Bullous pemphigoid is an autoimmune subepidermal blistering disease characterized by the development of tense bullae and is most frequently seen in the elderly. PD‐1/PD‐L1‐induced bullous pemphigoid (BP) has emerged as a potentially serious dermatologic toxicity. This article reports a case of a 72‐year‐old woman who developed BP shortly after initiating treatment with the PD‐1 inhibitor nivolumab for metastatic non‐small cell lung cancer.
Abstract
Immunotherapy has emerged as a highly effective treatment for numerous cancers. Use of checkpoint inhibitors against various molecules including programmed cell death protein‐1 (PD‐1), programmed death ligand‐1 (PD‐L1), and cytotoxic T‐lymphocyte‐associated protein‐4 have become widespread in clinical practice. Compared with conventional chemotherapy, immunotherapy is associated with a unique set of immune reactions known collectively as immune‐related adverse events (irAEs). Of known irAEs, cutaneous toxicity is among the most frequently observed in patients treated with immunotherapy. Although often mild, dermatologic toxicity can occasionally be high grade and potentially life‐threatening. In this article, we report a case of PD‐1 inhibitor‐induced bullous pemphigoid—a serious adverse event that has been increasingly observed with use of PD‐1/PD‐L1 inhibitors. We will also review diagnosis and management of low‐grade cutaneous irAEs and bullous disease with checkpoint inhibitors.
Key Points.
PD‐1/PD‐L1 inhibitor‐induced bullous pemphigoid (BP) is a rare but potentially serious dermatologic toxicity associated with checkpoint inhibitors
In patients with pruritus or rash that is refractory to topical steroids, physicians should have a greater index of suspicion for higher‐grade cutaneous immune‐related adverse events.
There is no standardized treatment algorithm for management of PD‐1/PD‐L1 inhibitor‐induced BP, but patients frequently require topical and systemic steroids.
Introduction
Immune checkpoint inhibitors have rapidly become first‐line therapy for a variety of advanced malignancies. Monoclonal antibodies against programmed cell death protein‐1 (PD‐1) and programmed death ligand‐1 (PD‐L1) have demonstrated durable anticancer effects and have drastically improved patient outcomes for several cancers [1], [2], [3], [4]. Although these drugs have been associated with a number of adverse events (AEs), cutaneous immune‐related adverse events (irAEs) are among the most common [5].
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease characterized by the development of tense bullae and is most frequently seen in the elderly. PD‐1/PD‐L1‐induced BP has recently emerged as a potentially serious dermatologic toxicity and has been observed with some degree of frequency. Herein, we report a case of a 72‐year‐old woman who developed BP shortly after initiating treatment with PD‐1 inhibitor nivolumab for metastatic non‐small cell lung cancer (NSCLC). In addition to adding to the existing literature regarding PD‐1 inhibitor‐induced BP, we will use this case to highlight diagnosis and management of cutaneous irAEs associated with checkpoint inhibitors.
Case Report
A 72‐year‐old woman with metastatic NSCLC presented for evaluation of new onset pruritic blisters. Three months prior, the patient was found to have a 4‐cm right upper lobe lung mass and numerous smaller pulmonary nodules during a workup for progressive dyspnea. Percutaneous biopsy at that time demonstrated CK5/p40‐positive and PD‐L1‐negative squamous cell carcinoma (SCC). Positron emission tomography‐computed tomography revealed an FDG‐avid soft tissue prominence between ribs 11 and 12 as well as FDG‐avid nodular thickening of the left adrenal gland, which were suspicious for metastasis. Past medical history was notable for a remote history of laryngeal SCC successfully treated with chemoradiation, complicated by partial vocal cord paralysis and tracheoesophageal fistula requiring tracheostomy and percutaneous endoscopic gastrostomy placement.
The patient declined chemotherapy but was amenable to treatment with immunotherapy and was started on intravenous nivolumab 3 mg/kg every 2 weeks. Following her first infusion, the patient noted new onset of generalized itching. Symptoms peaked immediately after infusion and improved over the following days to week until her second infusion, when symptoms again increased after treatment, following a similar pattern. Following cycle 3, the patient reported worsening pruritus and was found to have new blisters on her arms and legs. She was thus promptly referred to our clinic for evaluation.
