Interventional oncology uses image‐guided procedures to enhance cancer care. This article reviews challenges and opportunities for growth within interventional oncology.
Keywords: Cancer, Intervention, Minimally invasive intervention, Imaging
Abstract
Interventional oncology uses image‐guided procedures to enhance cancer care. Today, this specialty plays an increasingly critical role in cancer diagnosis (e.g., biopsy), cancer therapy (e.g., ablation or embolization), and cancer symptom palliation (e.g., nephrostomies or biliary drainages). Although the number of procedures and technical capabilities has improved over the last few years, challenges remain. In this article we discuss the need to advance existing procedures, develop new ones, and focus on several operational aspects that will dictate future interventional techniques to enhance cancer care, particularly by accelerating drug development and improving patient outcomes.
Implications for Practice.
Interventional oncology is vital for cancer diagnosis, therapy, and symptom palliation. This report focuses on current interventional procedures and techniques with a look toward future improvements that will improve cancer care and patient outcomes.
Introduction
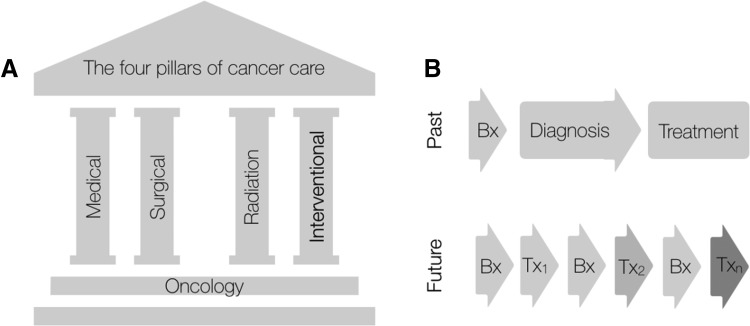
Image‐guided cancer interventions have steadily increased over the last decade as technical capabilities have improved, new procedures have been developed, and minimally invasive interventions have become generally preferred over more invasive alternatives. Today, the annual number of interventional oncology procedures in the U.S. alone are estimated in the millions. Nearly every patient with cancer will undergo at least one image‐guided biopsy prior to treatment, and many more patients will undergo palliative and symptomatic image‐guided treatments. For example, there were approximately 126,000 hospitalizations for malignant pleural effusion management in 2012 alone [1]. Interventional oncology can now be considered the fourth pillar of modern oncology care (Fig. 1).
Figure 1.
Interventional oncology plays a key role in integrated cancer care. (A): The four pillars of cancer care: medical oncology, surgical oncology, radiation oncology, and interventional oncology. (B): The old approach to intervention was largely linear (biopsy → diagnosis → treatment). The emerging promise of IO is one of multiple rebiopsies to optimize therapeutic approaches including interventions.
Abbreviations: Bx, Biopsy; Tx, Treatment.
Although cardiovascular interventional radiology was made possible by advances in fluoroscopy, interventional oncology (IO) is built primarily on advances in cross‐sectional imaging, including ultrasound, computed tomography (CT), magnetic resonance (MR), and positron emission tomography (PET) imaging. As a specialty, IO has leveraged many of interventional radiology's technical advances so that practitioners can provide increasingly differentiated cancer treatment strategies. Broadly speaking, IO comprises three interventional areas: (a) diagnosis (e.g., tissue sampling for molecular diagnostics and drug development), (b) therapy (e.g., tumor ablation and catheter delivered therapies), and (c) symptom palliation (Fig. 1). As “precision oncology” matures, using frequent tissue harvests to understand cancer's precise genetic and immune composition is critical. Furthermore, both catheter‐directed therapies and image‐guided ablative treatments have been shown to prolong survival in patients with hepatocellular carcinoma [2], [3], [4]. These treatments have been incorporated into international cancer care guidelines [5]. Finally, maintaining quality of life is critical to managing patients with cancer. IO techniques such as ablating painful bone metastases, preventing pathologic fractures, and draining malignant effusions and ascites can greatly improve patient quality of life.
The field of IO has largely self‐organized over the last decade, leading to broader use [6], established subdivisions in many hospitals, and, in 2017, the formation of the Society of Interventional Oncology (SIO). Given the current and potential future value of image‐guided intervention, the SIO embraces more forward‐looking and deliberative planning to develop IO's capacity to improve cancer care. Yet such enthusiasm is tempered by the tremendous constraints on different aspects of patient care. Integrative and multidisciplinary solutions are often needed to further the field. The goal of this article is to review current challenges and opportunities for growth within IO. The beneficiaries of future IO developments will always include patients with cancer, but health care systems may also benefit as IO specialists strive to provide a continuum of care, avoid unnecessary interventions, implement cost‐effective treatments, and further improve patient care across the globe.
Challenges and Limitations of Current Practice
Many interventional procedures are now well described, commonly practiced, and fully embraced by the oncology community (Table 1). Providing these services has presented a number of opportunities to meet existing challenges. We identify five major areas for improvement: (a) biopsy yields and their information content, (b) therapeutic procedures and coupling with immunotherapy, (c) existing hardware and device limitations, (d) operational workflows, and (e) training and research specific to interventional oncology.
