Abstract
Despite major advances in the understanding and treatment of hepatitis C, a preventive vaccine remains elusive. The marked genetic diversity and multiple mechanisms of persistence of hepatitis C virus, combined with the relatively poor immune response of the infected host against the virus, are major barriers. The lack of robust and convenient model systems further hampers the effort to develop an effective vaccine. Advances in our understanding of virus- host interactions and protective immunity in hepatitis C virus infection provide an important roadmap to develop potent and broadly directed vaccine candidates targeting both humoral and cellular immune responses. Multiple approaches to generating and testing viral immunogens have met with variable success. Several candidates have advanced to clinical trials based on promising results in chimpanzees. The ultimate path to a successful preventive vaccine requires comprehensive evaluations of all aspects of protective immunity, innovative application of state- of-the-art vaccine technology and properly designed vaccine trials that can affirm definitive endpoints of efficacy.
Ever since the early 1970s, hepatitis C was recognized as a form of infectious hepatitis distinct from that caused by the already known hepatitis A and B viruses, but the virus’s identity remained elusive for many years. The much celebrated cloning and characterization of hepatitis C virus (HCV) in 1989 was the first triumphant chapter in the chronicle of hepatitis C1 and augured the rapid use of science and technology to advance the goals of understanding and treating this viral infection. Subsequent epidemiological and natural history studies firmly established the global impact and public health burden of HCV2. In the ensuing two decades, we made major strides in understanding the structures and functions of HCV gene products, the key steps of the viral life cycle, the interplays between the virus and host cells, and the complex host immune responses3,4. These advances led to a rapid progress in therapeutic development5,6—since the virus’s discovery, we have leapfrogged from interferon monotherapy, with a less than 10% response rate, to molecularly targeted direct antiviral agents that can achieve up to 70% of treatment response1,7. These areas of major advancement will be discussed by other accompanying reviews in this issue of Nature Medicine. Unfortunately, one area of research and development has not kept pace with the other accomplishments: prophylactic vaccines. It is ironic that the HCV vaccine has lagged behind the rest of the field, considering that vaccination to prevent infection is one of the foundations of combating infectious diseases. This Review highlights and discusses the barriers and current progress in developing preventive vaccines for HCV.
Prevention strategies
Historically, vaccination to prevent infection has been the most effective medical strategy to control infections of major public health importance. The relatively low cost and high benefit of vaccines has made them a cornerstone of modern public health policy. In addition to the eradication of smallpox by vaccination several decades ago, the more recent success in reducing HBV infection and its complications by vaccination is a testament to the effectiveness of a successfully implemented universal vaccination program8. Although one might argue that HBV is much more infectious and prevalent than HCV, HCV infection has a higher chronicity and worse disease progression compared to HBV infection. In fact, in certain countries, such as Egypt, where HCV infection is endemic and decades of public health measures have failed to reduce the overall rate of HCV transmission9,10, an effective HCV vaccine would have an enormous impact on reducing the disease burden.
Recent advances in the treatment of hepatitis C raise the prospect that a preventive vaccine for HCV might not be necessary. This view, however, is shortsighted in light of the enormous public health burden and the current state of therapy for this infection. It is well known that the current optimal regimen, a triple combination of peginterferon, ribavirin and a direct-acting antiviral, has substantial side effects and is applicable to only a minority of HCV-infected persons7. Most HCV-infected individuals do not even know they are infected, and the disease tends to be insidious until it progresses to the late stage, whereupon patients seek medical attention11–13. The high price of therapy (~$50,000) even in wealthy countries such as the US and Western Europe makes the prospect of treating millions of people daunting. Therefore, it is unlikely that the current treatment paradigm and clinical practice will have much impact on the overall disease burden of HCV infection.
From a global perspective, not until the price of the therapy drops substantially, the regimen becomes considerably easier and the side effects are appreciably lessened can we realistically believe that treatment will make a dent on the worldwide burden of this infection. The recent application of antiviral drugs in preventing HIV infection has received much attention as an alternative means to prevent viral infection14. However, the success of these approaches hinges entirely on the provision of low-cost therapy to the masses and on a well-designed strategy to prevent sexual transmission15. These approaches are not possible with the current state of therapy for HCV.
Protective immunity
The fundamental tenet of preventive vaccination is to expose the host to a microbe-derived immunogen with the hypothesis that the induced immunity will protect the host from subsequent infection by the microbe. Much of the success in early vaccine development was based on empirical testing of candidate vaccines for effectiveness in preventing infection, but recent advances in biomedical sciences have allowed us to further understand the key components of protective immunity in vaccine-induced prevention. The comprehensive elucidation of dendritic cell functions, B and T cell immune responses, innate immunity, antigen presentation and other fundamental immunologic concepts provide the necessary knowledge to systematically decipher how one vaccine works and why another one fails.
