Abstract
Background
Breast cancer and its treatments are associated with a detrimental effect on bone health. Here we report the results of an exploratory analysis assessing changes in levels of biomarkers of bone metabolism in patients enrolled in the phase IIIb 4EVER study.
Methods
The 4EVER trial investigated everolimus in combination with exemestane in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer. In this prespecified exploratory analysis, changes in biomarkers of bone turnover were assessed in patients from baseline to weeks 4, 12, and 24. The serum bone markers assessed were procollagen type 1 N-terminal propeptide (P1NP), C-terminal cross-linking telopeptide of type 1 collagen (CTX), osteocalcin, parathyroid hormone (PTH), and 25-hydroxyvitamin D (25-OH-vitamin D). On-treatment changes in bone markers over time were described per subgroup of interest and efficacy outcomes.
Results
Bone marker data were available for 241 of 299 enrolled patients. At the final assessment, P1NP, osteocalcin, PTH, 25-OH-vitamin D (all P < 0.001), and CTX (P = 0.036) were significantly decreased from baseline values per the Wilcoxon signed-rank test. At the last assessment (24 weeks or earlier), levels of serum CTX and PTH were significantly lower (P = 0.009 and P = 0.034, respectively) among patients with vs. without prior antiresorptive treatment (ART). Serum CTX levels were significantly lower (P < 0.001), and 25-OH-vitamin D concentrations significantly higher (P = 0.029), at the last postbaseline assessment in patients receiving concomitant ART vs. those without ART. Changes from baseline in PTH and 25-OH-vitamin D concentrations to the final assessment were significantly smaller in patients with prior ART. Lower baseline serum concentrations of osteocalcin and PTH were associated with clinical response (partial vs. non-response) at 24 weeks. High serum levels of CTX and P1NP at baseline were risk factors for progression at 12 weeks.
Conclusions
These exploratory analyses support use of everolimus plus exemestane for the treatment of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer, and add to the body of evidence suggesting a potentially favorable impact of everolimus on bone turnover.
Trial registration
NCT01626222. Registered 22 June 2012, https://clinicaltrials.gov/ct2/show/NCT01626222.
Keywords: Bone health, Bone marker, Breast cancer, Everolimus, Hormone receptor-positive, Mammalian target of rapamycin
Abbreviations: 25-OH-vitamin D, 25-hydroxyvitamin D; ART, antiresorptive therapy; BSAP, bone-specific alkaline phosphatase; CI, confidence interval; CTX, C-terminal cross-linking telopeptide of type 1 collagen; HER2, human epidermal growth factor receptor 2; HR+, hormone receptor-positive; mTOR, mammalian target of rapamycin; NSAI, non-steroidal aromatase inhibitor; OR, overall response; ORR, overall response rate; ORR24w, overall response rate within the first 24 weeks of treatment; P1NP, procollagen type 1 N-terminal peptide; PFS, progression-free survival; PTH, parathyroid hormone; SD, standard deviation; SRE, skeletal-related event
1. Introduction
The integrity of the skeleton can be negatively influenced by metastatic involvement and cancer therapy-related adverse effects on bone turnover [1]. In women with advanced breast cancer, poor bone health can lead to skeletal-related events (SREs), such as pathological fracture, spinal cord compression, hypercalcemia, and an increased need for palliative radiotherapy or orthopedic surgery to bone, that may severely compromise quality of life [2]. Preserving bone health while maximizing treatment outcomes is therefore an important objective of therapy for patients with advanced breast cancer [2], [3].
The phase III BOLERO-2 trial demonstrated that addition of the mammalian target of rapamycin (mTOR) inhibitor everolimus to endocrine therapy with exemestane significantly prolonged the response period in postmenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer that had progressed on prior non-steroidal aromatase inhibitor (NSAI) treatment [4], [5]. Progression-free survival (PFS) was extended in patients who received the combination vs. exemestane alone (7.8 months vs. 3.2 months, hazard ratio = 0.45; 95% confidence interval [CI]: 0.38–0.54; P < 0.0001) [4]. Regulatory approval was granted for this combination, based on the results of the aforementioned study [4]. Exploratory analyses of BOLERO-2 indicated that treatment with everolimus had beneficial effects on bone homeostasis, assessed by a decrease in bone turnover markers (bone-specific alkaline phosphatase [BSAP], procollagen type 1 N-terminal propeptide [P1NP], and C-terminal cross-linking telopeptide of type 1 collagen [CTX]) relative to baseline, and reduced progressive disease in bone vs. exemestane alone [6].
The single-arm phase IIIb 4EVER trial (ClinicalTrials.gov identifier: NCT01626222) investigated the combination of everolimus and exemestane for the treatment of postmenopausal women with HR+, HER2-negative locally advanced or metastatic breast cancer. Compared with BOLERO-2, the inclusion criteria for the 4EVER trial did not restrict the number of prior chemotherapy lines, the time of recurrence and progression after NSAI therapy, and allowed prior exemestane treatment. The primary results of this trial have been presented previously [7]. Here, we report on a prespecified exploratory endpoint of the 4EVER trial analyzing markers of bone turnover to assess the impact of everolimus on bone.
2. Methods
2.1. Study design and patients
4EVER (ClinicalTrials.gov identifier: NCT01626222) was a multicenter, open-label, single-arm, phase IIIb trial conducted in Germany. The study design has been described previously [7].