Table 1. Image‐guided interventional procedures: Past and future.
Abbreviations: FNA, fine needle aspirate; HCC, hepatocellular carcinoma; ID, infectious disease analysis; MRI, magnetic resonance imaging; PDX, patient‐derived xenograft; SIRT, selective internal radiotherapy.
Evolving Paradigms in Biopsies
Although acquiring enough tissue sample to render morphologic diagnoses was once considered adequate, today's biopsy requirements are quite different. There is a shift from a single core to high throughput, multicore biopsies in which specimens are processed for histopathology, immunohistochemistry, fluorescence in situ hybridization analysis, genomic analyses, protein and phosphoprotein biomarker testing, and establishment of patient‐derived models (e.g., patient‐derived xenografts, cell lines, organoids, and mouse xenografts). Readouts from such biopsies have even become integral to entering certain “state‐of‐the‐art” clinical trials. In an ideal setting, future biopsies will have even higher diagnostic yields, become more comprehensive by sampling more biomarkers, occur more frequently to provide ongoing evaluation of the cancer and tumor microenvironment adaptations to therapeutic pressures, and be even less invasive and thus better tolerated by patients. Tissue sampling will be further informed by circulating biomarkers (e.g., liquid biopsies), whereas new technical capabilities will speed up the currently slow diagnostic cycle (i.e., from biopsy to end result).
What to Biopsy?
Currently, most biopsy planning is dictated by anatomic imaging. Targets are primarily chosen based on accessibility, surrounding structures, obstacles avoidance, and patient conditions (e.g., sedation requirements, pain control). Although this anatomic approach has generally worked well, there is increasing interest in specifically targeting high‐interest lesions that can be identified by molecular imaging. Biopsying “hot spots” assures molecular readouts of the most active cancer areas rather than those of quiescent or necrotic lesions. This specificity directly addresses cancer researchers and clinicians’ increasing concerns about intrapatient tumor heterogeneity.
In an ideal setting, future biopsies will have even higher diagnostic yields, become more comprehensive by sampling more biomarkers, occur more frequently to provide ongoing evaluation of the cancer and tumor microenvironment adaptations to therapeutic pressures, and be even less invasive and thus better tolerated by patients.
There is also ongoing debate about the number of cores required for testing. On one hand, analytical advances have ushered in an era of single‐cell sequencing and protein pathway analysis. On the other hand, requests for additional cores have increased substantially. One recent study [7] evaluated a large number of research biopsies (average of 5.5 cores per procedure) that were distributed to trial sponsors, internal research laboratories, and pathology services. The study concluded that harvesting extra tissue cores through coaxial needles during focal liver biopsies did not increase complication rates and yielded valuable tissue for additional experimental testing. Standardization of biopsy materials for molecular testing, however, has not been done universally and has been problematic in some studies [8].
Rapid Molecular Analyses of Fine Needle Aspirates
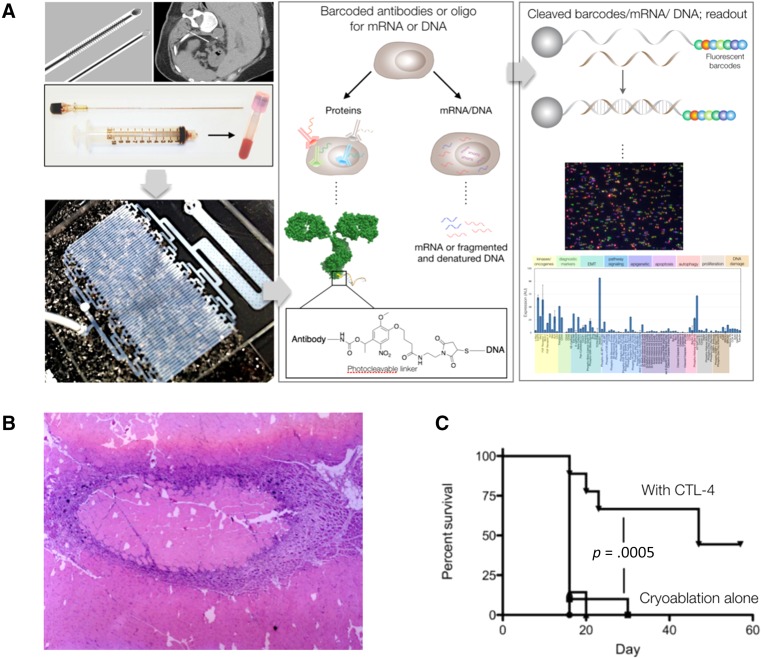
There is a need to perform broad, pathway‐centric immunohistochemistry on more limited sample sizes, such as fine needle aspirates (FNAs), rather than core biopsies. FNAs are obtained with 21 G needles, as opposed to 17 G core biopsies, and generally have fewer procedural complications and perhaps even lower sedation requirements. The challenge has been to obtain sufficient numbers of cells for diagnostic purposes. One recently described technology can analyze hundreds of proteins in cells from FNA samples (Fig. 2). The method capitalizes on DNA‐barcoded antibody sensing, in which barcodes can be digitally detected without any amplification steps. In one study, the method was used to profile approximately 90 proteins in cells, map patient heterogeneity, and demonstrate pathway responses to molecularly targeted drugs [9]. Methods based on this and similar principles [10], [11], [12] are now being developed and will ultimately allow deeper sample analyses in scant materials.
