This article provides context for the value and risks of adjuvant anthracyclines, focusing on recently reported evidence of disease and patient characteristics that influences decision making regarding the use of adjuvant anthracyclines.
Keywords: Breast cancer, Adjuvant chemotherapy, Anthracycline
Abstract
Anthracyclines have been a mainstay of breast cancer therapy for decades, with strong evidence demonstrating their impact on breast cancer survival. However, concerns regarding rare but serious long‐term toxicities including cardiotoxicity and hematologic malignancies have driven interest in alternative adjuvant therapy options with more favorable toxicity profiles. This article provides an update of data that help inform clinicians of the role anthracyclines should play in adjuvant breast cancer therapy. Two recently reported large randomized trials—the Anthracycline in Early Breast Cancer and Western German Study Plan B studies—compared a taxane and cyclophosphamide regimen with an anthracycline, taxane, and cyclophosphamide regimen. Although the studies had conflicting results, together these studies suggest that the benefit of adjuvant anthracycline therapy over a nonanthracycline taxane‐containing regimen is modest at best and may be primarily seen in patients with especially high‐risk disease (i.e., triple‐negative breast cancer, involvement of multiple lymph nodes). A third study—the MINDACT study—compared an anthracycline‐based regimen to a nonanthracycline regimen, with similar outcomes in both groups. Despite the toxicities, no adjuvant breast cancer regimen has been shown to be superior to an anthracycline‐taxane regimen in high‐risk patients. These data can directly inform clinical decision‐making in determining which patients warrant use of adjuvant anthracycline therapy. Future research may focus on confirming subgroups for whom it is reasonable to forgo adjuvant anthracyclines and validating predictive biomarkers or scores for anthracycline benefit.
Implications for Practice.
In patients with early breast cancer, the choice of adjuvant chemotherapy should be based on its effectiveness in reducing breast cancer recurrences and its short‐ and long‐term toxicities. Although adjuvant anthracycline and taxane chemotherapy has the most data supporting its effectiveness, anthracyclines carry a small but important increased risk for cardiotoxicity and leukemia. Two recent clinical trials help describe the degree of benefit with adjuvant anthracycline therapy compared with taxane therapy alone. They suggest that in patients with hormone receptor‐positive breast cancer and limited lymph node involvement, nonanthracycline taxane‐based adjuvant therapy may be adequate.
摘要
背景。数十年来,蒽环类一直是乳腺癌治疗的主要药物,来自各方的有力证据表明,此类药物对乳腺癌患者的存活率产生了积极的影响。然而,此类药物罕有但却十分严重的长期毒性反应(包括心脏毒性与血液系统恶性肿瘤)令人担忧,因此,促使人们将注意力转向寻找具有更良好毒性特征的替代辅助治疗方案。本文提供了最新数据,可帮助临床医生了解蒽环类在乳腺癌辅助治疗中所发挥的作用。最近报告的两项大型随机试验(早期乳腺癌中使用蒽环类研究和西德研究集团B计划研究)将紫杉烷和环磷酰胺治疗方案与蒽环类、紫杉烷和环磷酰胺治疗方案进行了比较。尽管研究结果存在矛盾,但这些研究同时表明,相对于含有紫杉烷的非蒽环类治疗方案,蒽环类辅助治疗的疗效在最好的情况下也只是略有改善,且主要见于患有特别高危疾病(即三阴性乳腺癌,肿瘤累及多个淋巴结)的患者。第三项研究(MINDACT 研究)将基于蒽环类的治疗方案与非蒽环类治疗方案进行了对比,两组方案得出了相似的结果。尽管存在毒性,但研究表明,在治疗高危患者的过程中,没有一项乳腺癌辅助治疗方案的疗效优于蒽环类‐紫杉烷治疗方案。这些数据可直接指导临床决策,以确定哪些患者需要使用蒽环类辅助治疗。未来的研究可能会侧重于确定哪些患者亚群可合理放弃蒽环类辅助治疗,并针对蒽环类的疗效获益验证预测性生物标志物或评分。
实践意义
对于早期乳腺癌患者而言,应基于降低乳腺癌复发的有效性及短期和长期毒性来选择辅助化疗。尽管有大量数据支持蒽环类和紫杉烷辅助化疗的有效性,但蒽环类会增加心脏毒性和罹患白血病的风险(此类情况并不常见)。近期开展的两项临床试验可有助于了解,与仅采用紫杉烷的治疗方案相比,蒽环类辅助治疗是否产生了更显著的疗效。研究显示,激素受体阳性乳腺癌和淋巴结受累有限的患者,可能适合采用基于紫杉烷的非蒽环类辅助疗法。
Introduction
The value of adjuvant chemotherapy to reduce recurrence risk by eradicating micrometastatic disease gained credence in the 1970s [1], [2]. Studies demonstrating anthracycline‐containing adjuvant regimens further improved disease‐free survival (DFS) and overall survival (OS) compared with other chemotherapy regimens led to their use as standard adjuvant chemotherapy in early‐stage breast cancer [3]. Among other factors, these advances in adjuvant breast cancer therapy have resulted in improvements in breast cancer survival over the last 40 years [4], [5]. Various modeling methods estimate adjuvant chemotherapy improves breast cancer survival by about 6%–10% [5].
However, as the toxicities of anthracyclines and efficacy of alternative therapies such as taxanes and HER2‐targeted agents were better appreciated, adjuvant anthracycline use has been called into question. This article aims to provide context for the value and risks of adjuvant anthracyclines. It will focus on recently reported evidence that sheds light on disease and patient characteristics that influence decision‐making regarding the use of adjuvant anthracyclines.
Evidence for the Use of Adjuvant Anthracyclines
The benefit of adjuvant chemotherapy was well established by the 1980s. A meta‐analysis including 40 adjuvant chemotherapy trials in over 13,000 breast cancer patients showed multiagent chemotherapy reduced the annual odds of death by about one quarter in the initial 5 years after treatment for women under 50 [2]. Although many of these early data supported the use of the combination of cyclophosphamide, methotrexate, and fluorouracil (CMF), advanced breast cancer studies in the 1980s suggested greater activity of anthracycline‐containing regimens based on higher response rates and response durations [6]. Subsequently, multiple randomized trials compared adjuvant anthracycline‐based chemotherapy regimens with CMF and suggested a DFS and OS benefit with adjuvant anthracyclines [7], [8], [9].
