This article reports the incidence of new‐onset thyroid function test abnormalities in patients undergoing treatment with ipilimumab, nivolumab, pembrolizumab, and combined therapy with ipilimumab and nivolumab and describes the clinical course of these patients.
Keywords: Anti‐PD1, Anti‐CTLA 4, Thyroid, Ipilimumab, Nivolumab, Pembrolizumab
Abstract
Background.
With the advent of immune‐checkpoint inhibitor (ICI) therapy (anti‐CTLA‐4, anti‐PD‐1), immune‐related adverse events such as thyroid function test abnormalities (TFTAs) are common, with a reported incidence range of 2%–15% depending upon the ICI used. The aim of this study is to describe the incidence of TFTAs retrospectively in patients who received ICI therapy.
Methods.
A total of 285 patients were reviewed (178 male, 107 female; 16–94 years of age), of whom 218 had no baseline TFTAs, 61 had baseline TFTAs, and 6 had a history of thyroidectomy (excluded). At least one dose of ipilimumab and/or nivolumab or pembrolizumab was administered. Post‐ICI therapy TFTAs were classified according to standard definitions of thyroid conditions when possible.
Results.
A total of 35% (76/218) patients had new‐onset TFTAs on ICI therapy. Of note, 70.5% (43/61) had baseline TFTAs that were exacerbated by ICI therapy. The median times to new‐onset or exacerbated baseline TFTA were 46 and 33 days, respectively. Of note, 64.5% (20/31) of patients on both ipilimumab and nivolumab had new‐onset TFTAs, compared with 31.3% (15/48) on ipilimumab, 31.5% (28/89) on nivolumab, and 26% (13/50) on pembrolizumab.
Conclusion.
The incidence of TFTAs with ICI therapy was higher than previously reported. Patients with baseline TFTAs and/or who were receiving ipilimumab and nivolumab combination therapy had a higher incidence of TFTAs than patients receiving single‐agent ICI therapy. We recommend more frequent evaluation of thyroid function in the first 8 weeks, especially in patients with baseline TFTAs.
Implications for Practice.
Increased use of immune‐checkpoint inhibitors in cancer treatment has highlighted the importance of monitoring for and treating immune‐related adverse events. This study was conducted to assess the incidence of thyroid function test abnormalities retrospectively in patients with cancer on immune‐checkpoint inhibitors, which is not known exactly. This study is unique in that it included patients with a variety of histologic subtypes of cancer and also followed the clinical course of patients with baseline thyroid function test abnormalities. This study can help make oncologists aware that the incidence of thyroid function test abnormalities is higher than anticipated. Early identification and timely treatment can help ameliorate symptoms for patients and improve their overall quality of life.
Introduction
The advent of immune‐checkpoint inhibitors (ICIs) heralds unique side effects, termed immune‐related adverse events (irAEs). The three most commonly used ICIs include ipilimumab, a cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4) inhibitor, along with nivolumab and pembrolizumab, both of which are anti‐programmed cell death protein 1 (PD‐1) inhibitors. The spectrum of irAEs encompasses dermatologic, gastrointestinal, hepatic, endocrine, and pulmonary systems [1], [2], [3]. The majority of data on incidence of irAEs comes from advanced melanoma clinical trials [4], [5], [9]. The exact mechanism by which ICI therapy causes thyroid function test abnormalities (TFTAs) is unknown. Interestingly, CTLA‐4 was the first non‐human leukocyte antigen system identified to have a role in the development of Graves’ disease. The CTLA‐4 gene polymorphisms are associated with Hashimoto's thyroiditis, type 1 diabetes mellitus and Addison's disease. CTLA‐4 regulates early T‐cell activity and development, limiting initiation of a T‐cell response to self‐antigens. In contrast, PD‐1 inhibits T‐cell signaling in peripheral tissue and limits T‐cell cytotoxic activity, proliferation, and survival. It also promotes the differentiation of CD4+ T cells into T regulatory cells and induces T‐cell apoptosis [10].
