A new fluoropyrimidine recently entered the scene of metastatic colorectal cancer (mCRC): trifluridine/tipiracil, also known as TAS‐102. Improving the cost/efficacy ratio of TAS‐102 in mCRC is needed to avoid toxicities in a definitely palliative setting. ECOG performance score, LDH levels, and time from diagnosis of metastatic disease may help identify patients most likely to benefit. Properly designed prognostic tools, such as the "ColonLife" nomogram, may allow for better treatment decisions for patients with limited life expectancy.
Keywords: Trifluridine/tipiracil, TAS‐102, Regorafenib, Refractory colorectal cancer
Abstract
Background.
TAS‐102 is indicated for patients with metastatic colorectal cancer (mCRC) previously treated with, or not considered candidates for, available therapies. Given the complete inefficacy in half of patients, the lack of predictive factors, the palliative setting, and the financial and clinical toxicity, optimizing the cost‐benefit ratio is crucial. The “ColonLife” nomogram allows an estimate of the 12‐week life expectancy of patients with refractory mCRC.
Materials and Methods.
We collected data from patients treated at eight Italian centers in the compassionate use program. Baseline characteristics of patients who were or were not progression free at 6 months were compared. The discriminative ability of the ColonLife nomogram was assessed. Among patients who received both TAS‐102 and regorafenib, clinical outcomes of the two sequences were compared.
Results.
This study included 341 patients. Six (2%) and 93 (27%) patients achieved response and disease stabilization, respectively. The median progression‐free survival (PFS) was 2.4 months with an estimated 6‐month PFS rate of 19%; the median overall survival (OS) was 6.2 months. An Eastern Cooperative Oncology Group performance status (ECOG PS) of 0, normal lactate dehydrogenase (LDH), and a time from the diagnosis of metastatic disease of >18 months were independently associated with higher chances of a patient being progression free at 6 months. The discriminative ability of ColonLife was confirmed. Among 121 patients who received both regorafenib and TAS‐102, no differences in first or second PFS or OS were reported between the two sequences.
Conclusion.
One out of five patients achieves clinical benefit with TAS‐102. ECOG PS, LDH, and time from diagnosis of metastatic disease may help to identify these patients. Excluding patients with very short life expectancy appears a reasonable approach.
Implications for Practice.
Improving the cost‐efficacy ratio of TAS‐102 in metastatic colorectal cancer is needed to spare useless toxicities in a definitely palliative setting. Eastern Cooperative Oncology Group performance status, lactate dehydrogenase levels, and time from the diagnosis of metastatic disease may help to identify patients more likely to achieve benefit. Properly designed prognostic tools (i.e., the “ColonLife” nomogram) may enable excluding from further treatments patients with very limited life expectancy.
摘要
背景。TAS‐102 适用于先前接受过现有疗法治疗或者被认定无法接受现有疗法治疗的转移性结直肠癌(mCRC)患者。由于对半数患者完全无效,缺乏预测因素,采取姑息治疗以及经济因素和临床毒性因素,因此,优化成本效益比显得至关重要。“ColonLife”列线图可以估算出难治性mCRC患者的预期寿命为 12 周。
材料和方法。我们收集了在8家参与同情使用计划的意大利中心接受治疗的患者的数据,并在 6 个月时对病情有进展与病情无进展的患者的基本特征进行了比较。我们还对 ColonLife 列线图的鉴别能力进行了评估。在同时服用 TAS‐102 和瑞格非尼的患者中,我们对两种服药顺序的临床效果进行了比较。
结果。本研究入组341名患者。其中分别有6名患者(2%)和93名患者(27%)的病情缓解及疾病稳定。中位无进展生存期(PFS)为2.4个月,估算的6个月PFS率为19%;中位总生存期(OS)为6.2个月。东部肿瘤协作组体能状态(ECOG PS)评分为0,乳酸脱氢酶(LDH)正常,诊断转移的时间超过18个月,均与患者在6个月时无疾病进展独立相关。ColonLife的鉴别能力得到了证实。在服用瑞格非尼和TAS‐102的121名患者中,未收到两种服药顺序在PFS或OS存在差异的报告。
结论。五分之一的患者在服用TAS‐102后得到临床受益。ECOG PS、LDH和诊断转移的时间可帮助确定这些患者。排除预期寿命很短的患者似乎是一种合理的方法。
实践意义
需要提高TAS‐102在治疗转移性结直肠癌过程中的成本疗效比率,进而消除其在明确姑息治疗环境中的无用毒性。东部肿瘤协作组体能状态评分、乳酸脱氢酶水平以及诊断转移的时间可帮助确定更有可能获得疗效的患者。设计合理的预后工具(如“ColonLife”列线图)可排除预期寿命非常有限的患者去接受进一步的治疗。
Introduction
A new fluoropyrimidine recently entered the scene of metastatic colorectal cancer (mCRC): trifluridine/tipiracil, also known as TAS‐102. Unlike other uracil‐based fluoropyrimidines, this compound consists of the combination of the thymidylate synthase inhibitor trifluridine with the thymidine phosphorylase inhibitor tipiracil, able to prevent the rapid degradation of trifluridine by thymidine phosphorylase [1], [2].
Preclinical data also demonstrated the efficacy of trifluridine/tipiracil in 5‐fluorouracil‐refractory models; this was then confirmed by the clinical evidence [3], [4], [5], [6], [7], [8], [9], [10]. The phase III, pivotal, double‐blind, placebo‐controlled RECOURSE trial led to the approval of TAS‐102 by both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for patients with mCRC previously treated with, or not considered candidates for, available therapies, including fluoropyrimidine‐, oxaliplatin‐, and irinotecan‐based chemotherapies and anti‐vascular endothelial growth factor and anti‐epidermal growth factor receptor (EGFR) agents [11]. RECOURSE met its primary endpoint, demonstrating a statistically significant improvement in overall survival (OS) with TAS‐102 versus placebo, with a 32% reduction in the risk of death (hazard ratio [HR], 0.68; p < .001). Even if the activity according to RECIST was minimal, a significant benefit was observed in terms of progression‐free survival (PFS), with a reduction in the risk of progression or death of 52% (HR, 0.48; p < .001). The safety profile mainly evidenced hematological adverse events (neutropenia, anemia, and thrombocytopenia) with limited subjective toxicities affecting patients’ quality of life. The randomized TERRA study confirmed similar safety and efficacy results in the Asian population [12]. Although the shape of the PFS curves clearly showed that half of treated patients do not derive any benefit from TAS‐102, no predictive biomarkers are currently available for clinicians. Therefore, a proper clinical selection of patients, mainly based on prognostic considerations, seems today the most efficient tool to optimize the treatment cost‐benefit ratio.
