Abstract
A new era has begun in the treatment of hepatitis C virus (HCV) infection with powerful yet expensive therapies. New treatments are emerging that target the entry step of HCV and could potentially block reinfection after liver transplant. These treatments include antibodies, which target the virus or host receptors required by HCV. Additionally, several new and previously approved small-molecule compounds have been described that target unique aspects of HCV entry. Overall, the blocking entry represents an attractive strategy that could yield powerful combination therapies to combat HCV.
Keywords: viral entry, HCV, antiviral, antibodies, small molecules
Graphical abstract
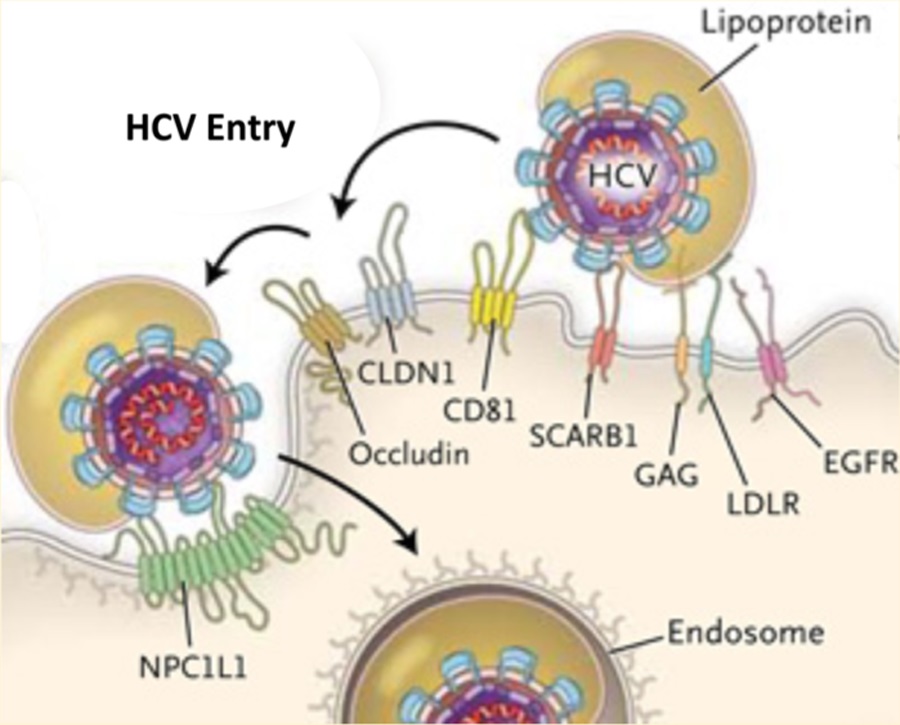
The first step in viral replication is binding and entry into the target cell and therefore the first potential target for antiviral therapy. Historically, targeting the entry process was relegated to the realm of vaccine discovery instead of antiviral development. This approach required the identification of viral and host receptors and designing antigens to elicit antibodies with neutralizing activity that block entry. However, new host-targeting antibodies as well as compounds that interfere with this step are beginning to emerge as powerful therapeutics to combat viral infection. Targeting these initial steps is advantageous as it can prevent the entry of viral genomic material that can persist in infected cells.1 This is especially true for viruses that are associated with chronic infections such as HIV, HBV, and HCV. Recently the HIV field has witnessed great strides toward blocking entry with one entry inhibitor already approved and several more in the pipeline.2
The therapeutic landscape for HCV infection has rapidly evolved since the approval of two direct-acting antivirals (DAAs) in 2011. Telaprevir and boceprevir are viral protease inhibitors that have clearance rates of 70% in genotype 1 patients in combination with peginterferon (peg-IFN) and ribavirin but are limited by side effects and the rapid emergence of resistance and are not approved for liver transplantation.3 Newly approved nucleotide analogs such as sofosbuvir have very high clearance rates (>90%) but are limited by their high cost.4 Therefore, there is still a great need to develop a larger pool of drugs that are affordable and safe and possess activity against all genotypes. Furthermore, drugs that inhibit entry are highly desirable for preventing reinfection after liver transplant.
