ABSTRACT
Aim: We aimed to validate the Arabic and Tunisian Arabic versions of diabetes- specific quality of life (QOL) instrument KINDL-R Diabetes Module for Tunisian children population with type 1 diabetes.
Patients and methods: This a cross-sectional study to validate Arabic and Tunisian KINDL QOL instrument that we translate in literary and dialectal Arabic. Both forward and backward translations from the German version of KINDL QOL into Arabic version were performed. Our project received a GPED grant in August 2014. After the face validity of the Arabic version was established, it was then pilot-tested. Finally, the validity and reliability of the final version of the Arabic KINDL questionnaire were evaluated.
Results: The KINDL-R Diabetes Module (DM) questionnaire of QOL was given to 212 persons : 108 children (aged 3–17 years) with T1DM and 104 parents. The Cronbach’s alpha coefficients of the overall items and the main domains was about 0.7.
The mean total score of the KINDL-R DM was 69,56  ± 14,01 in children aged 7–13 years, 59.93± 15.17 in children aged 13–17 years and 56.6± 9.9 in parents (higher scores indicate better QOL). The parents reported lower diabetes-specific HRQOL than the children themselves (p < 0.01).Emotional score was correlated to environment (p = 0,03). Self-esteem was reported to environment (p = 0,02) and mother’s instruction level’s (p = 0,014).
Conclusions: The KINDL-R Diabetes Module (DM) of QOL in literary and dialectal Arabic have sufficient acceptability, reliability and validity so as to be used for the purposes of a comparative in Tunisian and Arabic populations.
KEYWORDS: Quality of life, Type 1 diabetes, children, validation, questionnaire
1. Introduction
Health-related quality of life (HRQOL) is a multidimensional concept including well-being in terms of patient’s physical, emotional, mental, and social behaviors and is defined as the way the effects of a disease and/or its treatment are perceived by the patient [1]. Assessment of HRQOL in clinical practice is important in order to evaluate the course of the disease, early detection of problems, and to determine what type of therapy would be adequate to maintain acceptable metabolic control with less impact on HRQOL in each patient [1]. HRQOL has increasingly been acknowledged as an essential health outcome measure in pediatric medicine [2]. The development and use of pediatric HRQOL measures are important for identifying at-risk children and applying early intervention programs [3].
Type 1 diabetes mellitus (T1DM) is one of the most common chronic childhood illnesses, affecting approximately 1 in every 400–600 children and adolescents [4]. It affects all aspects of patient’s life, especially psychologically. Its management is complex, requiring a high degree of responsibility and self-control to achieve an adequate metabolic control. Key aspects to succeed are the support of a multidisciplinary team, education in disease management with decision-making capacity, the possibilities offered by new technologies and the emotional sphere of the patient and family [5].
In fact, treatment guidelines recommend routine screening for emotional status and family relationships, mainly during puberty characterized by hormonal and psychosocial changes [6].
Until recently, all HRQOL research in patients with diabetes had been conducted mostly in North America, Australia and the UK [7]. No study from the Maghrebin region has been reported. Since there is a great difference in the health care delivery system, religion, culture and family dynamics in different societies, there is a need to do HRQOL studies in different communities [2].
The aim of our study is to assess reliability and accomplish a limited validation of the diabetes- specific quality of life (QOL) instrument KINDL-R Diabetes Module (DM) to the population of Tunisian and Arabic diabetic children. In addition, a second purpose was to understand the relationship between the QOL in youths with type 1 diabetes and its associated clinical and sociodemographic factors.
2. Methods
2.1. Participants and instruments
The study has received the approval of the Ethical Committee on 1 October 2014.
One hundred and eight children and adolescents, aged 3–17, diagnosed with type 1 diabetes for at least 6 months and their parents referring to our diabetes Center’s, in Children’s Hospital Bechir Hamza of Tunisia, pediatric emergency department and external consultation, were enrolled in the study, between January and June 2016.
All the parents and children aged more than 6 years have given their informed consent. For children under 6 years, we only had their parents’ informed consent.
Exclusion criteria were less than 6 months from diagnosis of T1DM, cognitive problems that prevented comprehension of the questionnaires, and patients who declined to participate in the study. After agreeing to participate in the study, parents and child filled in the KINDL-R Diabetes Module (DM) questionnaire of QOL.