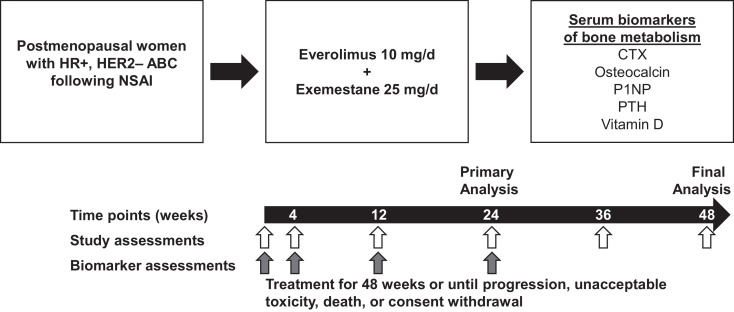
Patients were postmenopausal women with HR+, HER2-negative locally advanced or metastatic breast cancer that had progressed following treatment with an NSAI (letrozole or anastrozole; Fig. 1). Progression was defined as recurrence while on, or following completion of adjuvant treatment with an NSAI, or progression while on, or following completion of NSAI therapy for locally advanced or metastatic breast cancer. Other eligibility criteria have been described previously [7].
Fig. 1.
Study design. Abbreviations: ABC, advanced breast cancer; CTX, C-terminal cross-linking telopeptide of type 1 collagen; d, day; HER2–, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; NSAI, non-steroidal aromatase inhibitor; P1NP, procollagen type 1 N-terminal peptide; PTH, parathyroid hormone.
The protocol was reviewed and approved by the institutional review board at each participating center, each of which provided ethical approval for the collection of the data described in this report. The study was conducted in accordance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice, with applicable local regulations, and with the ethical principles laid down in the Declaration of Helsinki. All patients provided written informed consent for inclusion in this research.
2.2. Treatment and dose modifications
Patients received 10 mg/day everolimus and 25 mg/day exemestane orally for 48 weeks, or until disease progression, unacceptable toxicity, death, or discontinuation for any other reason. Dose adjustments were performed as described previously [7].
2.3. Efficacy and safety assessments
The primary study endpoint was overall response rate (ORR), defined as the proportion of patients with a best overall response of complete response or partial response within the first 24 weeks of treatment (ORR24w), and has been reported elsewhere [7].
2.4. Bone marker assessments
Assessment of percentage change in serum biomarkers of bone turnover was a protocol-defined exploratory objective to evaluate the potential protective effects of everolimus on bone. The bone markers measured allowed for assessment of: bone formation (P1NP, osteocalcin); osteoclast activation and bone resorption (CTX); parathyroid hormone [PTH]; and markers of overall bone health (25-hydroxyvitamin D [25-OH-vitamin D]).
Changes in blood levels of bone metabolism biomarkers were assessed from baseline to weeks 4, 12, and 24, or the last postbaseline assessment (24 weeks or earlier); samples were to be optimally collected in the morning following an overnight fast of at least 8 h, and performed consistently during study visits. Samples were stored at –80 °C and shipped to the central laboratory at the Department of Gynecological Endocrinology, Reproductive Medicine and Osteoporosis at the Philipps University of Marburg, Germany. All analyses (P1NP, osteocalcin, CTX, PTH, and 25-OH-vitamin D) were assessed using commercially available ELISA kits (COBAS device, Roche Diagnostics GmbH, Germany), in a single batch at the end of the study.
Subgroup analyses of bone marker changes over time were performed considering the following variables: presence of bone metastasis; prior antiresorptive therapy (ART; selected using the World Health Organization Drug Dictionary, Anatomical Therapeutic Chemical index, M05B classification [drugs affecting bone structure and mineralization]); ART concomitant with study medication; prior radiation to bone; prior bone fracture; and bone fracture while on study (yes/no for all).
2.5. Statistical analyses
Analysis of the primary endpoint of ORR24w has been reported elsewhere [7].
Differences in bone marker serum concentrations between baseline time-point measurements and the first postbaseline value (4 weeks) or the last postbaseline value (24 weeks or earlier) were evaluated using the Wilcoxon signed-rank test. The Wilcoxon rank-sum test was used to evaluate differences in bone marker serum concentrations according to patient and treatment characteristics. P-values are two-sided, with P less than 0.05 indicating statistical significance. The Kruskal–Wallis test was used to determine any association between baseline bone marker concentrations and best clinical response by weeks 24 and 48. Cox regression analyses were used to evaluate the presence of any relationship between PFS and baseline bone marker concentrations and any changes in bone marker concentrations at weeks 4 and 12. A logistic regression model was used to analyze any relationship between bone marker concentrations and disease progression after 12 weeks (yes/no) presented as odds ratio (OR) with associated Wald 95% CI.
3. Results
A total of 299 patients were enrolled in the 4EVER study from 25 June 2012 to 26 November 2013 at 82 study centers in Germany. For the primary assessment, all 299 patients were included in the safety analyses, and 281 patients were included in the full analysis set. Data validity issues led to the exclusion of 18 patients, as described elsewhere [7].
Data on bone markers were available for 241/281 (86%) patients. Reasons for missing samples included samples not being collected or analyzed per the protocol. Median treatment exposure for patients included in the bone biomarker analysis was 3.8 months for everolimus and 4.2 months for exemestane.
The median age of women in the bone marker analysis was 67 years (35–85 years), most were Caucasian (99.2%), and the majority had an Eastern Cooperative Oncology Group performance status of 0 at baseline (60.8%; Table 1). All patients were postmenopausal, as required by the protocol. The characteristics of patients in the bone marker population were consistent with those included in the full population. A total of 143 (59.3%) patients had bone metastases at baseline. Fifty-eight patients (24.1%) had previous ART use for a median of 30 months before starting everolimus, including zoledronic acid in 42 patients (17.4%), ibandronic acid in 11 patients (4.6%), and disodium pamidronate in seven patients (2.9%); 152 patients (63.1%) were taking ART concomitantly with study medication. Overall, 56 patients (23.2%) had received prior radiation to bone; 11 patients (4.6%) had experienced a bone fracture prior to study entry; and four patients (1.7%) experienced a bone fracture while on study.
Table 1.
