This article reports results of the SafeHer subgroup analysis, which assessed the safety of fixed‐dose subcutaneous trastuzumab as an adjuvant therapy in HER2‐positive early breast cancer by body weight.
Keywords: Body weight, Breast cancer, Human epidermal growth factor receptor 2‐positive, Subcutaneous injections, Trastuzumab, Asian population
Abstract
Background.
This SafeHer subgroup analysis assessed the safety of fixed‐dose subcutaneous trastuzumab (H SC) as an adjuvant therapy in HER2‐positive early breast cancer (EBC) by body weight.
Patients and Methods.
Patients with HER2‐positive EBC not previously treated with anti‐HER2 therapy received H SC 600 mg (every 3 weeks for 18 cycles), with neoadjuvant or adjuvant chemotherapy or without adjuvant chemotherapy. Adverse events (AEs) were assessed throughout treatment and at final follow‐up (28 ±5 days after last treatment). Subgroups were categorized by body weight, Asian origin, and chemotherapy administration. All analyses were descriptive.
Results.
Of 2,577 patients enrolled, 2,573 received ≥1 dose of study medication and were included in this safety analysis. Median body weight at baseline was 67.0 kg (range 33.6–150.0 kg). Any‐grade AEs occurred in 88.7% (2,282/2,573) of the overall population, versus 87.1% (590/677) of the lowest bodyweight quartile (≤59 kg), 90.0% (561/623) of the highest quartile (>77 kg), and 86.5% (327/378) of the Asian population. Grade ≥3 AEs occurred in 23.2% (596/2,573) of the overall population, 17.9% (121/677) of the lowest bodyweight quartile, 26.8% (167/623) of the highest quartile, and 15.3% (58/378) of the Asian population. The highest bodyweight quartile had the highest incidence of medical conditions at baseline (highest quartile, 75.6%; lowest quartile, 56.1%).
Conclusion.
These data support the use of fixed‐dose H SC as an adjuvant therapy in HER2‐positive EBC and confirm the comparable safety profile of H SC in patients with low body weight or of Asian origin versus the overall population in SafeHer. ClinicalTrials.gov: NCT01566721.
Implications for Practice.
The safety profile of fixed‐dose subcutaneous trastuzumab (H SC) was comparable between patients in the lowest bodyweight subgroup and the overall patient population, and also between patients of Asian origin (of whom a higher proportion often fall within the lower bodyweight quartiles) and the overall population. The safety data from this SafeHer subgroup analysis therefore support the use of fixed‐dose H SC 600 mg administered every 3 weeks as an adjuvant therapy for patients with HER2‐positive early breast cancer across different bodyweight subgroups and in the Asian patient population.
摘要
背景。此项SafeHer亚组分析主要按体重评估固定剂量皮下注射曲妥珠单抗(H SC)在HER2 阳性早期乳腺癌(EBC) 辅助治疗中的安全性。
患者和方法。先前未曾接受抗HER2治疗的HER2阳性早期乳腺癌患者接受了H SC给药(每3周一次,共18个周期),同时结合新辅助或辅助化疗或不结合辅助化疗。在整个治疗期间及末次随访(最后一次治疗后28±5天)时,对不良反应事件(AE)进行了评估。亚组按体重、亚洲血统及化疗给药方案进行分类。所有分析均为描述性分析。
结果。在2 577 名入组患者中,有2 573名患者接受了不少于1剂的研究药物,并被纳入到此项安全性分析中。基线中位体重是67.0kg(范围为33.6–150.0kg)。在所有患者中,任何级别AE发生率为 88.7% (2 282/2 573),而在体重最低的四分位组(≤59 kg)中的发生率为 87.1% (590/677),在体重最高的四分位组(高于77kg)中的发生率为90.0% (561/623),在亚洲患者人群中的发生率为86.5% (327/378)。在所有患者人群中,≥3 级AE的发生率为23.2% (596/2 573),在体重最低的四分位组中的发生率为17.9% (121/677),在体重最高的四分位组中的发生率为26.8% (167/623),在亚洲患者人群中的发生率为15.3% (58/378)。在基线,体重最高的四分位组医疗疾病发生率最高(最高四分位组为 75.6%;最低四分位组为 56.1%)。
结论。上述数据支持使用固定剂量的H SC辅助治疗HER2 阳性早期乳腺癌,并确认低体重或亚洲血统患者与参与 SafeHer 研究的所有患者群体接受H SC的安全谱相似。ClinicalTrials.gov: NCT01566721。
实践意义