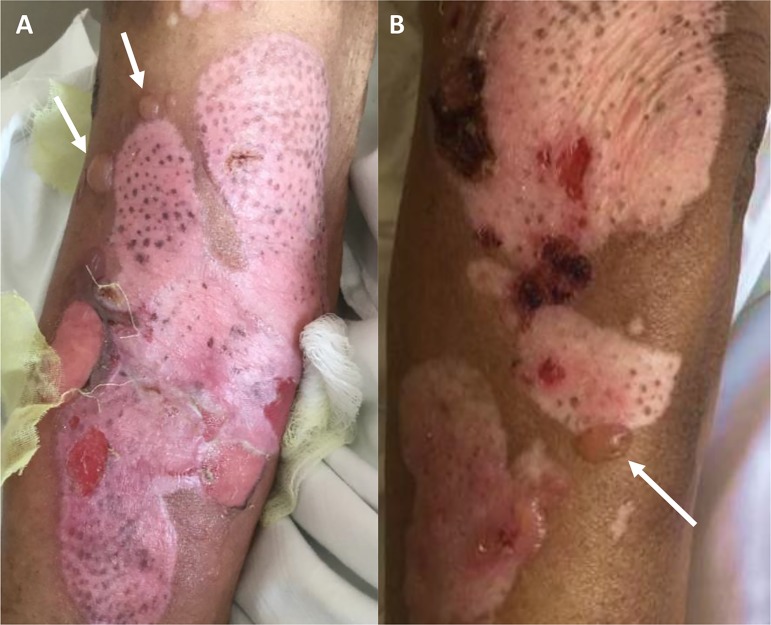
On exam, there were numerous superficial erythematous erosions and tense blisters on chest, arms, legs, and abdomen (Fig. 1). There was no involvement of palms or mucosal surfaces. Two 3.0‐mm punch biopsies of the lower leg were performed and sent to pathology for evaluation by hematoxylin and eosin (H&E) and immunofluorescence. H&E stain was remarkable for a perivascular lymphocytic and eosinophilic infiltrate, which was consistent with subepidermal bullous dermatitis. Direct immunofluorescence (DIF) showed linear IgG and C3 along basement membrane zone, confirming the diagnosis of BP.
Figure 1.
Tense bullae (arrows), erythematous superficial erosions, and healing ulcers on the right arm (A) and left leg (B). Re‐epithelialization and repigmentation is present in the areas of former blisters.
The patient was started on 60 mg of oral prednisone daily and topical clobetasol 0.05% cream twice daily, and nivolumab therapy was held. After 2 weeks of therapy with systemic steroids and high‐dose topical steroids, the prednisone dose was decreased to 50 mg/day as new blister formation ceased and the patient had marked improvement in pruritus and existing skin lesions. However, she subsequently developed recurrence of blisters/pruritus, and oral prednisone was increased back to 60 mg/day. In addition, the patient was also started on oral minocycline 100 mg/day and oral niacinamide 500 mg/day as adjunctive therapies. The patient was maintained on this regimen for 6 weeks, after which steroid taper was successful without BP recurrence. Following goals of care discussions, nivolumab therapy was not restarted.
Discussion
Within the past several years, our increased understanding of tumor immunity has led to the successful development of immunotherapy and has completely transformed the field of cancer therapeutics. Immune checkpoint inhibitors targeting PD‐1/PD‐L1 and cytotoxic T‐lymphocyte‐associated protein‐4 (CTLA‐4) have demonstrated exceptional antitumor activity in numerous solid and hematologic malignancies, resulting in marked survival benefits [1], [2], [3], [4]. Ipilimumab, an anti‐CTLA‐4 monoclonal antibody, was the first U.S. Food and Drug Administration‐approved immunotherapy in 2011 for metastatic melanoma [6]. Following this, agents targeted against other immune checkpoint molecules, including PD‐1 inhibitors pembrolizumab and nivolumab, have since become first‐line therapies for advanced melanoma and NSCLC [7]. As a result, research to evaluate the efficacy of checkpoint inhibitors in other cancers has exploded, with a push to expand approved indications of use [8], [9]. With anticipated growth in the number of patients eligible to receive checkpoint inhibitor therapy, it is critical for physicians to be familiar with associated drug toxicities and management.
Increased use of checkpoint inhibitors has revealed a unique set of inflammatory toxicities termed irAEs. Although the mechanism of irAEs is incompletely understood, it is widely believed that most irAEs develop secondary to nonspecific activation of the immune system [10]. Checkpoint inhibitors work primarily by restoring antitumor immune responses by disrupting coinhibitory T‐cell signaling. Overexpression of PD‐L1 by tumor cells is a major mechanism of tumor immune evasion via inhibition of T cell function [11]. Its receptor, PD‐1, is primarily found on regulatory T cells (Tregs), which foster a highly immunosuppressive environment by attenuating the immune response. In contrast, CTLA‐4 is expressed widely by T cells and interacts with its ligand on antigen‐presenting cells during the early phase of immune response [12]. The CTLA‐4 immune checkpoint functions to upregulate the immunosuppressive activity of Tregs and downregulate CD4+ T effector cells, resulting in a global impact on immune tolerance [13]. Although blocking these pathways with checkpoint inhibitors results in profound antitumor effects, the PD‐1/PD‐L1 and CTLA‐4 pathways are both crucial for the maintenance of normal immunologic homeostasis [14]. Therefore, dysregulation of these pathways can impair peripheral tolerance and alter the delicate balance within the immune system, resulting in the development of off‐target effects and autoimmunity. Risk factors for development of severe irAEs include personal or family history of autoimmune diseases, brisk inflammatory response at the tumor site, and concomitant use of medications with known autoimmune toxicities [15].