Patient baseline and disease characteristics of the bone analysis population.
| Baseline parameters | N = 241 |
|---|---|
| Median age (range), years | 67 (35–85) |
| Age group, n (%) | |
| <65 years | 111 (46.1) |
| ≥65 years | 130 (53.9) |
| Race, n (%) | |
| Caucasian | 239 (99.2) |
| Asian | 2 (0.8) |
| ECOG PS, n (%)a,b | |
| 0 | 146 (60.8) |
| 1 | 85 (35.4) |
| 2 | 9 (3.8) |
| Missing | 1 (0.4) |
| Disease status, n (%) | |
| Metastatic | 231 (95.9) |
| Locally advanced | 4 (1.7) |
| Metastatic + locally advanced | 6 (2.5) |
| HR status, n (%)c | |
| ER+, PgR+ | 192 (79.7) |
| ER+, PgR– | 47 (19.5) |
| ER–, PgR+ | 1 (0.4) |
| ER–, PgR– | 1 (0.4) |
| HER2 status, n (%)d | |
| HER2-negative | 240 (99.6) |
| Unknown/missing | 1 (0.4) |
| Baseline bone metastases, n (%) | |
| Yes | 143 (59.3) |
| No | 98 (40.7) |
| Baseline ART, n (%) | |
| Yes | 58 (24.1) |
| No | 183 (75.9) |
| Concomitant ART and study treatment, n (%) | |
| Yes | 152 (63.1) |
| No | 89 (36.9) |
| Prior radiation to bone, n (%) | |
| Yes | 56 (23.2) |
| No | 185 (76.8) |
| Prior fracture, n (%) | |
| Yes | 11 (4.6) |
| No | 230 (95.4) |
| Fracture during study, n (%) | |
| Yes | 4 (1.7) |
| No | 237 (98.3) |
Abbreviations: ART, antiresorptive treatment; ECOG PS, European Cooperative Oncology Group performance status; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; PgR, progesterone receptor.
At screening.
No patient had an ECOG PS ≥ 3 at screening; however, one patient had an ECOG PS = 3 at baseline.
Assessed in primary tumor in 70.5% of patients, in metastatic sites in 29.5% of patients.
Assessed in primary tumor in 66.7% of patients, in metastatic sites in 33.3% of patients, in unknown tumor stage for one (0.4%) patient.
3.1. Efficacy
The primary objective of the study (ORR24w) has been reported previously (8.9% [95% CI: 5.8–12.9]) [7].
3.2. Bone marker assessments
For the overall population of patients with bone marker assessments, serum bone marker concentrations and changes from baseline to week 4 and the last postbaseline visit (last assessment at week 24 or earlier) are shown in Table 2. At baseline, mean serum concentrations of key bone markers were within relevant reference ranges [8], including P1NP (mean: 84.09 ng/ml [standard deviation; SD: 91.63]; reference range: 16–96 ng/ml), osteocalcin (mean: 10.71 ng/ml [SD: 9.04]; reference range: 9–42 ng/ml), CTX (mean: 0.15 ng/ml [SD: 0.34]; reference range: 0.10–1.01 ng/ml), PTH (mean: 42.99 pg/ml [SD: 204.36]; reference range: 15–65 pg/ml), and 25-OH-vitamin D (mean: 24.62 ng/ml [SD: 13.76]; reference range: 20–50 ng/ml; Table 2).
Table 2.
Changes from baseline in bone biomarker values to first (4 weeks) and last postbaseline values.
| Baseline |
First postbaseline value (4 weeks) |
Change from baseline to first postbaseline value (4 weeks) |
Last postbaseline value (24 weeks or earlier) |
Change from baseline to last postbaseline value |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (range) | n | Mean (SD) | Median (range) | n | Mean (SD) | Median (range) | P-valuea | n | Mean (SD) | Median (range) | n | Mean (SD) | Median (range) | P-valuea | |
| CTX (ng/ml) | 230 | 0.15 (0.34) | 0.08 (0.01‒4.95) |
211 | 0.14 (0.33) | 0.08 (0.01‒4.44) |
195 | −0.01 (0.12) | 0.00 (−0.51‒0.96) |
0.322 | 214 | 0.13 (0.12) | 0.10 (0.01‒0.72) |
196 | −0.01 (0.36) | 0.01 (−4.76‒0.50) |
0.036 |
| Osteocalcin (ng/ml) | 241 | 10.71 (9.04) | 8.20 (0.97‒77.18) | 221 | 11.24 (12.15) | 8.04 (0.69‒134.90) | 210 | 0.12 (8.40) | −0.29 (−42.94‒82.88) | 0.395 | 219 | 9.85 (8.21) | 7.81 (1.00‒77.90) | 208 | −1.45 (7.05) | −0.96 (−40.88‒25.88) | < 0.001 |
| P1NP (ng/ml) | 241 | 84.09 (91.63) | 53.70 (10.82‒619.40) | 221 | 60.56 (62.54) | 39.62 (6.68‒323.30) | 210 | −22.16 (62.18) | −10.44 (−594.15‒108.93) | < 0.001 | 218 | 66.83 (122.59) | 37.06 (6.20‒1603.00) | 207 | −24.29 (78.68) | −11.53 (−589.95‒381.71) | < 0.001 |
| PTH (pg/ml) | 239 | 42.99 (204.36) | 20.59 (1.35‒3158.00) | 220 | 45.20 (143.25) | 23.73 (2.37‒2072.00) | 208 | 1.55 (82.67) | 2.38 (−1086.00‒206.34) | 0.008 | 220 | 40.84 (47.08) | 28.70 (1.36‒447.50) | 208 | −3.18 (220.51) | 6.30 (−3103.47‒381.74) | < 0.001 |
| 25-OH-vitamin D (ng/ml) | 239 | 24.62 (13.76) | 21.36 (3.92‒65.03) | 221 | 22.91 (13.16) | 20.81 (3.26‒66.83) | 209 | −1.29 (3.83) | −1.28 (−12.71‒9.93) | < 0.001 | 218 | 22.29 (14.16) | 18.62 (3.40‒69.64) | 207 | −1.96 (5.81) | −1.89 (−28.99‒21.78) | < 0.001 |
Abbreviations: 25-OH-vitamin D, 25-hydroxyvitamin D; CTX, C-terminal cross-linking telopeptide of type 1 collagen; P1NP, procollagen type 1 N-terminal peptide; PTH, parathyroid hormone; SD, standard deviation.
