge the support from all field respondents, and various government officials and P4P implementing agency.
Funding
This work was supported by the Government of Norway, which funded the data collection for the programme evaluation that was used in this paper (grant numbers: TAN-3108 and TAN 13/0005. http://www.norad.no/en/). The UK Department for International Development (DFID) as part of the Consortium for Research on Resilient and Responsive Health Systems (RESYST) also supported the data analysis and writing of this paper. This study is part of a PhD thesis at the University of Bergen for Peter Binyaruka, who is financially supported by the Norwegian State Education Loan Fund. The funding bodies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest statement. The authors of this manuscript have the following competing interests: two authors (P.B. and J.B.) were funded by the Government of Norway to undertake the data collection associated with this research. The Government of Norway also funded the P4P programme in Pwani region of Tanzania. The funder of the study had no role in data analysis, data interpretation or writing of the manuscript.
References
- Alderman H, Lavy V.. 1996. Household responses to public health services: cost and quality tradeoffs. The World Bank Research Observer 11: 3–22. [Google Scholar]
- Alshamsan R, Majeed A, Ashworth M, Car J, Millett C.. 2010. Impact of pay for performance on inequalities in health care: systematic review. Journal of Health Services Research & Policy 15: 178–84. [DOI] [PubMed] [Google Scholar]
- Anselmi L, Binyaruka P, Borghi J.. 2017. Understanding causal pathways within health systems policy evaluation through mediation analysis: an application to payment for performance (P4P) in Tanzania. Implementation Science 12: 10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AG, van der Pols JC, Dobson AJ.. 2005. Regression to the mean: what it is and how to deal with it. International Journal of Epidemiology 34: 215–20. [DOI] [PubMed] [Google Scholar]
- Binyaruka P, Borghi J.. 2017. Improving quality of care through payment for performance: examining effects on the availability and stock-out of essential medical commodities in Tanzania. Tropical Medicine & International Health 22: 92–102. [DOI] [PubMed] [Google Scholar]
- Binyaruka P, Patouillard E, Powell-Jackson T.. 2015. Effect of paying for performance on utilisation, quality, and user costs of health services in Tanzania: a controlled before and after study. PLoS One 10: e0135013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binyaruka P, Robberstad B, Torsvik G, Borghi J.. 2018. Who benefits from increased service utilisation? Examining the distributional effects of payment for performance in Tanzania. International Journal for Equity in Health 17: 14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blustein J, Borden WB, Valentine M.. 2010. Hospital performance, the local economy, and the local workforce: findings from a US National Longitudinal Study. PLoS Medicine 7: e1000297.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi J, Mayumana I, Mashasi I. et al. 2013. Protocol for the evaluation of a pay for performance programme in Pwani region in Tanzania: a controlled before and after study. Implementation Science 8: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AC, Miller DL.. 2015. A practitioner’s guide to cluster-robust inference. Journal of Human Resources 50: 317–72. [Google Scholar]
- Castro-Leal F, Dayton J, Demery L, Mehra K.. 2000. Public spending on health care in Africa: do the poor benefit? Bulletin of the World Health Organization 78: 66–74. [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Kang N, Juarez DT, Hodges KA, Chung RS, Legorreta AP.. 2010. Impact of a pay-for-performance program on low performing physicians. Journal for Healthcare Quality: Official Publication of the National Association for Healthcare Quality 32: 13–22. [DOI] [PubMed] [Google Scholar]
- Chien AT, Wroblewski K, Damberg C. et al. 2012. Do physician organizations located in lower socioeconomic status areas score lower on pay-for-performance measures? Journal of General Internal Medicine 27: 548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Gopalan SS, Chandramohan D.. 2016. Effect of pay for performance to improve quality of maternal and child care in low- and middle-income countries: a systematic review. BMC Public Health 16: 321.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian A. 1988. The quality of care: how can it be assessed? JAMA 260: 1743–8. [DOI] [PubMed] [Google Scholar]
- Doran T, Fullwood C, Kontopantelis E, Reeves D.. 2008. Effect of financial incentives on inequalities in the delivery of primary clinical care in England: analysis of clinical activity indicators for the quality and outcomes framework. Lancet (London, England) 372: 728–36. [DOI] [PubMed] [Google Scholar]
- Eijkenaar F. 2013. Key issues in the design of pay for performance programs. The European Journal of Health Economics 14: 117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkenaar F, Emmert M, Scheppach M, Schoffski O.. 2013. Effects of pay for performance in health care: a systematic review of systematic reviews. Health Policy (Amsterdam, Netherlands) 110: 115–30. [DOI] [PubMed] [Google Scholar]
- Fritsche G, Soeters R, Meessen B.. 2014. Performance-Based Financing Toolkit. Washington, DC: The World Bank. [Google Scholar]
- Gertler P, Vermeersch C.. 2013. Using Performance Incentives to Improve Health Outcomes. NBER Working Paper No. 19046.