We used a questionnaire that was originally designed in German (originally developed by Bullinger et al. (1994) revised by Ravens-Sieberer & Bullinger [8], for use in clinical populations but also with healthy children and adolescents. Three versions of the KINDL-R DM questionnaire are available as self-report measures for different age groups: KIDDY KINDL (3–6 years) (n = 19), KID KINDL (7–13 years) (n = 62) and KIDDO KINDL (13−17 years). In addition, the questionnaire is available in two proxy versions for parents (3–6-year-olds and 7–17-year-olds). The KINDL-R Diabetes Module (DM) of QOL consisted of 24 items divided into six subscales including physical well-being, emotional well-being, self-esteem, family, friends and everyday functioning (school or nursery school/kindergarten). The questionnaires took approximately 15 min to be completed. Likert response scale with four categories was used, ranging from never a problem (0) to usually a problem (4). All the subscales can be combined to produce a total score (0–100 score). Higher scores represented better QOL. One of the authors was responsible for completing the questionnaire for children by face-to-face interview and she was available to clarify the possible questions of the parents about the instrument.
We obtained permission to translate the KINDL-R Diabetes Module (DM) of QOL in literary and dialectal Arabic. The goal of this double translation was to expand the population to investigate. As a matter of fact, literal Arabic is learn at school and is accessible for parents and children who have been to school; Tunisian Arabic is the language spoken in Tunisia (and easy to understand for Libyan, Algerian and Moroccan people), it’s thus more adapted for illiterate parents and young children.
Translation was performed by the Tunisian National Translation Center and was approved by a third translator and 2 pediatricians.
The Arabic version was back translated to German by another translator and the team of the KINDL questionnaires send us a file comparing German original to German back translation with one of four remarks: no differences in wording/slight changes in wording but no difference in meaning/ bigger changes in wording, maybe difference in meaning/ grave changes in wording and meaning. We changed sentences with bigger and grave changes. The final accepted version was used and is available on the site www.kindl.org (language versions: Arabic and Tunisian Arabic).
A pre-test was administrated to a sample of patients and their parents to verify that the items are understandable.
2.2. Statistical analysis
The reliability of the QOL subscales was tested using the Cronbach’s alpha coefficient. An internal consistency of 0.70 was set for measures used to detect differences between groups and greater than 0.90 for interpreting individual scores. Construct validity was examined through an analysis of the intercorrelations among KINDL-R Total score and KINDL-R scale scores. Concurrent validity was assessed by calculating the intercorrelations between children’s and parents’ responds. The exploratory factor analysis with Varimax rotation was used to determine the construct validity of the KINDL-R. Convergent and discriminant validity was checked using Spearman correlation. The value of a correlation coefficient of greater than 0.40 between an item and its own scale is regarded as an adequate evidence of convergent validity.
Statistical analysis was performed with SPSS 12.0 for Windows. In order to assess any relationship between diabetes related QOL and sociodemographic factors, body mass index, metabolic control (HbA1c), type of family, age at onset and duration of diabetes, type of insulin regimen and educational level of the parents, the Pearson correlation coefficient was used. Unpaired t-tests were used for comparisons between the scores. One-way ANOVA was used for comparisons between different age groups. For all analyses, a value of p ≤ 0.05 was considered to provide statistical significance.
3. Results
Patient demographic and diabetes- specific data are presented in Tables 1 and 2. The average age of participants was 11.02 years; 50% were girls; 4.8% came from single parent families, and 29.8% of parents had university degree.
Table 1.
Demographic characteristics of the participants.
| Variable | N | Mean | Standard deviation | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Weight (SD) | Male | 54 | [+ 1,M] | 13.54 | 18 | 70.1 |
| Female | 54 | + 1 | 14.69 | 17 | 72.2 | |
| Height (SD) | Male | 54 | [M,-1] | 24.09 | 90 | 179 |
| Female | 54 | −1 | 22.01 | 88 | 167 | |
| IMC (kg/m2) | Male | 54 | 97% | 8.52 | 15.96 | 24.23 |
| Female | 54 | > 97% | 7.74 | 15.01 | 25.3 | |
| AGE (n) | 3–6 years | 19 | 4.82 | 0.68 | 4.40 | 6.70 |
| 7–13years | 62 | 11.2 | 1.12 | 7.00 | 13.90 | |
| 14–17 years | 27 | 14.7 | 1.33 | 14.00 | 17.80 | |
| Incomes | 104 | 816.92 | 691.32 | 100.00 | 4500.00 | |
| Uniparental families | 5 | – | – | – | – | |
| Mother’s age | 108 | 43.81 | 4.382 | 24.00 | 56.00 | |
| Father’s age | 108 | 46.74 | 6.61 | 32.00 | 60.00 | |
| Mother’s education | No education | 4 | – | – | – | – |
| Primary | 40 | – | – | – | – | |
| Secondary | 50 | – | – | – | – | |
| University | 14 | – | – | – | – | |
| Father’s education | No education | 10 | – | – | – | – |
| Primary | 35 | – | – | – | – | |
| Secondary | 46 | – | – | – | – | |
| University | 17 | – | – | – | – | |
Table 2.