在实施皮下注射固定剂量的曲妥珠单抗(H SC) 后,最低体重亚组与所有患者群体所呈现的药物安全性相似;同样地,亚洲血统(通常低体重四分位组患者所占比例较高)患者与所有患者群体所呈现的药物安全性也很相似。因此,来自此 SafeHer 亚组分析的安全性数据支持使用固定剂量的H SC(600 mg,每 3 周一次)作为 HER2 阳性早期乳腺癌患者(涵盖不同体重的亚组和亚洲患者群体)的辅助治疗。
Introduction
Fixed‐dose subcutaneous (SC) trastuzumab (Herceptin SC [H SC]; F. Hoffmann‐La Roche Ltd, Basel, Switzerland) administered every 3 weeks (q3w) for 18 cycles/1 year from an H SC Vial via handheld syringe is approved for the treatment of HER2‐positive breast cancer [1]. H SC for injection comprises 600 mg trastuzumab plus recombinant human hyaluronidase, an excipient known to reversibly degrade SC hyaluronan and facilitate the injection of large volumes with minimal damage to the SC fascia [1], [2].
The HannaH phase III study investigating the use of SC and intravenous (IV) trastuzumab (Herceptin [H IV]; F. Hoffmann‐La Roche) as neoadjuvant–adjuvant therapy in patients with HER2‐positive early breast cancer (EBC) established the noninferiority of pathological complete response and serum trough concentration for H SC versus H IV [3] and showed that H SC had an overall safety profile consistent with the established safety profile of H IV in EBC [3], [4], [5]. The phase III, nonrandomized, multinational, open‐label SafeHer study (NCT01566721) further confirmed the safety and tolerability of fixed‐dose H SC 600 mg in patients with HER2‐positive EBC [6]. No new safety signals were identified, and the adjuvant safety profile of H SC given with concurrent or sequential chemotherapy was consistent with previous studies of H SC and H IV [3], [5], [6], [7], [8].
Exploratory analyses from the HannaH study have suggested that although body weight has a modest effect on H exposure with the H SC 600 mg q3w fixed‐dose regimen, there is no association between body weight or pharmacokinetic (PK) drug exposure and toxicity [5], [9]. Given that fixed‐dose H SC may result in increased drug exposure compared with H IV weight‐based q3w dosing in patients with lower body weight, and conversely reduced drug exposure in patients with higher body weight, an exploratory analysis was conducted to examine the overall safety profile of H SC in combination with either concurrent or sequential chemotherapy by bodyweight subgroups in the SafeHer study. Patients’ active medical conditions at baseline were also examined for their potential impact on the observed safety profile of H SC. In addition, we specifically investigated the safety profile of H SC in the Asian population, as a high proportion of patients within the lower bodyweight subgroups were of Asian origin.
Subjects, Materials, and Methods
Patients and Study Design
The design of the SafeHer study has been published previously [6]. Briefly, patients with HER2‐positive EBC who had not previously received anti‐HER2 therapy were enrolled into this prospective, nonrandomized, multinational, open‐label, phase III study and allocated to one of two cohorts: H SC administered from (a) an H SC Vial via handheld syringe or (b) via a single‐use injection device [6]. Patients were excluded if they had a history of other malignancy that might affect compliance or the interpretation of the results, serious cardiac illness, medical conditions that precluded the use of H, or impaired renal or hepatic function. Enrolled patients received fixed‐dose H SC 600 mg q3w for up to 18 cycles, in combination with neoadjuvant or adjuvant chemotherapy (concurrent or sequential chemotherapy) or without chemotherapy (<10% of the study population) at the investigators’ discretion. Concomitant hormonal therapy/radiotherapy could be given as per local guidelines.