- Gillam SJ, Siriwardena AN, Steel N.. 2012. Pay-for-performance in the United Kingdom: impact of the quality and outcomes framework: a systematic review. Annals of Family Medicine 10: 461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravelle H, Sutton M, Ma A.. 2008. Doctor behaviour under a pay for performance contract: further evidence from the quality and outcomes framework. CHE Research Paper 34
- Heath C, Larrick RP, Wu G.. 1999. Goals as reference points. Cognitive Psychology 38: 79–109. [DOI] [PubMed] [Google Scholar]
- Ireland M, Paul E, Dujardin B.. 2011. Can performance-based financing be used to reform health systems in developing countries? Bulletin of the World Health Organization 89: 695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Joynt KE, Orav EJ, Epstein AM.. 2012. The long-term effect of premier pay for performance on patient outcomes. The New England Journal of Medicine 366: 1606–15. [DOI] [PubMed] [Google Scholar]
- Kakwani N, Wagstaff A, Van Doorslaer E.. 1997. Socioeconomic inequalities in health: measurement, computation, and statistical inference. Journal of Econometrics 77: 87–103. [Google Scholar]
- Khandker SR, Koolwal GB, Samad HA.. 2010. Handbook on Impact Evaluation: Quantitative Methods and Practices. Washington, DC: The World Bank. [Google Scholar]
- Kitchen CM. 2009. Nonparametric vs parametric tests of location in biomedical research. American Journal of Ophthalmology 147: 571–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontopantelis E, Buchan I, Reeves D, Checkland K, Doran T.. 2013. Relationship between quality of care and choice of clinical computing system: retrospective analysis of family practice performance under the UK's quality and outcomes framework. BMJ Open 3: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt S, List J, Neckermann S, Sadoff S.. 2012. The Behavioralist Goes to School: Leveraging Behavioral Economics to Improve Educational Performance. National Bureau of Economic Research, Inc. NBER Working Paper 18165, doi: 10.3386/w18165. [Google Scholar]
- Li J, Hurley J, DeCicca P, Buckley G.. 2014. Physician response to pay-for-performance: evidence from a natural experiment. Health Economics 23: 962–78. [DOI] [PubMed] [Google Scholar]
- Lindenauer PK, Remus D, Roman S. et al. 2007. Public reporting and pay for performance in hospital quality improvement. The New England Journal of Medicine 356: 486–96. [DOI] [PubMed] [Google Scholar]
- Markovitz AA, Ryan AM.. 2016. Pay-for-performance: disappointing results or masked heterogeneity? Medical Care Research and Review 74: 3–78. doi: 10.1177/1077558715619282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumana I, Borghi J, Anselmi L, Mamdani M, Lange S.. 2017. Effects of Payment for Performance on accountability mechanisms: evidence from Pwani, Tanzania. Social Science & Medicine (1982) 179: 61–73. [DOI] [PubMed] [Google Scholar]
- Meessen B, Soucat A, Sekabaraga C.. 2011. Performance-based financing: just a donor fad or a catalyst towards comprehensive health-care reform? Bulletin of the World Health Organization 89: 153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra A, Sorbero ME, Damberg CL.. 2010. Using the lessons of behavioral economics to design more effective pay-for-performance programs. The American Journal of Managed Care 16: 497–503. [PMC free article] [PubMed] [Google Scholar]
- Mendelson A, Kondo K, Damberg C. et al. 2017. The effects of pay-for-performance programs on health, health care use, and processes of care: a systematic review. Annals of Internal Medicine 166: 341–53. [DOI] [PubMed] [Google Scholar]
- Miller G, Babiarz KS.. 2013. Pay-for-performance incentives in low- and middle-income country health programs. NBER Working Paper No. 18932.