The exact incidence of irAEs has been difficult to determine given the variable methods of assessing, diagnosing, and monitoring in clinical trials. Increased incidence of irAEs has been reported with the combined use of ipilimumab and nivolumab. A recently published meta‐analysis of 38 randomized clinical trials demonstrated that the incidence of endocrine irAEs is higher in patients on combined ipilimumab and nivolumab therapy [11]. Other studies assessing incidence of TFTAs have only looked at subgroups of patients treated with ICI therapy, such as patients with melanoma [7] or non‐small cell lung cancer [12], [13]. However, this retrospective cohort study includes patients with a wide variety of malignancies. In addition, previous studies have excluded patients with baseline TFTAs.
The purpose of this retrospective cohort study was to assess the incidence of new‐onset TFTAs in patients undergoing treatment with ipilimumab, nivolumab, pembrolizumab, and combined therapy with ipilimumab and nivolumab. Secondary aims were to determine if ICI therapy exacerbated baseline TFTAs and to describe the clinical course of these patients.
Materials and Methods
A total of 285 individual patients were identified through a medical record database search based on any combination of ipilimumab, nivolumab, and pembrolizumab between the years 2010 and 2016 at Moores Cancer Center at the University of California San Diego (UCSD). The median age was 63 years (range, 16–94). Of 285 patients, 218 patients did not have baseline TFTAs and 61 patients had baseline TFTAs. Six patients (one male and five female) had history of total thyroidectomy and were excluded, which resulted in 279 (177 male and 102 female) individual patients who were included. The ICI used was determined based on chart review with corresponding treatment administration dates recorded for each patient. All patients received at least one dose of ipilimumab, nivolumab, or pembrolizumab or a combination of ipilimumab and nivolumab. Two separate reviewers manually reviewed each chart for data, and disagreements between reviewers were resolved after deliberate discussion.
Normal thyroid‐stimulating hormone (TSH) and free thyroxine (FT4) were defined as 0.27–4.20 mIU/ml and 0.93–1.7 ng/dL, respectively, based on the Elecsys assay (Roche, Basel, Switzerland) used at UCSD. Pretreatment baseline TSH and FT4 were recorded. The majority of patients had both a baseline TSH and FT4 simultaneously reported; however, a small number had either a baseline TSH or a FT4.
Post‐ICI thyroid function tests (TFTs) were followed while patients were on ICI therapy and up to 3 months after discontinuation of therapy. The treatment plan at our institution includes assessing TFTs every 2 or 3 weeks while patients are on treatment, depending on the ICI used. The TFTA was attributed to the specific ICI the patient was on when the TFTA occurred or to the most recent ICI when the TFTA occurred within 3 months of discontinuation.
TFTA in patients with no baseline TFTAs was characterized by any deviation from the normal reference range and classified according to standard definitions of thyroid dysfunction when possible (Table 1). The clinical course was described by noting if the TFTA resolved without intervention or if patients were treated (e.g., hormonal replacement with levothyroxine in primary hypothyroidism). The time to onset of the TFTA was recorded in number of days. Any histologic diagnosis of cancer treated with immune checkpoint blockade was included in this study (supplemental online Table 1).
Table 1. Classification of thyroid function test abnormalities.
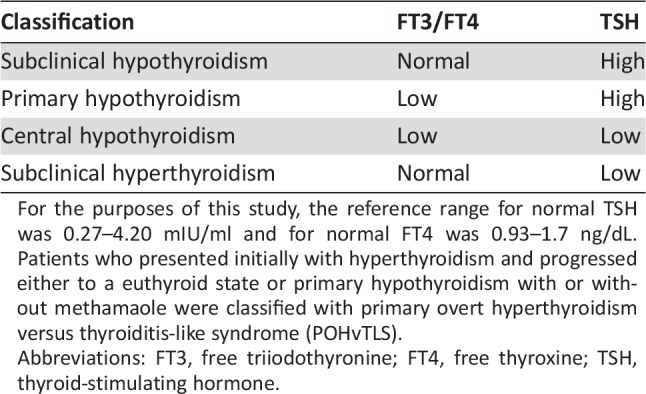
For the purposes of this study, the reference range for normal TSH was 0.27–4.20 mIU/ml and for normal FT4 was 0.93–1.7 ng/dL. Patients who presented initially with hyperthyroidism and progressed either to a euthyroid state or primary hypothyroidism with or without methamaole were classified with primary overt hyperthyroidism versus thyroiditis‐like syndrome (POHvTLS).