After the FDA approval in September 2015 and while awaiting the EMA marketing authorization in April 2016, a compassionate use program was launched in Italy in order to allow access to this new therapeutic option to patients with refractory mCRC.
By collecting data from patients treated in the compassionate use program at eight participating centers, we aimed to describe the safety and efficacy outcomes of TAS‐102 in the real‐world setting, to evaluate baseline prognostic characteristics, and to explore the role of sequences of TAS‐102 followed by regorafenib or vice versa, being regorafenib the other drug approved in the later line setting.
Finally, the “ColonLife” nomogram was recently built to predict the probability of death within 12 weeks of patients with refractory mCRC, based on four easy‐to‐collect clinical variables: Eastern Cooperative Oncology Group performance status (ECOG PS), primary tumor resection, lactate dehydrogenase (LDH) levels, and peritoneal metastases. The nomogram was built and validated in a heterogeneous series that included refractory patients treated with regorafenib, TAS‐102, or other systemic treatments [13]. Data from patients homogeneously treated with TAS‐102 in the compassionate use program allowed us to further validate the nomogram and to corroborate its usefulness as a potential tool to select patients with refractory mCRC with the chance of meaningful benefit from this new option.
Materials and Methods
Data from patients who received at least 1 day of treatment with TAS‐102 in the compassionate use program at centers adhering to the present registry were included. Patients that fulfilled all the criteria for the drug label at investigators’ judgment were eligible.
A starting dose of 35 mg/m2 twice daily, after morning and evening meals, 5 days a week with 2 days of rest, for 2 weeks, then followed by a 2‐week rest period, was planned. Treatment cycles were repeated every 28 days. Guidance for physicians was provided in terms of treatment management. Dose modifications, delays, and interruptions were suggested in response to adverse events, in line with those recommended in the RECOURSE trial [11]. Each dose reduction consisted of a 5 mg/m2 dose decrease. Adverse events occurring during the treatment were registered and graded according to the Common Terminology Criteria for Adverse Events, version 4.0 [14].
No specific recommendation was provided with regard to disease assessment during the treatment. However, investigators involved in the present registry agreed to perform disease assessments by means of contrast‐enhanced computed tomography scan every 8 weeks (two cycles) of treatment. RECIST version 1.1 criteria were adopted for the evaluation of responses [15].
The following baseline demographic, clinical, and molecular characteristics were collected: age, gender, ECOG PS, sites of metastases, number of metastatic sites, time to metastasis, primary tumor location, previous lines of treatment, previous administered regimens including their duration and outcome, time from the start of the first‐line treatment, RAS and BRAF mutational status, and microsatellite instability.
Statistical Methods
All time‐to‐event variables were calculated from the date of first trifluridine/tipiracil administration. OS time was the interval to the date of death from all causes, with censoring at the date of last follow‐up in living patients. PFS time was the interval to first progression or death, whichever came first, with censoring at the date of last follow‐up in patients alive and without progression. OS and PFS curves were estimated by the Kaplan‐Meier method, with the log‐rank test used to statistically compare subgroups.
All the patients were followed up for at least 6 months; thus we studied the association between the probability of progression events (considering progression or death, whichever occurred first within 6 months of follow‐up) and baseline characteristics of patients as categorical variables by means of the chi‐squared test or Fisher's exact test as appropriate, and odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated as well. Variables significantly (p < .10) affecting the probability of progression events were included in a multivariable binary logistic regression model.
The association of baseline characteristics and OS was first assessed in univariable analyses by means of the log‐rank test, and significantly prognostic variables (p < .10) were included in a multivariable Cox proportional hazard model.
Among patients who received both trifluridine/tipiracil and regorafenib during their disease history, treatment outcome was compared in the subgroup treated with regorafenib followed by trifluridine/tipiracil and in the subgroup receiving the reverse sequence. The first PFS was defined as the interval from the beginning of the treatment with the first administered agent to disease progression; the second PFS was defined as the interval from the beginning of the treatment with the second administered agent to progression or death, whichever came first, and with censoring at the date of last follow‐up in patients alive and without progression. OS was defined as the interval from the beginning of the treatment with the first administered agent to death from all causes, with censoring at the date of last follow‐up in living patients.
Finally, we externally validated the ColonLife nomogram [13] by examining calibration (how close the predictions were to the actual outcome; calibration plots and the Hosmer‐Lemeshow test were used [16]) and evaluating the discriminative ability by the Harrell C index [17].
Results
A total of 341 patients with mCRC received TAS‐102 in the compassionate use program at eight Italian centers. Baseline characteristics are summarized in Table 1. Most patients had ECOG PS 0 or 1 (98%) and more than one metastatic site (96%). At least three previous systemic regimens for metastatic disease had been administered in 67% of cases. A large percentage of patients were clearly fluoropyrimidine refractory (91%), and 66% were fluoropyrimidine refractory at the time of the last exposure to a fluoro‐containing regimen. Regorafenib had been previously received by 121 (35%) patients. RAS and BRAF mutations were found in 201 (63%) and 18 (6%) cases, respectively. All RAS and BRAF wild‐type patients had received an anti‐EGFR monoclonal antibody as part of their previous treatments.
Table 1. Baseline characteristics.