Clinical characteristics of the participants.
| Characteristics | N | Mean | Standard deviation | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Last HBA1C | 108 | 8.37 | 9.48 | 7.08 | 13.00 | |
| Annual HBA1C | 102 | 9.15 | 2.07 | 6.00 | 17.00 | |
| Duration of diabetes (years) | 104 | 4.4 | 5.74 | 0.1 | 13.00 | |
| Hypoglycemia/months | 98 | 8.74 | 1.57 | 6.00 | 13.00 | |
| Convulsion/years | 106 | 2.38 | 3.37 | 0 | 3.00 | |
| Ketoacidosis | No | 72 | – | – | – | – |
| Yes | 36 | – | – | – | – | |
| Insulin injection/day | 2 | 92 | – | – | – | – |
| 3 | 2 | – | – | – | – | |
| 4 | 9 | – | – | – | – | |
| 5 | 5 | – | – | – | – | |
The mean time of disease progression was 4.4 years, with 30.77% (n = 32) longstanding (> 5 years), and 18.26% less than 1 year. The mean annual HbA1c level was 9.15% (SD ± 2.07); 26.53% of patients had good metabolic control (HbA1c < 7%); 92 (85.18%) were taking 2 injections of insulin per day, 16 (14.81%) 3–4 injections, and no patient used an insulin pump. Although HbA1c values tended to increase, as children grew older we could not find a statistically significant difference between the different age’s groups and between the genders (p ≤ 0.01).
Cronbach’s alpha coefficient for all subscales and total scores of both patient and parent reports of the Arabic and dialectal versions of KINDL-R DM approached or exceeded the reliability standard of 0.70, which is considered satisfactory. It was equally high for all age groups and subscales (0.69–0.77). Patient and parent reports had an adequate level of concordance, with coefficient values of at least 0.684 (Table 3). In Table 4, underlined values represent correlations between child and parent proxy reports. Parents showed adequate level of concordance with their children and most of the correlation coefficients reached or exceeded 0.50. All of the above are statistically significant using Pearson correlation coefficient (p < 0.01).
Table 3.
Cronbach’s α coefficients values for KINDL-R diabetes module scales: parent proxy-report and child self-report.
| Variables |
||
|---|---|---|
| Correlation with total | Alpha | |
| Physical | 0.331150 | 0.736057 |
| Emotional | 0.542495 | 0.695491 |
| Self-esteem | 0.547057 | 0.694577 |
| Family | 0.550736 | 0.693838 |
| Friends | 0.185177 | 0.762089 |
| School | 0.286129 | 0.744255 |
| Disease | 0.215705 | 0.756775 |
| Total_subscale_score | 0.918512 | 0.684336 |
Table 4.
Individual correlations between the total scores and the subscales of the KINDL-R DM.
| Correlation’s coefficient of Pearson, Prob > |r| under H0: Rho = 0 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Physical | Emotional | Self_esteem | Family | Friends | School | Disease | Total_subscale_score | |
| physical | 1.00000 | 0.26279 | 0.26732 | 0.32513 | −0.04295 | 0.22871 | −0.04225 | 0.44337 |
| 0.0526 | 0.0485 | 0.0154 | 0.7556 | 0.0930 | 0.7594 | 0.0007 | ||
| emotional | 0.26279 | 1.00000 | 0.36592 | 0.35307 | 0.16461 | 0.17236 | 0.32138 | 0.61841 |
| 0.0526 | 0.0060 | 0.0082 | 0.2298 | 0.2083 | 0.0167 | ≤ .0001 | ||
| self_esteem | 0.26732 | 0.36592 | 1.00000 | 0.37146 | 0.28528 | 0.11764 | 0.15559 | 0.71211 |
| 0.0485 | 0.0060 | 0.0052 | 0.0348 | 0.3924 | 0.2567 | ≤ .0001 | ||
| family | 0.32513 | 0.35307 | 0.37146 | 1.00000 | −0.03448 | 0.40438 | 0.28589 | 0.58338 |
| 0.0154 | 0.0082 | 0.0052 | 0.8027 | 0.0022 | 0.0344 | ≤ .0001 | ||
| friends | −0.04295 | 0.16461 | 0.28528 | −0.03448 | 1.00000 | −0.11970 | −0.03185 | 0.61105 |
| 0.7556 | 0.2298 | 0.0348 | 0.8027 | 0.3841 | 0.8174 | ≤ .0001 | ||
| school | 0.22871 | 0.17236 | 0.11764 | 0.40438 | −0.11970 | 1.00000 | 0.08261 | 0.37211 |
| 0.0930 | 0.2083 | 0.3924 | 0.0022 | 0.3841 | 0.5488 | 0.0052 | ||
| disease | −0.04225 | 0.32138 | 0.15559 | 0.28589 | −0.03185 | 0.08261 | 1.00000 | 0.19146 |
| 0.7594 | 0.0167 | 0.2567 | 0.0344 | 0.8174 | 0.5488 | 0.01614 | ||
| Total_subscale_score | 0.44337 | 0.61841 | 0.71211 | 0.58338 | 0.61105 | 0.37211 | 0.19146 | 1.00000 |
| 0.0007 | ≤ .0001 | ≤ .0001 | ≤ .0001 | ≤ .0001 | 0.0052 | 0.1614 | ||
Underlined values represent correlations between patient report and parent proxy report. All correlations are significant (P ≤ 0.01, by two-tailed Pearson’s correlation).