The entry of HCV is highly complex, and numerous host receptors and pathways have been implicated in the entry step.5 Entry begins with the HCV envelope proteins (E1 and E2) binding to host factors on the hepatocyte. The current list of entry factors includes heparan sulfate proteoglycans, scavenger receptor class B type I (SR-BI), CD81, claudin-1 (CLDN1), occludin (OCLN), Niemann-Pick C1-like 1 (NPC1L1), transferrin receptor 1 (TfR1), epidermal growth factor receptor (EGFR), and ephrin receptor A2 (EphA2). Thus, HCV entry provides several potential targets to interrupt HCV infection and remains an attractive area for drug development. Furthermore, viral kinetic studies have determined that HCV in the serum is rapidly cleared after treatment, followed by a loss of infected cells.6,7 Therefore, blocking new infections of naivë hepatocytes may be important in interrupting and curing persistent infections. In this perspective, we will focus on newly emerging strategies to block HCV entry. This strategy includes antibodies targeting HCV and host factors as well as newly identified compounds that interfere with the entry process.
HCV- AND HOST-TARGETING ANTIBODIES
HCV envelope protein E2 is responsible for binding to host cell receptors CD81 and SR-BI.8 Therefore, an attempt to hinder or neutralize this binding has been the primary goal for treatment with HCV-specific antibodies. The first neutralizing epitopes described were found within hypervariable region 1 (HVR1), and the protective potential was validated in chimpanzees infected with a specific HCV variant.9 However, due to the high variability of the region, there was no protection against other variants. Besides HVR1, other neutralizing epitopes have been found using monoclonal antibodies. Many broadly neutralizing antibodies have been described and were able to provide passive protection in a small animal model of HCV infection.10–12 Currently there are HCV-targeting antibodies undergoing clinical trials to determine the efficacy of blocking reinfection after liver transplantation.13,14 There is also a polyclonal antibody preparation, Civacir, currently undergoing a phase III trial that has the potential to block reinfection after liver engraftment.15 Despite neutralizing activity, HCV is still able to persist even in the presence of these antibodies, and several mechanisms have been proposed including escape mutations, shielding by glycans, and direct cell–cell transmission.8 Overall, more work needs to be done before neutralizing antibodies can be used as an effective treatment option.
Perhaps a more promising avenue is the use of antibodies that target host receptors. Currently there are several entry antibody inhibitors in preclinical development that target CD81, SR-BI, and CLDN1. Research using a liver-uPA-SCID mouse model has indicated that a monoclonal anti-CD81 antibody can effectively prevent HCV infection in a dose-dependent manner.16 Furthermore, anti-CD81 antibodies can inhibit HCV infection across all genotypes.17
Similar to CD81, monoclonal antibodies targeting SR-BI are able to block cell-free virus infection and cell−cell spread, both in vitro and in vivo.18,19 Once again these antibodies efficiently blocked the infection of different HCV genotypes, validating the advantage of targeting a conserved host factor rather than the variable virus receptor. Interestingly, a follow-up study in humanized mice indicated that treatment with SR-BI antibody is effective even when initiated several days after HCV exposure.20
Finally, new evidence is emerging that the tight junction protein CLDN1 is a viable target to prevent and block the spread of HCV.21 Further work has confirmed this antiviral activity in a mouse model and demonstrated efficacy in highly infectious escape mutants that were resistant to neutralizing antienvelope antibodies.22,23 Overall, these antibodies offer a unique strategy to target the host rather than the virus, thus greatly reducing the emergence of viral resistance. However, these studies are still in their initial stages, and it remains to be seen if this efficacy will translate into a clinical setting. In addition, concerns for toxicity in targeting ubiquitous host factors needs to be addressed.
HCV ENTRY AND CHOLESTEROL
HCV entry is a highly complex process that not only requires receptor interactions but also appears to be dependent on other host pathways in a postattachment step. For instance, in vivo the HCV particle exists as a “lipoviral” particle, meaning that it is closely associated with low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL).24 LDL and VLDL are also highly enriched in cholesterol, which has been shown to be necessary for HCV cell entry.25,26 Using this knowledge, Sainz, Jr. et al.27 identified NPC1L1 as a receptor for HCV entry. The authors have shown that by using an NPC1L1 antibody, HCV infection can be blocked similarly to what has been described using anti-CD81 or anti-SR-BI antibodies. Furthermore, they used a known inhibitor of NPC1L1, ezetimibe, to block HCV entry at or before the process of viral fusion. The authors went on to demonstrate in a mouse model that treatment with ezetimibe could significantly delay but did not prevent the establishment of HCV infection. Furthermore, much of the orally administered ezetimibe initially binds intestinal enterocytes due to a high expression of NPC1L1 on their surface. Nevertheless, this study highlights the intricate nature of HCV entry in vivo and identifies a potential target for blocking HCV infection.