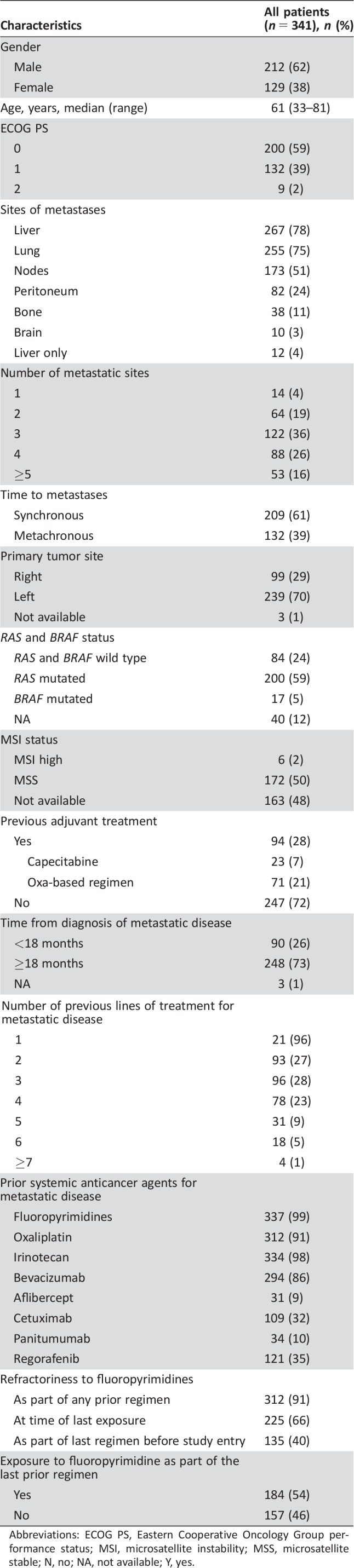
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; MSI, microsatellite instability; MSS, microsatellite stable; N, no; NA, not available; Y, yes.
A median of two cycles (range, 1–14) of TAS‐102 was administered, and 17% of patients received at least six cycles of treatment. At the data cutoff, April 2017, treatment was still ongoing for 13 (4%) patients. The most frequent reason for treatment discontinuation was disease progression (88%), and permanent interruptions because of adverse events occurred only in 11 (3%) cases. In 136 (40%) patients, at least one treatment cycle was delayed because of an adverse event. TAS‐102 dose was reduced because of toxicity by one or two dose levels in 60 (18%) and 26 (8%) cases, respectively.
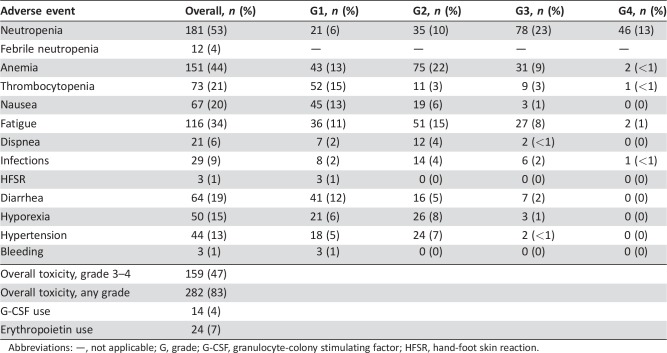
One hundred fifty‐nine (47%) patients experienced at least one grade 3–4 adverse event, mainly consisting of hematological toxicities (Table 2). The most frequent grade 3–4 toxicities were neutropenia (46%), anemia (10%), thrombocytopenia (3%), and fatigue (9%). The incidence of febrile neutropenia was 4%. Granulocyte‐colony stimulating factor (G‐CSF) and erythropoietin were used as secondary prophylaxis in 14 (4%) and 24 (7%) patients, respectively.
Table 2. Safety profile.
Abbreviations: —, not applicable; G, grade; G‐CSF, granulocyte‐colony stimulating factor; HFSR, hand‐foot skin reaction.
With regard to treatment activity, six (2%) patients achieved a RECIST response, whereas in 93 (27%) cases, disease stabilization was reported as the best response, for an overall disease control rate of 29% (Table 3).
Table 3. Treatment activity and efficacy (n = 341).
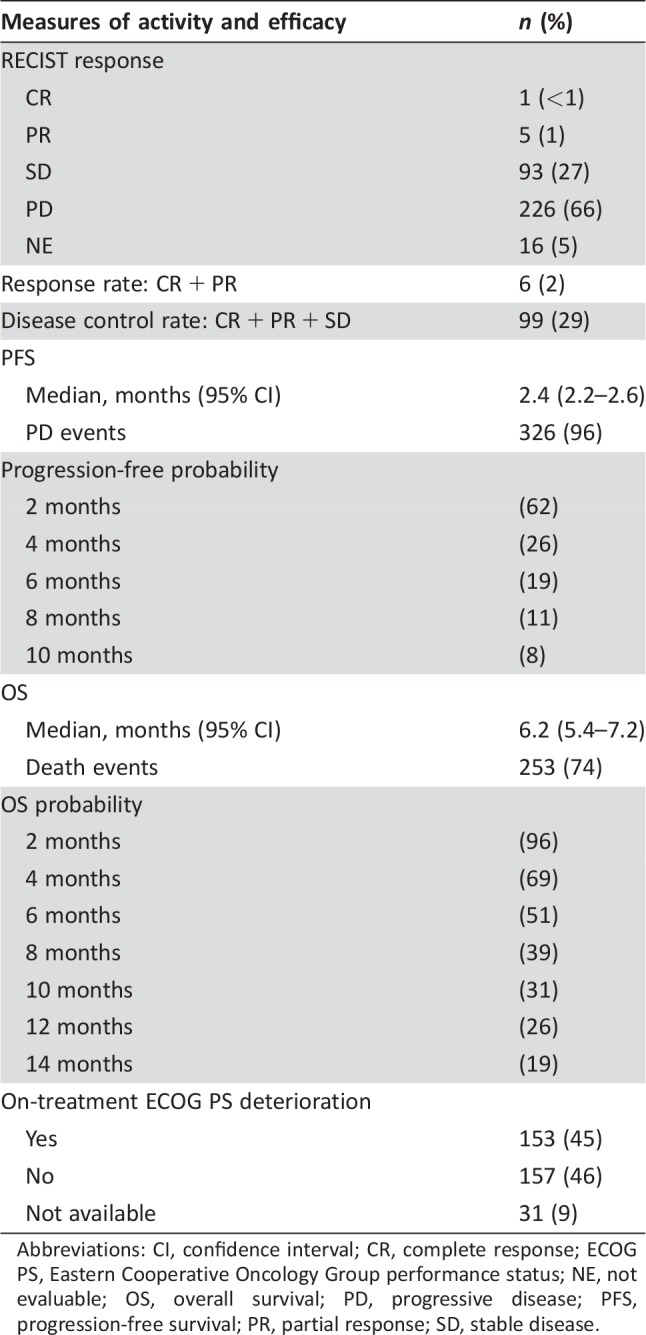
Abbreviations: CI, confidence interval; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; NE, not evaluable; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
At a median follow‐up of 13.3 months, 253 (74%) deaths and 326 (96%) progression events were registered. The median OS was 6.2 months (95% CI, 5.4–7.2), and the estimated 1‐year OS was 26%. The median PFS was 2.4 months (95% CI, 2.2–2.6), and the estimated 6‐month PFS was 19%.