No significant correlation was found between the total or all subscales score of the diabetic children and gender, BMI, HbA1c, type of family, age at onset and duration of diabetes, insulin therapy and residence. Figures 1 and 2 display the mean and range of item scores of the child and parent proxy Arabic KINDL-R DM. Paired-samples t-test revealed that there was a statistically significant difference between the generic HRQOL of children and adolescents with diabetes as reported by themselves and their parents as shown in Table 5.
Figure 1.
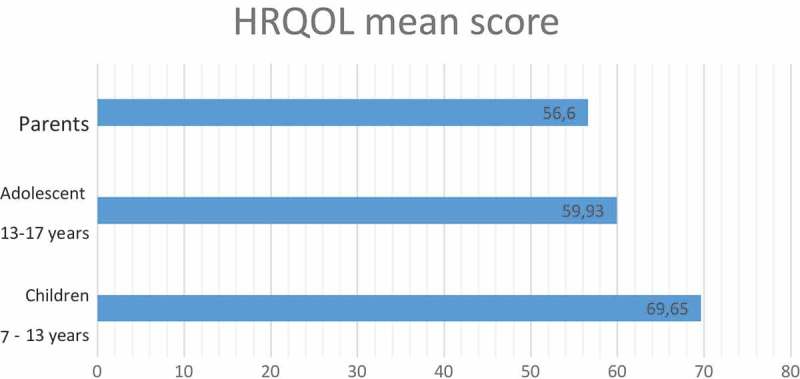
HRQOL mean score.
Figure 2.
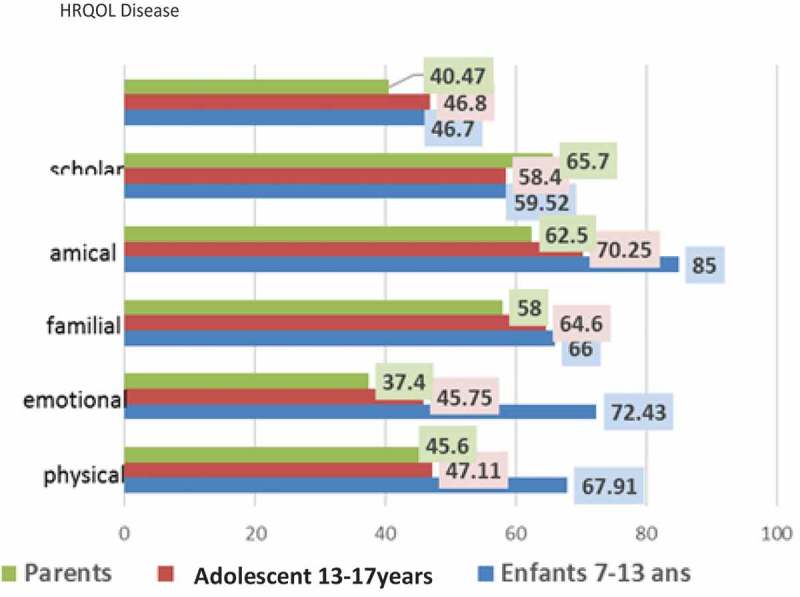
The mean of item scores of the child and parent.
Table 5.
Results from dependent t-test (paired sample): Differences between parents and children in how they estimate children’s HRQOL.