The primary objective of the SafeHer study was to assess the safety and tolerability of H SC using two different methods of administration. Here, we report safety data from the SafeHer study treatment period (from the first H SC dose until 28 ±5 days after the last dose) by bodyweight subgroup [6]. The SafeHer study was carried out in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and all patients provided written informed consent. Approval for the protocol and all accompanying materials provided to the patients was obtained from independent ethics committees at the participating institutions.
Assessments
Adverse events (AEs) and serious AEs (SAEs) were assessed throughout the SafeHer study treatment period and at the safety follow‐up visit, planned for 28 days after the last treatment (plus a 5‐day window) [6]. AEs were coded by system organ class (SOC) and preferred term (PT) as per Medical Dictionary for Regulatory Activities version 18.0 and graded as per the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI‐CTCAE) version 4.0 [10]. Congestive heart failure (CHF) was assessed by NCI‐CTCAE version 4.0 and New York Heart Association functional classification [11]. Body weight was assessed at baseline. A subset of patients also had their body weight measured at Cycle 9 (on‐treatment assessment). Physical examinations, vital sign assessments, and cardiac monitoring (echocardiography, multigated acquisition scan, or magnetic resonance imaging and echocardiogram) were performed at baseline, every four cycles, and at the safety follow‐up visit.
Statistical Analysis
All analyses performed were descriptive. Safety outcomes were evaluated in the combined H SC Vial and H SC single‐use injection device cohorts grouped according to body weight. Analyses were conducted in the individual cohorts of the SafeHer study; however, as the general safety profiles were very similar between cohorts [6], this paper focuses on data from the combined population, which provides greater statistical power to observe differences across bodyweight subgroups. Patient subgroups were defined according to bodyweight quartiles (≤59, >59 to ≤67, >67 to ≤77, and >77 kg) and the lowest weight decile (≤53 kg) at baseline, race (Asian origin), and chemotherapy use (sequential or concurrent). Active medical conditions at baseline were evaluated in the bodyweight and chemotherapy use subgroups.
Results
Baseline Characteristics
In total, 2,577 patients were enrolled into the SafeHer study between May 2012 and January 2014 [6]. Of these, 2,573 patients received at least one dose of study medication and were included in the safety population.
Patient demographics and tumor characteristics of the safety population according to body weight are shown in Table 1 (patient demographics and tumor characteristics according to chemotherapy usage per bodyweight subgroup are provided in supplemental online Table 1). Median body weight at baseline was 67.0 kg (range, 33.6–150.0 kg) in the overall SafeHer study patient population [6]. There were no notable changes in patients’ body weights from baseline to Cycle 9 (n = 637; data not shown). The median number of cycles of H SC received in the overall safety population was 18.0 (range, 1–19); 90% of patients received 18 cycles of H SC [6]. The proportion of patients who completed treatment according to the study protocol was 90.4% (612/677) in the lowest bodyweight quartile, 88.9% (554/623) in the highest quartile, and 89.9% (340/378) in patients of Asian origin.
Table 1. Patient demographics and tumor characteristics in the overall population and by bodyweight subgroup.
Quartile 1 group includes the lowest decile.
Data for the overall population reported as in Gligorov et al. [6]
n = 2,566.