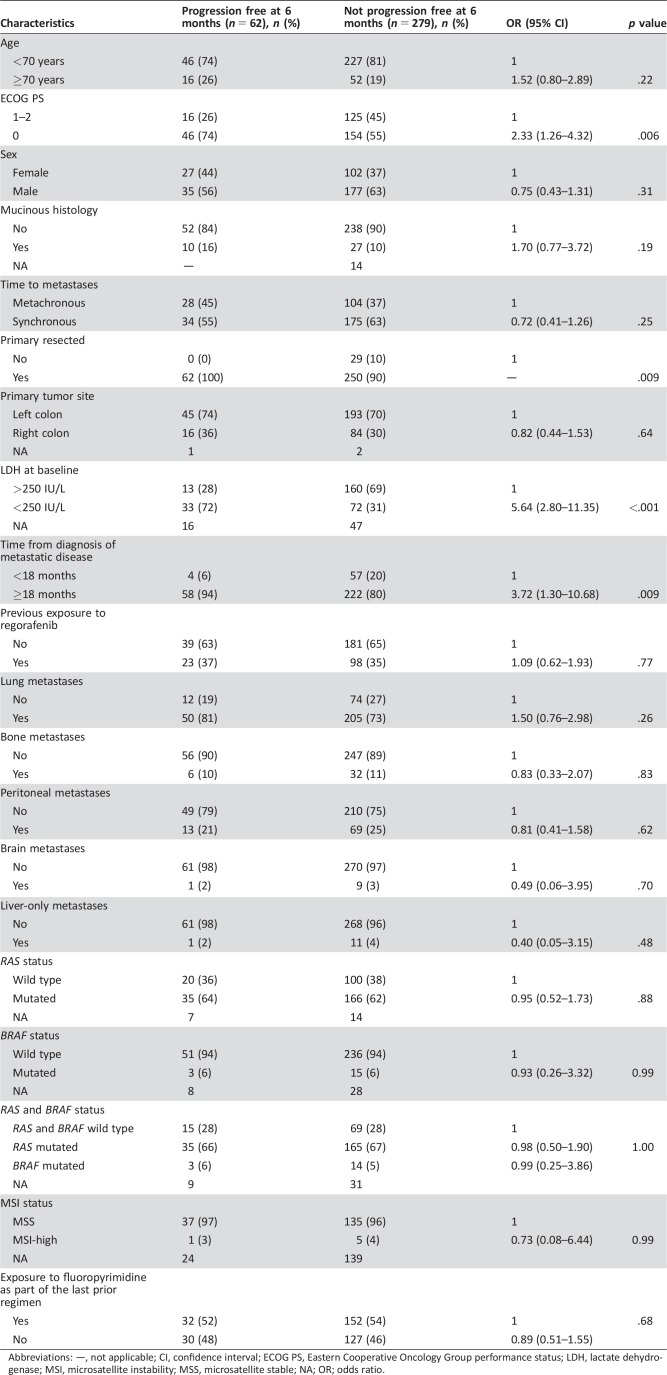
As shown in Table 4, patients with ECOG PS 0 (OR, 2.33; 95% CI, 1.26–4.32; p = .006), normal LDH levels (OR, 5.64; 95% CI, 2.80–11.35; p < .001), resected primary tumor (p = .009) and a time interval of at least 18 months from the diagnosis of metastatic disease to the beginning of TAS‐102 (OR, 3.72; 95% CI, 1.30–10.68; p = .009) had a significantly higher chance of being progression free at 6 months. No association with other putative prognostic variables was evident, including exposure to a fluoropyrimidine in the last prior regimen before the beginning of TAS‐102.
Table 4. Association of baseline characteristics with the probability of being progression free at 6 months.
Abbreviations: —, not applicable; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; MSI, microsatellite instability; MSS, microsatellite stable; NA; OR; odds ratio.
In the multivariable model, ECOG PS (OR, 3.36; 95% CI, 1.48–7.63; p = .004), LDH levels (OR, 5.79; 95% CI, 2.79–12.02; p < .001), and time from the diagnosis of metastatic disease (OR, 4.27; 95% CI, 1.18–15.44; p = .027) were still associated with higher chances of being progression free at 6 months.
Table 5 shows the association of baseline characteristics OS at the univariable analyses. In the multivariable model, only the following variables retained their significant association with OS: ECOG PS (HR, 0.51; 95%,CI; 0.38–0.68; p < .001), time to metastasis (HR, 0.62; 95% CI, 0.32–0.99) LDH levels (HR, 0.34; 95% CI, 0.24–0.47; p < .001), and time from the diagnosis of metastatic disease (HR, 0.53; 95% CI, 0.37–0.75; p < .001).
Table 5. Association of baseline characteristics with OS.