Figure 2.
Example of emerging interventional procedures. (A): Novel diagnostics developed for fine needle aspirate sampling under image guidance. Using a 21 G needle alone it is now possible to quickly obtain thousands of single cells for protein, mRNA, and DNA analysis. This has the potential to replace cutting biopsies and reduce complications while improving information content. Reprinted with permission from [9]. (B): Combination of local therapy, drug delivery, and systemic immunotherapy potentiates therapeutic efficacy. Hematoxylin and eosin staining of a 7‐day‐old ablation lesion created with high intensity focused ultrasound in a rabbit muscle. The image demonstrates the intense inflammatory response surrounding the ablation zone and the opportunity for antigen presentation. This type of response suggests the possibility for combining ablation therapy with immunotherapy treatments. Reprinted with permission from [48]. (C): The graph demonstrates the synergy between cryoablation and anti‐CTLA‐4 combination therapy to mediate rejection of a second tumor challenge. Reprinted with permission from AACR [31].
Tissue versus “Liquid Biopsy” for Molecular Analyses
Liquid biopsies, that is, the analysis of tumor‐derived material in peripheral blood, have exploded in popularity over the last few years. Different approaches include analyzing scant circulating tumor cells [13], circulating tumor DNA [14], and extracellular vesicles (“exosomes”) [15]. These highly specific methods are generally viewed as complementary to image‐guided, minimally invasive biopsies. Despite their lower sensitivity, liquid biopsies seem to be particularly useful for screening and for serially monitoring treatment response or relapse. Percutaneous biopsies, however, continue to remain the gold standard in procuring sufficient malignant tissue for detailed molecular and cellular analysis to inform routine clinical care. Combining information from the two different sources could more deeply mine data points via integrated diagnostics. The complementary strengths of tissue and liquid biopsies will likely drive the next wave of influential cancer diagnostics and profiling strategies.
Low‐Cost Molecular Analyses in Developing Countries
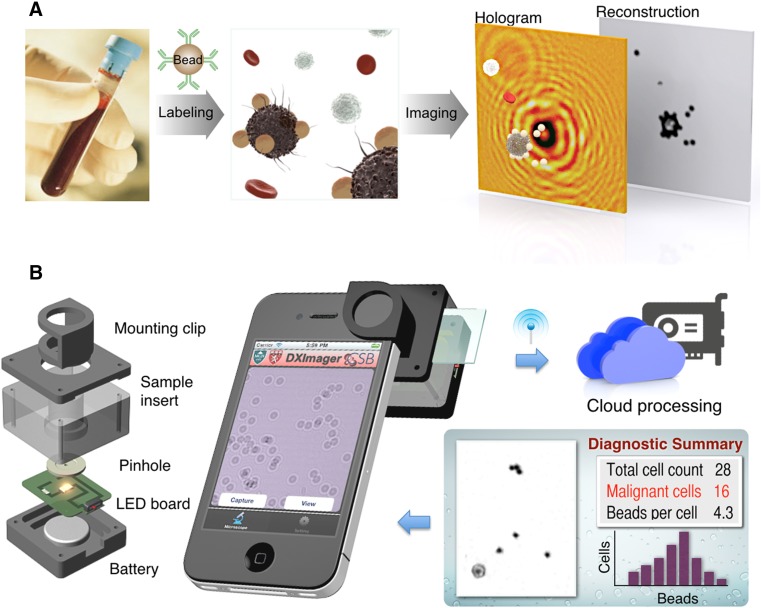
One of the critical health challenges in low‐ and middle‐income countries (LMICs) is identifying patients with aggressive cancer who require imminent therapy. Because of limited pathology resources and large caseloads, there has been interest in developing advanced stand‐alone diagnostics. An automated point‐of‐care technology was recently developed to molecularly diagnose lymphoma in a resource‐limited setting [16], [17], [18]. Namely, a contrast‐enhanced microholography method integrating a deep learning algorithm was developed to directly analyze percutaneously obtained FNAs of palpable lesions (Fig. 3). The method demonstrated high accuracy in a lymphoma‐focused diagnostic clinical study. Similar technology is also being developed for palpable breast lesions. We believe that these and other low‐cost portable devices along with automated analysis will allow accurate classification of patients in resource‐limited settings for prompt triage into appropriate treatment regimens. Deploying innovative, robust, “low tech” strategies in LMICs should help catalyze efforts to reduce the vast global disparities in cancer care and outcomes. Moreover, engaging historically underserved populations could help us understand both the epidemiology and biological underpinnings of their cancers to refine resources and tailor care.
Figure 3.