In 2012, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) conducted a patient‐level meta‐analysis of over 100,000 patients in trials of adjuvant polychemotherapy for early breast cancer, providing the most comprehensive view of the value of adjuvant anthracycline therapy [3]. It showed anthracycline‐containing adjuvant chemotherapy compared with no chemotherapy decreased the 10‐year risk of breast cancer recurrence from 47.4% to 39.4% (relative risk [RR] 0.73, 95% confidence interval [CI] 0.68–0.79). Additionally, it reduced 10‐year overall mortality from 39.6% to 34.6% (RR 0.84, 95% CI 0.78–0.91). The same analysis also compared anthracycline‐containing regimens with CMF. “Standard dose” anthracycline regimens (cumulative dose: doxorubicin 240 mg/m2 or epirubicin 360 mg/m2) were equivalent to CMF in terms of recurrence rate, breast‐cancer‐specific mortality rate, and overall mortality rate at 10 years. However, regimens with higher doses of anthracyclines demonstrated a reduction of about 4% in 10‐year mortality (RR 0.84, 95% CI 0.76–0.92). Furthermore, the EBCTCG meta‐analysis demonstrated that the addition of a taxane to an anthracycline regimen provided additional reduction in the 8‐year recurrence rate (34.8% vs. 30.2%) and mortality rates (26.7% vs. 23.5%). This benefit was diminished in trials that increased the number of cycles of nontaxane chemotherapy to mirror the number of cycles given in the anthracycline‐taxane arm. The proportional risk reduction was relatively unaffected by age, nodal status, tumor differentiation, estrogen receptor status, or tamoxifen use [3].
These analyses helped establish anthracycline‐ and taxane‐based chemotherapy as a standard for adjuvant treatment. Notably, the absolute benefit is relatively small, indicating only a small subset of patients with invasive breast cancer derive benefit from adjuvant anthracyclines when compared with other adjuvant chemotherapy regimens.
Toxicity of Anthracyclines
Despite their ability to improve survival for women with early breast cancer, anthracyclines can result in severe short‐ and long‐term toxicities. As supportive care has improved, short‐term toxicities, such as nausea and neutropenia, can be better managed. Nevertheless, these side effects may pose problems, particularly for elderly patients and patients with comorbidities. Rare but important long‐term toxicities include cardiotoxicity and secondary hematologic malignancy.
Cardiotoxicity
The advances in long‐term survival for women with early breast cancer make late and chronic toxicities a particular concern. In an analysis of women diagnosed with breast cancer in the Surveillance, Epidemiology, and End Results Program database, death from cardiac causes were just slightly more common than death from breast cancer (15.9% vs. 15.1%, respectively) [10]. Anthracyclines can damage the myocardium and cause a variety of cardiac effects including a dilated cardiomyopathy, supraventricular tachycardia, myopericarditis, electrocardiogram changes, and sudden death [11]. The cardiomyopathy can run the spectrum from severe congestive heart failure (CHF) to subclinical, subtle echocardiographic changes. Risk factors for anthracycline‐induced cardiotoxicity include age (>65 years), higher cumulative anthracycline dose, mediastinal radiation, pre‐existing cardiac disorders, and other cardiac risk factors (i.e., hypertension) [12], [13], [14]. Radiation therapy and trastuzumab can have additive or synergistic cardiotoxic effects with anthracyclines [15].
Inconsistent definitions of cardiotoxicity have resulted in significant variation in the estimated incidence of anthracycline‐induced cardiotoxicity. The risk for cardiotoxicity with anthracyclines is dose‐dependent and increases dramatically at doses higher than doxorubicin 400 mg/m2 or epirubicin 800 mg/m2 [12], [16]. Overall, the incidence of CHF at 5 years with modern anthracycline‐containing adjuvant breast cancer regimens is relatively low, ranging from 0% to 1.6% [17]. Severe toxicity is uncommon. For example, in one study of adjuvant doxorubicin (total dose 240 mg/m2) and cyclophosphamide (AC) versus paclitaxel in lymph node‐positive breast cancer, only 2 of 1,107 patients receiving AC developed symptomatic CHF due to a drop in left ventricular ejection fraction (LVEF) responsive to intervention (≥grade 3 LV systolic dysfunction) at a median follow‐up of 6 years [18]. However, when all degrees of cardiotoxicity are considered in patients receiving AC followed by paclitaxel (AC→T), there is about a 10% incidence of post‐treatment cardiotoxicity and decline in LVEF [19]. Additionally, anthracyclines result in evidence of subclinical cardiovascular disease, including increase in LV end systolic volume, myocardial strain, pulse wave velocity, and other cardiac MRI measures [13], [20], [21]. These parameters are associated with future clinical cardiovascular events, and may contribute to “multiple hits” in patients likely to experience long‐term survival and other cardiovascular risk factors [15].
The addition of trastuzumab to anthracyclines substantially increased the incidence of cardiac dysfunction. In BCIRG 006, patients with HER2‐positive early breast cancer were randomized to AC→docetaxel, AC→docetaxel + trastuzumab (H), or docetaxel, carboplatin, and trastuzumab (TCH). The rates of symptomatic CHF were 0.7%, 2.0%, and 0.4%, respectively. Late occurrence of CHF is rare [22]. However, incidence of asymptomatic decline in LVEF by >10% was 11.2%, 18.6%, and 9.4%, respectively [23]. The significance of asymptomatic cardiac toxicity is not well understood in the context of other cardiac risk factors.
Therapy‐Related Marrow Neoplasms
Anthracyclines are topoisomerase II inhibitors and thought to cause secondary leukemia and myelodysplastic syndrome (marrow neoplasms) by interfering with ligation after single and double strand breaks. Therapy‐related marrow neoplasms are also an important concern in patients receiving anthracyclines in the adjuvant setting. The 5‐year rate of acute leukemia after adjuvant breast cancer therapy ranged from 0% to 1.4% [17]. These rates dramatically increase with higher‐dose therapy [24]. One case‐control study estimated an RR of 3.11 (95% CI 1.96–4.96) [25]. An analysis of over 20,000 patients with stage I–III breast cancer treated through the National Comprehensive Cancer Network showed the RR of a marrow neoplasm with adjuvant chemotherapy was 6.8. The incidence was 0.46 per 1,000 patient years compared with 0.16 per 1,000 patient years in the surgery alone group. Importantly, the risk continued to rise beyond 5 years, with the incidence at 10 years about double that at 5 years [26]. Breast cancer patients receiving adjuvant anthracyclines are also frequently exposed to other risk factors for therapy‐related marrow neoplasms (i.e., radiation, growth factors, alkylating agents) that present an additive or synergistic risk [25], [26]. Unfortunately, therapy‐related neoplasms that develop are more likely to have adverse features: Patients often have increased toxicities given prior therapies, and patients are more likely to have poor outcomes independent of other established prognostic factors [27].