P-values for change from baseline used the Wilcoxon signed-rank test for differences in biomarker concentrations. Statistical significance was accepted when P < 0.05 (bold text).
At the first postbaseline assessment (4 weeks), significant decreases from baseline were observed for serum levels of P1NP (mean [SD] change: –22.16 ng/ml [62.18]; P < 0.001) and 25-OH-vitamin D (mean [SD] change: –1.29 ng/ml [3.83]; P < 0.001), while a significant increase from baseline was observed for PTH (mean [SD] change: 1.55 pg/ml [82.67]; P = 0.008; Table 2). At the last postbaseline assessment, significant reductions from baseline were reported for all of these bone markers: P1NP (mean [SD] change: –24.29 [78.68] ng/ml; P < 0.001); osteocalcin (mean [SD] change: –1.45 [7.05] ng/ml; P < 0.001); PTH (mean [SD] change: –3.18 [220.51] pg/ml; P < 0.001), 25-OH-vitamin D (mean [SD] change: –1.96 [5.81] ng/ml; P < 0.001); and CTX (mean [SD] change: –0.01 [0.36] ng/ml; P = 0.036; Table 2). All mean values remained within relevant reference ranges.
Differences in serum levels of bone biomarkers according to baseline patient disease and treatment characteristics were evaluated at the last postbaseline time-point (Table 3). Mean (SD) serum CTX levels were significantly lower for patients with prior ART compared with those without prior ART (0.11 [0.13] vs. 0.14 [0.12] ng/ml, respectively; P = 0.009) and for patients taking concomitant ART compared with those not receiving concomitant ART (0.11 [0.10] vs. 0.18 [0.14] ng/ml, respectively; P < 0.001). Mean (SD) serum PTH was also significantly lower among patients who had prior ART compared with those without (32.74 [41.69] vs. 44.15 [49.65] pg/ml, respectively; P = 0.034). Mean 25-OH-vitamin D concentrations were significantly higher in patients taking concomitant ART vs. those without (23.72 [14.66] vs. 19.21 [12.68] ng/ml; P = 0.029; Table 3). Among the bone markers reported here, no other patient or treatment variables significantly impacted serum levels at the last postbaseline assessment, including bone metastasis, prior bone irradiation, or prior fracture (Table 3).
Table 3.
Differences in bone biomarker values at last postbaseline assessment according to key baseline patient or treatment characteristics.
| Bone metastases |
Prior AR treatment |
Concomitant AR treatment |
Prior bone radiation |
Prior fracture |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without | With | Without | With | Without | With | Without | With | Without | With | ||
| CTX (ng/ml) | n | 82 | 121 | 152 | 51 | 72 | 131 | 157 | 46 | 195 | 8 |
| Mean (SD) | 0.14 (0.13) | 0.13 (0.12) | 0.14 (0.12) | 0.11 (0.13) | 0.18 (0.14) | 0.11 (0.10) | 0.14 (0.13) | 0.11 (0.09) | 0.13 (0.12) | 0.11 (0.10) | |
| Median (range) | 0.10 (0.01‒0.65) |
0.10 (0.01‒0.72) |
0.10 (0.01‒0.65) |
0.07 (0.02‒0.72) |
0.14 (0.01‒0.65) |
0.08 (0.01‒0.72) |
0.10 (0.01‒0.72) |
0.09 (0.01‒0.45) |
0.10 (0.01‒0.72) |
0.06 (0.03‒0.32) |
|
| P-valuea | 0.375 | 0.009 | < 0.001 | 0.372 | 0.446 | ||||||
| Osteocalcin (ng/ml) |
n | 82 | 126 | 156 | 52 | 73 | 135 | 161 | 47 | 200 | 8 |
| Mean (SD) | 9.76 (6.85) | 10.13 (9.15) | 9.68 (6.38) | 10.89 (12.47) | 10.54 (7.07) | 9.69 (8.92) | 9.97 (8.54) | 10.05 (7.52) | 10.08 (8.44) | 7.48 (3.14) | |
| Median (range) | 7.76 (1.28‒42.49) | 7.81 (1.00‒77.90) | 7.97 (1.28‒42.49) | 7.56 (1.00‒77.90) |
7.86 (2.31‒42.49) | 7.81 (1.00‒77.90) | 7.67 (1.00‒77.90) | 8.40 (2.33‒43.87) |
7.81 (1.00‒77.90) | 6.98 (3.26‒11.46) |
|
| P-valuea | 0.902 | 0.373 | 0.200 | 0.556 | 0.498 | ||||||
| P1NP (ng/ml) | n | 81 | 126 | 155 | 52 | 72 | 135 | 160 | 47 | 199 | 8 |
| Mean (SD) | 54.86 (59.78) | 63.91 (67.78) | 59.98 (63.40) | 61.53 (69.33) | 55.50 (49.13) | 62.97 (71.77) | 57.12 (61.44) | 71.44 (74.67) | 61.19 (65.62) | 39.80 (34.24) | |
| Median (range) | 37.17 (9.22‒458.20) | 37.12 (6.20‒323.30) | 38.34 (6.20‒458.20) | 34.00 (11.52‒302.80) | 39.09 (6.20‒302.80) | 36.06 (9.22‒458.20) | 37.06 (6.20‒458.20) | 38.41 (11.52‒323.30) | 37.60 (6.20‒458.20) | 29.97 (12.66‒114.30) | |