Abbreviations: FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid‐stimulating hormone.
Patients with baseline TFTAs were included in this study. Commonly cited etiologies of baseline TFTA included a history of primary hypothyroidism or primary hyperthyroidism, previous cancer therapy known to cause TFTA (e.g., tyrosine kinase inhibitors such as sunitinib and axitinib), prior interleukin‐2 therapy, and radiation to the head and neck area. Any exacerbation of a TFTA while patients were on ICI was recorded. It was noted if a patient was on levothyroxine hormone replacement therapy at baseline and whether the dose required adjustment on ICI therapy. It was also noted if a patient required a new start of levothyroxine hormone replacement therapy while on ICI therapy.
Statistical Analysis
All statistical analysis was performed using the IBM SPSS Statistics version 24. A Mann‐Whitney test was performed to determine statistical significance in comparing median TSH and FT4 values.
Results
Pooled Analysis
Of 218 patients with no baseline TFTAs, 76 patients developed new‐onset TFTAs. Of 61 patients with baseline TFTAs, 43 patients had exacerbation of TFTAs while on ICI therapy. There was no significant difference seen between male and female patients.
Total Incidence of New‐Onset TFTAs in Patients with No Prior Baseline TFTAs
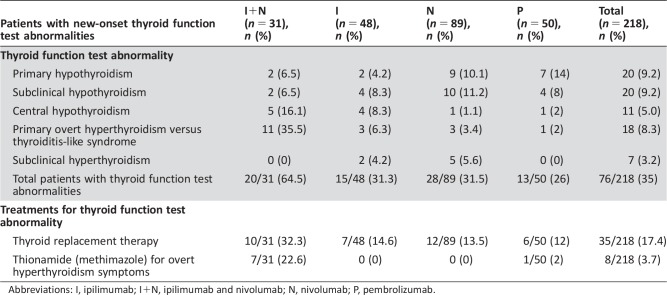
The primary objective was to assess the incidence of new‐onset TFTAs in those with no baseline TFTAs. For this group, the median baseline TSH was 2.0 mIU/ml, and FT4 was 1.20 ng/dL (Table 1). For comparison, in those patients who did not have baseline or post‐ICI therapy TFTAs, the median baseline TSH was 1.64 mIU/ml, and FT4 was 1.26 ng/dL (Table 2). Of 218 patients with no baseline TFTAs, 76 developed post‐ICI TFTAs. The incidence according to classification (Table 1) was as follows: 9.2% developed primary hypothyroidism (20 patients), 5.0% developed central hypothyroidism (11 patients), 8.3% developed a primary overt hyperthyroidism versus thyroiditis‐like syndrome (POHvTLS; 18 patients), 9.2% developed sub‐clinical hypothyroidism (20 patients), and 3.2% developed subclinical hyperthyroidism (7 patients). The total incidence of new‐onset TFTAs was 35.0% (76/218 patients; Table 3).
Table 2. Median baseline TFTs.
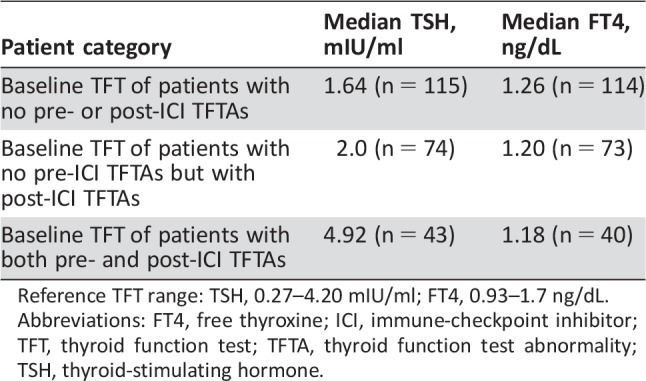
Reference TFT range: TSH, 0.27–4.20 mIU/ml; FT4, 0.93–1.7 ng/dL.