Alternatives to Anthracyclines
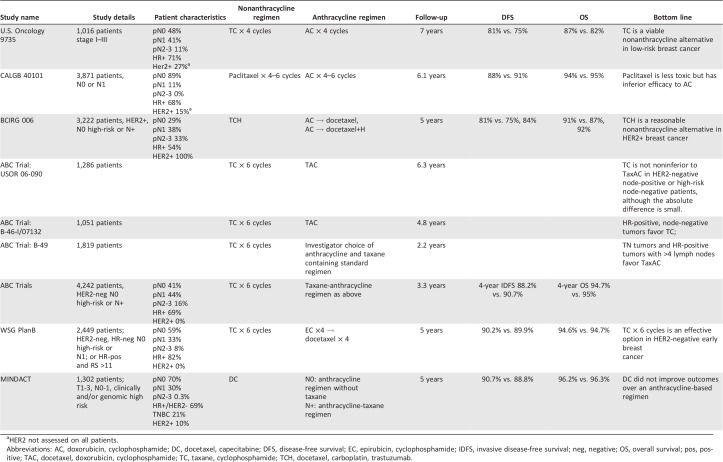
The benefits of anthracyclines must be weighed against their relatively rare but severe long‐term toxicities in these patients with expected long‐term survival [28]. As taxanes were incorporated into standard adjuvant regimens, there was interest in determining if they can achieve similar clinical benefits (i.e., DFS and OS) and be anthracycline sparing. Although data for nonanthracycline regimens do not have the same depth, a growing body of evidence supports their use (Table 1).
As taxanes were incorporated into standard adjuvant regimens, there was interest in determining if they can achieve similar clinical benefits and be anthracycline sparing. Although data for non‐anthracycline regimens does not have the same depth, a growing body of evidence supports their use.
Table 1. Important randomized trials comparing anthracycline and nonanthracycline adjuvant regimens in early breast cancer.
HER2 not assessed on all patients.
Abbreviations: AC, doxorubicin, cyclophosphamide; DC, docetaxel, capecitabine; DFS, disease‐free survival; EC, epirubicin, cyclophosphamide; IDFS, invasive disease‐free survival; neg, negative; OS, overall survival; pos, positive; RS,; TAC, docetaxel, doxorubicin, cyclophosphamide; TC, taxane, cyclophosphamide; TCH, docetaxel, carboplatin, trastuzumab; TCx6, .
The U.S. Oncology 9735 trial evaluated four cycles of docetaxel and cyclophosphamide (TC) versus four cycles of AC [29]. The study included 1,016 women with operable stage I–III breast cancer. About 70% were hormone receptor (HR) positive, and half were lymph node positive. At a median follow‐up of 7 years, the cohort receiving TC had improved DFS (81% vs. 75%, p = .033) and OS (87% vs. 82%, p = .032). Of note, only 170 patients were assessed for HER2 status, and only 11% of patients had four or more lymph nodes involved. In unplanned subgroup analyses, the benefit of TC was seen in all age subgroups, HR status, and HER2 status.
The comparator arm in the U.S. Oncology 9735 trial of AC was shown to be at least equivalent to CMF in the EBCTCG analysis [30]. These results led to the acceptance of TC as a viable nonanthracycline adjuvant regimen in patients with low‐risk breast cancer. However, given the strong evidence seen throughout several global trials of the benefit of taxanes in adjuvant therapy, this comparison did not answer the important question of whether a nonanthracycline regimen was superior to an anthracycline‐ and taxane‐based regimen [3].
The CALGB 40101 study also compared adjuvant taxanes with anthracycline‐based chemotherapy for women with early‐stage breast cancer. In the trial, 3,871 breast cancer patients with ≤3 positive lymph nodes were randomized to paclitaxel or AC [18]. About 90% of patients had lymph node‐negative disease, and 68% were HR positive. HER2 status was only assessed in a subgroup of patients, of whom 15% had HER2‐positive breast cancer. At a median follow‐up of 6.1 years, the relapse‐free survival was 88% versus 91% with a hazard ratio of 1.26 (one‐sided upper bound CI 1.48). This follow‐up was enough to demonstrate that single‐agent paclitaxel was inferior to AC in women with early‐stage breast cancers.
Exploring options for taxane‐based, nonanthracycline adjuvant regimens was of special interest in HER2‐positive breast cancer, with the observation of a multiple‐fold increase in cardiotoxicity when trastuzumab was added to an anthracycline [31], [32], [33]. The BCIRG 006 study evaluated TCH as a nonanthracycline, alternative adjuvant regimen in HER2‐positive breast cancer [23]. The study included 3,222 women with HER2‐positive, stage I–III breast cancer who were randomized to AC followed by docetaxel, AC→ docetaxel + H, and TCH, stratified by nodal and HR status. Patients had high‐risk, lymph node‐negative disease (ER negative, tumor >2 cm, grade 2 or 3, or age <35) or lymph node‐positive disease. More than half had HR‐positive disease. About 29% of patients had lymph node‐negative disease, 38% had one to three lymph nodes, and 33% had four or more lymph nodes involved. The 5‐year DFS was 75% with AC→docetaxel, 84% with AC→docetaxel + H, and 81% with TCH. The OS was 87%, 92%, and 91% in each arm, respectively. Both trastuzumab‐containing regimens led to a significant improvement in DFS and OS at 5 and 10 years. Although the difference in efficacy outcomes between AC→docetaxel + H and TCH was not statistically significant at 5 or 10 years, the study was not powered to compare the anthracycline arm with the nonanthracycline arm [34]. Nevertheless, there was a trend toward improved DFS and OS in the anthracycline arm. Total critical clinical events (distant breast cancer recurrence, grade 3 or 4 CHF, and acute leukemia) were similar (146 vs. 149 events) between AC→docetaxel + H and TCH. Toxicities, including neutropenia, CHF, and sensory neuropathy, were lower in the nonanthracycline arm than the anthracycline arm. Subgroup analyses suggest that similar efficacy is seen even in the lymph node‐positive patients, with 5‐year DFS of 80% and 78% with AC→docetaxel + H and TCH, respectively. These data have led TCH to be a preferred regimen along with AC→taxane + H for adjuvant therapy in HER2‐positive breast cancers [23], [34].