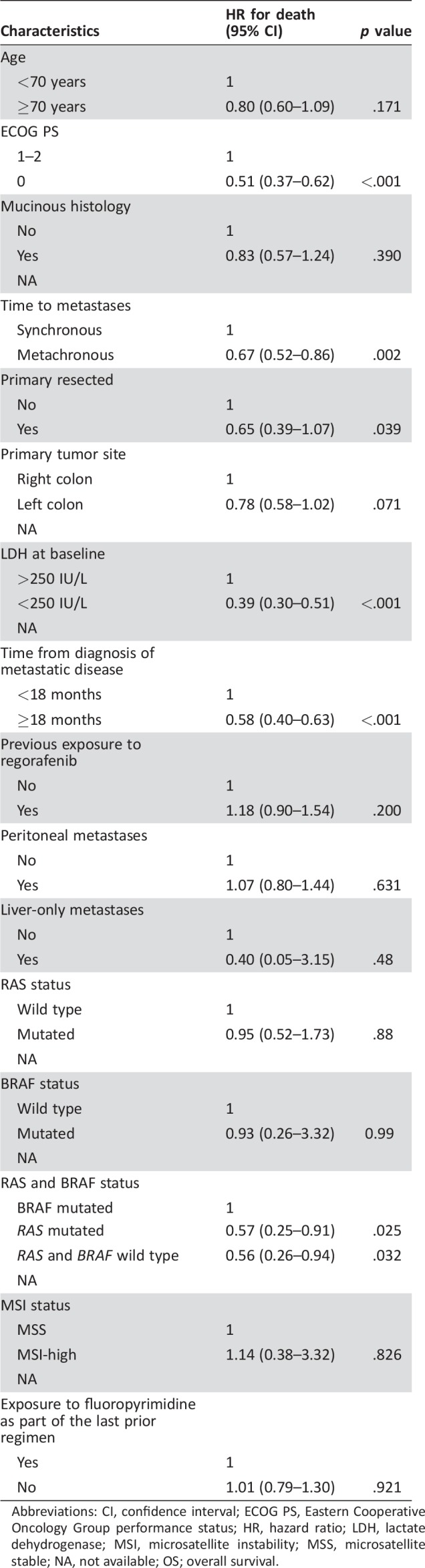
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase; MSI, microsatellite instability; MSS, microsatellite stable; NA, not available; OS; overall survival.
Of note, the development of grade 3–4 neutropenia at any time of the treatment was associated with longer PFS (HR, 0.44; 95% CI; 0.35–0.55; p < .001) and OS (HR, 0.35; 95% CI, 0.28–0.45; p < .001). When adjusting for the number of cycles received, this association was still significant in terms of OS (p < .001) with a trend toward significance in PFS (p = .095).
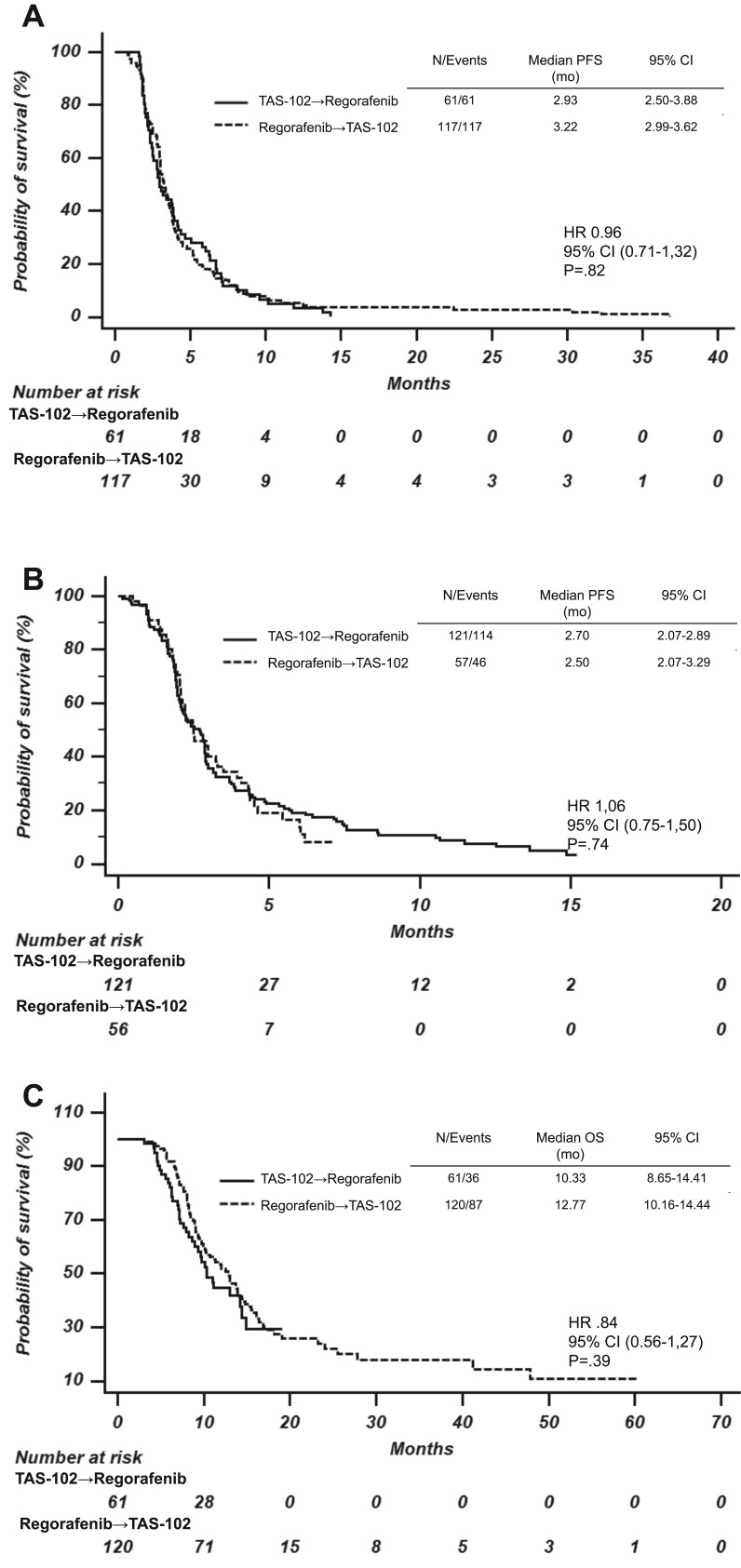
One hundred eighty‐two (53.4%) out of 341 patients in the present series received both TAS‐102 and regorafenib in their treatment history. One hundred twenty‐one patients received regorafenib followed by TAS‐102, and 61 patients received the reverse sequence. No differences were reported in terms of the first PFS (HR, 0.96; 95% CI, 0.71–1.31; p = .808), second PFS (HR, 1.06; 95% CI, 0.75–1.50; p = .736), and OS (HR, 0.85; 95% CI, 0.85–1.56; p = .388) between the two subgroups (Fig. 1).