Digital diffraction diagnosis (D3) platform for rapid diagnosis of biopsy material in resource‐limited settings. (A): Assay schematic for cellular detection. Target cells in patient samples (e.g., blood or biopsy) are immunolabeled with microbeads, and their diffraction patterns are recorded. The diffraction images are then digitally reconstructed into object images wherein bead‐labeled target cells are identified. (B): The snap‐on module for a smartphone consists of a light‐emitting diode powered by a coin battery, a pinhole for uniform illumination with partial coherence, and a sample mount. (C): The D3‐mounted smartphone's embedded phone camera is used to record the diffraction images of the specimen. The recorded images are transferred to a server via the cloud service for real‐time image reconstruction and analyses, which can be returned to the smartphone in less than 2 minutes. Reprinted with permission from PNAS [17].
New Therapeutics for Intratumoral Delivery
Over the past two decades, the opportunities to help patients with both catheter‐directed therapies and image‐guided ablative treatments have grown exponentially. As technology has progressed, the original gelfoam‐based transarterial chemoembolizations have been replaced by emulsions and embolic beads with drug elution [19], [20], [21]. Furthermore, yttrium‐90‐laden beads for radioembolization are another effective tool in the armamentarium, and holmium‐166 for selective internal radiotherapy is a promising therapeutic agent that can be visualized in both x‐ray and MR imaging (MRI) [22]. A recent phase II study demonstrated hepatic disease control, with an acceptable toxicity profile, in 49% of patients [23].
Tumor Ablations
Ablative tools have advanced as well. Initially, ablation was performed with ethanol, but given the liquid's variable spread, most practitioners now favor thermal tumor ablation using radiofrequency, microwave, laser, cryotherapy, and focused ultrasound. Today, the most commonly used techniques include radiofrequency, microwave, or cryotherapy, depending on the location and operator [24], [25], [26]. A more recently developed and adopted alternative to thermal ablation is irreversible electroporation, which transiently disrupts cell membranes through electric pulses that kill cancer cells, although this technology is still in its infancy [27]. As different ablative technologies have emerged, their use has been supported by advances in imaging tools such as ultrasound fusion and contrast, CT fluoroscopy, MR thermometry, and PET guidance.
Imaging Guides Intratumoral Delivery
Precisely delivering novel therapeutics into tumors is gaining interest. These deliveries often occur through either catheter‐based approaches or direct intratumoral injections. Furthermore, image guidance has enabled other novel techniques, such as oncolytic viral therapies [28] and bacterial therapies [29], [30]. In many cases, the effects of direct intratumoral injection of these therapeutics have been better than systemic intravenous approaches. This in turn indicates that image‐guided administration will continue to play an important and expanded role in the future.
The field of immuno‐oncology has blossomed in response to our increasing knowledge of the interactions between cancer and the immune system. Antibodies against checkpoint inhibitors have become exciting new systemic therapies for treating many cancers. However, with the improved antigen presentation that can be accomplished with ablative or catheter‐directed therapies (Fig. 2), these newer systemic therapies may be further improved [31], [32], [33], [34], [35]. As such, there are several ongoing treatment trials that combine immunotherapy with IO.
Chimeric antigen receptor (CAR) T‐cell treatment is another exciting treatment modality. In this case, too, studies show that localized tumoral delivery of CAR T cells may be more effective than systemic delivery [36]. The potential to expand CAR T‐cell treatment opens up future synergistic opportunities for IO that could further cement its role in cancer care.
New Technical Capabilities
Although the vast majority of IO procedures are performed using ultrasound, x‐ray fluoroscopy, or CT imaging modalities, a number of new technical advances are on the horizon.
Interventional MRI, Including Hybrid Imaging Systems
MRI is well suited to interventions because of its superior soft tissue contrast and capacity to monitor treatment response in real time using functional imaging techniques (e.g., MR thermometry). However, because of the restricted patient access in high‐field systems, IO has been largely limited to open‐bore systems, which so far have had inferior performance in terms of spatial resolution, speed, and functional monitoring. In addition, the development of MRI‐compatible interventional devices has never reached the level or performance of mainstream product lines for x‐ray‐based interventional procedures. More recently, hybrid systems (XMRI) with separate two‐room MRI and x‐ray fluoroscopy installations have increased procedure flexibility and spectrum. The systems are interlinked by an automated floating table for exact and reproducible patient positioning. One growing indication for XMRI is interventional brachytherapy to locally control nonresectable, infiltrative tumors that cannot be effectively reached by either external beam radiation therapy or standard thermal ablative techniques. Radioactive seed placement has also shown promising tumor control results for recurrent intrahepatic cholangiocarcinoma [37]. XMRI has the potential to integrate state‐of‐the‐art morphologic and functional MRI, x‐ray, and MR image fusion for precise interventional needle placement and immediate follow‐up of tumor response including diffusion, perfusion, thermometry, and advanced image analysis. Such an integrated approach has the potential to shorten the diagnostic‐therapeutic information continuum and to reduce the diagnostic uncertainty of long‐term follow‐up (Fig. 1).