Although there are a wide range of known irAEs associated with anti‐PD‐1/PD‐L1 and anti‐CTLA‐4 therapy, cutaneous AEs are among the most commonly observed toxicities associated with checkpoint inhibitors [16]. Although the majority of cutaneous irAEs are mild or moderate, checkpoint inhibitor‐induced BP has emerged as a rare but serious potential cutaneous AE of checkpoint inhibitor therapy. Other potentially severe cutaneous irAEs that have been associated with anti‐PD‐1 inhibitor therapy include Stevens‐Johnson Syndrome, erythema multiforme, and drug rash with eosinophilia and systemic symptoms (DRESS syndrome; Panel 1) [17], [18], [19], [20]. If left untreated, these dermatologic conditions can result in significant morbidity and may even be life‐threatening.
To date, 22 cases of BP associated with PD‐1/PD‐L1 inhibitors have been reported in the literature [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. In contrast, BP associated with anti‐CTLA‐4 therapy has only been reported in two cases, suggesting this irAE is more specific to anti‐PD‐1/PD‐L1 therapy [37], [38]. Although the mechanism of PD‐1/PD‐L1 inhibitor‐induced BP is unknown, it is thought to be driven by autoantibody production against hemidesmosomal structural proteins BP180 and BP230 [36, 39]. Although this is the same pathomechanism believed to cause conventional BP, it is unclear how anti‐PD‐1/PD‐L1 immunotherapy facilitates this reaction. Of reported cases, BP most frequently developed within the first 6–8 months of treatment; however, a smaller subset of patients did not present until 1–1.5 years later [27], [31]. In many patients, development of bullae was preceded by prodromal pruritus and nonspecific rash, as observed in this case [23], [24], [25], [26], [28], [30], [31], [32], [34], [35], [36].
Making the diagnosis of BP can be challenging, as its clinical presentation is heterogeneous. In some instances, pruritus may be the predominant symptom and blisters or rash may never develop [40]. In addition, pruritus is one of the hallmark symptoms of the prodromal BP phase, which can precede the development of bullae by weeks to months [41]. Unfortunately, pruritus is also one of the most commonly observed low‐grade AEs with PD‐1 inhibitors and has been reported in approximately 15%–30% of patients [1], [42], [43], [44], [45]. Although new onset pruritus with PD‐1 inhibitor therapy is not usually a harbinger of severe cutaneous toxicity, there are currently no predictive biomarkers to aid in the diagnosis of irAEs [46], [47], [48]. Therefore, differentiating PD‐1 inhibitor‐induced BP from other low‐grade cutaneous toxicity is not always straightforward, and biopsy is generally required. Diagnosis can be confirmed with DIF on perilesional skin biopsy. Although DIF is the gold standard for the diagnosis of BP, serum testing via indirect immunofluorescence and enzyme‐linked immunosorbent assay can be useful in combination to support the diagnosis of BP in a patient in whom clinical and histopathologic features suggest BP but DIF is negative [49], [50].
In addition to distinguishing prodromal BP from other common AEs, differentiating drug‐induced BP from idiopathic BP in the elderly can be difficult, as the vast majority of idiopathic cases of BP occur in individuals over the age of 60 [51]. The clinical picture is further complicated in oncologic populations in which some evidence suggests BP can arise as a paraneoplastic condition, although this is controversial [52], [53]. However, the numerous reports of BP arising in the setting of PD‐1 inhibitor therapy has served to strengthen the association between medication exposure and development of this autoimmune disease [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. In this case, our patient developed BP 7 weeks after initiation of therapy with nivolumab. Given the temporal relationship between initiation of immunotherapy and the onset of BP, paraneoplastic and idiopathic BP are less likely.
Although the incidence of high‐grade cutaneous toxicity with PD‐1 inhibitors is low (approximately 1%–2%), “rash,” pruritus, and vitiligo are the three most common cutaneous toxicities observed with pembrolizumab and nivolumab [54]. In a recent meta‐analysis of phase I–III monotherapy trials with nivolumab and pembrolizumab, the relative risk for developing any dermatologic AE for pembrolizumab or nivolumab was 2.95 and 2.3 respectively. All‐grade incidence of rash with pembrolizumab was 16.7% (95% confidence interval [CI]: 11.9%–23.0%) and 14.3% (95% CI: 8.7%–22.7%) with nivolumab. The incidence of all‐grade pruritus was also frequent calculated at 13.2% (95% CI: 8.9%–19.2%) and 20.2% (95% CI: 14.8%–26.9) for nivolumab and pembrolizumab, respectively. The observed incidence of vitiligo was reported to be 7.5% (95% CI: 5.9%–9.5%) with nivolumab and 8.3% (95% CI: 4.4%–15.2%) with pembrolizumab [54]. Interestingly, all AEs of vitiligo were noted in trials with patients being treated for melanoma, and it has been suggested as a prognostically favorable AE [42], [43], [55], [56], [57]. Although the evidence is mixed, development of cutaneous AEs for both pembrolizumab and nivolumab has been associated with longer progression‐free survival when compared with those who did not experience cutaneous toxicity [58], [59].