Figure 1.
Kaplan‐Meier estimates of first PFS (A), second PFS (B), and overall survival (C) in patients who received trifluridine/tipiracil followed by regorafenib (continuous line) or the reverse sequence (dashed line).
Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression‐free survival.
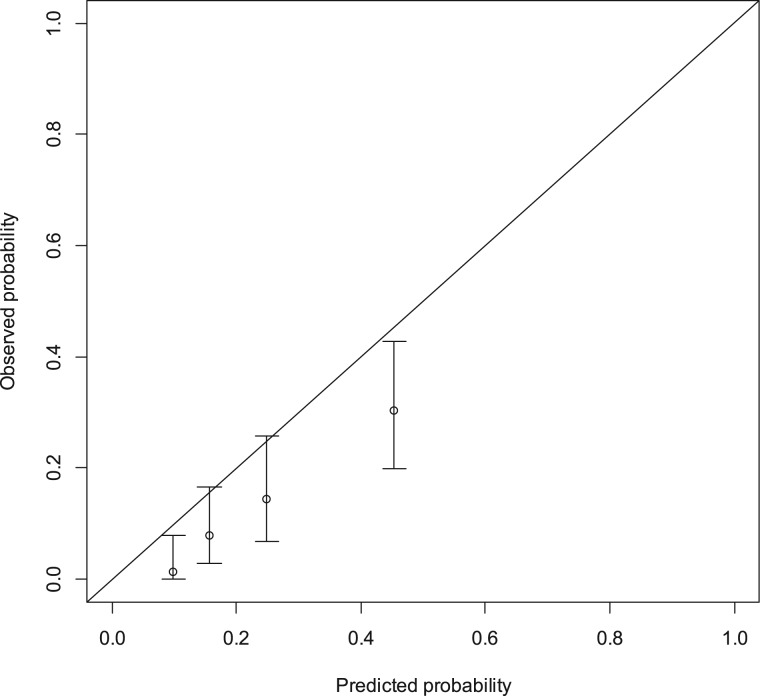
The Colon Life nomogram's discriminative ability in the present series was quite good, having a Harrell C index as high as 0.788. The calibration plot for external validation is shown in Figure 2; the predicted probability tended to be higher than the observed proportion of deaths within 12 weeks, and this produced a significant result for the Hosmer–Lemeshow calibration test (p = .0001).
Figure 2.
Calibration plot for external validation of the “Colon Life” nomogram. The nomogram‐predicted probabilities were stratified in equally sized subgroups. For each subgroup, the average 12‐week nomogram predicted probability of death (x‐axis) was plotted against the observed proportion of 12‐week deaths (y‐axis). 95% confidence intervals of the estimates are indicated with vertical lines. The dashed reference line indicates where an ideal nomogram would lie.
Discussion
The approval of TAS‐102 for the treatment of patients with refractory mCRC added a new therapeutic option able to confer an additional incremental gain in OS. The introduction of new agents in clinical practice always opens the way to new opportunities for affected patients but also to new challenges for treating physicians, who need to acquire expertise to manage a new molecule in daily practice.
Whereas the results of pivotal trials are of paramount importance to estimate the magnitude of benefit provided by a new agent compared with the standard of care, the collection of data closer to the real‐life setting may add relevant information to answer some of clinicians’ frequently asked questions: Is the toxicity profile manageable, and how can adverse events be handled? What efficacy can be expected outside of the framework of the clinical trials’ selection criteria? How can candidate patients be selected? What is the best positioning of the new agent in the landscape of available options? In order to answer these questions, we registered and analyzed data from patients treated with TAS‐102 at eight Italian centers in the compassionate use program.
Baseline characteristics of our patients’ population were similar to those of patients enrolled in randomized studies of TAS‐102 versus placebo, with the only exception of lower percentages of previous exposure to other active agents, probably as a consequence of the higher frequency of contraindications to one of these drugs in patients treated in the real‐life setting [10], [11].
First, safety results were largely consistent: the toxicity profile evidenced the need to monitor hematological adverse events, especially neutropenia, but was also reassuring about the low incidence of febrile cases and the limited need to use G‐CSF as secondary prophylaxis. Overall, the treatment was manageable, requiring permanent discontinuation because of adverse events only in a limited percentage of cases (3%). A significant association of grade ≥3 neutropenia with PFS and OS was reported at the univariate analyses and confirmed after adjusting for the number of cycles received, thus supporting the potential meaning of neutropenia as a surrogate marker for adequate antitumor doses of TAS‐102, as previously hypothesized [18].
Second, efficacy and activity results did not differ from those previously achieved in randomized studies [10], [11]. The minimal activity of TAS‐102 in terms of RECIST response was confirmed, with a responses rate as low as 2%, and disease stabilization was achieved in one out of three treated patients. Consistently, the median duration of PFS of 2.4 months further underlines that about half of patients do not derive any benefit from the treatment. The pre‐emptive identification of these individuals would be crucial in such a palliative setting to minimize both the financial and clinical toxicity burden of the drug, thus improving its cost‐efficacy ratio. To this end, we compared the clinical and molecular characteristics of patients who were or were not progression free at 6 months and found significant differences in terms of ECOG PS, LDH levels, prior resection of the primary tumor, and time from the diagnosis of metastatic disease (<18 months vs. >18 months). These variables coincide, with the exception of the time from the diagnosis of metastatic disease, with those previously identified in a clinical nomogram, ColonLife, built to predict the probability of death within 12 weeks in heavily pretreated patients with mCRC [13]. The discriminative ability of the nomogram, applied to the present series by using information collected before the beginning of the treatment with TAS‐102, was confirmed, thus corroborating the effectiveness of this prognostic tool for a population homogeneously treated with TAS‐102. At the same time, as observed in a previous validation cohort, the early mortality of patients treated with TAS‐102 was lower than predicted by the ColonLife variables, thus indirectly confirming the efficacy of the drug in prolonging survival. The lack of a control group of untreated patients prevented drawing any conclusion about the potential predictive ability of the ColonLife nomogram or of other clinical or molecular factors that should be properly investigated in randomized trials [13]. However, even if the nomogram does not permit identifying an individual patient who should not be treated with later‐line options, it is clinically arguable that refractory patients with a very low probability of being alive after 12 weeks may benefit less from any further treatment, including TAS‐102, so that in these cases the cost‐efficacy balance of available therapies should be carefully weighed.