| Parents | Children | t | p | |||
|---|---|---|---|---|---|---|
| HRQOL disease | 40.47 | 12.9 | 46.8 | 12.5 | 4.78 | 0.01 |
| Scholar | 65.7 | 16.5 | 58.72 | 17.8 | 4.31 | 0.01 |
| Friends | 62.5 | 18.4 | 75.75 | 19.2 | 5.82 | 0.001 |
| Familial | 58 | 15.9 | 65.3 | 15.7 | 6.91 | 0.001 |
| Emotional | 37.4 | 13.2 | 64.07 | 13.9 | 7.87 | 0.0001 |
| Physical | 45.6 | 19.3 | 57.01 | 15.8 | 6.85 | 0.001 |
We did not find any differences in HRQOL reports among different age and gender groups. Table 6 demonstrates the comparison between HRQOL reports of male and female based on the KINDL-R DM responds. HRQOL total and all subscales scores were regressed on gender, age, number of hypoglycemic episodes, environment, parent’s instruction level’s, socio-economic condition and HbA1c (Table 7). Emotional score was correlated to environment (p = 0.03). Physical score was related to father’s instruction level’s (p = 0.04). Self-esteem was reported to environment (p = 0.02) and mother’s instruction level’s (p = 0.014). Gender, large BMI, poor metabolic control, school level and intensity of treatment did not influence QOL of children with diabetes (Tables 6 and 7).
Table 6.
Comparison between HRQOL reports of boys and girls with diabetes type 1.
| Gender |
|||||||
|---|---|---|---|---|---|---|---|
| Male |
Female |
Total |
|||||
| Mean | St D | Mean | St D | p | Mean | St D | |
| Physical | 3.7 | 0.7 | 3.7 | 0.7 | 0.8693 | 3.7 | 0.7 |
| Emotional | 3.9 | 0.9 | 3.9 | 0.8 | 0.9652 | 3.9 | 0.9 |
| Self_esteem | 3.5 | 1.1 | 3.7 | 0.8 | 0.5904 | 3.6 | 1.0 |
| Family | 3.4 | 0.9 | 4.0 | 0.8 | 0.0136 | 3.6 | 0.9 |
| Friends | 4.5 | 2.3 | 4.3 | 0.5 | 0.9475 | 4.4 | 1.7 |
| School | 3.3 | 0.8 | 3.4 | 0.6 | 0.5745 | 3.4 | 0.7 |
| Disease | 2.7 | 0.8 | 3.1 | 0.8 | 0.0811 | 2.9 | 0.8 |
| Sum_score | 89.6 | 15.1 | 92.1 | 11.4 | 0.5139 | 90.8 | |
| Total_subscale_score | 3.7 | 0.6 | 3.8 | 0.5 | 0.5139 | 3.8 | 0.6 |
Table 7.
Correlation between total and all subscales of HRQOL and sociodemographic variable.
| VARIABLE | Physical | Emotional | Self esteem | Family | Friend | School | Disease | Total sub score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Father’s education | Primary school | M | 3.5 | 4.0 | 3.6 | 3.8 | 4.7 | 3.5 | 3.2 | 3.8 |
| SD | 0.6 | 0.7 | 0.8 | 0.9 | 2.6 | 0.7 | 0.8 | 0.6 | ||
| Secondary school | M | 3.9 | 3.9 | 3.5 | 3.5 | 4.2 | 3.3 | 2.7 | 3.8 | |
| SD | 0.7 | 1.0 | 1.2 | 1.0 | 0.8 | 0.7 | 0.8 | 0.6 | ||
| University degree | M | 3.6 | 3.6 | 3.6 | 3.9 | 4.2 | 3.4 | 2.6 | 3.7 | |
| SD | 0.6 | 0.9 | 0.6 | 0.4 | 0.4 | 006 | 0.7 | 0.4 | ||
| P | 0.0439 | 0.5096 | 0.8313 | 0.3682 | 0.6940 | 0.4956 | 0.1165 | 0.7925 | ||
| Mother’s education | Analphabete | M | 4.0 | 4.5 | 4.3 | 3.3 | 3.6 | 4.2 | 2.9 | 4.0 |
| SD | 1.1 | 0.9 | 0.5 | 0.7 | 0.8 | 0.5 | 1.2 | 0.5 | ||
| Primary school | M | 3.6 | 3.7 | 3.0 | 3.6 | 4.1 | 3.4 | 3.0 | 3.6 | |
| SD | 0.7 | 0.8 | 1.0 | 1.1 | 0.7 | 0.8 | 0.9 | 0.6 | ||
| Secondary school | M | 3.8 | 3.9 | 3.7 | 3.7 | 4.6 | 3.3 | 2.8 | 3.9 | |
| SD | 0.6 | 1.0 | 1.0 | 0.9 | 2.3 | 0.7 | 0.8 | 0.6 | ||
| University degree | M | 3.8 | 4.3 | 4.0 | 3.6 | 4.5 | 3.2 | 3.0 | 3.9 | |
| SD | 0.5 | 0.5 | 0.6 | 0.7 | 0.5 | 0.6 | 0.7 | 0.3 | ||
| p | 0.4873 | 0.1722 | 0.0147 | 0.7021 | 0.2898 | 0.1753 | 0.9353 | 0.3994 | ||
| Children’s school level’s | Primary school | M | 3.7 | 3.9 | 3.6 | 3.7 | 4.3 | 3.4 | 2.8 | 3.8 |
| SD | 0.7 | 0.9 | 0.9 | 0.9 | 0.6 | 0.6 | 0.8 | 0.5 | ||