SMALL-MOLECULE INHIBITORS OF KNOWN ENTRY FACTORS
A second study using an siRNA screen identified a network of kinases involved in HCV entry.28 Silencing of EGFR and EphA2 significantly reduced the entry of HCV into both hepatoma cells and primary human hepatocytes. Interestingly, the receptor kinase activity was required for entry as blocking this step with known inhibitor erlotinib (EGFR) or dasatinib (EphA2) impaired entry in a dose-dependent manner. Further characterization of this inhibition demonstrated that it occurred at a postbinding step that disrupted the CD81-CLDN1 coreceptor interaction. The authors also observed an inhibition of membrane fusion, which suggests EGFR and EphA2 impair both early and late postattachment steps. Finally, these receptor kinases were also required for cell−cell spread and blocked infection in the uPA-SCID mouse model. Similar to ezetimibe, treatment with these compounds only delayed HCV infection; thus there is a need to develop more potent compounds.
ITX5061, a small-molecule inhibitor of SR-BI in lipoprotein metabolism, is currently undergoing clinical trials for efficacy and safety in HCV treatment.29,30 Recent data suggests that ITX5061 was not effective in chronically infected HCV patients but could be useful in combination therapy. These types of treatments could be beneficial for preventing reinfection after liver transplantation.
EMERGING THERAPIES WITH COMPOUNDS HAVING AN UNKNOWN MECHANISM OF ACTION
The development of high-throughput cell culture screening systems has greatly increased the discovery of novel inhibitors of HCV replication.31 This technological advance led to the large-scale screening of compound libraries and the identification of potential therapeutics. Our group and others recently published reports identifying antihistamine chlorcyclizine HCl (CCZ) as a potent inhibitor of HCV replication.32,33 High-throughput screening of a library consisting of FDA-approved drugs identified CCZ and its derivatives as having anti-HCV activity. We further characterized the inhibition and determined that CCZ was able to block HCV entry in a postattachment step similar to what was observed for ezetimibe and/or erlotinib. Additionally, CCZ was able to significantly reduce HCV genotype 1b and 2a replication in the uPA-SCID mouse model. Since CCZ has already been used extensively in humans, it is considered to be safe, and our studies confirm the low toxicity in vitro and in vivo. Furthermore, CCZ showed a strong synergism with current HCV drugs such as sofosbuvir, boceprevir, and telaprevir. Overall, this provides a strong rationale to develop CCZ to combine with current inhibitors to produce more powerful treatment options.
Overall, a great deal of momentum is building to develop entry inhibitors of HCV infection. This early step is critical for reinfection and entry inhibitors may pave the way for prevention of reinfection after liver transplantation in HCV patients. Furthermore, targeting host factors and the use of combinations with different targets will significantly reduce the risk of developing resistance. Current treatment regimens are effective in treating HCV but require long treatment durations, are costly, and are associated with drug resistance. Practically, these new possibilities could be incorporated into the standard of care to reduce duration and cost. A new era has begun for HCV treatment, and we will likely see a major impact on the public health burden of HCV in the next decade.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsinfecdis.5b00060.