The TRYPHAENA study was a phase II clinical trial of neoadjuvant therapy in HER2‐positive breast cancer (tumor >2 cm and/or lymph node‐positive disease) designed to assess cardiac safety as the primary endpoint [35]. In this study, 225 patients were randomized to TCH + pertuzumab; 5‐fluorouracil, epirubicin, and cyclophosphamide (FEC) followed by docetaxel, trastuzumab, and pertuzumab; or FEC followed by docetaxel with concurrent trastuzumab and pertuzumab. About half of patients had HR‐positive disease, and the majority of patients had zero to three involved lymph nodes. The pathologic complete response (pCR) rates in the breast (ypT0/is) were 66.2%, 45.3%, and 51.9% in the respective treatment arms. Although the study was not powered to assess differences in pCR, it did show high rates of pCR (which is correlated with favorable long‐term outcomes) in the nonanthracycline arm, adding evidence in support of nonanthracycline adjuvant options [36].
The first two studies presented (U.S. Oncology 9735 and CALBG 40101) were conducted prior to the incorporation of trastuzumab into therapy for HER2‐positive breast cancers and routine HER2 testing (HER2 status is known only for 17% and 48% of patients, respectively). Anthracyclines have shown clear benefit for patients with HER2‐positive disease in the era before the standard use of adjuvant trastuzumab [37]. However, as indicated by BCIRG 006, with the incorporation of trastuzumab, the anthracycline benefit is less evident [23]. The incomplete knowledge of HER2 status and its influence on response in these two trials limit their generalizability, as it is unclear whether the benefit of anthracyclines was primarily in the HER2‐positive patients and if that benefit is obviated with the incorporation of trastuzumab.
With the growing literature demonstrating comparable outcomes with taxane‐based adjuvant therapies, their use in clinical practice has grown steadily [38]. However, none of these studies included an anthracycline and taxane regimen, for which there is the strongest data. In the past year, data have matured and been reported from two large randomized trials, the Anthracycline in Early Breast Cancer (ABC) and Western German Study (WSG) Plan B trials, offering the greatest insight into this comparison. Additionally, the MINDACT trial provided further insight from a modern patient population comparing an anthracycline versus nonanthracycline adjuvant regimen.
The ABC study was a joint analysis of three adjuvant breast cancer trials comparing TC for six cycles with an anthracycline‐ and taxane‐containing adjuvant regimen (TaxAC) [39]. The comparison arms were docetaxel, doxorubicin, and cyclophosphamide every 21 days in USOR 06‐090 and NSABP B‐46‐I/USOR 07132 and investigator's choice of several standard anthracycline‐ and taxane‐containing regimens in B‐49 (including dose‐dense regimens and regimens with sequential anthracycline and taxane use). The NSABP B‐46‐I/USOR 07132 also included a TC and bevacizumab arm that was not included in the analysis. The primary objective was to determine if TC was noninferior to TaxAC as evaluated by a 5‐year invasive disease‐free survival (IDFS). A hazard ratio of ≥1.18 corresponding to an absolute difference of ≥2% was prespecified as indicating inferior efficacy.
Together, there were 4,242 patients with lymph node‐positive or high‐risk lymph node‐negative HER2‐negative breast cancer across the trials who were randomized to TaxAC or TC. Patients were stratified by HR status, nodal involvement, and parent trial. Details of study‐specific patient populations are included in Table 1. Overall, 69% of patients had HR‐positive disease. Most patients had limited nodal involvement: 41% were lymph node negative, 44% had one to three lymph nodes, and only 16% had four or more lymph nodes involved. Over half of patients had high histologic grade tumors.
At the planned interim analysis, when 334 IDFS events have occurred (over half of an anticipated 668), the hazard ratio exceeded the 1.18 prespecified hazard ratio cutoff and indicated TC was statistically inferior to TaxAC. At the cutoff time, the hazard ratio was 1.23 (95% CI 1.01–1.50), which translated into an absolute difference in 4‐year IDFS of 2.5% (TC 88.2% and TaxAC 90.7%). At this cutoff, there was no significant difference in overall survival (hazard ratio 1.08, 95% CI 0.82–1.41, p = .60). Toxicities were consistent with those previously described with each regimen. In the TaxAC arm, five patients developed leukemia, compared with none in the TC arm.
Tests for interaction by parent protocol, HR status, and nodal status were negative. However, in the exploratory analysis, TaxAC was favored in the studies with longer follow‐up, although this did not meet statistical significance (USOR 06–090 hazard ratio 1.31, 95% CI 0.97–1.78 and B46/07132 hazard ratio 1.34, 95% CI 0.94–1.91). Additionally, TaxAC was favored in patients with HR‐negative disease (hazard ratio 1.42, 95% CI 1.04–1.94) and more nodal involvement, although this did not meet statistical significance.
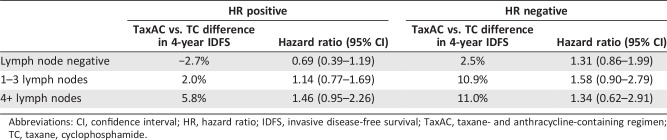
In unplanned exploratory analysis, HR and nodal status were jointly analyzed (Table 2). Hazard ratios for these subgroup analyses did not show a statistically significant advantage for either arm. Nevertheless, the hazard ratio for patients with HR‐positive, lymph node‐negative breast cancer favored TC (hazard ratio 0.69, 95% CI 0.39–1.19), with an absolute 4‐year IDFS difference of 2.7%. In contrast, the greatest advantage to TaxAC appeared to be in HR‐negative cancers (hazard ratio ranged between 1.3 and 1.6 for various degrees of nodal involvement). Patients with HR‐negative, lymph node‐positive tumors had an absolute difference in 4‐year IDFS of about 11%. Additionally, HR‐positive cancers with more than four lymph nodes involved favored TaxAC (hazard ratio 1.49, 95% CI 0.95–2.26; absolute difference in 4‐year IDFS 5.8%).
Table 2. Exploratory analysis from the anthracycline in early breast cancer trials.
Abbreviations: CI, confidence interval; HR, hazard ratio; IDFS, invasive disease‐free survival; TaxAC, taxane‐containing adjuvant regimen; TC, taxane, cyclophosphamide.
