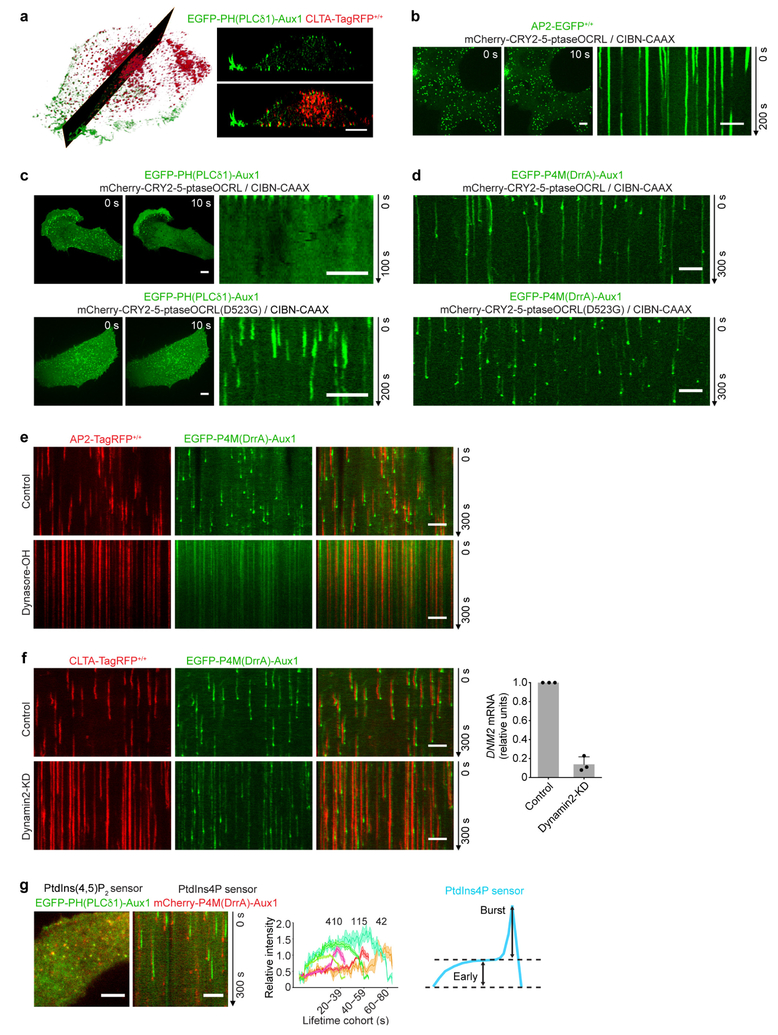
Extended Data Figure 2 |. Restricted localization and lipid specificity of the Aux1-based phosphoinositide sensors.
a, Intracellular distribution of the Auxl-based PtdIns(4,5)P2 sensor recorded by lattice light-sheet microscopy. The representative images are from a CLTA-TagRFP+/+ (red) cell expressing the Auxl-based PtdIns(4,5)P2 sensor EGFP-PH(PLCδl)-Auxl. The left panel shows a deconvolved 3D rendering from a single time point and illustrates the location of the optical section shown in the right panels. The images contrast the full colocalization throughout the plasma membrane of the fluorescent signals corresponding to the sensor and clathrin with the absence of colocalization in endosomal clathrin-containing structures. The EGFP channel in the right lower panel was shifted laterally by six pixels. b, Bottom surface of a gene-edited AP2-EGFP+/+ cell, bearing endocytic AP2 complexes labelled by σ2-EGFP and co-expressing CIBN-CAAX and the mCherry-tagged inositol 5-phosphatase module of OCRL (mCherry-CRY2–5-ptaseOCRL), imaged at 1-s intervals for 200 s by spinning-disk confocal microscopy. Acute depletion of plasma membrane PtdIns(4,5)P2, by recruitment of mCherry-CRY2–5-ptaseOCRL from the cytosol to the plasma membrane, was triggered by illumination with 488-nm light beginning at t = 0 s. The kymograph shows that partially formed coated pits stalled and new ones failed to initiate owing to loss of PtdIns(4,5)P2. c, Bottom surface of a SUM159 cell co-expressing the PtdIns(4,5)P2 sensor EGFP-PH(PLCδl)-Auxl together with CIBN-CAAX and mCherry-CRY2–5-ptaseOCRL imaged at 2-s intervals for l00 s by spinning-disk confocal microscopy. Acute, light-mediated depletion of plasma membrane PtdIns(4,5)P2 was initiated at t = 0 s. The kymograph illustrates loss of membrane recruitment of the Auxl-based PtdIns(4,5)P2 sensor (top). Recruitment of the Auxl-based PtdIns(4,5)P2 sensor to assembling endocytic coated pits was not affected when a mutant of the phosphatase module of OCRL, unable to hydrolyse PtdIns(4,5)P2, was targeted to the plasma membrane by light activation (bottom). SUMl59 cells co-expressing the PtdIns(4,5)P2 sensor EGFP-PH(PLCδl)-Auxl, together with CIBN-CAAX and the catalytically inactive mCherry-CRY2–5-ptaseOCRL(D523G) (bottom). Images at single time points and the corresponding kymograph are shown. d, Early recruitment of the Auxl-based PtdIns4P sensor to stalled coated pits was not affected when the active phosphatase module of OCRL was targeted to the plasma membrane by light activation (top). The late burst of the PtdIns4P sensor was absent, however, consistent with failure