- MoHSW. 2013. Tanzania Service Availability and Readiness Assessment (SARA) 2012. Dar es Salaam: Ministry of Health and Social Welfare and Ifakara Health Institute. [Google Scholar]
- MoHSW. 2015. Tanzania Health Sector Strategic Plan (HSSP IV) 2015–2020. Dar es Salaam: Ministry of Health and Social Welfare (MoHSW; ). [Google Scholar]
- Mullen KJ, Frank RG, Rosenthal MB.. 2010. Can you get what you pay for? Pay-for-performance and the quality of healthcare providers. The Rand Journal of Economics 41: 64–91. [DOI] [PubMed] [Google Scholar]
- Munga MA, Maestad O.. 2009. Measuring inequalities in the distribution of health workers: the case of Tanzania. Human Resources for Health 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NBS. 2013. Tanzania Population and Housing Census: Population Distribution by Administrative Areas 2012. Dar es Salaam: National Bureau of Statistics (NBS; ). [Google Scholar]
- O'Donnell O, Van Doorsslaer E, Wagstaff A, Lindelöw M.. 2008. Analyzing Health Equity Using Household Survey Data: A Guide to Techniques and Their Implementation. Washington, DC: World Bank Publications. [Google Scholar]
- O’Neill K, Takane M, Sheffel A, Abou-Zahr C, Boerma T.. 2013. Monitoring service delivery for universal health coverage: the service availability and readiness assessment. Bulletin of the World Health Organization 91: 923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renmans D, Holvoet N, Orach CG, Criel B.. 2016. Opening the ‘black box’ of performance-based financing in low- and lower middle-income countries: a review of the literature. Health Policy and Planning 31: 1297–309. [DOI] [PubMed] [Google Scholar]
- Rittenhouse DR, Shortell SM, Gillies RR. et al. 2010. Improving chronic illness care: findings from a national study of care management processes in large physician practices. Medical Care Research and Review 67: 301–20. [DOI] [PubMed] [Google Scholar]
- Rosenthal MB, Dudley RA.. 2007. Pay-for-performance: will the latest payment trend improve care? JAMA 297: 740–4. [DOI] [PubMed] [Google Scholar]
- Rosenthal MB, Frank RG.. 2006. What is the empirical basis for paying for quality in health care? Medical Care Research and Review 63: 135–57. [DOI] [PubMed] [Google Scholar]
- Rosenthal MB, Frank RG, Li Z, Epstein AM.. 2005. Early experience with pay-for-performance: from concept to practice. JAMA 294: 1788–93. [DOI] [PubMed] [Google Scholar]
- Ryan AM, Blustein J.. 2011. The effect of the MassHealth hospital pay-for-performance program on quality. Health Services Research 46: 712–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AM, Blustein J, Doran T, Michelow MD, Casalino LP.. 2012. The effect of phase 2 of the premier hospital quality incentive demonstration on incentive payments to hospitals caring for disadvantaged patients. Health Services Research 47: 1418–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry TB, Bauhoff S, Mohanan M.. 2017. Multitasking and heterogeneous treatment effects in pay-for-performance in health care: evidence from Rwanda. American Journal of Health Economics 3: 192–226. [Google Scholar]
- Sutton M, Nikolova S, Boaden R. et al. 2012. Reduced mortality with hospital pay for performance in England. The New England Journal of Medicine 367: 1821–8. [DOI] [PubMed] [Google Scholar]
- TDHS. 2016. Tanzania Demographic and Health Survey and Malaria Indicator Survey 2015–16. Dar es Salaam: National Bureau of Statistics (NBS: ). [Google Scholar]
- Van de Poel E, Flores G, Ir P, O'Donnell O.. 2016. Impact of performance-based financing in a low-resource setting: a decade of experience in Cambodia. Health Economics 25: 688–705. [DOI] [PubMed] [Google Scholar]
- Van Herck P, De Smedt D, Annemans L. et al. 2010. Systematic review: effects, design choices, and context of pay-for-performance in health care. BMC Health Services Research 10: 247.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora CG, Vaughan JP, Barros FC, Silva AC, Tomasi E.. 2000. Explaining trends in inequities: evidence from Brazilian child health studies. Lancet (London, England) 356: 1093–8. [DOI] [PubMed] [Google Scholar]
- Vyas S, Kumaranayake L.. 2006. Constructing socio-economic status indices: how to use principal components analysis. Health Policy and Planning 21: 459–68. [DOI] [PubMed] [Google Scholar]
- WHO. 2004. Equitable Access to Essential Medicines: A Framework for Collective Action. Geneva: World Health Organization. [Google Scholar]
- WHO. 2013. Handbook on Health Inequality Monitoring: With a Special Focus on Low- and Middle-Income Countries. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Witter S, Fretheim A, Kessy FL, Lindahl AK.. 2012. Paying for performance to improve the delivery of health interventions in low- and middle-income countries. Cochrane Database of Systematic Reviews, Cd007899. [DOI] [PubMed] [Google Scholar]
- Witter S, Toonen J, Meessen B. et al. 2013. Performance-based financing as a health system reform: mapping the key dimensions for monitoring and evaluation. BMC Health Services Research 13: 367.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.