New Auxiliary Robotic Systems to Increase Spatial Precision
Robotic technologies have slowly been implemented in the operating room over the last 2 decades, with variable outcomes. Although visually driven systems have advanced more quickly, one of the success‐limiting steps in IO has been the difficulty of seamlessly integrating intraprocedural imaging and the device at frame rates to account for physiologic motion (e.g., breathing, organ motion). Interdisciplinary development sites have recently presented innovative concepts for interactive robotic navigation by aligning the stereotactic imaging frame of the cone beam CT and MRI systems with the coordinates of the robotic arm with real‐time needle position update. This induces a paradigm shift from a fixed robotic device to an integrated assistant manipulator, which in turn allows the advancement of percutaneous biopsy or thermal ablation probes with immediate image‐guided feedback. Initial studies have shown that this approach reduces both overall procedure time and radiation exposure while also increasing precision for out‐of‐plane needle placement, particularly for less experienced users [38]. Broader availability of these systems will allow the IO community to expand its user pool. In addition, diagnostic and treatment‐related imaging can be comprehensively assessed, reducing overall procedure‐related time (Fig. 1).
Advances in Ultrasound
In IO, ultrasound has recently experienced a “rebirth” because of its real‐time feedback, improved image quality, and portability along with the introduction of 3D imaging, the approval of ultrasound contrast agents, and the development of therapeutic ultrasound. Ultrasound contrast agents can more precisely visualize difficult‐to‐see lesions and assess organ perfusion. 3D fusion techniques combine ultrasound, CT, and MRI images in real time to further improve spatial orientation and localization. Agent reinjection offers immediate surrogate markers of radiologic‐interventional tumor ablation. Ultrasound agents with target‐specific molecules recognizing angiogenesis are being applied in studies to guide stereotactic biopsies of aggressive hypervascular tumor areas [39]. High‐intensity focused ultrasound ablation is another emerging therapeutic modality wherein noninvasive, pinpoint ablations can be performed [40]. This modality also allows tumor regional increases in drug delivery through either selective opening of the vascular barrier or nanoparticle disruption [41].
Theranostics
Theranostics (Rx/Dx) refers to the development of targeted therapeutics and combined molecular diagnostics with the goal of personalizing treatment by targeting therapy to an individual's specific disease subtype and genetic profile. The premise is that the linked companion diagnostic test will monitor therapy in real time or determine whether a patient will benefit from a specific treatment. Examples include nuclear medicine approaches [23], [42], [43], ultrasound contrast agents, and MR‐imageable, drug‐loaded nanostructures [44], [45]. Theranostics is an actively growing field with its own journal and rapid advances, several of which can now enter clinical testing.
Redesigning Clinical Operations to Provide a Care Continuum
In an ideal scenario, interventional procedures are performed in state‐of‐the‐art imaging suites and patients are cared for in modern perioperative recovery areas and followed in clinics. Increasingly, it has also become routine for IO specialists to round on inpatients, and many institutions even offer them admission privileges. Despite these advances, the reality of the emerging specialty is often different. For example, in many hospitals, radiology departments were designed in the preinterventional era; newer imaging suites are often scattered across different buildings. This invariably leads to workflow, scheduling, and communication problems. It is thus apparent that critical review of practical and patient‐centric workflows needs to be implemented.
Measuring Interventions: Level of Clinical Evidence
IO is maturing and the number of different procedures available to patients with cancer is growing. This raises important issues regarding the safe and efficient deployment of emerging procedures to guide appropriate care. The level of evidence for IO procedures is still dominated by single‐arm studies largely reporting technical successes or clinical efficacy and hence a relatively low level of evidence. Although such early studies are important in popularizing new treatment approaches, they are not sufficient to change clinical practice uniformly across health care systems. The challenge for trial investigators in the future will be to incorporate cost‐effectiveness measures and patient‐reported outcomes into larger‐scale studies to change clinical practice. Endpoints of clinical efficacy (e.g., procedure lengths and wait times, avoidable and unavoidable procedure‐related complications, patient satisfaction, measurable disease response, progression‐free survival, disease‐free survival) and effectiveness (e.g., overall survival, hospitalization‐free period, quality of life, health care costs) have been published [47].
The level of evidence for IO procedures is still dominated by single‐arm studies largely reporting technical successes or clinical efficacy and hence a relatively low level of evidence. Although such early studies are important in popularizing new treatment approaches, they are not sufficient to change clinical practice uniformly across health care systems.
What is likely required at this stage is to convene a National Comprehensive Cancer Network task force to define challenges surrounding diagnostic, curative, and palliative procedures and building clinical evidence. This would also help address other impending changes to the health care system, including Centers for Medicare and Medicaid Services regulations, changes in reimbursement models, and other governmental pressures. In the future it will, therefore, become essential to measure outcomes and benefits not only for continued care improvement but also to document for appropriate reimbursement.
Limited Clinical Trial Infrastructure
The clinical trial infrastructure for image‐guided interventions is currently quite limited for several reasons. First, IO trials often lack industry funding at the scale required for large phase II and III trials. Although medical and surgical fields are often backed by multi‐billion‐dollar companies, the IO commercial landscape is much more modest. However, there are opportunities to expand this by integrating pharma‐supported systemic therapies with IO therapies to achieve synergy, for example in the field of immuno‐interventional oncology (Fig. 2) [31], or the use of advanced diagnostic readouts of pathway inhibition [12]. Second, there is an absence of cooperative group structure that could create and accrue successful IO trials. The American College of Radiology Imaging Network (ACRIN) is one such groups, but with the exception of a few IO studies (e.g., ACRIN trial 6673, radiofrequency ablation of hepatocellular carcinoma in cirrhosis) they have focused more on diagnostic imaging. Third, we need to use registries to gather large data sets on IO procedures, such as through the IO Clinical Outcomes Registry.