Table summarizing the current landscape of HCV entry inhibitors (PDF)
REFERENCES
- (1).Fofana I, Jilg N, Chung RT, and Baumert TF (2014) Entry inhibitors and future treatment of hepatitis C. Antiviral Res 104, 136–142. [DOI] [PubMed] [Google Scholar]
- (2).Flexner C, and Saag M (2013) The antiretroviral drug pipeline: prospects and implications for future treatment research. Curr. Opin. HIV AIDS 8, 572–578. [DOI] [PubMed] [Google Scholar]
- (3).Liang TJ, and Ghany MG (2013) Current and future therapies for hepatitis C virus infection. N. Engl. J. Med 368, 1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Schiff L (2015) Finding Truth in a World Full of Spin: Myth-Busting in the Case of Sovaldi. Clin. Ther 37, 1092. [DOI] [PubMed] [Google Scholar]
- (5).Zeisel MB, Felmlee DJ, and Baumert TF (2013) Hepatitis C virus entry. Curr. Top Microbiol Immunol 369, 87–112. [DOI] [PubMed] [Google Scholar]
- (6).Chatterjee A, Smith PF, and Perelson AS (2013) Hepatitis C viral kinetics: the past, present, and future. Clin Liver Dis 17, 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, and Perelson AS (1998) Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282, 103–107. [DOI] [PubMed] [Google Scholar]
- (8).Wahid A, and Dubuisson J (2013) Virus-neutralizing antibodies to hepatitis C virus. J. Viral Hepat 20, 369–376. [DOI] [PubMed] [Google Scholar]
- (9).Farci P, Shimoda A, Wong D, Cabezon T, De Gioannis D, Strazzera A, Shimizu Y, Shapiro M, Alter HJ, and Purcell RH (1996) Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc. Natl. Acad. Sci. U. S. A 93, 15394–15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, Bukh J, Rice CM, Ploss A, Burton DR, and Law M (2012) Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc. Natl. Acad. Sci. U. S. A 109, 6205–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Keck ZY, Sung VM, Perkins S, Rowe J, Paul S, Liang TJ, Lai MM, and Foung SK (2004) Human monoclonal antibody to hepatitis C virus E1 glycoprotein that blocks virus attachment and viral infectivity. J. Virol 78, 7257–7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Owsianka A, Tarr AW, Juttla VS, Lavillette D, Bartosch B, Cosset FL, Ball JK, and Patel AH (2005) Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J. Virol 79, 11095–11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Schiano TD, Charlton M, Younossi Z, Galun E, Pruett T, Tur-Kaspa R, Eren R, Dagan S, Graham N, Williams PV, and Andrews J (2006) Monoclonal antibody HCV-AbXTL68 in patients undergoing liver transplantation for HCV: results of a phase 2 randomized study. Liver Transpl 12, 1381–1389. [DOI] [PubMed] [Google Scholar]
- (14).Galun E, Terrault NA, Eren R, Zauberman A, Nussbaum O, Terkieltaub D, Zohar M, Buchnik R, Ackerman Z, Safadi R, Ashur Y, Misrachi S, Liberman Y, Rivkin L, and Dagan S (2007) Clinical evaluation (Phase I) of a human monoclonal antibody against hepatitis C virus: safety and antiviral activity. J. Hepatol 46, 37–44. [DOI] [PubMed] [Google Scholar]
- (15).Davis GL, Nelson DR, Terrault N, Pruett TL, Schiano TD, Fletcher CV, Sapan CV, Riser LN, Li Y, Whitley RJ, and Gnann JW Jr. (2005) A randomized, open-label study to evaluate the safety and pharmacokinetics of human hepatitis C immune globulin (Civacir) in liver transplant recipients. Liver Transpl 11, 941–949. [DOI] [PubMed] [Google Scholar]
- (16).Meuleman P, Hesselgesser J, Paulson M, Vanwolleghem T, Desombere I, Reiser H, and Leroux-Roels G (2008) Anti-CD81 antibodies can prevent a hepatitis C virus infection in vivo. Hepatology 48, 1761–1768. [DOI] [PubMed] [Google Scholar]
- (17).Fofana I, Xiao F, Thumann C, Turek M, Zona L, Tawar RG, Grunert F, Thompson J, Zeisel MB, and Baumert TF (2013) A novel monoclonal anti-CD81 antibody produced by genetic immunization efficiently inhibits Hepatitis C virus cell-cell transmission. PLoS One 8, e64221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lacek K, Vercauteren K, Grzyb K, Naddeo M, Verhoye L, Slowikowski MP, Fafi-Kremer S, Patel AH, Baumert TF, Folgori A, Leroux-Roels G, Cortese R, Meuleman P, and Nicosia A (2012) Novel human SR-BI antibodies prevent infection and dissemination of HCV in vitro and in humanized mice. J. Hepatol 57, 17–23. [DOI] [PubMed] [Google Scholar]