of the stalled (that is, persistent) coated pits to finish assembly and hence failure to bud into coated vesicles. As expected, membrane targeting of the mutant phosphatase module of OCRL had no effect on recruitment of the PtdIns4P sensor to coated pits and vesicles (bottom). The cells co-expressed the PtdIns4P sensor EGFP-P4M(DrrA)-Aux1, together with CIBN-CAAX and mCherry-CRY2–5-ptaseOCRL (top) or the catalytically inactive mCherry-CRY2–5-ptaseOCRL(D523G) (bottom). The bottom surface of the cells was imaged using spinning-disk confocal microscopy. e, Blocking dynamin activity prevented the acute burst but not the binding of the PtdIns4P sensor to arrested (that is, persistent) coated structures at the plasma membrane. AP2-TagRFP+/+ cells expressing the PtdIns4P sensor EGFP-P4M(DrrA)-Aux1 were treated for 10 min without or with dynasore-OH (30 μM) and then imaged using TIRF microscopy. f, CLTA-TagRFP+/+ cells stably expressing the PtdIns4P sensor EGFP-P4M(DrrA)-Auxl were treated with siRNA targeting dynamin2 (DNM2) or with a control sequence, and imaged by TIRF microscopy. The kymograph from a time series of the cell depleted of dynamin2 shows stalling of coated pits, absence of acute PtdIns4P burst and maintenance of the binding of the PtdIns4P sensor to arrested (that is, persistent) coated structures at the plasma membrane. The right panel shows the efficiency of DNM2 mRNA depletion measured by real-time quantitative PCR (n = 3 independent experiments, mean ± s.d.). g, Direct comparison of the recruitment dynamics at endocytic clathrin-coated structures of the Auxl-based PtdIns(4,5)P2 and PtdIns4P sensors. The left panels show a representative image and the corresponding kymograph from a time series, obtained by TIRF microscopy from the bottom surface of a SUM159 cell coexpressing the PtdIns(4,5)P2 sensor (green) and the PtdIns4P sensor (red). The central panel shows the averaged fluorescence intensity traces (mean ± s.e.m.) of the PtdIns(4,5)P2 sensor (green) and the PtdIns4P sensor (red), including the numbers of traces analysed for each lifetime cohort. The right panel is a schematic representation of the early (Early) and late (Burst) recruitment of the PtdIns4P sensor during clathrin-coated pit formation. Note that although the PtdIns4P and PtdIns(4,5)P2 sensors are both recruited early during coated pit formation, only the PtdIns4P sensor then appears as a transient burst coinciding with decline of the PtdIns(4,5)P2 signal. The EGFP channel in all the kymographs with EGFP and TagRFP overlaid was shifted laterally by six pixels. Data are representative of at least two independent experiments. Scale bars, 5 μm.