Abbreviations: FT4, free thyroxine; ICI, immune‐checkpoint inhibitor; TFT, thyroid function test; TFTA, thyroid function test abnormality; TSH, thyroid‐stimulating hormone.
Table 3. Summary of the incidence of new‐onset thyroid function test abnormalities by immune‐checkpoint inhibitor treatment.
Abbreviations: I, ipilimumab; I+N, ipilimumab and nivolumab; N, nivolumab; P, pembrolizumab.
Of 20 patients with new‐onset primary hypothyroidism, 17 were started on levothyroxine therapy. Of 11 patients with central hypothyroidism, 10 were started on levothyroxine therapy. The patients not started on levothyroxine were transitioned to hospice because of cancer progression. Based on the Common Terminology Criteria for Adverse Events, version 4.0, for endocrine dysfunction [6], the individuals who were started on levothyroxine replacement therapy were grade 2.
Of the 18 patients with POHvTLS, 8 patients required levothyroxine therapy when they progressed to primary hypothyroidism. The remaining ten patients who had a suppressed TSH and elevated FT4 concerning for primary overt hyperthyroidism to their health care providers were treated as follows: seven patients were treated with methimazole therapy by their providers (grade 2), one patient required hospitalization (grade 3), and two patients achieved a euthyroid state without treatment (grade 1). The 20 patients with subclinical hypothyroidism and 7 patients with subclinical hyperthyroidism were not started on further treatment during the course of our review (grade 1; Table 3). The median time to the onset of TFTA was 46 days.
Incidence of New‐Onset TFTAs by Immune‐Checkpoint Inhibitor Regimen (Table 4)
Table 4. Number of patients per ICI used in patients with no baseline thyroid function test abnormalities.
Abbreviation: ICI, immune‐checkpoint inhibitor.
Ipilimumab and Nivolumab.
Of 31 patients on ipilimumab and nivolumab therapy, 6.5% (2 patients) had primary hypothyroidism, 6.5% (2 patients) had subclinical hypothyroidism, 16.1% (5 patients) had central hypothyroidism, and 25.5% (11 patients) had POHvTLS. The total incidence on combined ipilimumab and nivolumab was 64.5% (20 patients).
Of note, one of eight patients with POHvTLS required discontinuation of ipilimumab and nivolumab and required hospitalization because of severe symptoms of hyperthyroidism treated with both steroids and methimazole. This patient subsequently tolerated pembrolizumab therapy.
Pembrolizumab.
Of 50 patients on pembrolizumab, 14% (7 patients) had primary hypothyroidism, 8.0% (4 patients) had subclinical hypothyroidism, 2.0% (1 patient) had POHvTLS, and 2.0% (1 patient) had central hypothyroidism. The total incidence in patients on pembrolizumab was 26% (13 patients).
Nivolumab.
Of 89 patients on nivolumab therapy, 10.1% (9 patients) had primary hypothyroidism, 11.2% (10 patients) had subclinical hypothyroidism, 1.1% (1 patient) had central hypothyroidism, 5.6% (5 patients) had subclinical hyperthyroidism, and 3.4% (3 patients) had POHvTLS. The total incidence in patients on nivolumab was 31.5% (28 patients).
Ipilimumab.
Of 48 patients on ipilimumab therapy, 4.2% (2 patients) had primary hypothyroidism, 8.3% (4 patients) had subclinical hypothyroidism, 8.3% (4 patients) had central hypothyroidism, 4.2% (2 patients) had subclinical hyperthyroidism, and 6.3% (3 patients) had POHvTLS. The total incidence in patients on ipilimumab was 31.3% (15 patients).