The efforts in HBV and HIV vaccine development highlight a contrasting case in point regarding the evolving strategies of vaccinology over the last 20 years. The HBV vaccine was successfully developed through the classical approach of generating a viral vaccine by purifying the virus from infected sources and inactivating it. The results of initial vaccine trials were impressive and led to the subsequent implementation of a worldwide HBV vaccination program8. In contrast, HIV vaccine development has had a difficult path and has stalled with several recent high-profile failures16,17. These challenges led many in the field back to the fundamental questions of what protective immunity is and how it can be induced by innovative technologies. These two examples are great lessons for the HCV vaccine field. The path to a successful HCV vaccine is fraught with challenges and barriers, similar to the path of the HIV vaccine. Use of recombinant envelope proteins, similar to the approach for HBV vaccine, was met with limited success. However, HCV, unlike HIV, can be cleared either naturally or therapeutically from infected per- sons. Therefore, it is imperative to define and understand protective immunity in HCV infection before embarking on wide clinical testing of putative vaccine candidates.
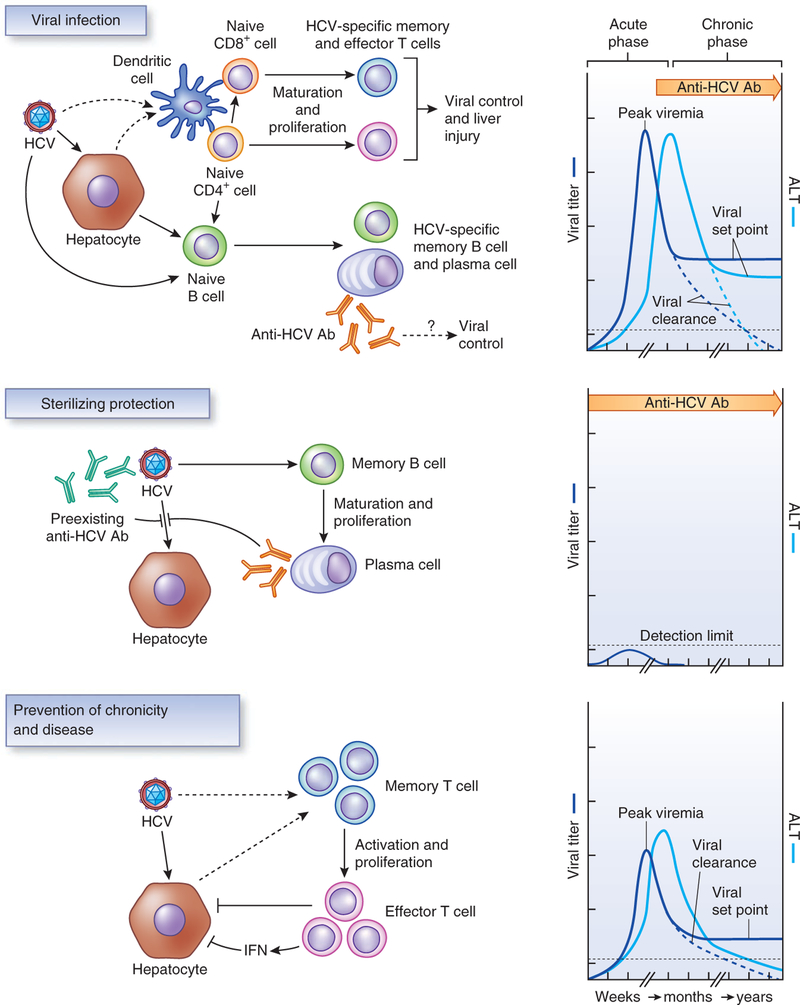
Protective immunity induced by vaccination results in an alteration of the natural course of infection leading to a more favorable clinical outcome (Fig. 1). Sterilizing protection, defined by the absence of serologic evidence of infection after exposure, may not be a realistic or attainable goal for viruses that cause chronic infection, such as HIV, HCV and herpes viruses. Therefore, the goal of an effective vaccine for HCV has shifted from sterilizing immunity to immunity that is capable of preventing chronic infection and disease. This type of protective immunity can lower the peak viremia and viral set point, with a higher likelihood of subsequent viral clearance (for HCV) or a milder disease (for HIV). Indeed, the word ‘sterilizing’ is probably a misnomer even for a classical protective vaccine such as that for HBV, as many vaccinated persons after exposure may experience subclinical infection that cannot be detected by standard serologic and virologic assays18.