In fact, all the characteristics negatively affecting the outcome of patients treated with TAS‐102 in our series are markers of suboptimal general conditions (i.e., ECOG PS 1), disease aggressiveness (high LDH levels, time from the diagnosis of metastatic disease <18 months), or risk of complications or deterioration (not resected primary tumor). Unlike other previous experiences, we did not find a significant prognostic impact of the tumor burden, described in terms of number of metastatic sites or specific organ involvement.
Finally, because both TAS‐102 and regorafenib share the same indication, and they seem to provide a similar magnitude of efficacy in absence of predictive biomarkers, clinicians currently wonder which drug should be used first. In the present series, as already reported in the RECOURSE trial, no difference in clinical outcome was evident according to the previous exposure to regorafenib [11]. Moreover, among patients who received both agents sequentially, no differences in PFS or OS were observed according to the drugs’ sequence. Although we acknowledge the limitation of our analysis, which was biased by the lack of a prospective design and thus did not follow the intention‐to‐treat principle, a preferable strategy cannot be recommended based on our results. Therefore, today, patients’ comorbidities and drugs’ safety profiles are the major drivers of the regorafenib versus TAS‐102 choice. In the next future, further steps in tumors’ molecular characterization will lead to other treatment options in selected patients (such as HER2 dual blockade in HER2‐amplified tumors [19], alkylating agents in MGMT methylated tumors [20], [21], [22], and combined anti‐BRAF/anti‐EGFR/anti‐MEK strategies in BRAF V600E mutants [23], [24], [25], [26]), thus making more and more crowded the therapeutic landscape of chemorefractory mCRC.
Conclusion
The safety and efficacy of TAS‐102 in a real‐life setting are consistent with results of pivotal clinical trials. In the absence of validated predictive factors, the selection of candidate patients may rely on an accurate prognostic assessment, including ECOG PS, LDH levels, and time from the diagnosis of metastatic disease. The reliability of the ColonLife nomogram in predicting the 12‐week life expectancy is further confirmed [13]. Although excluding patients with a very short life expectancy from receiving further treatments seems reasonable, the role of the nomogram as predictor of benefit from TAS‐102 should be evaluated in post hoc analyses of randomized trials.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Author Contributions
Conception/design: Chiara Cremolini, Daniele Rossini, Erika Martinelli, Filippo Pietrantonio, Sara Lonardi, Silvia Noventa, Emiliano Tamburini, Giovanni Luca Frassineti, Stefania Mosconi, Federico Nichetti, Sabina Murgioni, Teresa Troiani, Beatrice Borelli, Gemma Zucchelli, Alessandro Dal Maso, Vincenzo Sforza, Gianluca Masi, Carlotta Antoniotti, Maria Di Bartolomeo, Rosalba Miceli, Fortunato Ciardiello, Alfredo Falcone
Provision of study material or patients: Chiara Cremolini, Daniele Rossini, Erika Martinelli, Filippo Pietrantonio, Sara Lonardi, Silvia Noventa, Emiliano Tamburini, Giovanni Luca Frassineti, Stefania Mosconi, Federico Nichetti, Sabina Murgioni, Teresa Troiani, Beatrice Borelli, Gemma Zucchelli, Alessandro Dal Maso, Vincenzo Sforza, Gianluca Masi, Carlotta Antoniotti, Maria Di Bartolomeo, Rosalba Miceli, Fortunato Ciardiello, Alfredo Falcone
Collection and/or assembly of data: Chiara Cremolini, Daniele Rossini, Erika Martinelli, Filippo Pietrantonio, Sara Lonardi, Silvia Noventa, Emiliano Tamburini, Giovanni Luca Frassineti, Stefania Mosconi, Federico Nichetti, Sabina Murgioni, Teresa Troiani, Beatrice Borelli, Gemma Zucchelli, Alessandro Dal Maso, Vincenzo Sforza, Gianluca Masi, Carlotta Antoniotti, Maria Di Bartolomeo, Rosalba Miceli, Fortunato Ciardiello, Alfredo Falcone
Data analysis and interpretation: Chiara Cremolini, Daniele Rossini, Erika Martinelli, Filippo Pietrantonio, Sara Lonardi, Silvia Noventa, Emiliano Tamburini, Giovanni Luca Frassineti, Stefania Mosconi, Federico Nichetti, Sabina Murgioni, Teresa Troiani, Beatrice Borelli, Gemma Zucchelli, Alessandro Dal Maso, Vincenzo Sforza, Gianluca Masi, Carlotta Antoniotti, Maria Di Bartolomeo, Rosalba Miceli, Fortunato Ciardiello, Alfredo Falcone
Manuscript writing: Chiara Cremolini, Daniele Rossini, Erika Martinelli, Filippo Pietrantonio, Sara Lonardi, Silvia Noventa, Emiliano Tamburini, Giovanni Luca Frassineti, Stefania Mosconi, Federico Nichetti, Sabina Murgioni, Teresa Troiani, Beatrice Borelli, Gemma Zucchelli, Alessandro Dal Maso, Vincenzo Sforza, Gianluca Masi, Carlotta Antoniotti, Maria Di Bartolomeo, Rosalba Miceli, Fortunato Ciardiello, Alfredo Falcone
Final approval of manuscript: Chiara Cremolini, Daniele Rossini, Erika Martinelli, Filippo Pietrantonio, Sara Lonardi, Silvia Noventa, Emiliano Tamburini, Giovanni Luca Frassineti, Stefania Mosconi, Federico Nichetti, Sabina Murgioni, Teresa Troiani, Beatrice Borelli, Gemma Zucchelli, Alessandro Dal Maso, Vincenzo Sforza, Gianluca Masi, Carlotta Antoniotti, Maria Di Bartolomeo, Rosalba Miceli, Fortunato Ciardiello, Alfredo Falcone
Disclosures