In general, PD‐1/PD‐L1 inhibitors have been associated with fewer side effects than anti‐CTLA‐4 therapy [60]. This can potentially be explained by greater T cell proliferation and reduced Treg‐mediated immunosuppression with CTLA‐4 blockade when compared with PD‐1 inhibition [13]. Although anti‐CTLA‐4 therapy is associated with a myriad of irAEs, a pooled analysis of ipilimumab clinical trials demonstrated that dermatologic irAEs were the most common at 44.9% (all grade) [16]. This is of particular relevance as combination immunotherapy has become an emerging paradigm in cancer therapeutics. In particular, combination treatment with anti‐PD‐1 and anti‐CTLA‐4 therapies has become a hot area of research as synergistic effects may result in better outcomes [57], [61], [62], [63]. When ipilimumab is used in combination therapy, cutaneous toxicity has been reported to be as high as 64.3%, which is greater than the observed incidence of dermatologic toxicity of either agent alone [64]. Combination treatments of PD‐1 inhibitors with ipilimumab have also shown relatively higher rates of grade 3–4 cutaneous toxicity [65]. Although this evidence suggests that cutaneous risk may be additive when ipilimumab is used in combination with PD‐1/PD‐L1 inhibitors, the relationship between irAEs and dose, duration of exposure, and drug combinations has yet to be fully elucidated [66].
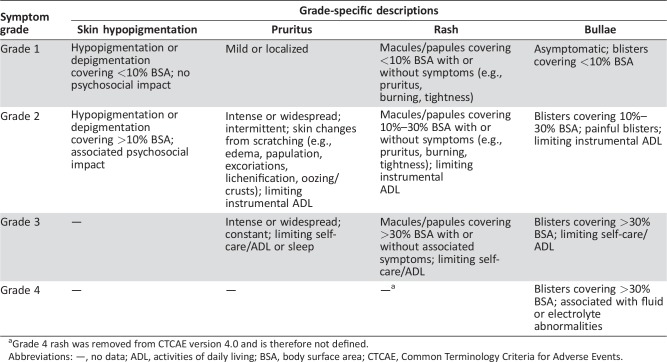
Therapy for cutaneous irAEs is primarily based on grade severity. The National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) is the most widely used severity grading scale for adverse event reporting in clinical trials. In the oncologic setting, “rash” may be used to encompass a myriad of cutaneous reactions. Released in 2009, CTCAE version 4.0 defines a variety of distinct dermatologic AEs that were not present in previous iterations (selected toxicities shown in Table 1) [67]. To better understand the cutaneous irAE profile of PD‐1/PD‐L1, providers should attempt to describe cutaneous reactions in as much detail as possible to best categorize the reaction. Although the majority of PD‐1/PD‐L1 clinical trial data does not provide more specific information regarding “rash” features, patient evaluation by dermatologists have described some of these cutaneous eruptions as eczema, lichenoid dermatitis, stomatitis, and urticaria [68], [69].
Table 1. Descriptions of select cutaneous immune‐related adverse events as defined by the CTCAE version 4.0.
Grade 4 rash was removed from CTCAE version 4.0 and is therefore not defined.
Abbreviations: —, no data; ADL, activities of daily living; BSA, body surface area; CTCAE, Common Terminology Criteria for Adverse Events.
There is currently no standardized treatment for many irAEs, and recommendations are largely based on case reports, case series, personal experience, and expert consensus. Low‐grade cutaneous toxicity associated with checkpoint inhibitors can generally be treated with topical steroids. In patients with pruritus or rash that is refractory to topical steroids, physicians should have a higher index of suspicion for higher‐grade cutaneous irAE, and skin biopsy is recommended.
The treatment approach to checkpoint inhibitor‐induced BP has largely been derived from studies conducted in patients with conventional BP. In a multicenter, randomized trial, 341 patients with BP were treated with either topical clobetasol propionate 0.05% cream (total 40 g/day over twice daily application) or oral prednisone (0.5 mg/kg/day for moderate disease or 1 mg/kg/day for severe disease). Both groups received treatment for 15 days after disease control and were tapered off topical and oral steroids until treatment was discontinued after 12 months. At study termination, they found that patients treated with clobetasol responded more quickly and had less severe/frequent complications than those treated with systemic steroids [70]. Although the findings of this study suggest that topical corticosteroids are preferable, systemic steroids are still highly effective and are widely accepted as another first‐line therapy for BP in patients for whom topical corticosteroids are not practical or feasible. Although the optimal dosing of systemic steroids is unclear, studies suggest that 0.75–1.25 mg/kg/day of prednisolone (or steroid equivalent) is necessary to effectively control BP [71], [72]. In our experience, we have found monotherapy with high‐dose topical steroids in this population to be ineffective for rapid disease control and prefer upfront combination treatment with high‐dose topical corticosteroids and oral steroids. In addition, holding immunotherapy is recommended in patients actively receiving treatment in order to halt further progression of BP. We attempt to rapidly taper patients off of systemic steroids after 2 weeks of treatment; however, this is not always possible, as in the case described here.