Safety Profile of H SC by Body Weight
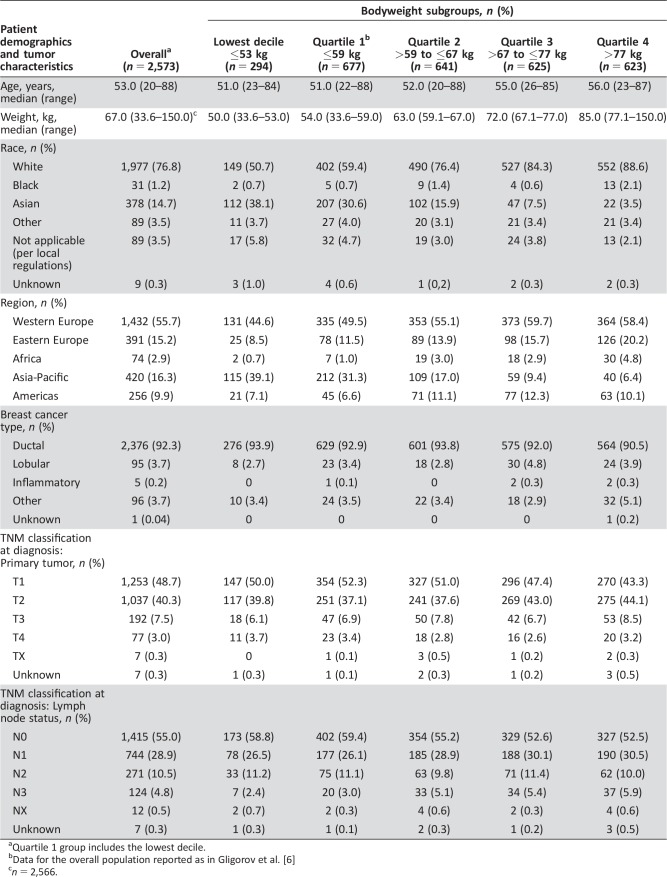
The proportion of patients with any‐grade AEs recorded during the SafeHer study treatment period was 87.8% in the lowest bodyweight decile and 87.1% in the lowest bodyweight quartile, versus 88.7% in the overall population (Fig. 1).
Figure 1.
Adverse events of interest by body weight and chemotherapy subgroups. System organ class categories of adverse events of interest were determined and agreed upon by the authors. Abbreviations: AE, adverse event; CHF, congestive heart failure.
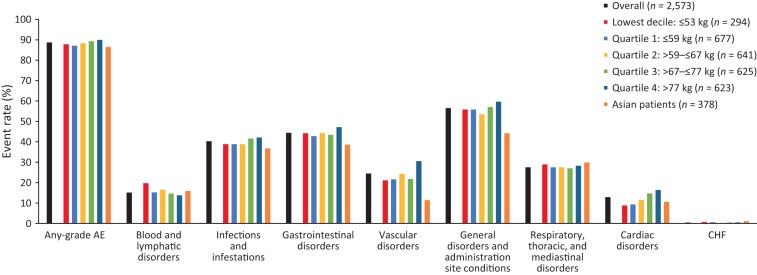
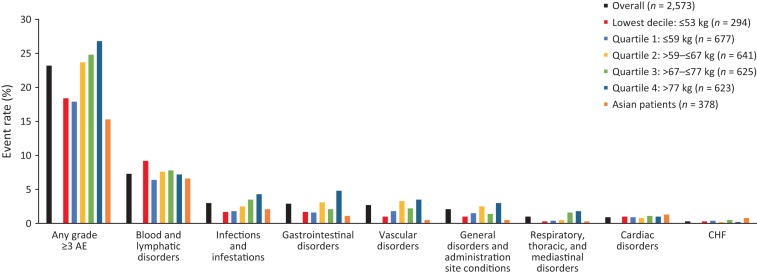
The proportion of patients with grade ≥3 AEs was 18.4% in the lowest bodyweight decile and 17.9% in the lowest bodyweight quartile, versus 23.2% in the overall safety population. The proportion was 26.8% in the highest bodyweight quartile (Fig. 2). The proportion of patients with SAEs was 12.7% in the overall population, 11.2% in the lowest bodyweight decile, 10.2% in the lowest bodyweight quartile, and 16.2% in the highest bodyweight subgroup (Fig. 3). No clear pattern of differences in administration‐site reactions was observed (data not shown).
Figure 2.
Grade ≥3 adverse events of interest by body weight and chemotherapy subgroups. System organ class categories of adverse events of interest were determined and agreed by the authors. Adverse events graded per National Cancer Institute's Common Terminology Criteria for Adverse Events version 4.0 [10]. Abbreviations: AE, adverse event; CHF, congestive heart failure.
Figure 3.
Serious adverse events of interest by body weight and chemotherapy subgroups. System organ class categories of adverse events of interest were determined and agreed by the authors. Abbreviations: CHF, congestive heart failure; SAE, serious adverse event.