The difference noted in outcomes between the treatment arms in the ABC trials contrasts with the recently reported results of a study also comparing a taxane‐based regimen with an anthracycline and taxane regimen. The WSG Plan B trial was a phase III trial of women with HER2‐negative lymph node‐positive and high‐risk lymph node‐negative (T2‐4, G2‐3, <35 years, or high uPA/PAI‐1) breast cancer [40]. The 21‐gene assay (OncotypeDx) was run for patients with HR‐positive, pN0–1 breast cancer. Those with a recurrence score ≤11 had chemotherapy omitted and were treated only with local therapy and endocrine therapy. The trial was designed with a noninferiority margin of 4.4% for the nonanthracycline compared with the anthracycline regimen. Over 2,400 patients with high‐risk disease were randomized to TCx6 or epirubicin and cyclophosphamide followed by docetaxel for four cycles each (ECx4→Tx4). Forty‐one percent of patients had lymph node‐positive disease, 82% were HR positive, and 42% had grade 3 tumors. There was no difference observed in 5‐year DFS (90.2% vs. 89.9%) or 5‐year OS (94.7% vs. 94.6%). Even among those with the highest recurrence scores (RS > 25), the 5‐year DFS was 86% versus 85%. Subgroup analyses did not suggest a particular subset derived greater benefit from the anthracycline‐containing arm. There were more treatment‐related deaths in the TCx6 arm (0.4 vs. 0.1%) but more serious adverse events in the anthracycline‐containing arm (397 vs. 358 events). These results suggest TCx6 is noninferior to ECx4→Tx4 and patients are “sufficiently treated” with TCx6. Additionally, the 21‐gene recurrence score was not predictive of efficacy of anthracycline therapy.
The MINDACT trial evaluated the utility of the 70‐gene assay, MammaPrint, in addition to clinical risk assessment by Adjuvant! Online. Patients with clinical or genomic high‐risk disease receiving chemotherapy underwent a second randomization to chemotherapy versus no chemotherapy, and those with disease considered high risk by both measures received chemotherapy [41]. The 1,301 patients receiving chemotherapy on study had a second randomization to a standard anthracycline‐based regimen (including a taxane only for patients with node‐positive disease) or docetaxel and capecitabine (DC) × six cycles. The study was designed to detect superiority of DC over the traditional anthracycline regimens. Seventy percent did not have lymph node involvement, and the remaining had N1 disease. Sixty‐nine percent had HR‐positive, HER2‐negative disease, and 20% had triple‐negative breast cancer. At a median of 5 years follow‐up, DC was not superior to the anthracycline regimens, with a DFS of 90.7% versus 88.8%. For patients with both clinical and genomic high‐risk disease, the DFS was not statistically different (86.1% vs. 88.1%).
Several differences between the trials may account for the divergent outcomes. The ABC trials collectively enrolled over 4,200 patients, compared with 2,400 and 1,300 in the WSG Plan B and MINDACT studies, respectively. The ABC study also included more clinically high‐risk patients, with more lymph node‐positive patients (60% vs. 41% and 30%) and more triple‐negative patients (31% vs. 18% and 21%), compared with the WSG PlanB and MINDACT trials, respectively. The MINDACT study did not include N2 or N3 disease. Additionally, as the ABC studies with longer follow‐up favored the anthracycline regimen, and the B‐49 with the shortest follow‐up did not, it will be important to update data with longer follow‐up given the sustained risk for recurrence, particularly in HR‐positive disease [42]. Furthermore, whereas both the ABC and PGS Plan B studies were powered for noninferiority, the ABC trials were powered for a smaller absolute difference.
Although these studies had different outcomes, the data in total suggest the absolute benefit of an anthracycline‐ and taxane‐containing regimen compared with a nonanthracycline adjuvant regimen is at best small and likely limited to the higher‐risk patients. Further studies and longer follow‐up are needed to confirm these findings and reconcile the differences in outcomes. Pooled analyses and additional data may help identify clinical factors or biomarker‐based subgroups that glean the greatest benefit or stand the greatest risk with adjuvant anthracycline therapy. Of note, these trials utilized six cycles of taxane‐based, nonanthracycline chemotherapy. This contrasts with the frequently used four cycles of TC supported by two prior clinical trials and an effort to balance efficacy with toxicities. The U.S. Oncology 9735 trial suggested TC × 4 was superior to AC × 4 [29]. In CALGB 40101, over 3,000 women with zero to three involved lymph nodes were randomized to either four or six cycles of chemotherapy and treatment with either paclitaxel or AC. At a median follow‐up of 5.3 years, the hazard ratio for RFS was 1.02 (95% CI 0.84–1.49), with six cycles associated with increasing toxicities [43]. However, we lack high‐quality data comparing a modern anthracycline‐taxane regimen with four cycles of TC.
Strategies to Limit Anthracycline Use and Toxicity
Ultimately, the goal is to optimize risk reduction for recurrence and limit serious adverse events in patients who receive adjuvant therapy for stage I–III breast cancers. This means findings the therapies that will result in the fewest total events including disease recurrence, death, and severe toxicity (i.e., cardiotoxicity, leukemia). One strategy for achieving this goal is to limit adjuvant chemotherapy to those likely to have a clinically meaningful benefit as predicted by the clinical scenario and the use of genomic risk prediction tools for HR‐positive disease (i.e., 21‐gene and 70‐gene assays) [44], [45]. Similarly, molecular subtyping may help predict tumors more or less likely to be chemotherapy responsive [46]. However, none of these tools predict sensitivity specifically to anthracyclines.