- (19).Meuleman P, Catanese MT, Verhoye L, Desombere I, Farhoudi A, Jones CT, Sheahan T, Grzyb K, Cortese R, Rice CM, Leroux-Roels G, and Nicosia A (2012) A human monoclonal antibody targeting scavenger receptor class B type I precludes hepatitis C virus infection and viral spread in vitro and in vivo. Hepatology 55, 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Vercauteren K, Van Den Eede N, Mesalam AA, Belouzard S, Catanese MT, Bankwitz D, Wong-Staal F, Cortese R, Dubuisson J, Rice CM, Pietschmann T, Leroux-Roels G, Nicosia A, and Meuleman P (2014) Successful anti-scavenger receptor class B type I (SR-BI) monoclonal antibody therapy in humanized mice after challenge with HCV variants with in vitro resistance to SR-BI-targeting agents. Hepatology 60, 1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Fofana I, Krieger SE, Grunert F, Glauben S, Xiao F, Fafi-Kremer S, Soulier E, Royer C, Thumann C, Mee CJ, McKeating JA, Dragic T, Pessaux P, Stoll-Keller F, Schuster C, Thompson J, and Baumert TF (2010) Monoclonal anti-claudin 1 antibodies prevent hepatitis C virus infection of primary human hepatocytes. Gastroenterology 139, 953–964. [DOI] [PubMed] [Google Scholar]
- (22).Fukasawa M, Nagase S, Shirasago Y, Iida M, Yamashita M, Endo K, Yagi K, Suzuki T, Wakita T, Hanada K, Kuniyasu H, and Kondoh M (2015) Monoclonal Antibodies against Extracellular Domains of Claudin-1 Block Hepatitis C Virus Infection in a Mouse Model. J. Virol 89, 4866–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Mailly L, Xiao F, Lupberger J, Wilson GK, Aubert P, Duong FH, Calabrese D, Leboeuf C, Fofana I, Thumann C, Bandiera S, Lutgehetmann M, Volz T, Davis C, Harris HJ, Mee CJ, Girardi E, Chane-Woon-Ming B, Ericsson M, Fletcher N, Bartenschlager R, Pessaux P, Vercauteren K, Meuleman P, Villa P, Kaderali L, Pfeffer S, Heim MH, Neunlist M, Zeisel MB, Dandri M, McKeating JA, Robinet E, and Baumert TF (2015) Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat. Biotechnol 33, 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M Jr., and Ye J (2007) Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. U. S. A 104, 5848–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Aizaki H, Morikawa K, Fukasawa M, Hara H, Inoue Y, Tani H, Saito K, Nishijima M, Hanada K, Matsuura Y, Lai MM, Miyamura T, Wakita T, and Suzuki T (2008) Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J. Virol 82, 5715–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kapadia SB, Barth H, Baumert T, McKeating JA, and Chisari FV (2007) Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J. Virol 81, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Sainz B Jr., Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Marsh KA, Yu X, Chayama K, Alrefai WA, and Uprichard SL (2012) Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat. Med 18, 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, Royer C, Fischer B, Zahid MN, Lavillette D, Fresquet J, Cosset FL, Rothenberg SM, Pietschmann T, Patel AH, Pessaux P, Doffoel M, Raffelsberger W, Poch O, McKeating JA, Brino L, and Baumert TF (2011) EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat. Med 17, 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Syder AJ, Lee H, Zeisel MB, Grove J, Soulier E, Macdonald J, Chow S, Chang J, Baumert TF, McKeating JA, McKelvy J, and Wong-Staal F (2011) Small molecule scavenger receptor BI antagonists are potent HCV entry inhibitors. J. Hepatol 54, 48–55. [DOI] [PubMed] [Google Scholar]
- (30).Sulkowski MS, Kang M, Matining R, Wyles D, Johnson VA, Morse GD, Amorosa V, Bhattacharya D, Coughlin K, Wong-Staal F, and Glesby MJ (2014) Safety and antiviral activity of the HCV entry inhibitor ITX5061 in treatment-naive HCV-infected adults: a randomized, double-blind, phase 1b study. J. Infect. Dis 209, 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hu Z, Lan KH, He S, Swaroop M, Hu X, Southall N, Zheng W, and Liang TJ (2014) Novel cell-based hepatitis C virus infection assay for quantitative high-throughput screening of anti-hepatitis C virus compounds. Antimicrob. Agents Chemother 58, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).He S, Lin B, Chu V, Hu Z, Hu X, Xiao J, Wang AQ, Schweitzer CJ, Li Q, Imamura M, Hiraga N, Southall N, Ferrer M, Zheng W, Chayama K, Marugan JJ, and Liang TJ (2015) Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci. Transl. Med 7, 282ra49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Chamoun-Emanuelli AM, Pecheur EI, and Chen Z (2014) Benzhydrylpiperazine compounds inhibit cholesterol-dependent cellular entry of hepatitis C virus. Antiviral Res 109, 141–148. [DOI] [PubMed] [Google Scholar]