Grade ≥3 or serious cardiac disorders or CHF occurred in 0.3%–1.2% of patients in the overall safety population; rates across the bodyweight subgroups are shown in Figures 2 and 3 (also in supplemental online Tables 3 and 4).
AEs within each SOC were comparable across the bodyweight quartiles; no distinct pattern of PTs or SOCs was observed for patients with the lowest body weight.
The incidence of any‐grade AEs, grade ≥3 AEs, and SAEs was consistently lower in patients receiving H SC with sequential chemotherapy compared with those receiving H SC with concurrent chemotherapy, across all bodyweight subgroups (Figs. 1, 2, 3; supplemental online Tables 2–4).
Safety of H SC in the Asian Population
In general, the proportion of Asian patients increased with decreasing bodyweight quartile (Table 1). Over one third of patients in the lowest bodyweight decile (112/294; 38.1%) and 30.6% of patients in the lowest bodyweight quartile (207/677) were of Asian origin, compared with 14.7% of the overall population (378/2,573) and 3.5%–15.9% of the higher bodyweight groups (Quartiles 2–4; Table 1). Rates of any‐grade AEs, grade ≥3 AEs, and SAEs were lower or comparable in Asian patients versus the overall safety population (any‐grade AEs, 86.5% versus 88.7%; grade ≥3 AEs, 15.3% versus 23.2%; SAEs, 11.4% versus 12.7%, respectively; Figs. 1, 2, 3; supplemental online Tables 2–4).
Active Medical Conditions at Baseline by Body Weight
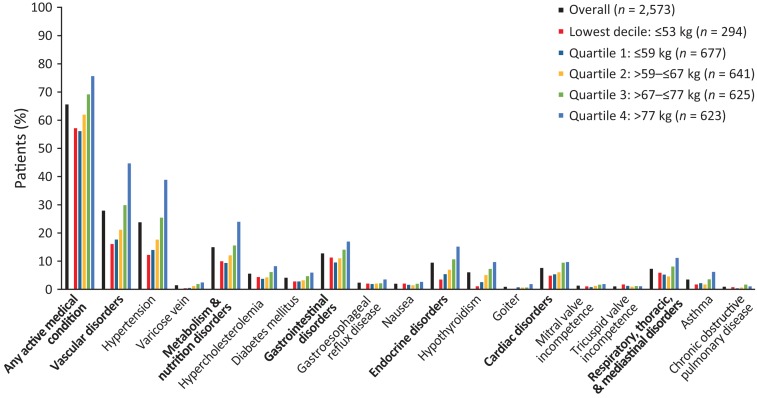
In total, 1,686/2,573 (65.5%) patients in the overall safety population had an active medical condition at baseline (Fig. 4; supplemental online Table 5). The most frequently reported baseline active medical conditions by SOC were vascular disorders, metabolism/nutrition disorders, and gastrointestinal disorders (Fig. 4). Hypertension was the most common active medical condition at baseline. Patients in the highest bodyweight quartile had higher rates of hypertension (242/623 [38.8%]) compared with the overall population (609/2,573 [23.7%]) and lower bodyweight quartiles (94/677 [13.9%], 113/641 [17.6%], and 159/625 [25.4%] in bodyweight quartiles 1, 2, and 3, respectively). No other single active medical condition was reported in more than 6% of patients.
Figure 4.
Active medical conditions at baseline by body weight and chemotherapy subgroups. The two most common individual diagnoses overall are listed for each system organ class.
Patients in the lowest decile and quartile bodyweight subgroups had lower rates of active medical conditions at baseline, compared with the overall population (Fig. 4). Conversely, patients in the two highest bodyweight quartiles had higher rates of active medical conditions versus the overall population (Fig. 4). This trend was observed for the majority of the baseline active medical condition SOCs of interest, including vascular disorders and metabolism/nutrition disorders (Fig. 4; supplemental online Table 5).