HER2 amplification and TOP2A have been shown to predict for benefit from anthracyclines. TOP2A encodes DNA topoisomerase IIα, which is involved in DNA replication and repair and is a target of anthracyclines. TOP2A is located on chromosome 17q near HER2 and is frequently coamplified with HER2 [47]. In fact, in the BCIRG 005 study of over 1,600 patients with HER2‐negative disease, no tumors had TOP2A amplification. In contrast, in BCIRG 006, over one third of almost 3,000 patients with HER2‐amplified breast cancer also had TOP2A overexpression [48]. Retrospective analyses of several studies found patients with HER2 positivity and TOP2A gene aberrations had increased efficacy when treated with adjuvant anthracycline therapy [49], [50], [51]. A subsequent pooled analysis compiled data from eight studies that randomized 5,354 patients with known HER2 status to anthracycline‐ or nonanthracycline‐containing adjuvant chemotherapy [37]. For patients with HER2‐positive breast cancer, DFS and OS were improved with anthracyclines (hazard ratio 0.71, 95% CI 0.61–0.83; and hazard ratio 0.73, 95% CI 0.62–0.85). However, no improvement was seen in HER2‐negative patients, (hazard ratio 1.00 and 1.03, respectively). Of note, methods of HER2 evaluation and interpretation were not standardized across studies. Furthermore, these data were from the pre‐trastuzumab era. A later patient‐level meta‐analysis with standardized fluorescent in‐situ hybridization for HER2 and TOP2A suggested patients with HER2 positivity and TOP2A aberrations did have greater advantage of anthracycline‐based therapy than those without (event‐free survival hazard ratio for HER2 amplification 0.71, 95% 0.58–0.86 and for TOP2A altered 0.64, 95% CI 0.50–0.81) [52]. However, those with HER2 nonamplified and TOP2A normal still had a hazard ratio that suggested benefit from anthracyclines, although of a smaller magnitude. Because of these data suggesting HER2 and TOP2A‐normal patients still derive some benefit from anthracyclines, they are not used clinically as predictive biomarkers for anthracycline benefit [52]. Another patient‐level pooled analysis of five studies comparing anthracycline‐based chemotherapy with CMF adjuvant therapy also showed TOP2A aberrations predicted for recurrence‐free survival and OS, as did CEP17 duplication [53].
To further evaluate TOP2A expression as a predictive biomarker for response to anthracycline therapy, the DBCG 07‐READ study evaluated outcomes in 2012 TOP2A‐normal early‐stage breast cancer [54]. In this open‐label, randomized phase III trial, patients received docetaxel and cyclophosphamide for six cycles or epirubicin and cyclophosphamide for four cycles followed by docetaxel for four cycles. About half of patients were lymph node negative, 38% had one to three lymph nodes, and only 15% had four or more lymph nodes involved. The estrogen receptor was positive in 71% of patients. The 5‐year DFS was 87.9% versus 88.3% in each arm, with a hazard ratio of 1.00. OS was similar in both treatment arms. This study further supports the hypothesis that the anthracycline benefit is derived primarily from patients with abnormal TOP2A. Importantly, there were limited patients with high‐risk features (N2‐3 or triple‐negative disease) that have been noted to derive the greatest anthracycline benefit.
In addition to TOP2A, CEP17 duplication, homologous recombination deficiency, and other biomarkers are associated with greater anthracycline benefit [53], [55]. To reflect the more complex activity of anthracyclines on breast cancer, anthracycline sensitivity signatures or scores have been developed to help enhance the predictive ability of TOP2A alone. These show promise, particularly to help determine which patients may avoid anthracycline therapy given a high negative predictive value. However, these tests still need independent prospective validation and are not used in routine clinical practice [56], [57]. Ongoing molecular analyses from the ABC trials may provide additional insight into predictors of benefit from anthracyclines [39].
Conclusion
Anthracyclines have been a mainstay of adjuvant breast cancer therapy for decades, with extensive data supporting their value in improving DFS and OS. However, their toxicities, the efficacy of taxane‐based nonanthracycline adjuvant chemotherapy, and the relatively small incremental benefit of anthracyclines have brought their use into question. Recently, the ABC, WSG PlanB, and MINDACT randomized trials provided high‐quality randomized data regarding the direct comparison of nonanthracycline taxane‐based and anthracycline‐ and taxane‐based adjuvant chemotherapy regimens in HER2‐negative breast cancer. Despite the discordant study results, it is clear that the magnitude of benefit of adjuvant anthracyclines compared with a taxane regimen is small and may be primarily concentrated in patients with triple‐negative tumors or extensive lymph node involvement. Longer‐term follow‐up and confirmatory data from subgroups at highest and lowest risk may help determine which patients stand to benefit from adjuvant anthracycline therapy. Future studies' randomized trials may evaluate other alternatives to an anthracycline regimen such as a platinum‐based nonanthracycline therapy for triple‐negative breast cancer, which has shown promising pCR rates as neoadjuvant therapy [58], [59].
Despite the discordant study results, it is clear that the magnitude of benefit of adjuvant anthracyclines compared with a taxane regimen is small and may be primarily concentrated in patients with triple negative tumors or extensive lymph node involvement.
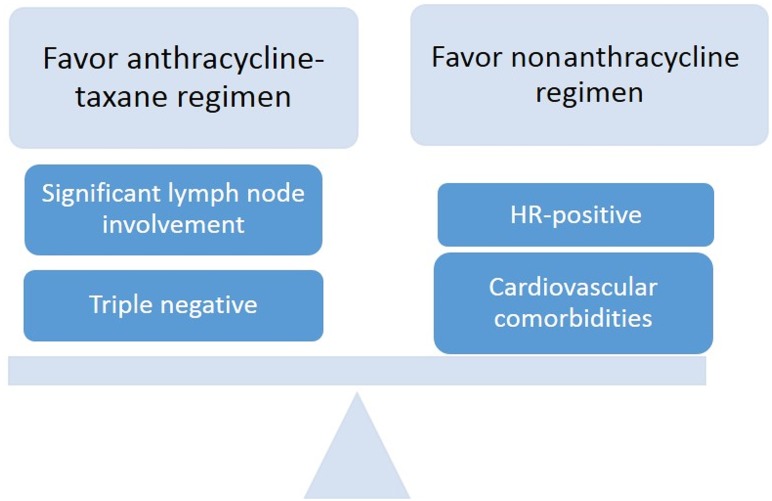
In our practice, after considering a patient's clinical risk and relevant comorbidities, if appropriate, we continue to recommend an adjuvant anthracycline in those with significant nodal involvement or higher‐risk triple‐negative disease (Fig. 1). For lymph node‐negative HR‐positive tumors being treated with chemotherapy, we often utilize a nonanthracycline‐based regimen. For these patients, we often utilize TC × 4 given more limited absolute benefit in a lower‐risk patient population and a desire to limit toxicities.
Figure 1.
Factors influencing decisions regarding anthracycline use for adjuvant breast cancer therapy. Abbreviation: HR, hormone receptor.
For almost 15 years, there has been speculation that the use of adjuvant anthracyclines would come to an end [60], [61]. As data for nonanthracycline regimens grow, the role of adjuvant anthracyclines in breast cancer is becoming more limited. Nevertheless, anthracyclines remain an important part of adjuvant therapy for some high‐risk patients.