Developing Patient‐Friendly Workflows
Most patients with cancer are monitored by imaging on a routine basis. When abnormalities are detected, rapid and seamless integration with the IO service facilitates rapid preprocedure planning. In some hospitals, this occurs at tumor board meetings and through electronic communications (electronic medical records, flowcharts) or personal visits in others. Irrespective of the mechanism, a continuous dialogue of neighboring clinical disciplines will ultimately help the patient and allow for rapid treatment changes based on new results.
From a patient and workflow perspective, it is essential to implement periprocedural areas where patients can give consent, be examined, and undergo recovery. Currently, these recovery areas are often at capacity during peak hours, leading to unnecessary backlogs in procedure rooms and delays for other patients. Time‐based scheduling, more efficient turnover, and expansion facilities are some mechanisms that can improve workflows. Going forward, we can prospectively plan these receiver areas to avoid mistakes of the past.
Finally, there are opportunities to improve patient‐physician interactions by using existing and developing future Health Insurance Portability and Accountability Act‐compliant smartphone apps. Simplifying appointment setups, controlling and managing pain, and using telemedicine are just a few examples.
Improving Oncology Integration and Developing New Partnerships
Although IO has been dubbed the fourth pillar of oncology, there are locoregional differences in integration between this and other oncologic fields. IO procedures can be better integrated with other multimodality treatments. Examples of such integration include enabling leukapheresis for CAR T‐cell therapy by placing large bore venous catheters, facilitating the use of radiotherapy by placing fiducial markers, and partnering with urologists who place ureteral stents as protective devices during renal ablation. These types of partnerships and alliances may also prove useful to continue the growth of the specialty.
Can Robotic Approaches Be Used to Address the Workforce Bottleneck?
IO is a fast‐growing specialty with high demand for new trainees. This is aggravated by the rising number of elderly, multimorbid oncologic patients across the globe. The specialist shortage is particularly pronounced in Europe, Asia, and rural areas. A key question then becomes whether technology could one day augment or replace a less‐skilled work force. For example, simulation systems, robots, and navigation systems could be designed to assist younger physicians in simpler procedures. Innovative assisting devices might be the way to address these tasks in the future. Coupling such hardware with emerging artificial intelligence and machine learning could make significant inroads. Much work remains to see if indeed the strategies mentioned above are feasible and cost‐effective.
Research and Training
Research in interventional oncology is critical to assure continued growth and development of next‐generation technologies. In the past, basic research has taken a backseat to clinical procedure development. This is not entirely unexpected given the clinical nature of the specialty, the absence of formal research training in curricula, and the often close relationships between physicians and device and equipment manufacturers. In the future, basic and outcomes research will hopefully blossom. For example, there are extraordinary opportunities in developing next‐generation diagnostics, miniaturized sensors, integrating diagnostics, artificial intelligence, novel ablative therapies, early detection strategies, and adjuvant immunotherapies, to name a few. Increased involvement of the IO community in development of new procedures would be beneficial, and incorporating research time into IO fellowships, similar to surgery and medical oncology fellowships, might be possible.
Training the next generation of interventional specialists is well under way, now that the American Board of Radiology administers Initial Certifications and then Maintenance of Certification exams. Similar curricula have been established by Cardiovascular and Interventional Radiological Society of Europe in the European community and require training not only in interventional radiology but also in pathology, tumor biology, pharmacology, bioinformatics (e.g., artificial intelligence, machine learning), and technical skills. One specific route for providing online continuing medical education‐accredited education in the fundamentals of oncologic disease management for interventional radiologists is the IO University Curriculum provided by the SIO (SIO‐central.org). Another opportunity is the Radiological Society of North America's Clinical Trials Methodology Workshop, a week‐long intensive boot camp, similar to the American Society of Clinical Oncology's Vail course, in which junior faculty attend lectures on clinical trial design, statistics, and logistics while developing their own trial protocols in small group sessions.
Continued education and understanding of how IO procedures can better integrate with other multimodality treatments is needed. Similarly, the level of molecular and pharmacologic training is still subpar in comparison with other oncologic specialties. Future clinical trials and practice should integrate disciplines and crystallize synergies for better patient care.
Conclusion
The future of IO is exciting: two decades of improving techniques and experience have demonstrated significant value to the patient with cancer for diagnosis, therapy, and symptom palliation. These advances have generated further opportunities for growth, as outlined above. Going forward, one of the most interesting directions will be the emergence of new therapeutic procedures and technical capabilities and their prospective efficacy testing in larger trials. Similarly, better interventional tools are on the horizon and will ultimately enable more accurate and safer procedures. The availability of IO services in individual countries [6] and across the globe is somewhat uneven. In the U.S. and some European countries, another challenge has been the increasing number of procedures, leading to resource constraints, often suboptimal integration of IO into oncologic teams, and reduced time in training the next generation of specialists. These locoregional challenges can be addressed by more efficiently integrating IO into cancer management teams. Such positioning would enable IO to accommodate emerging cancer advances and practices. The beneficiaries will clearly include patients as well as cancer clinicians and researchers.