Patients with Baseline Thyroid Function Test Abnormalities
Of 61 patients with baseline TFTAs, 43 had TFTAs that were exacerbated while patients were on ICI therapy. The median baseline TSH was 4.92 mIU/ml, and FT4 was 1.18 ng/dL in this group. Of 43 patients, there were 16 who had baseline primary hypothyroidism on levothyroxine therapy that required an increase in their levothyroxine doses on ICI therapy. An additional 10 patients with baseline primary hypothyroidism on levothyroxine therapy had worsening TFTAs (elevated TSH with or without lower FT4) but did not have their levothyroxine dose increased. The most common reason why dose adjustments were not made was cancer progression and the patient enrolling in hospice. There were seven patients with baseline hypothyroidism on levothyroxine therapy who developed a hyperthyroid state (elevated FT4 and suppressed TSH). There was one patient with baseline primary hypothyroidism on levothyroxine therapy who required a decrease in levothyroxine dose during the treatment period. A total of six patients had baseline subclinical hypothyroidism with increased TSH during the treatment period. One patient with baseline primary hypothyroidism developed overt hyperthyroidism and was treated with methimazole while on ICI therapy. One patient with baseline subclinical hypothyroidism developed a thyroiditis‐like syndrome. One patient with baseline subclinical hypothyroidism developed primary hypothyroidism and required levothyroxine replacement therapy.
One patient had a baseline TFTA from sunitinib therapy for the treatment of renal cell carcinoma [14]. Because of renal cell carcinoma progression, nivolumab was added to the sunitinib. The patient required an increase in her levothyroxine dose while on both therapies. Further research on the effects of combined tyrosine kinase inhibitors and ICI therapy on thyroid function is needed, as both these classes of medications are used to treat patients with renal cell carcinoma.
Overall, the total percent of exacerbated TFTAs in those with baseline TFTAs was 70.5% (43 patients). The medium time to onset of exacerbated TFTA was 33 days in this group.
Comparison of Baseline Median TSH and FT4
Based on the Mann‐Whitney test to compare median values, there was a statistically significant difference in the median TSH in those with no pre‐ or post‐ICI TFTAs versus those with new‐onset TFTAs (p = .031). There was no difference seen in FT4 between these two groups. There was also a statistically significant difference in the median TSH in those with no pre‐ or post‐ICI TFTAs versus those with both pre‐ and post‐ICI TFTAs (p < .001). There was no difference seen in FT4 between these two groups (p = .116). There was also a statistically significant difference in the median TSH in those with pre‐ and post‐ICI TFTAs versus those with only new‐onset post‐ICI TFTAs (p < .001). There was no difference seen in FT4 between these groups.
Symptoms
Chart review was completed to assess symptoms that may have been present during the time of TFTA, with the most commonly documented symptom being increased fatigue. Some patients with POHvTLS experienced anxiety, heart palpitations, insomnia, weight loss, diarrhea, and night sweats. Other less commonly documented symptoms included myalgias, constipation, and weight gain, occurring in those with hypothyroidism. As previously mentioned, only one patient had discontinuation of treatment because of severe symptomatic overt hyperthyroidism. The majority of patients discontinued therapy because of cancer progression or another irAE that was beyond the scope of this paper (e.g., colitis, adrenal insufficiency).
Discussion
The overall incidence of new‐onset TFTAs was higher than previously reported in clinical trials in this retrospective single‐institution cohort. In addition, patients with baseline TFTAs were included with the goal of attempting to describe their clinical courses if they had exacerbated TFTAs. Overall, 35% of patients with no baseline TFTAs developed TFTAs while on ICI therapy. In addition, 70.5% of patients with baseline TFTAs had an exacerbation of their TFTAs while on ICI therapy. At this time, it is unclear what length of time TFTs should be measured after ICI therapy discontinuation.
In those with new‐onset TFTAs, a higher incidence of TFTAs was found in patients on combined ipilimumab and nivolumab therapy, 64.5%. The most common new‐onset TFTAs overall were primary hypothyroidism and subclinical hypothyroidism, with an equal incidence of 9.2%. The highest incidence of primary hypothyroidism was seen in those receiving pembrolizumab therapy, 14%, which correlated with what has been reported in clinical trials. Further studies will be needed to determine if patients with subclinical hypothyroidism developed primary hypothyroidism or if their TSH elevation was transient. Primary hypothyroidism was managed by starting levothyroxine therapy in the majority of patients, achieving a euthyroid state with treatment.