Author Contributions
Conception/design: Ami N. Shah, William J. Gradishar
Collection and/or assembly of data: Ami N. Shah, William J. Gradishar
Data analysis and interpretation: Ami N. Shah, William J. Gradishar
Manuscript writing: Ami N. Shah, William J. Gradishar
Final approval of manuscript: Ami N. Shah, William J. Gradishar
Disclosures
The authors indicated no financial relationships.
References
- 1. Bonadonna G, Brusamolino E, Valagussa P et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976;294:405–410. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group . Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med 1988;319:1681–1692. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) , Peto R, Davies C et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta‐analyses of long‐term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park JH, Anderson WF, Gail MH. Improvements in US breast cancer survival and proportion explained by tumor size and estrogen‐receptor status. J Clin Oncol 2015;33:2870–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berry DA, Cronin KA, Plevritis SK et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784–1792. [DOI] [PubMed] [Google Scholar]
- 6. Aisner J, Weinberg V, Perloff M et al. Chemotherapy versus chemoimmunotherapy (CAF v CAFVP v CMF each +/‐ MER) for metastatic carcinoma of the breast: A CALGB study. Cancer and Leukemia Group B. J Clin Oncol 1987;5:1523–1533. [DOI] [PubMed] [Google Scholar]
- 7. Ejlertsen B, Mouridsen HT, Jensen MB et al. Improved outcome from substituting methotrexate with epirubicin: Results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 2007;43:877–884. [DOI] [PubMed] [Google Scholar]
- 8. Coombes RC, Bliss JM, Wils J et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil versus fluorouracil, epirubicin, and cyclophosphamide chemotherapy in premenopausal women with axillary node‐positive operable breast cancer: Results of a randomized trial. The International Collaborative Cancer Group. J Clin Oncol 1996;14:35–45. [DOI] [PubMed] [Google Scholar]
- 9. Levine MN, Bramwell VH, Pritchard KI et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node‐positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1998;16:2651–2658. [DOI] [PubMed] [Google Scholar]
- 10. Patnaik JL, Byers T, DiGuiseppi C et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res 2011;13:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singal PK, Iliskovic N. Doxorubicin‐induced cardiomyopathy. N Engl J Med 1998;339:900–905. [DOI] [PubMed] [Google Scholar]
- 12. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003;97:2869–2879. [DOI] [PubMed] [Google Scholar]
- 13. Drafts BC, Twomley KM, D'Agostino R Jr et al. Low to moderate dose anthracycline‐based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging 2013;6:87–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qin A, Thompson CL, Silverman P. Predictors of late‐onset heart failure in breast cancer patients treated with doxorubicin. J Cancer Surviv 2015;9:252–259. [DOI] [PubMed] [Google Scholar]
- 15. Jones LW, Haykowsky MJ, Swartz JJ et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 2007;50:1435–1441. [DOI] [PubMed] [Google Scholar]
- 16. Von Hoff DD, Layard MW, Basa P et al. Risk factors for doxorubicin‐induced congestive heart failure. Ann Intern Med 1979;91:710–717. [DOI] [PubMed] [Google Scholar]
- 17. Trudeau M, Charbonneau F, Gelmon K et al. Selection of adjuvant chemotherapy for treatment of node‐positive breast cancer. Lancet Oncol 2005;6:886–898. [DOI] [PubMed] [Google Scholar]
- 18. Shulman LN, Berry DA, Cirrincione CT et al. Comparison of doxorubicin and cyclophosphamide versus single‐agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (Alliance). J Clin Oncol 2014;32:2311–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henderson IC, Berry DA, Demetri GD et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node‐positive primary breast cancer. J Clin Oncol 2003;21:976–983. [DOI] [PubMed] [Google Scholar]
- 20. Jordan JH, Vasu S, Morgan TM et al. Anthracycline‐associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan‐Chiu E, Yothers G, Romond E et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node‐positive, human epidermal growth factor receptor 2‐overexpressing breast cancer: NSABP B‐31. J Clin Oncol 2005;23:7811–7819. [DOI] [PubMed] [Google Scholar]
- 22. Advani PP, Ballman KV, Dockter TJ et al. Long‐term cardiac safety analysis of NCCTG N9831 (Alliance) adjuvant trastuzumab trial. J Clin Oncol 2016;34:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slamon D, Eiermann W, Robert N et al. Adjuvant trastuzumab in HER2‐positive breast cancer. N Engl J Med 2011;365:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Praga C, Bergh J, Bliss J et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: Correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol 2005;23:4179–4191. [DOI] [PubMed] [Google Scholar]
- 25. Le Deley MC, Suzan F, Cutuli B et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony‐stimulating factor: Risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol 2007;25:292–300. [DOI] [PubMed] [Google Scholar]
- 26. Wolff AC, Blackford AL, Visvanathan K et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: The National Comprehensive Cancer Network experience. J Clin Oncol 2015;33:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kayser S, Döhner K, Krauter J et al. The impact of therapy‐related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood 2011;117:2137. [DOI] [PubMed] [Google Scholar]
- 28. Robson D, Verma S. Anthracyclines in early‐stage breast cancer: Is it the end of an era? The Oncologist 2009;14:950–958. [DOI] [PubMed] [Google Scholar]
- 29. Jones S, Holmes FA, O'Shaughnessy J et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7‐year follow‐up of US Oncology Research Trial 9735. J Clin Oncol 2009;27:1177–1183. [DOI] [PubMed] [Google Scholar]
- 30. Peto R, Davies C, Godwin J et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta‐analyses of long‐term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Romond EH, Perez EA, Bryant J et al. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med 2005;353:1673–1684. [DOI] [PubMed] [Google Scholar]
- 32. Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B et al. Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med 2005;353:1659–1672. [DOI] [PubMed] [Google Scholar]