| P-valuea | 0.999 | 0.500 | 0.475 | 0.505 | 0.283 | ||||||
| PTH (pg/ml) | n | 83 | 126 | 157 | 52 | 73 | 136 | 162 | 47 | 201 | 8 |
| Mean (SD) | 42.64 (56.54) | 40.43 (41.57) | 44.15 (49.65) | 32.74 (41.69) | 35.81 (28.91) | 44.26 (55.43) | 40.37 (41.82) | 44.54 (65.30) | 42.12 (48.68) | 20.99 (12.30) | |
| Median (range) | 29.49 (3.65‒447.50) | 29.00 (1.36‒289.50) | 30.40 (2.78‒447.50) | 23.75 (1.36‒289.50) | 28.45 (1.36‒119.00) | 29.67 (2.78‒447.50) | 28.59 (1.36‒289.50) | 30.70 (6.63‒447.50) | 29.49 (1.36‒447.50) | 21.93 (3.20‒33.26) |
|
| P-value a | 0.914 | 0.034 | 0.560 | 0.581 | 0.145 | ||||||
| 25-OH-vitamin D (ng/ml) | n | 82 | 125 | 155 | 52 | 72 | 135 | 160 | 47 | 200 | 7 |
| Mean (SD) | 21.10 (13.06) | 22.85 (14.81) | 21.80 (13.93) | 23.20 (14.82) | 19.21 (12.68) | 23.72 (14.66) | 22.42 (14.18) | 21.24 (14.10) | 22.11 (14.01) | 23.45 (18.64) | |
| Median (range) | 17.53 (4.08‒57.75) | 18.90 (3.40‒69.64) | 18.15 (3.40‒69.64) | 21.50 (3.65‒69.44) |
16.48 (3.40‒69.64) | 21.21 (3.91 ‒69.44) |
18.53 (3.65‒69.44) | 17.59 (3.40‒69.64) |
18.15 (3.40‒69.64) | 19.84 (8.30‒62.76) |
|
| P-value a | 0.461 | 0.542 | 0.029 | 0.551 | 0.987 | ||||||
Abbreviations: 25-OH-vitamin D, 25-hydroxyvitamin D; AR, antiresorptive; CTX, C-terminal cross-linking telopeptide of type 1 collagen; P1NP, procollagen type 1 N-terminal peptide; PTH, parathyroid hormone; SD, standard deviation.
P-values for differences in last postbaseline assessment values for each biomarker were established using the Wilcoxon rank-sum test. Statistical significance was accepted when P < 0.05 (bold text).
On-treatment changes from baseline in bone biomarker values were also evaluated according to the same patient disease and treatment characteristics at the last postbaseline assessment time-point. Patients receiving prior ART before starting study treatment experienced smaller changes from baseline in serum PTH (P = 0.026) and 25-OH-vitamin D (P = 0.039) than those without prior ART. No differences in absolute change from baseline were observed for any other bone biomarker reported here, by patient or treatment characteristic.
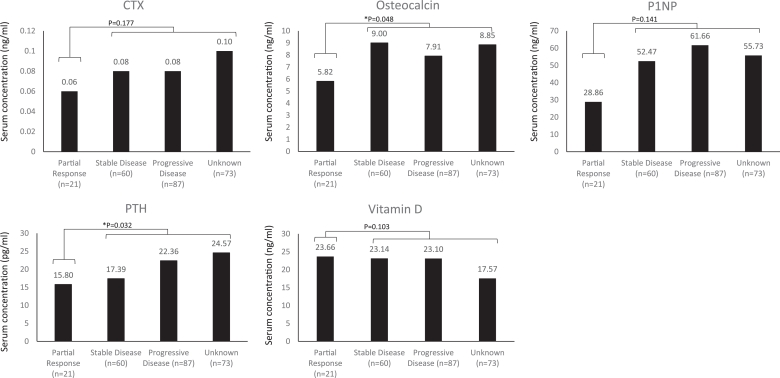
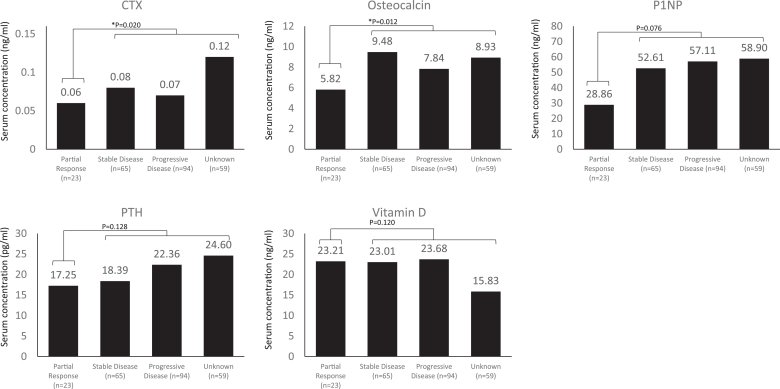
Baseline bone biomarkers were evaluated according to best overall response within the first 24 weeks of treatment. Baseline serum concentrations of osteocalcin (P = 0.048) and PTH (P = 0.032) were significantly lower in patients with better clinical responses (partial response vs. non-responders [unknown, progressive disease, stable disease]) after 24 weeks of treatment with everolimus plus exemestane (Fig. 2). Baseline serum concentrations of CTX (P = 0.020) and osteocalcin (P = 0.012) were significantly lower in patients with better clinical responses vs. non-responders, as assessed after 48 weeks of study treatment (Fig. 3). Of note, 73 (30.3%) and 59 (24.5%) patients had an unknown response status at 24 weeks and 48 weeks, respectively, due to early discontinuation of study treatment, missing Response Evaluation Criteria In Solid Tumors data at week 24 or 48, or loss to follow-up; these patients were regarded as non-responders.
Fig. 2.
Median baseline bone biomarker levels according to best overall response after 24 weeks of treatment. Statistical significance was accepted when P < 0.05. P-values for difference in baseline biomarker values based on overall response were established using the Kruskal–Wallis test. Patients were classified with an unknown response status at 24 weeks due to early discontinuation of study treatment, missing Response Evaluation Criteria In Solid Tumors data at week 24, or loss to follow-up. Abbreviations: CTX, C-terminal cross-linking telopeptide of type 1 collagen; P1NP, procollagen type 1 N-terminal peptide; PTH, parathyroid hormone.
Fig. 3.