The highest incidence of new‐onset central hypothyroidism was seen in those patients on combined ipilimumab and nivolumab therapy, 16.1%, followed by ipilimumab monotherapy, 8.3%. Although beyond the scope of this study, of the 11 patients diagnosed with central hypothyroidism, 4 patients did not have other pituitary hormones assessed at the time of diagnosis. Five patients had concurrent secondary adrenal insufficiency requiring steroid replacement therapy along with central hypothyroidism. Two patients had central hypothyroidism without concurrent secondary adrenal insufficiency, as their cortisol levels were normal. Although these numbers are small, there appears to be less hypophysitis associated with anti‐PD‐1 therapies compared with anti‐CTLA‐4 therapies. The reason for this is not entirely clear; however, a review by Joshi et al. suggests that anti‐CTLA‐4 can generate de novo pituitary reactive effector cells, whereas anti‐PD‐1 can make existing pituitary reactive effector cells more active [15]. Another study by Iwama et al. reports that the pituitary itself may express CTLA‐4, making it a direct target. There is no current evidence the pituitary expresses PD‐1 or PD‐L1 [15, 16].
As mentioned in the Results section, there was one patient on combined sunitinib and nivolumab therapy with frequent TFT fluctuations. This patient highlights an important point that as the use of ICI therapy combined with other agents known to cause TFTA increases, there is a need for closer monitoring of TFTs. Further research will be needed to assess long‐term effects of combined agents on TFTs.
To date, no study assessing TFTAs in patients on ICI therapy has included patients with baseline TFTAs. In those with baseline TFTAs, there is a high likelihood the TFTAs will be exacerbated while the patients are on ICI therapy. A new start or dose adjustments in levothyroxine therapy may be required to help mitigate symptoms. In addition, one patient with baseline subclinical hypothyroidism developed a thyroiditis‐like syndrome, and another patient with primary hypothyroidism developed overt hyperthyroidism. This highlights the need for clinicians to be prepared for a new TFTA that a patient may not have had previously.
Limitations
This study does have certain limitations. This study heavily depended on patient chart review, raising the possibility that available data could be incomplete. In order to increase thoroughness, two separate individuals (N.S.P. and A.O.) reviewed each patient chart. There is a possibility that a small number of patients who were not excluded may have been on other medications known to cause TFTAs, such as amiodarone. The reviewers only had access to the electronic medical record system at this institution and not those of outside clinics or hospitals.
Given the retrospective nature of this cohort study, we could not verify if patients with baseline TFTAs on levothyroxine replacement therapy were 100% compliant. Any adjustments in dose needed while patients were on ICI therapy could have been due to noncompliance versus irAE. This could have been especially true for patients with baseline primary hypothyroidism who developed an elevated FT4 with low TSH. This finding could have been explained by a need for decreased levothyroxine dose because of cancer‐associated weight loss or increased compliance with levothyroxine.
In regard to patients classified as having POHvTLS, we did not have testing to confirm increased thyroxine production, such as a radioactive iodine uptake (RAIU) scan. To our knowledge, antibody testing (anti‐thyroid peroxidase, antithyroglobulin, or TSH receptor antibodies) was not routinely performed when a patient had a TFTA on ICI therapy. Initial attempts were made to separately classify the patients in this group into primary overt hyperthyroidism versus thyroiditis. Because of the retrospective nature of this study, the classification would have been based on whether patients were treated with methimazole for primary overt hyperthyroidism, which was at the discretion of the oncologist. It was determined that given the lack of antibody testing and RAIU scans, patients could have been treated with methimazole in the setting of a fulminant thyroiditis, and the decision was made to classify these patients into one group. Of note, there were five patients who were treated with methimazole who actually progressed to primary hypothyroidism and required levothyroxine replacement therapy.
It should also be noted that a portion of the findings in this study are reported in a descriptive manner because of the nature of the study. Lastly, the incidence of TFTAs could be underestimated because of limited TFT testing after the discontinuation of ICI therapy at this institution.
Conclusion
The overall incidence of TFTAs is higher than previously reported in clinical trials. It is unclear what the long‐term effects of TFTAs in the setting of ICI therapy are or whether patients with new‐onset TFTAs will achieve a euthyroid state in the future. A wide range of TFTAs can occur with or without baseline TFTAs, and patients with baseline TFTAs should be monitored more frequently for aberrant thyroid function while on ICI, especially in the first 8 weeks.
See http://www.TheOncologist.com for supplemental material available online.