Given the numerous side effects associated with prolonged use of systemic steroids, eventual transition to alternative agents is ideal. Although there are limited data to support the use of glucocorticoid‐sparing drugs for the treatment of BP, anti‐inflammatory and immunosuppressive agents are frequently utilized in clinical dermatology practice. Studies and case reports have demonstrated azathioprine, mycophenolate mofetil, methotrexate, tetracycline antibiotics (i.e., tetracycline, doxycycline, minocycline), dapsone, and nicotinamide to be efficacious steroid‐sparing treatments for BP [73], [74], [75], [76], [77], [78], [79]. Given the potentially significant toxicity associated with azathioprine, mycophenolate mofetil, and methotrexate in already‐immunosuppressed oncologic patients, we prefer use of anti‐inflammatory glucocorticoid‐sparing agents for the treatment of checkpoint inhibitor‐induced BP. Because of their favorable side‐effect profile and tolerability, we generally initiate doxycycline and niacinamide immediately before tapering steroids. As in this case, we occasionally add doxycycline and niacinamide to oral and systemic steroid regimens in patients with disease relapse upon steroid taper. In patients with disease refractory to all regimens discussed above, rituximab is a recognized therapy for BP and has been used successfully in one case of nivolumab‐induced BP [23].
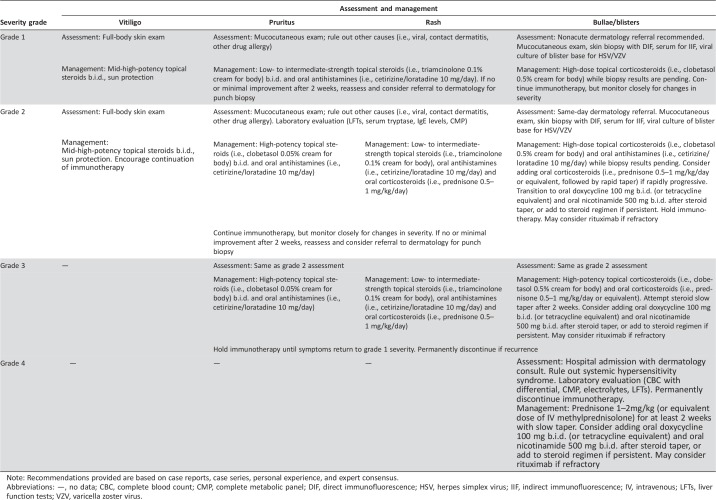
In general, high‐grade dermatologic toxicity secondary to checkpoint inhibitors will require systemic immunosuppression and temporary, if not permanent, cessation of immunotherapy [5], [54], [80], [81]. In patients actively receiving immunotherapy, obtaining disease control as quickly as possible is imperative if treatment is to be reinitiated in a timely manner. Our approach to such patients is outlined in detail in Table 2. Although there is the theoretical concern for reduced efficacy of immunotherapy with concomitant administration of immunosuppressive medications, the available evidence is limited and mixed [82], [83]. As no definitive conclusions can be drawn from existing data regarding cancer outcomes in patients treated with high‐dose systemic steroids, we recommend symptomatic treatment of high‐grade cutaneous toxicity with systemic immunosuppression. This may facilitate rapid reduction in symptoms and may enable prompt reinitiation of immunotherapy in patients in whom BP can be adequately controlled off systemic steroids.
Table 2. Columbia University Cutaneous Oncology Center recommendations for management of Common Terminology Criteria for Adverse Events‐based immune‐related adverse events associated with immune checkpoint inhibitors.
Note: Recommendations provided are based on case reports, case series, personal experience, and expert consensus.
Abbreviations: —, no data; CBC, complete blood count; CMP, complete metabolic panel; DIF, direct immunofluorescence; HSV, herpes simplex virus; IIF, indirect immunofluorescence; IV, intravenous; LFTs, liver function tests; VZV, varicella zoster virus.
Conclusion
Cutaneous toxicity is among the most common irAEs associated with checkpoint inhibitor therapy. Although most reactions are mild, some patients may develop severe or life‐threatening AEs. Prompt recognition of irAEs is critical in order to prevent and/or reduce interruptions in potentially life‐saving cancer therapy. Early treatment of reactions of immune dysregulation is important to limit duration and severity of toxicity. Keeping in mind cutaneous immune‐related AEs may have late onset, clinicians should carefully evaluate patients with new skin findings even after their therapy is completed. As PD‐1/PD‐L1 inhibitors remain relatively new, management of cutaneous toxicity is optimal under multidisciplinary care. Any new symptom should be evaluated and investigated further if not improving.
Author Contributions
Conception/design: Adriana T. Lopez, Larisa Geskin
Provision of study material or patients: Adriana T. Lopez, Larisa Geskin
Collection and/or assembly of data: Adriana T. Lopez, Larisa Geskin
Data analysis and interpretation: Adriana T. Lopez, Larisa Geskin
Manuscript writing: Adriana T. Lopez, Larisa Geskin
Final approval of manuscript: Adriana T. Lopez, Larisa Geskin
Disclosures
The authors indicated no financial relationships.