- 33. Smith I, Procter M, Gelber RD et al. 2‐year follow‐up of trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer: A randomised controlled trial. Lancet 2007;369:29–36. [DOI] [PubMed] [Google Scholar]
- 34. Slamon DJ, Eiermann W, Robert NJ et al. Abstract S5‐04: Ten year follow‐up of BCIRG‐006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. Cancer Res 2016:S5–04a. [Google Scholar]
- 35. Schneeweiss A, Chia S, Hickish T et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline‐containing and anthracycline‐free chemotherapy regimens in patients with HER2‐positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278–2284. [DOI] [PubMed] [Google Scholar]
- 36. von Minckwitz G, Untch M, Blohmer JU et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796–1804. [DOI] [PubMed] [Google Scholar]
- 37. Gennari A, Sormani MP, Pronzato P et al. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: A pooled analysis of randomized trials. J Natl Cancer Inst 2008;100:14–20. [DOI] [PubMed] [Google Scholar]
- 38. Lynce F, Potosky AL, Swain SM et al. Trends in the use of adjuvant therapy for women with breast cancer under age 65. J Clin Oncol 2015;33(suppl 15):e17541a. [Google Scholar]
- 39. Blum JL, Flynn PJ, Yothers G et al. Anthracyclines in early breast cancer: The ABC Trials‐USOR 06–090, NSABP B‐46‐I/USOR 07132, and NSABP B‐49 (NRG Oncology). J Clin Oncol 2017;35:2647–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harbeck N, Gluz O, Clemens MR et al. Prospective WSG phase III PlanB trial: Final analysis of adjuvant 4xEC→4x doc vs. 6x docetaxel/cyclophosphamide in patients with high clinical risk and intermediate‐to‐high genomic risk HER2‐negative, early breast cancer. J Clin Oncol 2017;35(suppl 15):504a. [Google Scholar]
- 41. Cardoso F, Piccart‐Gebhart MJ, Rutgers EJ et al. Standard anthracycline‐based vs. docetaxel‐capecitabine in early breast cancer: Results from the chemotherapy randomization (R‐C) of EORTC 10041/BIG 3–04 MINDACT phase III trial. J Clin Oncol 2017;35(suppl 15):516a. [Google Scholar]
- 42. Pan H, Gray R, Braybrooke J et al. 20‐year risks of breast‐cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017;377:1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shulman LN, Cirrincione CT, Berry DA et al. Six cycles of doxorubicin and cyclophosphamide or paclitaxel are not superior to four cycles as adjuvant chemotherapy for breast cancer in women with zero to three positive axillary nodes: Cancer and Leukemia Group B 40101. J Clin Oncol 2012;30:4071–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cardoso F, van't Veer LJ, Bogaerts J et al. 70‐gene signature as an aid to treatment decisions in early‐stage breast cancer. N Engl J Med 2016;375:717–729. [DOI] [PubMed] [Google Scholar]
- 45. Sparano JA, Gray RJ, Makower DF et al. Prospective validation of a 21‐gene expression assay in breast cancer. N Engl J Med 2015;373:2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prat A, Fan C, Fernández A et al. Response and survival of breast cancer intrinsic subtypes following multi‐agent neoadjuvant chemotherapy. BMC Med 2015;13:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jarvinen TA, Liu ET. Simultaneous amplification of HER‐2 (ERBB2) and topoisomerase IIalpha (TOP2A) genes–molecular basis for combination chemotherapy in cancer. Curr Cancer Drug Targets 2006;6:579–602. [DOI] [PubMed] [Google Scholar]
- 48. Press MF, Sauter G, Buyse M et al. Alteration of topoisomerase II‐alpha gene in human breast cancer: Association with responsiveness to anthracycline‐based chemotherapy. J Clin Oncol 2011;29:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Di Leo A, Gancberg D, Larsimont D et al. HER‐2 amplification and topoisomerase IIalpha gene aberrations as predictive markers in node‐positive breast cancer patients randomly treated either with an anthracycline‐based therapy or with cyclophosphamide, methotrexate, and 5‐fluorouracil. Clin Cancer Res 2002;8:1107–1116. [PubMed] [Google Scholar]
- 50. Di Leo A, Isola J. Topoisomerase II alpha as a marker predicting the efficacy of anthracyclines in breast cancer: Are we at the end of the beginning? Clin Breast Cancer 2003;4:179–186. [PubMed] [Google Scholar]
- 51. Knoop AS, Knudsen H, Balslev E et al. Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol 2005;23:7483–7490. [DOI] [PubMed] [Google Scholar]
- 52. Di Leo A, Desmedt C, Bartlett JM et al. HER2 and TOP2A as predictive markers for anthracycline‐containing chemotherapy regimens as adjuvant treatment of breast cancer: A meta‐analysis of individual patient data. Lancet Oncol 2011;12:1134–1142. [DOI] [PubMed] [Google Scholar]
- 53. Bartlett JM, McConkey CC, Munro AF et al. Predicting anthracycline benefit: TOP2A and CEP17—Not only but also. J Clin Oncol 2015;33:1680–1687. [DOI] [PubMed] [Google Scholar]
- 54. Ejlertsen B, Tuxen MK, Jakobsen EH et al. Adjuvant cyclophosphamide and docetaxel with or without epirubicin for early TOP2A‐normal breast cancer: DBCG 07‐READ, an open‐label, phase III, randomized trial. J Clin Oncol 2017;35:2639–2646. [DOI] [PubMed] [Google Scholar]
- 55. Sharma P, Barlow WE, Godwin AK et al. Impact of homologous recombination deficiency biomarkers on outcomes in patients with triple‐negative breast cancer treated with adjuvant doxorubicin and cyclophosphamide (SWOG S9313). Ann Oncol 2018;29:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Turner N, Forcato M, Nuzzo S et al. A multifactorial 'Consensus Signature' by in silico analysis to predict response to neoadjuvant anthracycline‐based chemotherapy in triple‐negative breast cancer. NPJ Breast Cancer 2015;1:15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Desmedt C, Di Leo A, de Azambuja E et al. Multifactorial approach to predicting resistance to anthracyclines. J Clin Oncol 2011;29:1578–1586. [DOI] [PubMed] [Google Scholar]
- 58. Sharma P, Lopez‐Tarruella S, Garcia‐Saenz JA et al. Efficacy of neoadjuvant carboplatin plus docetaxel in triple‐negative breast cancer: Combined analysis of two cohorts. Clin Cancer Res 2017;23:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Campos Gomez S, Campos Gomez KA, Garcia Garces M et al. Neoadjuvant carboplatin and docetaxel in locally advanced triple negative breast cancer: A Hispanic trial. J Clin Oncol 2016;34(suppl 15):e12554a. [Google Scholar]
- 60. Bonnefoi HR. Anthracyclines, HER2, and TOP2A: The verdict. Lancet Oncol 2011;12:1084–1085. [DOI] [PubMed] [Google Scholar]
- 61. Pritchard KI, Messersmith H, Elavathil L et al. HER‐2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol 2008;26:736–744. [DOI] [PubMed] [Google Scholar]