Discussion
The SafeHer study included more than 2,500 patients and is the largest study of H SC to date [6]. In this subgroup analysis of the SafeHer study, we show that the safety profile of fixed‐dose H SC in patients in the lower bodyweight subgroups was comparable with the overall study population. The safety profile of H SC in patients of Asian origin (who comprised a notably higher percentage of patients within the lower bodyweight subgroups compared with the overall safety population) was also comparable with that of the overall study population. Patients in the higher bodyweight subgroups had comparable or slightly higher rates of grade ≥3 AEs and SAEs compared with the overall population. The higher bodyweight subgroups also had increased rates of active medical conditions at baseline, compared with the overall population and the lowest bodyweight subgroups, suggesting that the safety profile of H SC in high‐bodyweight patients is potentially confounded by the presence of active medical conditions at baseline. The available data do not indicate any differences in grade ≥3 cardiac disorders and serious cardiac disorders between the overall population and the bodyweight subgroups or patients of Asian origin (although it is acknowledged that the study has low power to detect differences between subgroups for rare events).
Across all subgroups examined, the incidence of any‐grade AEs, grade ≥3 AEs, and SAEs in the SafeHer study was generally higher in patients who received H SC concurrently with chemotherapy, compared with those who received H SC sequentially after chemotherapy, across all bodyweight subgroups (supplemental online Tables 2–4), as expected based on previous analyses of SafeHer [6]. The proportion of patients who received concurrent versus sequential chemotherapy in the SafeHer study was consistent across the overall population and the bodyweight subgroups (supplemental online Table 1). Therefore, the comparison between AE profiles in the overall population and the bodyweight subgroups does not appear to be influenced by the sequence of chemotherapy use in relation to H SC treatment.
Trastuzumab is the standard of care in HER2‐positive EBC/invasive breast cancer [12], [13], [14]. The phase III HannaH study showed that H SC is noninferior to standard H IV in terms of pathologic complete response and serum trough concentration, with a similar safety profile [3]. Subsequent analyses of the study results supported the long‐term efficacy [4] of H SC and confirmed the consistency of its safety profile with that of H IV [4], [5]. These supportive safety data for H SC were reinforced by the PrefHer study, which also reported compelling patient preferences for, and health care professional satisfaction with, H SC (largely driven by the reduced time commitment required for patients and health care professionals) [7], [8], [15], [16].
The SafeHer study recruited the largest population of patients with HER2‐positive EBC for treatment with H SC of any study to date. The primary analysis of SafeHer did not identify any new safety signals associated with H SC, despite its broader eligibility criteria compared with previous studies, and the overall safety profile was consistent with previous data on H SC and H IV [1], [3], [5], [6]. With the fixed dosing of H SC, patients with lower body weight might be exposed to higher doses of H compared with the weight‐based dosing of H IV [9]. The current SafeHer subgroup analysis was performed to determine whether there was any evidence of safety issues associated with this potentially higher H exposure, particularly in patients with lower body weights and in Asian populations (which typically include a high proportion of patients with low body weight). We identified comparable or slightly lower rates of SAEs and cardiac disorders in the lowest bodyweight subgroups and comparable or slightly higher rates in the highest bodyweight subgroups, compared with the overall population. This trend was in line with previous findings from a follow‐up analysis of the HannaH study [4], in which the lowest bodyweight quartile (<59 kg) had a cardiac AE rate of 8.5%, compared with 14.1% in the overall population and 19.7% and 16.5% in the third and fourth bodyweight quartiles (≥68 to <79 kg and ≥79 kg), respectively.
The incidence of baseline active medical conditions in patients of the lowest bodyweight decile was comparable with that of patients in the lowest bodyweight quartile and lower than that of the overall population. The highest bodyweight quartile patients had a higher proportion of active medical conditions at baseline, compared with the overall population and with the lowest bodyweight quartiles. This trend was observed for most of the baseline active medical condition SOCs of interest, but was particularly marked for vascular disorders and metabolism/nutrition disorders. Taken together with the comparable or slightly higher AE rates reported in patients within the highest bodyweight subgroups versus the overall population, these data suggest that the observed safety profile in patients with HER2‐positive EBC treated with fixed‐dose H SC (600 mg q3w) might be affected by patients’ active medical conditions at baseline.