Figure 1.
Natural course of HCV infection and types of protective immunity. Top, the course of acute HCV infection and the induced adaptive immune responses to control HCV infection. During the acute phase of HCV infection, replication of the virus is unchecked and attains high-level viremia within a short period of time. Liver enzymes, such as alanine cell aminotransferase (ALT), become elevated. As the host immune response is activated, either viral clearance occurs or viral level drops and persists at a lower level as the viral set point during the chronic phase, which can be associated with elevated ALT. The classical vaccine strategy of inducing sterilizing immunity is to prevent acute infection and has been highly effective in viral infections that cause acute and severe disease. The mechanism of sterilizing protection mediated by vaccine-transient viremia after exposure to HCV that is below the limit of detection (middle)108.In contrast, cell-mediated immunity is crucial for viral clearance in the case of recovery or control of viral replication during the chronic phase of infection (bottom)79. Anti-HCV Ab, HCV-specific antibody.
Cell-mediated immunity.
Studies in chimpanzees and humans have shown that the majority of HCV infections result in chronicity despite the presence of an intact immune status2,19. This high rate of chronicity suggests that vaccine development for HCV will be potentially challenging. Evidence for protective immunity in HCV infection comes from studies of convalescent chimpanzees and humans who were re-exposed to HCV20–22. Although re-infection occurs commonly, it usually resolves more rapidly and persists less frequently as compared to primary infection in non-immune subjects. There does not seem to be a single correlate of protective immunity; how- ever, evidence suggests that a broadly directed CD4+ and CD8+ T cell response is linked to virologic control19. In particular, T cell responses that are sustained over time and are multifunctional, with interferon-γ (IFN- γ) and interleukin (IL-2) production (T helper type 1 (TH1) phenotype) and a high proliferative phenotype, are crucial3,19. Viral core and nonstructural gene products, such as NS3 and NS5, are often the targets of protective T cell response3,23,24. Direct evidence for protective immunity mediated by CD4+ or CD8+ T cells was demonstrated in chimpanzees by antibody-mediated depletion of either T cell population. This depletion resulted in prolonged or persistent HCV infection25,26. Another argument in favor of the T cell response being important in protective immunity is the strong associations of certain HLA class I and II alleles with infection outcomes27.
Humoral immunity.
Although B cell immunity, as represented by neutralizing antibodies, may contribute to host defense against HCV, its role in viral clearance is less clear21,28–31. In particular, the envelope proteins of HCV are not very immunogenic, as evidenced by a slow and weak antibody response during primary infection32,33. The induced antibodies tend to be non-neutralizing and often interfere with the effectiveness of broadly neutralizing antibodies in cell- based assays34. In addition, the antibodies commonly target the variable regions of the envelope proteins, which have a high mutational rate35,36. Furthermore, heavy glycosylation of the envelope proteins probably hinders the induction and action of neutralizing antibodies37. Finally HCV circulates in complex with host lipoproteins and plausibly exploits this mechanism to evade binding by neutralizing antibodies38. In this context, the preponderance of evidence suggests that T cell immunity has a major role in recovery from natural HCV infection and should be a primary target of vaccine-induced immunity3,39–42. Humoral immunity, however, may still play a part in viral clearance and should not be ignored in vaccine development21,28,30,31.
Innate immunity.
Natural killer (NK) and NKT cells probably contribute to the initial control of viral infection19,28, yet there is little evidence that innate immunity can be harnessed for vaccine-induced immunity against subsequent viral infection. Interestingly, a recent study suggested the possible existence of immunologic memory of NK cells43, raising the prospect of targeting this arm of immune response in vaccine development.
Unexpectedly, a pivotal correlate of protective immunity came from the unrelated field of genome-wide association studies. Several single nucleotide polymorphisms (SNPs) in the IL28B (interferon- λ −3) locus were recently shown to be highly associated (~P < 10−30) with recovery from acute HCV infection or with treatment-induced viral clearance44–47. IFN-λ, like IFN-α, is part of intrinsic innate immunity, is rapidly induced in response to HCV infection48 and represents the first responder in host antiviral response. This observation points to a crucial role of innate immunity in the control of HCV infection. Although the functional significance of this genetic association remains to be elucidated, such knowledge in protective immunity can potentially be harnessed for a new vaccine approach. For example, if activation of IFN- λ is crucial for HCV control, a strategy can be devised to target its robust induction by a vaccine candidate.