Median baseline bone biomarker levels according to best overall response after 48 weeks of treatment. Statistical significance was accepted when P < 0.05. P-values for difference in baseline biomarker values based on overall response were established using the Kruskal–Wallis test. Patients were classified with an unknown response status at 48 weeks due to early discontinuation of study treatment, missing Response Evaluation Criteria In Solid Tumors data at week 48, or loss to follow-up. Abbreviations: CTX, C-terminal cross-linking telopeptide of type 1 collagen; P1NP, procollagen type 1 N-terminal peptide; PTH, parathyroid hormone.
The correlation between PFS and baseline bone markers, change in bone markers at 4 weeks, and change in bone markers at 12 weeks was assessed. Multiple logistic regression analysis did not show any statistically relevant influences of baseline bone marker levels on progression status at week 12 (the analysis used progression status [yes/no] at week 12, rather than PFS, as a dependent variable). A logistic regression model for progression suggested that high values for CTX and P1NP at baseline tended to be higher risk factors for progression at 12 weeks (OR: 1.534 [1.042–2.258] for CTX and 1.072 [1.019–1.127] for P1NP), while high values for PTH and osteocalcin tended to be lower risk factors for progression at 12 weeks (OR: 0.928 [0.873–0.988] for PTH and 0.268 [0.120–0.598] for osteocalcin).
3.3. Safety
The safety profile of everolimus plus exemestane in the 4EVER study has been reported previously [7]. In the full safety population (N = 299), the most frequent all-grade adverse events were stomatitis, fatigue, diarrhea, nausea, decreased appetite, and dyspnea, which are consistent with the known safety profile of everolimus plus exemestane in this setting. Adverse events affecting the skeleton included: fracture (eight patients; 2.7%), osteonecrosis of the jaw (two patients; 0.7%), and osteoporosis (one patient; 0.3%).
4. Discussion
In this exploratory analysis of the phase IIIb single-arm 4EVER study, we studied the effect of everolimus plus exemestane on levels of bone metabolism biomarkers in a population of patients with advanced HR+, HER2-negative breast cancer. Patients included in this trial had no restrictions on the number of previous chemotherapy lines for advanced disease, time of recurrence or progression after NSAI therapy, or previous exemestane therapy. Over the course of the study, statistically significant changes relative to baseline were observed in mean levels of certain biomarkers indicative of bone turnover: decreases were observed in levels of bone formation biomarkers (P1NP and osteocalcin), bone resorption biomarkers (CTX and PTH), and 25-OH-vitamin D (an important regulator of bone turnover involved in mineralization and responsible for bone formation and resorption). The absence of a comparator arm limits the interpretation of the analyses. Nevertheless, these exploratory analyses support use of everolimus plus exemestane for the treatment of postmenopausal women with HR+, HER2-negative advanced breast cancer, and add to the body of evidence suggesting a potentially favorable impact of everolimus on bone turnover.
The findings presented here align with the pattern of bone biomarker changes observed in the experimental arm (everolimus plus exemestane) of the pivotal phase III BOLERO-2 study. In BOLERO-2, mean P1NP, CTX, and BSAP levels decreased over the course of the study in the everolimus plus exemestane arm, while they increased in the exemestane plus placebo arm [6]. The authors of that study proposed that these findings suggest a bone protective effect with everolimus that counters the well-documented negative effect of exemestane on skeletal integrity [6], [9], [10]. The additional bone biomarkers measured in the 4EVER study (PTH, osteocalcin, and 25-OH-vitamin D) relative to BOLERO-2 provide further information on the effect of everolimus plus exemestane on bone turnover. In particular, the observed decrease in PTH with everolimus plus exemestane may indicate lower levels of osteoclast activation.
The 4EVER study more closely represented a ‘real-world’ population of postmenopausal patients with HR+, HER2-negative advanced breast cancer compared with the selected group of patients included in the BOLERO-2 trial. Patients in the 4EVER study had more advanced disease and were more heavily pretreated than those in BOLERO-2 [4], [5]. For example, a larger proportion of patients recruited into the 4EVER study had received multiple lines of endocrine treatment vs. BOLERO-2, which may have adversely impacted bone health prior to study entry. Furthermore, over 50% of patients in the 4EVER study had already been exposed to palliative chemotherapy. Previous studies in patients receiving chemotherapy for early breast cancer in the neoadjuvant or adjuvant settings unequivocally show that modern regimens including alkylating agents as well as anthracyclines and taxanes are likely to exhibit detrimental effects on bone metabolism, which may be best classified as a direct inhibition of osteoblast function [11], [12]. Nevertheless, the effect of chemotherapy for advanced breast cancer on bone metabolism still needs to be clarified. A limitation of the present study was that no information on the prior use of anti-angiogenic agents, such as bevacizumab, was available for the bone marker population, as these compounds are likely to negatively impact bone metabolism.