| Secondary school | M | 3.7 | 4.0 | 3.4 | 3.6 | 4.8 | 3.4 | 3.1 | 3.9 | |
| SD | 0.6 | 1.0 | 1.2 | 1.0 | 3.4 | 0.8 | 0.8 | 0.8 | ||
| p | 0.5203 | 0.4634 | 0.5694 | 0.8631 | 0.6148 | 0.9449 | 0.3188 | 0.4560 | ||
| Monthly pay | < 1 minimum wage | M | 3.8 | 4.0 | 4.1 | 3.5 | 4.6 | 3.5 | 3.0 | 3.9 |
| SD | 0.8 | 0.8 | 0.9 | 0.5 | 0.5 | 0.8 | 0.9 | 0.3 | ||
| [1–2 minimum wage] | M | 3.6 | 4.0 | 3.4 | 3.6 | 4.6 | 3.3 | 2.9 | 3.8 | |
| SD | 0.7 | 0.6 | 1.1 | 1.1 | 2.7 | 0.6 | 0.9 | 0.7 | ||
| > 3 minimum wage | M | 3.8 | 3.9 | 3.5 | 3.8 | 4.3 | 3.4 | 2.8 | 3.8 | |
| SD | 0.6 | 0.9 | 0.9 | 0.8 | 0.6 | 0.7 | 0.7 | 0.5 | ||
| p | 0.4687 | 0.9530 | 0.1199 | 0.6649 | 0.3596 | 0.8040 | 0.9360 | 0.7670 | ||
| Number of poeple/ household | < 4 people | M | 3.6 | 3.7 | 3.4 | 3.4 | 4.7 | 3.2 | 2.6 | 3.7 |
| SD | 0.7 | 1.0 | 1.0 | 0.9 | 2.6 | 0.7 | 0.8 | 0.7 | ||
| > people | M | 3.8 | 4.0 | 3.7 | 3.8 | 4.3 | 3.5 | 3.0 | 3.8 | |
| SD | 0.6 | 0.8 | 1.0 | 0.8 | 0.7 | 0.7 | 0.8 | 0.5 | ||
| p | 0.7627 | 0.2281 | 0.2464 | 0.1094 | 0.7066 | 0.1171 | 0.0829 | 0.2052 | ||
| environment | Rural | M | 3.4 | 3.6 | 2.9 | 3.4 | 4.1 | 3.2 | 2.7 | 3.4 |
| SD | 0.6 | 0.5 | 1.0 | 0.9 | 0.6 | 0.6 | 0.6 | 0.4 | ||
| Urban | M | 3.8 | 4.0 | 3.7 | 3.7 | 4.5 | 3.4 | 2.9 | 3.9 | |
| SD | 0.7 | 0.9 | 0.9 | 0.9 | 1.8 | 0.7 | 0.8 | 0.6 | ||
| P | 0.1621 | 0.0362 | 0.0237 | 0.2952 | 0.4477 | 0.2985 | 0.5970 | 0.0126 | ||
4. Discussion
There has been an increasing need among clinicians for disease specific QOL scales in recent years [9]. While information about HRQoL is extremely important to the clinical practice of all clinicians, a diabetes-related QoL instrument was not available in Tunisia. This study indicates that the literary and dialectal Arabic version of the KINDL-R DM is a reliable instrument in Tunisian children with type 1 diabetes, with excellent convergent and good discriminant validity. Cultural adaptation of this instrument in the diabetic population of Tunisian children was completed by the evaluation of their psychometric properties. The KINDL-R DM child and parent proxy- report internal consistency reliabilities generally reached the recommended minimum coefficient standard of 0.70 for group comparisons. As Cronbach’s internal consistency coefficients represent the lower limit of the actual reliability of a measurement instrument, and a conservative estimate of actual reliability, it is concluded that Arabic and dialectal versions of the KINDL – R DM are reliable and can be used for the measurement of the QOL of youths with diabetes and for the conduction of a comparative study with non-diabetic youths in Northern Africa and Arab countries. However, it should be noted that the relatively small sample sizes possibly precluded better reliability estimates for this instrument. To additionally extend reliability and validation estimates, the KINDL-R DM could be tested with different types of methods such as test-retest [10].
In comparison with Greek [11,12], American [13], Dutch [14]and Iranian [15] children, Tunisian children with type 1 diabetes reported lower HRQOL total and all subscales scores, according to self- and proxy-reports using the KINDL-R DM questionnaire . It may be attributed to different cultures and languages. In fact, Ethnic disparities are important in determining the prevalence, care, treatment outcomes and QOL of diabetics as shown by many international studies including the San Antonio Heart Study (SAHS) [16].