Footnotes
For Further Reading: Elisa González‐Rodríguez, Delvys Rodríguez‐Abreu, on behalf of the Spanish Group for Cancer Immuno‐Biotherapy (GETICA). Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. The Oncologist 2016;21:804–816.
Implications for Practice: Immune checkpoint inhibitors are already part of oncologists' therapeutic arsenal as effective therapies for otherwise untreatable neoplasias, such as metastatic melanoma or lung cancer. Their use is expected to increase exponentially in the near future as additional agents become available and their approval is extended to different tumor types. Adverse events affecting the endocrine system are among the most frequent and complex toxicities oncologists may face, and some may be life‐threatening if not recognized. This study reviews endocrinopathies associated to immune checkpoint inhibitors available to date. Incidence, timing patterns, and clinical presentation are discussed, and practical recommendations for management are proposed.
Author Contributions
Conception/design: Nisha Subhash Patel, Sandip Pravin Patel
Provision of study material or patients: Nisha Subhash Patel, Gregory A. Daniels, Lyudmila Bazhenova, Sandip Pravin Patel
Collection and/or assembly of data: Nisha Subhash Patel, Anais Oury
Data analysis and interpretation: Nisha Subhash Patel, Anais Oury, Sandip Pravin Patel
Manuscript writing: Nisha Subhash Patel, Anais Oury, Gregory A. Daniels, Lyudmila Bazhenova, Sandip Pravin Patel
Final approval of manuscript: Nisha Subhash Patel, Anais Oury, Gregory A. Daniels, Lyudmila Bazhenova, Sandip Pravin Patel
Disclosures
The authors indicated no financial relationships.
References
- 1. Naidoo J, Page DB, Li BT et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corsello SM, Barnabei A, Marchetti P et al. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 2013;98:1361–1375. [DOI] [PubMed] [Google Scholar]
- 3. Abdel‐Wahab N, Shah M, Suarez‐Almazor M. Adverse events associated with immune checkpoint blockade in patients with cancer: A systematic review of case reports. PLoS One 2016;11:e0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Postow MA, Chesney J, Pavlick AC et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolchok J, Kluger H, Callahan M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchbinder E, Desai A. CTLA‐4 and PD‐1 pathways: Similarities, differences and implications of their inhibition. Am J Clin Oncol 2016;39:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barroso‐Sousa R, Barry W, Garrido‐Castro A et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta‐analysis. JAMA Oncol Published online September 28, 2017. Doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morganstein DL, Lai Z, Spain L et al. Thyroid abnormalities following the use of cytotoxic T‐lymphocyte antigen‐4 and programmed death receptor protein‐1 inhibitors in the treatment of melanoma. Clin Endocrinol 1‐7, 2017. [DOI] [PubMed] [Google Scholar]
- 10. Rossi E, Sgambato A, De Chiara G et al. Thyroid‐induced toxicity of check‐point inhibitors immunotherapy in the treatment of advanced non‐small cell lung cancer. J Endocrinol Diabetes 2016;3:1–10. [Google Scholar]
- 11. deFilette J, Jansen Y, Schreuer M et al. Incidence of thyroid‐related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab 2016;101:4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Common Terminology Criteria for Adverse Events . 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed Dec 16, 2016. [Google Scholar]
- 13. Lodish M, Stratakis C. Endocrine side effects of broad‐acting kinase inhibitors. Endocr Relat Cancer 2010;17:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joshi MN, Whitelaw BC, Palomar MT et al. Immune checkpoint inhibitor‐related hypophysitis and endocrine dysfunction: Clinical review. Clin Endocrinol 2016;85:331–339. [DOI] [PubMed] [Google Scholar]
- 15. Dillard T, Yedinak C, Alumkal J et al. Anti‐CTLA‐4 antibody therapy associated autoimmune hypophysitis: Serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 2010;13:29–38. [DOI] [PubMed] [Google Scholar]
- 16. Iwama S, De Remigis A, Callahn MK et al. Pituitary expression of CTLA‐4 mediates hypophysitis secondary to administration of CTLA‐4 blocking antibody. Sci Transl Med 2014;230–24515. [DOI] [PubMed] [Google Scholar]