References
- 1. Ribas A, Puzanov I, Dummer R et al. Pembrolizumab versus investigator‐choice chemotherapy for ipilimumab‐refractory melanoma (KEYNOTE‐002): A randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reck M, Rodriguez‐Abreu D, Robingson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 3. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peters S, Gettinger S, Johnson ML et al. Phase II trial of atezolizumab as first‐line or subsequent therapy for patients with programmed death‐ligand 1‐selected advanced non‐small‐cell lung cancer (BIRCH). J Clin Oncol 2017;35:2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Puzanov I, Diab A, Abdallah K et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lipson EJ, Drake CG. Ipilimumab: An anti‐CTLA‐4 antibody for metastatic melanoma. Clin Cancer Res 2011;17:6958–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alsaab HO, Sau S, Alzhrani R et al. PD‐1 and PD‐L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front Pharmacol 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frenel JS, Le Tourneau C, O'Neil B et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1–positive cervical cancer: Results from the phase Ib KEYNOTE‐028 trial. J Clin Oncol 2017;35:4035–4041. [DOI] [PubMed] [Google Scholar]
- 9. O'Neil BH, Wallmark JM, Lorente D et al. Safety and antitumor activity of the anti‐PD‐1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One 2017;12:e0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune‐related adverse events in immune checkpoint inhibitor treatments in cancer: A short review. Immunotargets Ther 2017;6:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boussiotis VA. Molecular and biochemical aspects of the PD‐1 checkpoint pathway. N Engl J Med 2016;375:1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015;27:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walunas TL, Lenschow DJ, Bakker CY et al. CTLA‐4 can function as a negative regulator of T cell activation. Immunity 1994;1:405–413. [PubMed] [Google Scholar]
- 14. Francisco LM, Sage PT, Sharpe AH. The PD‐1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Champiat S, Lambotte O, Barreau E et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol 2016;27:559–574. [DOI] [PubMed] [Google Scholar]
- 16. Ibrahim RA, Berman DM, DePril V et al., Ipilimumab safety profile: Summary of findings from completed trials in advanced melanoma. J Clin Oncol 2011;29(suppl 15):8583. [Google Scholar]
- 17. Salati M, Pifferi M, Baldessari C et al. Stevens–Johnson syndrome during nivolumab treatment of NSCLC. Ann Oncol 2018;29:283–284. [DOI] [PubMed] [Google Scholar]
- 18. Saw S, Lee HY, Ng QS. Pembrolizumab‐induced Stevens‐Johnson syndrome in non‐melanoma patients. Eur J Cancer 2017;81:237–239. [DOI] [PubMed] [Google Scholar]
- 19. Mirza S, Hill E, Ludlow SP et al. Checkpoint inhibitor‐associated drug reaction with eosinophilia and systemic symptom syndrome. Melanoma Res 2017;27:271–273. [DOI] [PubMed] [Google Scholar]
- 20. Nomura H, Takahashi H, Suzuki S et al. Unexpected recalcitrant course of drug‐induced erythema multiforme‐like eruption and interstitial pneumonia sequentially occurring after nivolumab therapy. J Dermatol 2017;44:818–821. [DOI] [PubMed] [Google Scholar]
- 21. Hirotsu K, Chiou, AS, Chiang A et al. Localized bullous pemphigoid in a melanoma patient with dual exposure to PD‐1 checkpoint inhibition and radiation therapy. JAAD Case Rep 2017;3:404–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wada N, Uchi H, Furue M. Bullous pemphigoid induced by pembrolizumab in a patient with advanced melanoma expressing collagen XVII. J Dermatol 2017;44:e240–e241. [DOI] [PubMed] [Google Scholar]
- 23. Sowerby L, Dewan AK, Granter S et al. Rituximab treatment of nivolumab‐induced bullous pemphigoid. JAMA Dermatol 2017;153:603–605. [DOI] [PubMed] [Google Scholar]
- 24. Russo I, Sacco G, Frega S et al. Immunotherapy‐related skin toxicity: Bullous pemphigoid in a lung adenocarcinoma patient treated with the anti‐PDL1 antibody atezolizumab. Eur J Dermatol 2017;27:205–208. [DOI] [PubMed] [Google Scholar]
- 25. Rofe O, Bar‐Sela G, Keidar Z et al. Severe bullous pemphigoid associated with pembrolizumab therapy for metastatic melanoma with complete regression. Clin Exp Dermatol 2017;42:309–312. [DOI] [PubMed] [Google Scholar]
- 26. Parakh S, Nguyen R, Opie JM et al. Late presentation of generalised bullous pemphigoid‐like reaction in a patient treated with pembrolizumab for metastatic melanoma. Australas J Dermatol 2017;58:e109–e112. [DOI] [PubMed] [Google Scholar]
- 27. Mochel MC, Ming ME, Imadojemu S et al. Cutaneous autoimmune effects in the setting of therapeutic immune checkpoint inhibition for metastatic melanoma. J Cutan Pathol 2016;43:787–791. [DOI] [PubMed] [Google Scholar]