Although efficacy data from the SafeHer study are currently immature, follow‐up analyses from the HannaH study have reported similar efficacy between H SC and H IV study arms in patients with high body weight who may experience reduced drug exposure, compared with the overall population [4], [5], [17], [18]. These suggest that efficacy, in addition to safety and tolerability, is not affected by body weight and further supports the use of fixed‐dose H SC 600 mg in patients in both low and high bodyweight subgroups.
PK assessments within the SafeHer study were limited to measurement of serum drug concentrations in a subset of patients. A previous PK analysis of data from the HannaH study confirmed that body weight had a statistically significant effect on drug exposure with both IV and SC dosing regimens and routes of administration. However, the analysis also showed that despite this there was a large overlap in the distribution of the steady‐state trough concentration and area under the curve PK parameters across the bodyweight range for both H IV and H SC. Importantly, most patients achieved or exceeded the historical therapeutic target of 20 μg/mL (H SC, 98%; IV, 99.5%) [9].
Conclusion
The safety data from this subgroup analysis of the SafeHer study support the use of fixed‐dose H SC 600 mg as adjuvant therapy for patients with HER2‐positive EBC across all bodyweight subgroups, as well as in the Asian patient population.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The authors thank the patients, their families, the nurses, and the investigators who participated in this study. Mona Shing was an employee of Genentech Inc. at the time of this study.
Funding for the SafeHer study was provided by F. Hoffmann‐La Roche Ltd.
Support for third‐party writing assistance for this manuscript (including medical writing, editorial, and fact‐checking assistance), furnished by Rachel Johnson, PhD, of Health Interactions, was provided by F. Hoffmann‐La Roche Ltd.
Author Contributions
Conception/design: Xavier Pivot, Joseph Gligorov
Provision of study material or patients: Kyung Hae Jung, Beyhan Ataseven, Mark Verrill, Xavier Pivot, Michelino De Laurentiis, Nedal Al‐Sakaff, Joseph Gligorov, Hamdy A. Azim
Collection and/or assembly of data: Beyhan Ataseven, Michelino De Laurentiis, Nedal Al‐Sakaff, Mona Shing, Hamdy A. Azim
Data analysis and interpretation: Kyung Hae Jung, Beyhan Ataseven, Xavier Pivot, Michelino De Laurentiis, Sabine Lauer, Mona Shing, Joseph Gligorov, Hamdy A. Azim
Manuscript writing: Kyung Hae Jung, Beyhan Ataseven, Mark Verrill, Xavier Pivot, Michelino De Laurentiis, Nedal Al‐Sakaff, Sabine Lauer, Mona Shing, Joseph Gligorov, Hamdy A. Azim
Final approval of manuscript: Kyung Hae Jung, Beyhan Ataseven, Mark Verrill, Xavier Pivot, Michelino De Laurentiis, Nedal Al‐Sakaff, Sabine Lauer, Mona Shing, Joseph Gligorov, Hamdy A. Azim
Disclosures
Mark Verrill: Roche (C/A, RF, H); Michelino De Laurentiis: Roche, Novartis, Eli Lilly & Co., Pfizer, AstraZeneca, Amgen, Eisai (C/A), Roche, Novartis, Eli Lilly & Co., Pfizer, Eisai (H); Nedal Al‐Sakaff: Roche (E); Sabine Lauer: Roche (C/A); Joseph Gligorov: Roche/Genentech, Eisai, Novartis, Pfizer, Daiichi, Genomic Health, MacroGenics, Samsung (C/A, H), Roche/Genentech, Eisai, Genomic Health (RF), Roche/Genentech, Eisai, Daiichi, Genomic Health (SAB); Hamdy A. Azim: Pfizer, Merck Sharp & Dohme, Bayer (RF), Novartis, Roche, Amgen, Pfizer, Merck Sharp & Dohme, Bristol‐Myers Squibb, AstraZeneca (H), Novartis, Roche, Amgen, Pfizer, Merck Sharp & Dohme, Bristol‐Myers Squibb, AstraZeneca (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Roche Registration Ltd. Herceptin (trastuzumab). Summary of Product Characteristics. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/hum an/000278/WC500074922.pdf. Accessed August 8, 2017.