The parents in our study reported that their children had lower diabetes-specific HRQOL than the children themselves. The discrepancy between patient and parent reports is consistent with previous findings [2,17,18]. It is possible that this result reflects that the parents have identified themselves with the child’s diabetes. In addition to this, this may indicate the burden of diabetes on the parent, which could affect family communication and lead to parental over involvement [10]. Also, parents may be more likely to take into consideration that their child might be restricted by their diabetes.
In terms of age differences, present study showed that adolescents (between 13–17 years), reported the lower diabetes-specific HRQOL than younger children. These findings have been reported in earlier studies [19]. This may indicates an enhanced experience of being different from their peers, because these children are living with a chronic disease. This result might also reveal that a chronic disease management might interfere and complicate the process of separation and development towards maturity.
According to the present study, analysis revealed that urban environment predict better total and emotional score mainly children’s self-esteem. In addition, socioeconomic condition such as parent’s level instruction was predictive factor of better physical score and self-esteem. In fact, other studies had already linked a worse HRQOL in children with specific characteristics such as those from families with disadvantaged socioeconomic status [19,20]. The results of the present study reinforce this finding and the importance of knowing the social situation of the patient.
Evidence shows that better HRQOL is associated with better metabolic control although this relationship is modest, in many studies [5,21]. In our study, it is not possible to establish the directionality of association between HRQOL and HbA1c. Also, gender, large BMI, poor metabolic control, school level and intensity of treatment was not predictor of HRQOL of children with diabetes.
This study has a number of potential limitations, which may minimally affect the power of its results. First, it had not included a control group similar in age and gender distribution to the study group, in order to compare them to diabetic children. Further Tunisian studies are required in the generic HRQOL-field comparing children and adolescents with T1DM and healthy children, or among children in different clinical groups concerning their chronic conditions.
Second, all patients were recruited from a single pediatric hospital; thus caution is necessary when generalizing the findings to children and adolescents being managed in other settings.
A larger study is also needed to further investigate the possible correlations between the predictive factors of HRQOL in diabetic children.
5. Conclusions
In conclusion, this study showed that the literary and dialectal Arabic version of the KINDL-R DM of QOL have sufficient acceptability, reliability and validity so as to be used for the purposes of a comparative in Tunisian and Arabic populations. Moreover, lower HRQOL in Tunisian children with diabetes indicates that youth with diabetes in Tunisia require intensive programs to increase their HRQOL, and more supportive resources should be allocated. In fact, we recommend that assessment of HRQOL after diagnosis of T1DM should be a routine practice in patients with diabetes to facilitate communication, identify early problems and implement early intervention.
Funding Statement
This study was funded by Global Pediatric Endocrinology and Diabetes (GPED, www.globalpedendo.org).
WHAT IS ALREADY KNOWN ON THIS TOPIC
An increasing need among clinicians for disease specific QoL scales.
Many researches have suggested that patients with diabetes have reduced HRQoL compared to the general population
Among these numerous generic and specific questionnaires validated for the teenagers diabetics, the best in the use are the KINDL-R and the PedsQL.
While information about HRQoL is extremely important to the clinical practice of all clinicians, a diabetes-related QoL instrument was not available in Arabic and Tunisian Arabic.
WHAT THIS STUDY ADDS:
A validated Arabic version of KINDL-R diabetes module to estimate the quality of life of the young diabetics from the Maghreb and Arabic-speaking population generally.
First evaluation of Young Diabetic Tunisian quality of life
Disclosure statement
No potential conflict of interest was reported by the authors.