- 28. Lomax AJ, Ge L, Anand S et al. Bullous pemphigoid‐like reaction in a patient with metastatic melanoma receiving pembrolizumab and previously treated with ipilimumab. Australas J Dermatol 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29. Kwon CW, Land AS, Smoller BR et al. Bullous pemphigoid associated with nivolumab, a programmed cell death 1 protein inhibitor. J Eur Acad Dermatol Venereol 2017;31:e349–e350. [DOI] [PubMed] [Google Scholar]
- 30. Jour G, Glitza IC, Ellis RM et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti‐PD‐1 antibody therapy: A report on bullous skin eruptions. J Cutan Pathol 2016;43:688–696. [DOI] [PubMed] [Google Scholar]
- 31. Hwang SJ, Carlos G, Chou S et al. Bullous pemphigoid, an autoantibody‐mediated disease, is a novel immune‐related adverse event in patients treated with anti‐programmed cell death 1 antibodies. Melanoma Res 2016;26:413–416. [DOI] [PubMed] [Google Scholar]
- 32. Damsky W, Kole L, Tomayko MM. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep 2016;2:442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cozzani E, Gasparini G, Burlando M et al. Atypical presentations of bullous pemphigoid: Clinical and immunopathological aspects. Autoimmun Rev 2015;14:438–445. [DOI] [PubMed] [Google Scholar]
- 34. Carlos G, Anforth R, Chou S et al. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma Res 2015;25:265–268. [DOI] [PubMed] [Google Scholar]
- 35. Bandino JP, Perry DM, Clarke CE et al. Two cases of anti‐programmed cell death 1‐associated bullous pemphigoid‐like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol 2017;31:e378–e380. [DOI] [PubMed] [Google Scholar]
- 36. Naidoo J, Schindler K, Querfeld C et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD‐1 and PD‐L1. Cancer Immunol Res 2016;4:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuwatsuka Y, Iwanaga A, Kuwatsuka S et al. Bullous pemphigoid induced by ipilimumab in a patient with metastatic malignant melanoma after unsuccessful treatment with nivolumab. J Dermatol 2018;45:e21–e22. [DOI] [PubMed] [Google Scholar]
- 38. Cavalcante L, Amin A, Lutzky J. Ipilimumab was safe and effective in two patients with metastatic melanoma and end‐stage renal disease. Cancer Manag Res 2015;7:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Z, Sui W, Zhao M et al. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. J Autoimmun 2008;31:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bakker CV, Terra JB, Pas HH et al. Bullous pemphigoid as pruritus in the elderly: A common presentation. JAMA Dermatol 2013;149:950–953. [DOI] [PubMed] [Google Scholar]
- 41. Lamb PM, Abell E, Tharp M et al. Prodromal bullous pemphigoid. Int J Dermatol 2006;45:209–214. [DOI] [PubMed] [Google Scholar]
- 42. Robert C, Ribas A, Wolchok JD et al. Anti‐programmed‐death‐receptor‐1 treatment with pembrolizumab in ipilimumab‐refractory advanced melanoma: A randomised dose‐comparison cohort of a phase 1 trial. Lancet 2014;384:1109–1117. [DOI] [PubMed] [Google Scholar]
- 43. Weber JS, D'Angelo SP, Minor D et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): A randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 44. Balar AV, Castellano D, O'Donnell PH et al. First‐line pembrolizumab in cisplatin‐ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE‐052): A multicentre, single‐arm, phase 2 study. Lancet Oncol 2017;18:1483–1492. [DOI] [PubMed] [Google Scholar]
- 45. Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 46. Schindler K, Harmankaya K, Kuk D et al. Correlation of absolute and relative eosinophil counts with immune‐related adverse events in melanoma patients treated with ipilimumab. J Clin Oncol 2014;32(suppl 15):9096. [Google Scholar]
- 47. Callahan MK, Yang A, Tandon S et al. Evaluation of serum IL‐17 levels during ipilimumab therapy: Correlation with colitis. J Clin Oncol 2011;29(suppl 15):2505. [Google Scholar]
- 48. Shahabi V, Berman D, Chasalow SD et al. Gene expression profiling of whole blood in ipilimumab‐treated patients for identification of potential biomarkers of immune‐related gastrointestinal adverse events. J Transl Med 2013;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sardy M, Kostaki D, Varga R et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme‐linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol 2013;69:748–753. [DOI] [PubMed] [Google Scholar]
- 50. Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev 2010;10:84–89. [DOI] [PubMed] [Google Scholar]
- 51.51 . Stafford MA, Patel SS, Boyers LN et al. Autoimmune bullous diseases and drugs In: Hall JC, Hall BJ, eds. Cutaneous Drug Eruptions: Diagnosis, Histopathology and Therapy. London: Springer; London, 2015:193–203. [Google Scholar]
- 52. Ogawa H, Sakuma M, Morioka S et al. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci 1995;9:136–141. [DOI] [PubMed] [Google Scholar]
- 53. Atzmony L, Mimouni I, Reiter O et al. Association of bullous pemphigoid with malignancy: A systematic review and meta‐analysis. J Am Acad Dermatol 2017;77:691–699. [DOI] [PubMed] [Google Scholar]