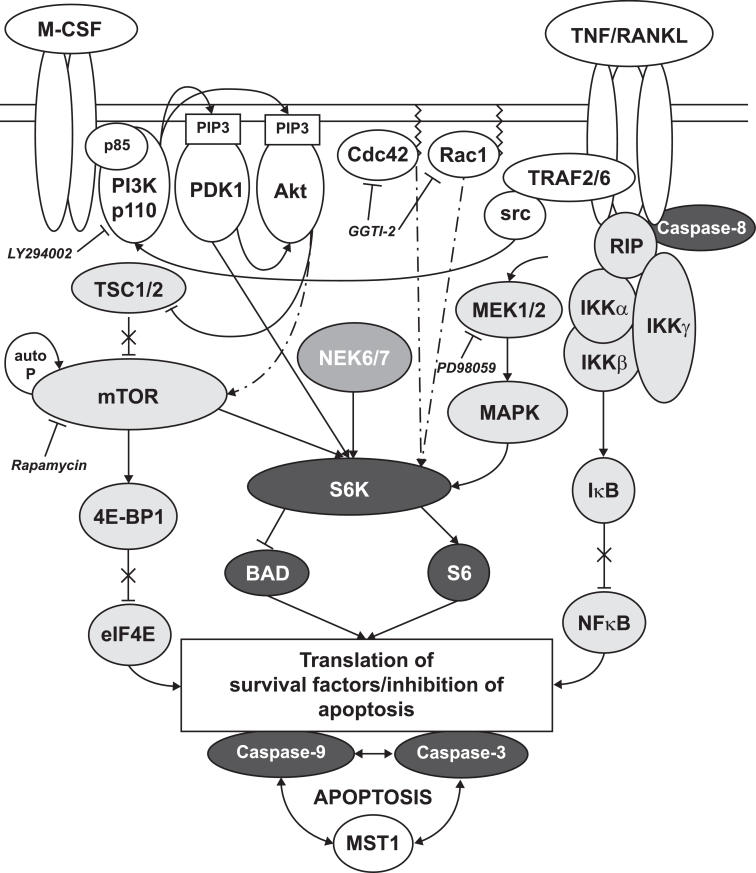
A bone protective effect with everolimus is supported by the results of preclinical studies. Everolimus was shown to impair osteoclastogenesis in vitro, but has little or no effect on osteoblastogenesis [13]. A further in vitro study showed that downregulation of mTOR reduced bone resorption, decreased osteoclast maturation, and increased osteoclast apoptosis [14]. Cell line models have indicated that this bone protective effect may be driven through upregulation of osteoprotegerin [15], a decoy receptor for receptor activator of nuclear factor kappa-B ligand (RANKL), which is required for osteoclastogenesis and osteoclast activation (Fig. 4) [16]. In vivo, in addition to preventing tumor growth, everolimus restored ovariectomized-induced bone loss in rat/murine models through reduced osteoclast-mediated bone resorption [13], [17]. Further assessment by bone histomorphometry confirmed that the positive effects of everolimus on skeletal integrity were achieved through inhibition of osteoclast-mediated bone resorption [13].
Fig. 4.
Schematic illustration of mTOR/S6K intracellular signal transduction pathways in the osteoclast [16]. M-CSF and RANKL are required for osteoclastogenesis and osteoclast activation. Upon activation of their respective receptors, M-CSF-R and RANK, downstream signaling pathways are activated. Signals converge on the mTOR/S6K axis. Upon mTOR inhibition, suppression of this pathway leads to osteoclast apoptosis and thereby reduced bone resorption. Abbreviations: Akt, protein kinase B; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; P, phosphate; RANK, receptor activator of nuclear factor kappa-B; RANKL, receptor activator of nuclear factor kappa-B ligand; S6K, 40 S ribosomal S6 kinase; src, steroid receptor coactivator; TNF, tumor necrosis factor; TSC tuberous sclerosis complex.
In the present study, it was expected that levels of bone metabolism biomarkers would change from baseline when bone metastasis were present to reflect the increase in osteoclast activity, especially in those patients not receiving ART; however, no interaction between bone metastasis and differences in bone biomarker serum levels was detected. Levels of CTX, a marker of bone resorption, were higher in patients without prior ART vs. patients with prior ART. Furthermore, patients who received concomitant ART had lower levels of CTX, and higher 25-OH-vitamin D. These effects are consistent with the expected impact of an antiresorptive therapy on osteoclast activity and improved overall bone health. A limitation of the present study was that no information on concomitant vitamin D supplementation was collected. Patients receiving ART are more likely to be taking supplemental vitamin D, and this in turn may affect the levels of bone biomarkers measured. Nevertheless, the possible influence of 25-OH-vitamin D on cancer-specific outcomes is irrespective of its origin (exogenous vs. endogenous).
When the association between bone biomarkers and patient clinical outcome was examined, lower baseline serum concentrations of CTX were observed in patients with better clinical response at 48 weeks; this was consistent with the finding using a logistic regression model identifying high CTX levels as a high-risk factor for progression at 12 weeks. Although high P1NP levels were found to be another high-risk factor for progression at 12 weeks by the logistic regression model, changes in P1NP were not associated with any of the variables tested. Although these findings are promising, the results should be interpreted carefully due to the small sample size of responders.
5. Conclusions
Endocrine therapy for breast cancer, particularly aromatase inhibitors, reduces bone mass by promoting osteoclastic bone resorption. In patients with advanced cancer, the consequent SREs can severely compromise quality of life. Preserving bone health while maximizing treatment outcomes is therefore an important objective of breast cancer therapy. Overall, the exploratory findings from the 4EVER trial support use of everolimus plus exemestane for the treatment of postmenopausal women with HR+, HER2-negative advanced breast cancer; they add to the body of preclinical and clinical evidence showing that everolimus may have an impact on bone mineral density, and thereby may prevent the SREs that can impact quality of life. Importantly, we provide supplementary clinical evidence to support our conclusions by presenting additional bone biomarkers not previously measured in BOLERO-2.
Acknowledgments
Acknowledgments
The authors would like to thank the patients involved with the 4EVER trial and their families.
Medical editorial assistance was provided by Alison Lovibond Ph.D. of Articulate Science and was funded by Novartis Pharmaceuticals Corporation.
Funding
This work was supported by Novartis Pharmaceuticals Corporation.
Declarations
PH has received speaker fees and unrestricted research grants from Novartis, Pfizer, Amgen, and Astra Zeneca. TD has received honoraria for advisory boards from Novartis. CMK reports personal fees from Novartis during the conduct of this study. Personal fees and non-financial support were received from Novartis, Amgen, Roche, and Teva; personal fees from Axios and Riemser; and non-financial support from Medac outside the submitted work. FM reports receiving consultancy fees from Novartis and Pfizer during the conduct of the study. AS has received honoraria for scientific talks from Roche, Celgene, Astra Zeneca, Novartis, and Pfizer, and research support from Celgene. PAF reports grants and personal fees from Novartis, and personal fees from Pfizer, Roche, and Celgene during the conduct of the study. MPL has received honoraria for lectures and advisory boards from Novartis. DL has received financial support for academic research by Novartis and is a member of the Speakers Bureau. WJ reports receiving grants and consultancy fees from Novartis during the conduct of the study. Grants and consultancy fees were also received from Novartis outside of the submitted work. MM, JK, and CQ are all employees of Novartis Pharma GmbH, Nürnberg. HT has received honoraria for lectures and advisory boards from Novartis Pharmaceuticals Corporation. AD, CM, EMG and OS have no conflicts of interest to disclose. For the duration of the study, AD was employed by Medical Healthcare Centre East Hessen GmbH, Fulda, Germany.