Acknowledgments
The authors thank members of the International Society for Strategic Studies in Radiology for active discussions. Special thanks go to Drs. Jonathan Carlson, Cesar Castro, and Hedvig Hricak for critical review and valuable suggestions.
Author Contributions
Conception/design: Stefan O. Schoenberg, Ulrike I. Attenberger, Stephen B. Solomon, Ralph Weissleder
Manuscript writing: Stefan O. Schoenberg, Ulrike I. Attenberger, Stephen B. Solomon, Ralph Weissleder
Final approval of manuscript: Stefan O. Schoenberg, Ulrike I. Attenberger, Stephen B. Solomon, Ralph Weissleder
Disclosures
The Institute of Clinical Radiology and Nuclear Medicine (S.O.S., U.I.A.) has research agreements with Siemens Healthcare. Stephen B Solomon: Medtronic, Johnson & Johnson, BTG, AstraZeneca, Adgero (C/A), GE Healthcare (RF). Ralph Weissleder: co‐founder of Lumicell and T2Biosystems (IP), scientific consultant to ModeRNA, Tarveda Therapeutics, Alivio Therapeutics, Bioanalytix (C/A). None of these entities contributed to this manuscript. Stefan O. Schoenberg, Ulrike I. Attenberger, and Stephen B. Solomon indicate no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: Data from the 2012 National Inpatient Sample. Chest 2017;151:845–854. [DOI] [PubMed] [Google Scholar]
- 2. Lo CM, Ngan H, Tso WK et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164–1171. [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429–442. [DOI] [PubMed] [Google Scholar]
- 4. Shiina S, Tateishi R, Arano T et al. Radiofrequency ablation for hepatocellular carcinoma: 10‐year outcome and prognostic factors. Am J Gastroenterol 2012;107:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benson AB 3rd, D'Angelica MI, Abbott DE et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw 2017;15:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhong J, Atiiga P, Alcorn DJ et al. Cross‐sectional study of the provision of interventional oncology services in the UK. BMJ Open 2017;7:e016631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frenk NE, Spring L, Muzikansky A et al. High‐content biopsies facilitate molecular analyses and do not increase complication rates in patients with advanced solid tumors. JCO Precis Oncol 2017;1:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Conley BA, Gray R, Chen A. NCI‐molecular analysis for therapy choice (NCI‐MATCH) clinical trial: Interim analysis. Cancer Res 2016;76(suppl 14):CT101A. [Google Scholar]
- 9. Ullal AV, Peterson V, Agasti SS et al. Cancer cell profiling by barcoding allows multiplexed protein analysis in fine‐needle aspirates. Sci Transl Med 2014;6:219ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin JR, Fallahi‐Sichani M, Sorger PK. Highly multiplexed imaging of single cells using a high‐throughput cyclic immunofluorescence method. Nat Commun 2015;6:8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peterson VM, Zhang KX, Kumar N et al. Multiplexed quantification of proteins and transcripts in single cells. Nat Biotechnol 2017;35:936–939. [DOI] [PubMed] [Google Scholar]
- 12. Giedt RJ, Pathania DC, McFarland P et al. Single cell barcode analysis (SCANT) to monitor clinical drug response. Nat Commun 2018. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haber DA, Velculescu VE. Blood‐based analyses of cancer: Circulating tumor cells and circulating tumor DNA. Cancer Discov 2014;4:650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen JD, Javed AA, Thoburn C et al. Combined circulating tumor DNA and protein biomarker‐based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci USA 2017;114:10202–10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang KS, Im H, Hong S et al. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci Transl Med 2017;9:pii.eaal3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Im H, Pathania D, McFarland P et al. Contrast enhanced microholography enables point‐of‐care diagnosis of lymphoma in resource‐limited settings. Nat Biomed Eng 2018. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Im H, Castro CM, Shao H et al. Digital diffraction analysis enables low‐cost molecular diagnostics on a smartphone. Proc Natl Acad Sci USA 2015;112:5613–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pathania D, Im H, Kilcoyne A et al. Holographic assessment of lymphoma tissue (HALT) for global oncology field applications. Theranostics 2016;6:1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: Current state of the art. World J Gastroenterol 2018;24:161–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu YS, Ou MC, Tsai YS et al. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol‐doxorubicin versus doxorubicin‐loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol 2015;16:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sandow TA, Arndt SE, Albar AA et al. Assessment of response to transcatheter arterial chemoembolization with doxorubicin‐eluting microspheres: Tumor biology and hepatocellular carcinoma recurrence in a 5‐year transplant cohort. Radiology 2018;286:1072–1083. [DOI] [PubMed] [Google Scholar]
- 22. Smits ML, Elschot M, van den Bosch MA et al. In vivo dosimetry based on SPECT and MR imaging of 166Ho‐microspheres for treatment of liver malignancies. J Nucl Med 2013;54:2093–2100. [DOI] [PubMed] [Google Scholar]