- 54. Belum VR, Benhuri B, Postow MA et al. Characterisation and management of dermatologic adverse events to agents targeting the PD‐1 receptor. Eur J Cancer 2016;60:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 56. Hua C, Boussemart L, Mateus C et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016;152:45–51. [DOI] [PubMed] [Google Scholar]
- 57. Larkin J, Hodi FS, Wolchok JD et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PubMed] [Google Scholar]
- 58. Sanlorenzo M, Vujic I, Daud A et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 2015;151:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Freeman‐Keller M, Kim Y, Cronin H et al. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kumar V, Chaudhary N, Garg M et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 2017;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Antonia SJ, Lopez‐Martin JA, Bendell J et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): A multicentre, open‐label, phase 1/2 trial. Lancet Oncol 2016;17:883–895. [DOI] [PubMed] [Google Scholar]
- 62. Weber JS, Gibney G, Sullivan RJ et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): An open‐label, randomised, phase 2 trial. Lancet Oncol 2016;17:943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Postow MA, Chesney J, Pavlick AC et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sznol M, Ferrucci PF, Hogg D et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 2017;35:3815–3822. [DOI] [PubMed] [Google Scholar]
- 65. Utsunomiya A, Oyama N, Iino S et al. A case of erythema multiforme major developed after sequential use of two immune checkpoint inhibitors, nivolumab and ipilimumab, for advanced melanoma: Possible implication of synergistic and/or complementary immunomodulatory effects. Case Rep Dermatol 2018;10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ernstoff MS, Gandhi S, Pandey M et al. Challenges faced when identifying patients for combination immunotherapy. Future Oncol 2017;13:1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0, June 2010. Available at https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accessed February 15, 2018.
- 68. Hwang SJ, Carlos G, Wakade D et al. Cutaneous adverse events (AEs) of anti‐programmed cell death (PD)‐1 therapy in patients with metastatic melanoma: A single‐institution cohort. J Am Acad Dermatol 2016;74:455–461.e1. [DOI] [PubMed] [Google Scholar]
- 69. Joseph RW, Cappel M, Goedjen B et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti‐PD‐1 therapy. Cancer Immunol Res 2015;3:18–22. [DOI] [PubMed] [Google Scholar]
- 70. Joly P, Roujeau JC, Benichou J et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med 2002;346:321–327. [DOI] [PubMed] [Google Scholar]
- 71. Morel P, Guillaume JC. Treatment of bullous pemphigoid with prednisolone only: 0.75 mg/kg/day versus 1.25 mg/kg/day. A multicenter randomized study [in French]. Ann Dermatol Venereol 1984;111:925–928. [PubMed] [Google Scholar]
- 72. Kirtschig G, Middleton P, Bennett C et al. Interventions for bullous pemphigoid. Cochrane Database Syst Rev 2010:CD002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wojnarowska F, Kirtschig G, Highet AS et al. Guidelines for the management of bullous pemphigoid. Br J Dermatol 2002;147:214–221. [DOI] [PubMed] [Google Scholar]
- 74. Fox JC, Kenkare S, Petronic‐Rosic V et al. Bullous pemphigoid in late childhood successfully treated with mycophenolate mofetil as an adjuvant therapy. Pediatr Dermatol 2010;27:537–539. [DOI] [PubMed] [Google Scholar]
- 75. Eskin‐Schwartz M, David M, Mimouni D. Mycophenolate mofetil for the management of autoimmune bullous diseases. Dermatol Clin 2011;29:555–559. [DOI] [PubMed] [Google Scholar]
- 76. Du‐Thanh A, Merlet S, Maillard H et al. Combined treatment with low‐dose methotrexate and initial short‐term superpotent topical steroids in bullous pemphigoid: An open, multicentre, retrospective study. Br J Dermatol 2011;165:1337–1343. [DOI] [PubMed] [Google Scholar]
- 77. Fivenson DP, Breneman DL, Rosen GB et al. Nicotinamide and tetracycline therapy of bullous pemphigoid. Arch Dermatol 1994;130:753–758. [PubMed] [Google Scholar]
- 78. Williams HC, Wojnarowska F, Kirtschig G et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: A pragmatic, non‐inferiority, randomised controlled trial. Lancet 2017;389:1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bouscarat F, Chosidow O, Picard‐Dahan C et al. Treatment of bullous pemphigoid with dapsone: Retrospective study of thirty‐six cases. J Am Acad Dermatol 1996;34:683–684. [DOI] [PubMed] [Google Scholar]
- 80. Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin Dermatol 2018;19:345–361. [DOI] [PubMed] [Google Scholar]
- 81. Hofmann L, Forschner A, Loquai C et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side‐effects of anti‐PD‐1 therapy. Eur J Cancer 2016;60:190–209. [DOI] [PubMed] [Google Scholar]
- 82. Garant A, Guilbault C, Ekmekjian T et al. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: A systematic review. Crit Rev Oncol Hematol 2017;120:86–92. [DOI] [PubMed] [Google Scholar]
- 83. Horvat TZ, Adel NG, Dang TO et al. Immune‐related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]