Chiara Cremolini: Roche, Bayer, Merck, Amgen, Eli Lilly & Co., Sirtex (C/A, H); Fortunato Ciardiello: Bayer, Eli Lilly & Co., Roche, Merck Serono, Pfizer, Bristol‐Myers Squibb, Servier, Amgen (C/A), Bayer, Merck Serono, Roche (RF); Alfredo Falcone: Amgen, Bayer, Merck, Roche, Sanofi, Servier, Merck Sharp & Dohme, Bristol‐Myers Squibb (C/A, RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Tanaka N, Sakamoto K, Okabe H et al. Repeated oral dosing of TAS‐102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep 2014;32:2319–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fukushima M, Suzuki N, Emura T et al. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2’‐deoxyribonucleosides. Biochem Pharmacol 2000;59:1227–1236. [DOI] [PubMed] [Google Scholar]
- 3. Emura T, Murakami Y, Nakagawa F et al. A novel antimetabolite, TAS‐102 retains its effect on FU‐related resistant cancer cells. Int J Mol Med 2004;13:545–549. [PubMed] [Google Scholar]
- 4. Emura T, Suzuki N, Yamaguchi M et al. A novel combination antimetabolite, TAS‐102, exhibits antitumor activity in FU‐resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol 2004;25:571–578. [PubMed] [Google Scholar]
- 5. Hong DS, Abbruzzese JL, Bogaard K et al. Phase I study to determine the safety and pharmacokinetics of oral administration of TAS‐102 in patients with solid tumors. Cancer 2006;107:1383–1390. [DOI] [PubMed] [Google Scholar]
- 6. Overman MJ, Varadhachary G, Kopetz S et al. Phase 1 study of TAS‐102 administered once daily on a 5‐day‐per‐week schedule in patients with solid tumors. Invest New Drugs 2008;26:445–454. [DOI] [PubMed] [Google Scholar]
- 7. Overman MJ, Kopetz S, Varadhachary G et al. Phase I clinical study of three times a day oral administration of TAS‐102 in patients with solid tumors. Cancer Invest 2008;26:794–799. [DOI] [PubMed] [Google Scholar]
- 8. Doi T, Ohtsu A, Yoshino T et al. Phase I study of TAS‐102 treatment in Japanese patients with advanced solid tumours. Br J Cancer 2012;107:429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bendell JC, Rosen LS, Mayer RJ et al. Phase 1 study of oral TAS‐102 in patients with refractory metastatic colorectal cancer. Cancer Chemother Pharmacol 2015;76:925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshino T, Mizunuma N, Yamazaki K et al. TAS‐102 monotherapy for pretreated metastatic colorectal cancer: A double‐blind, randomised, placebo‐controlled phase 2 trial. Lancet Oncol 2012;13:993–1001. [DOI] [PubMed] [Google Scholar]
- 11. Mayer RJ, Van Cutsem E, Falcone A et al. Randomized trial of TAS‐102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–1919. [DOI] [PubMed] [Google Scholar]
- 12. Kim TW, Shen L, Xu JM et al. TERRA: A randomized, double‐blind, placebo‐controlled phase 3 study of TAS‐102 in Asian patients with metastatic colorectal cancer. Ann Oncol 2016;27(suppl 6):465PD. [DOI] [PubMed] [Google Scholar]
- 13. Pietrantonio F, Miceli R, Rimassa L et al. Estimating 12‐week death probability in patients with refractory metastatic colorectal cancer: The Colon Life nomogram. Ann Oncol 2017;28:555–561. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute . Common Terminology Criteria for Adverse Events. Version 4.0. Rockville, MD: National Cancer Institute; 2009. [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 16. Hosmer DW, Hosmer T, Le Cessie S et al. A comparison of goodness‐of‐fit tests for the logistic regression model. Stat Med 1997;16:965–980. [DOI] [PubMed] [Google Scholar]
- 17. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 18. Hamauchi S, Yamazaki K, Masuishi T et al. Neutropenia as a predictive factor in metastatic colorectal cancer treated with TAS‐102. Clin Colorectal Cancer 2017;16:51–57. [DOI] [PubMed] [Google Scholar]
- 19. Sartore‐Bianchi A, Trusolino L, Martino C et al. Dual‐targeted therapy with trastuzumab and lapatinib in treatment‐refractory, KRAS codon 12/13 wild‐type, HER2‐positive metastatic colorectal cancer (HERACLES): A proof‐of‐concept, multicentre, open‐label, phase 2 trial. Lancet Oncol 2016;17:738–746. [DOI] [PubMed] [Google Scholar]
- 20. Calegari MA, Inno A, Monterisi S et al. A phase 2 study of temozolomide in pretreated metastatic colorectal cancer with MGMT promoter methylation. Br J Cancer 2017;116:1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pietrantonio F, Perrone F, de Braud F et al. Activity of temozolomide in patients with advanced chemorefractory colorectal cancer and MGMT promoter methylation. Ann Oncol 2014;25:404–408. [DOI] [PubMed] [Google Scholar]
- 22. Pietrantonio F, de Braud F, Milione M et al. Dose‐dense temozolomide in patients with MGMT‐silenced chemorefractory colorectal cancer. Target Oncol 2016;11:337–343. [DOI] [PubMed] [Google Scholar]
- 23. Corcoran RB, Atreya CE, Falchook GS et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600‐mutant colorectal cancer. J Clin Oncol 2015;33:4023–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Geel RMJM, Tabernero J, Elez E et al. A phase Ib dose‐escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF‐mutant colorectal cancer. Cancer Discov 2017;7:610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.BRAF/MEK/EGFR inhibitor combination study in colorectal cancer (CRC). ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/NCT01750918. Accessed September 11, 2017.
- 26.Study of encorafenib + cetuximab plus or minus binimetinib vs. irinotecan/cetuximab or infusional 5‐fluorouracil (5‐FU)/folinic acid (FA)/irinotecan (FOLFIRI)/cetuximab with a safety lead‐in of encorafenib + binimetinib + cetuximab in patients with BRAF V600E‐mutant metastatic colorectal cancer (BEACON CRC). ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/NCT02928224. Accessed September 11, 2017.