- 2. Bookbinder LH, Hofer A, Haller MF et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release 2006;114:230–241. [DOI] [PubMed] [Google Scholar]
- 3. Ismael G, Hegg R, Muehlbauer S et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2‐positive, clinical stage I‐III breast cancer (HannaH study): A phase 3, open‐label, multicentre randomised trial. Lancet Oncol 2012;13:869–878. [DOI] [PubMed] [Google Scholar]
- 4. Jackisch C, Hegg R, Stroyakovskiy D et al. HannaH phase III randomised study: Association of total pathological complete response with event‐free survival in HER2‐positive early breast cancer treated with neoadjuvant‐adjuvant trastuzumab after 2 years of treatment‐free follow‐up. Eur J Cancer 2016;62:62–75. [DOI] [PubMed] [Google Scholar]
- 5. Jackisch C, Kim SB, Semiglazov V et al. Subcutaneous versus intravenous formulation of trastuzumab for HER2‐positive early breast cancer: Updated results from the phase III HannaH study. Ann Oncol 2015;26:320–325. [DOI] [PubMed] [Google Scholar]
- 6. Gligorov J, Ataseven B, Verrill M et al. Safety and tolerability of subcutaneous trastuzumab for the adjuvant treatment of human epidermal growth factor receptor 2‐positive early breast cancer: SafeHer phase III study's primary analysis of 2573 patients. Eur J Cancer 2017;82:237–246. [DOI] [PubMed] [Google Scholar]
- 7. Pivot X, Gligorov J, Müller V et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2‐positive early breast cancer (PrefHer): An open‐label randomised study. Lancet Oncol 2013;14:962–970. [DOI] [PubMed] [Google Scholar]
- 8. Pivot X, Gligorov J, Müller V et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2‐positive early breast cancer: Final analysis of 488 patients in the international, randomized, two‐cohort PrefHer study. Ann Oncol 2014;25:1979–1987. [DOI] [PubMed] [Google Scholar]
- 9. Quartino AL, Hillenbach C, Li J et al. Population pharmacokinetic and exposure‐response analysis for trastuzumab administered using a subcutaneous “manual syringe” injection or intravenously in women with HER2‐positive early breast cancer. Cancer Chemother Pharmacol 2016;77:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Published May 28, 2009; updated June 14, 2010. Available at https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed August 8, 2017. [Google Scholar]
- 11.The Criteria Committee for the New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed Boston, MA: Little, Brown and Company; 1994:253–255. [Google Scholar]
- 12. Coates AS, Winer EP, Goldhirsch A et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 2. Fort Washington, PA: National Comprehensive Cancer Network; 2016. [DOI] [PubMed] [Google Scholar]
- 14. Senkus E, Kyriakides S, Ohno S et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2015;26 (suppl 5):v8–v30. [DOI] [PubMed] [Google Scholar]
- 15. De Cock E, Pivot X, Hauser N et al. A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2‐positive early breast cancer. Cancer Med 2016;5:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pivot X, Verma S, Fallowfield L et al. Efficacy and safety of subcutaneous trastuzumab and intravenous trastuzumab as part of adjuvant therapy for HER2‐positive early breast cancer: Final analysis of the randomized, two‐cohort PrefHer study. Ann Oncol 2016;27(suppl 6):209P. [DOI] [PubMed] [Google Scholar]
- 17. Jackisch C, Hegg R, Stroyakovskiy D et al. Phase III HannaH study of subcutaneous or intravenous trastuzumab for HER2‐positive early breast cancer: Exploratory subgroup analyses of pathological complete response and 3‐year event‐free survival according to body weight and anti‐drug antibody status. Eur J Cancer 2015;51(suppl 3):1945. [Google Scholar]
- 18. Ahn JS, Jackisch C, Hegg R et al. Phase III HannaH study of subcutaneous or intravenous trastuzumab for HER2‐positive early breast cancer: Exploratory subgroup analyses of pathological complete response and 3‐year event‐free survival by body weight and anti‐drug antibody status. Ann Oncol 2015:26(suppl 9):550. [Google Scholar]