Authorship
MM, JK, CQ, and HT designed the study. PH, OS, TD, CMK, FM, AS, CM, AD, PAF, MPL, DL, WJ, EMG, and HT conducted the study and collected data. PH, MM, JK, and CQ analyzed and interpreted data. All authors drafted and revised the manuscript. PH takes responsibility for the integrity of the data analysis. All authors have read and approved the final manuscript.
Contributor Information
Peyman Hadji, Email: hadji.peyman@khnw.de.
Oliver Stoetzer, Email: ojstoetzer@aol.com.
Thomas Decker, Email: decker@onkonet.eu.
Christian M. Kurbacher, Email: kurbacher@web.de.
Frederik Marmé, Email: frederik.marme@med.uni-heidelberg.de.
Andreas Schneeweiss, Email: andreas.schneeweiss@med.uni-heidelberg.de.
Christoph Mundhenke, Email: Christoph.mundhenke@uksh.de.
Andrea Distelrath, Email: docdistel@gmail.com.
Peter A. Fasching, Email: peter.fasching@uk-erlangen.de.
Michael P. Lux, Email: michael.lux@uk-erlangen.de.
Diana Lüftner, Email: diana.lueftner@charite.de.
Wolfgang Janni, Email: wolfgang.janni@uniklinik-ulm.de.
Mathias Muth, Email: mathias.muth@novartis.com.
Julia Kreuzeder, Email: julia.kreuzeder@novartis.com.
Claudia Quiering, Email: claudia.quiering@novartis.com.
Eva-Marie Grischke, Email: eva-maria.grischke@med.uni-tuebingen.de.
Hans Tesch, Email: hans.tesch@chop-studien.de.
References
- 1.Lipton A., Uzzo R., Amato R.J. The science and practice of bone health in oncology: managing bone loss and metastasis in patients with solid tumors. J. Natl. Compr. Cancer Netw. 2009;7(Suppl 7):S1–29. doi: 10.6004/jnccn.2009.0080. (Quiz S30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman R., Body J.J., Aapro M., Hadji P., Herrstedt J., Guidelines Working Group. ESMO. Bone health in cancer patients: ESMO clinical practice guidelines. Ann. Oncol. 2014;25(Suppl 3):iii124–iii137. doi: 10.1093/annonc/mdu103. [DOI] [PubMed] [Google Scholar]
- 3.Choksi P., Williams M., Clark P.M., Van Poznak C. Skeletal manifestations of treatment of breast cancer. Curr. Osteoporos. Rep. 2013;11:319–328. doi: 10.1007/s11914-013-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yardley D.A., Noguchi S., Pritchard K.I. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv. Ther. 2013;30:870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baselga J., Campone M., Piccart M. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnant M., Baselga J., Rugo H.S. Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. J. Natl. Cancer Inst. 2013;105:654–663. doi: 10.1093/jnci/djt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tesch H., Stoetzer O., Decker T. Efficacy and safety of everolimus plus exemestane in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer: results of the single-arm phase IIIB 4EVER trial. Int. J. Cancer. 2018 doi: 10.1002/ijc.31738. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayo Clinic, Mayo Medical Laboratories. Test catalog. http://www.mayomedicallaboratories.com/test-catalog/index.html. Accessed 21 May 2018.
- 9.Hadji P., Asmar L., van Nes J.G. The effect of exemestane and tamoxifen on bone health within the tamoxifen exemestane adjuvant multinational (TEAM) trial: a meta-analysis of the US, German, Netherlands, and Belgium sub-studies. J. Cancer Res. Clin. Oncol. 2011;137:1015–1025. doi: 10.1007/s00432-010-0964-y. [DOI] [PubMed] [Google Scholar]
- 10.Coleman R.E., Banks L.M., Girgis S.I. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8:119–127. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y., Xu G., Yang F. Effect of neoadjuvant chemotherapy on the serum levels of bone turnover markers in women with early-stage breast cancer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurbacher C.M., Rauschenbach N., Kurbacher A.T. Changes of bone turnover markers during perioperative anthracycline- and/or taxane-based chemotherapy in pre- and postmenopausal patients with primary breast cancer. Cancer Res. 2016;76(Suppl 4) abstract P4-10-17. [Google Scholar]
- 13.Browne A.J., Kubasch M.L, Göbel A. Concurrent antitumor and bone-protective effects of everolimus in osteotropic breast cancer. Breast Cancer Res. 2017;19:92–106. doi: 10.1186/s13058-017-0885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glantschnig H., Fisher J.E., Wesolowski G., Rodan G.A., Reszka A.A. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 15.Mogi M., Kondo A. Down-regulation of mTOR leads to up-regulation of osteoprotegerin in bone marrow cells. Biochem. Biophys. Res. Commun. 2009;384:82–86. doi: 10.1016/j.bbrc.2009.04.084. [DOI] [PubMed] [Google Scholar]
- 16.Hadji P., Coleman R., Gnant M. Bone effects of mammalian target of rapamycin (mTOR) inhibition with everolimus. Crit. Rev. Oncol. Hematol. 2013;87:101–111. doi: 10.1016/j.critrevonc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Kneissel M., Luong-Nguyen N.H., Baptist M. Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone. 2004;35:1144–1156. doi: 10.1016/j.bone.2004.07.013. [DOI] [PubMed] [Google Scholar]