- 23. Prince JF, van den Bosch MAAJ, Nijsen JFW et al. Efficacy of radioembolization with 166Ho‐microspheres in salvage patients with liver metastases: A phase 2 study. J Nucl Med 2018;59:582–588. [DOI] [PubMed] [Google Scholar]
- 24. Higgins LJ, Hong K. Renal ablation techniques: State of the art. AJR Am J Roentgenol 2015;205:735–741. [DOI] [PubMed] [Google Scholar]
- 25. Nault JC, Sutter O, Nahon P et al. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26. Vroomen LGPH, Petre EN, Cornelis FH et al. Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: What are the differences? Diagn Interv Imaging 2017;98:609–617. [DOI] [PubMed] [Google Scholar]
- 27. Narayanan G, Hosein PJ, Beulaygue IC et al. Percutaneous image‐guided irreversible electroporation for the treatment of unresectable, locally advanced pancreatic adenocarcinoma. J Vasc Interv Radiol 2017;28:342–348. [DOI] [PubMed] [Google Scholar]
- 28. Pease DF, Kratzke RA. Oncolytic viral therapy for mesothelioma. Front Oncol 2017;7:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang CZ, Kazmierczak RA, Eisenstark A. Strains, mechanism, and perspective: Salmonella‐based cancer therapy. Int J Microbiol 2016;2016:5678702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wall DM, Srikanth CV, McCormick BA. Targeting tumors with salmonella Typhimurium ‐ potential for therapy. Oncotarget 2010;1:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Waitz R, Solomon SB, Petre EN et al. Potent induction of tumor immunity by combining tumor cryoablation with anti‐CTLA‐4 therapy. Cancer Res 2012;72:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hickey RM, Kulik LM, Nimeiri H et al. Immuno‐oncology and its opportunities for interventional radiologists: Immune checkpoint inhibition and potential synergies with interventional oncology procedures. J Vasc Interv Radiol 2017;28:1487–1494. [DOI] [PubMed] [Google Scholar]
- 33. Li G, Staveley‐O'Carroll KF, Kimchi ET. Potential of radiofrequency ablation in combination with immunotherapy in the treatment of hepatocellular carcinoma. J Clin Trials 2016;6:pii.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McArthur HL, Diab A, Page DB et al. A pilot study of preoperative single‐dose ipilimumab and/or cryoablation in women with early‐stage breast cancer with comprehensive immune profiling. Clin Cancer Res 2016;22:5729–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silvestrini MT, Ingham ES, Mahakian LM et al. Priming is key to effective incorporation of image‐guided thermal ablation into immunotherapy protocols. JCI Insight 2017;2:e90521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adusumilli PS, Cherkassky L, Villena‐Vargas J et al. Regional delivery of mesothelin‐targeted CAR T cell therapy generates potent and long‐lasting CD4‐dependent tumor immunity. Sci Transl Med 2014;6:261ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seidensticker R, Seidensticker M, Doegen K et al. Extensive use of interventional therapies improves survival in unresectable or recurrent intrahepatic cholangiocarcinoma. Gastroenterol Res Pract 2016;2016:8732521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diehl SJ, Rathmann N, Kostrzewa M et al. Irreversible electroporation for surgical renal masses in solitary kidneys: Short‐term interventional and functional outcome. J Vasc Interv Radiol 2016;27:1407–1413. [DOI] [PubMed] [Google Scholar]
- 39. Kiessling F, Fokong S, Bzyl J et al. Recent advances in molecular, multimodal and theranostic ultrasound imaging. Adv Drug Deliv Rev 2014;72:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tempany CM, McDannold NJ, Hynynen K et al. Focused ultrasound surgery in oncology: Overview and principles. Radiology 2011;259:39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park J, Aryal M, Vykhodtseva N et al. Evaluation of permeability, doxorubicin delivery, and drug retention in a rat brain tumor model after ultrasound‐induced blood‐tumor barrier disruption. J Control Release 2017;250:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jadvar H, Chen X, Cai W et al. Radiotheranostics in cancer diagnosis and management. Radiology 2018;286:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yordanova A, Eppard E, Kürpig S et al. Theranostics in nuclear medicine practice. Onco Targets Ther 2017;10:4821–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller MA, Gadde S, Pfirschke C et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci Transl Med 2015;7:314ra183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaittanis C, Bolaender A, Yoo B et al. Targetable clinical nanoparticles for precision cancer therapy based on disease‐specific molecular inflection points. Nano Lett 2017;17:7160–7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Franklin JM, Gebski V, Poston GJ et al. Clinical trials of interventional oncology‐moving from efficacy to outcomes. Nat Rev Clin Oncol 2015;12:93–104. [DOI] [PubMed] [Google Scholar]
- 47. Solomon SB, Nicol TL, Chan DY et al. Histologic evolution of high‐intensity focused ultrasound in rabbit muscle. Invest Radiol 2003;38:293–301. [DOI] [PubMed] [Google Scholar]