Compliance with Ethical Standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- [1].Clarke SA, Eiser C. The measurement of health-relatedquality of life (QOL)in pediatric clinical trials: a systematic review. Health Qual Life Outcomes.2004;2:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abdul-Rasoul M, AlOtaibi F, Abdulla A, et al. Quality of life of children and adolescents with type 1 diabetes in Kuwait. Med Principles Pract. 2013;22:379–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Wit M, Delemarre-van de Waal H, Bokma J, et al. Monitoring and discussing health-related quality of life in adolescents with type 1diabetes improve psychological well-being: a randomized controlled trial. Diabetes Care. 2008;31:1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kalyva E, Malakonaki E, Eiser C, et al. Health-related quality of life (HRQoL) of children with type 1 diabetes mellitus (T1DM): self and parental perceptions. Pediatr Diabetes. 2011;12:34–40. [DOI] [PubMed] [Google Scholar]
- [5].Murillo M, Bel J, Pérez J, et al. Health-related quality of life (HRQOL) and its associated factors in children with type 1 diabetes mellitus (T1DM). Bio Med Central Pediatrics. 2017;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Delamater AM, de Wit M, McDarby V, et al. ISPAD clinical practice consensus guidelines 2014 compendium. Psychological care of children and adolescents with type 1 diabetes. Pediatr Diabetes. 2014;15(supp 20):232–244. [DOI] [PubMed] [Google Scholar]
- [7].Varni J, Burwinkle T, Jacobs J, et al. The peds-QL TM in type 1 and type 2 diabetes. Reliability and validity of the pediatric quality of life inventory TM generic core scales and type 1 diabetes module. Diabetes Care. 2003;26:631–637. [DOI] [PubMed] [Google Scholar]
- [8].Ravens-Sieberer U, Bullinger M.. Assessing health-related quality of life in chronically ill children with the German KINDL: first psychometric and content analytical results. Qual Life Res. 1998;7(5):399–407. [DOI] [PubMed] [Google Scholar]
- [9].Yildirim A, Akinci F, Gozu H, et al. Translation, cultural adaptation, cross-validation of the Turkish diabetes quality-of-life (DQOL) measure. Translation, cultural adaptation, cross-validation of the Turkish diabetes quality-of-life (DQOL) measure. Qual Life Res. 2007;16(5):873–879. [DOI] [PubMed] [Google Scholar]
- [10].Sand P, Kljajić M, Schaller J, et al. The reliability of the health related quality of life questionnaire PedsQL 3.0 Diabetes Module™ for Swedish children with type 1 diabetes. ActaPaediatr. 2012;101(8):344–349. [DOI] [PubMed] [Google Scholar]
- [11].Gkoltsiou K, Dimitrakaki C, Tzavara C, et al. Measuring health-related quality of life in Greek children: psychometric properties of the Greek version of the pediatric quality of life inventory (TM) 4.0 generic core scales. Qual Life Res. 2008;17(2):299–305. [DOI] [PubMed] [Google Scholar]
- [12].Emmanouilidou E, Galli-Tsinopoulou A, Karavatos A, et al. Quality of life of children and adolescents with diabetes of Northern Greek origin. Hippokratia. 2008;12(3):168–175. [PMC free article] [PubMed] [Google Scholar]
- [13].Varni JW, Limbers CA, Burwinkle TM, et al. TheePedsQL in type 1 and type 2 diabetes: feasibility, reliability, and validity of the pediatric quality of life inventory internet administration. Diabetes Care. 2008;31(4):672–677. [DOI] [PubMed] [Google Scholar]
- [14].Jafari P, Forouzandeh E, Bagheri Z, et al. Health related quality of life of Iranian children with type 1 diabetes: reliability and validity of the Persian version of the PedsQL™ generic core scales and diabetes module. Health Qual Life Outcomes. 2011;23(9):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nuboer R, Borsboom GJ, Zoethout JA, et al. Effects of insulin pump vs. injection treatment on quality of life and impact of disease in children with type 1 diabetes mellitus in a randomized, prospective comparison. Pediatr Diabetes. 2008;28:291–296. [DOI] [PubMed] [Google Scholar]
- [16].Goh SG, Rusli BN, Khalid BA. Development and validation of the Asian diabetes quality of life (AsianDQOL) questionnaire. Diabetes Res Clin Pract. 2015;108(3):489–498. [DOI] [PubMed] [Google Scholar]
- [17].Kalyva E, Malakonaki E, Eiser C, et al. Health related quality of life (HRQoL) of children and adolescents with type 1 diabetes (T1DM): self and parental perception. Pediatr Diabetes. 2011;12:34–40. [DOI] [PubMed] [Google Scholar]
- [18].Nardi L, Zucckini S, D’Alberton F, et al. Quality of life, psychological adjustment and metabolic control in youths with type 1 diabetes: a study with self- and parent-report questionnaires. Pediatr Diabetes. 2008;9:496–503. [DOI] [PubMed] [Google Scholar]
- [19].Anderson BJ, McKay SV. Barriers to glycemic control in youth with type 1 diabetes and type 2 diabetes. Pediatr Diabetes. 2011;12:197–205. [DOI] [PubMed] [Google Scholar]
- [20].Silverstein J, Cheng P, Ruedy KJ, et al. For the pediatric diabetes consortium. Depressive symptoms in youth with type 1 or type 2 diabetes: results of the pediatric diabetes consortium screening assessment of depression in diabetes study. Diabetes Care. 2015;38(12):2341–2343. [DOI] [PubMed] [Google Scholar]
- [21].Hilliard ME, Mann KA, Peugh JL, et al. How poorer quality of life in adolescence predicts subsequent type 1 diabetes management and control. Patient Educ Couns. 2013;91:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]