Protective immunity in the chimpanzee model.
Many of the chimpanzee studies mentioned above, although elegantly conducted, have several caveats for vaccine development. Although chimpanzees are genetically close to humans, they are divergent from humans in several gene clusters, some of which contain genes involved in immunologic responses to infection49. Therefore, it is not surprising that chimpanzees respond quite differently from humans to chronic viral infection in terms of pathology, disease progression and viral clearance, including in HIV, HBV and HCV infection50,51. In addition, chimpanzees used for experimental studies are bred in captivity and represent a relatively inbred population, whereas human populations are much more evolutionarily divergent and biologically diverse. Therefore, the results from the chimpanzee studies need to be interpreted with caution, especially those related to protective immunity51. Despite these limitations, the chimpanzee is the only animal model that is suitable for preclinical testing of HCV vaccines and will continue to be valuable for HCV vaccine development.
Biological barriers and solutions to HCV vaccine development
HCV genetic diversity.
HCV has a high genetic diversity and is classified into seven major genotypes, which differ by more than 30% sequence diversity. HCV circulates in infected individuals as multiple closely related but distinct viruses, referred to as a ‘quasispecies’ population, with sequence variations up to 10% (ref. 52). The viral polymerase’s lack of a proofreading capacity accounts for the high mutational rate of 10−5–10−4 nucleotides per replication cycle, which is an order of magnitude higher than that for HIV and HBV53. HCV has two envelope glycoproteins, gpE1 and gpE2, and gpE2 has a highly variable region, hypervariable region-1, which is under constant immunologic pressure because it is a main target of the host antibody response32,36. Other regions of the envelope proteins that are also targets of antibody response probably exist and can be subject to immunologic selection. In addition, cellular immunity targeting the envelope proteins can contribute to immunologic escape54.
HCV can rapidly mutate its envelope proteins to escape the neutralizing antibody response in humans, which accounts for the lack of an effective antibody response in HCV infection35,36,55. The coexistence of viremia and envelope-specific antibodies that are neutralizing for another viral species, but not the prevailing one, is often observed in HCV-infected individuals55. Escape mutations in T cell epitopes have also been described in HCV-infected persons54. The patterns and types of T cell escape mutations are often determined by the host genetic background, as T cell epitopes are recognized in the context of specific HLA molecules56. Interestingly, HLA-B27 confers a protective effect in HCV infection because of a genotype-specific conserved immunodominant CD8+ T cell epitope57.
The vastly mutable nature of HCV not only contributes to its high persistence rate but also makes vaccine design difficult. Nevertheless, there are regions of viral gene products that are highly conserved, such as regions on the core proteins NS3 and NS5B, probably because of the functional integrity of these regions52. Effective targeting of these regions could minimize the problem of genetic diversity, but the question remains as to how to target them. For T cell–based vaccines, these conserved sequences can be genetically engineered as part of a candidate vaccine because the T cell epitopes are presented to cell-mediated immunity as linear and discontinuous targets58.
Targeting conserved B cell epitopes proves to be more difficult. First, the target would have to be in the envelope proteins because of the functionality of antibody-mediated immunity, but the virus can mutate the envelope proteins quickly to escape humoral immunity32,35,36. The difficulties in inducing broadly neutralizing antibodies, as discussed above, are major impediments to exploiting antibody responses for protective immunity. Furthermore, neutralizing anti- bodies may be targeting conformational epitopes with discontinuous sequences, and correctly designing antigens with this type of epitope may be difficult. HCV, like other viruses capable of causing chronic infection in humans, has evolved to be less visible to the repertoire of human immune responses59,60.
Despite these caveats, if we fully understand the structure and function of the viral envelope proteins in the context of virion structure, cell binding and entry mechanism, we can design antigens that mimic certain critically conserved domains on the virus and induce broadly active neutralizing antibodies with high potency. These potentially conserved structures can be either on the native virion surface or buried deep in the virus but become exposed during viral entry. Envelope-specific antibodies capable of neutralizing HCV infection at the post–cell-binding stage have been described61. Such an approach is currently under way to target conserved structural intermediates of HIV during its entry and fusion process62, but this strategy is not possible for HCV now as the three-dimensional structures of the virus and envelope proteins remain unknown63.
The relatively weak immunogenicity of HCV envelope proteins, as discussed above, can be overcome with a highly immunogenic formulation that is designed to induce a strong B cell response and production of antibodies. Improvements in vaccine formulation and development of potent adjuvants can confer high immunogenicity to previously poor antigens64.
Regardless of the HCV vaccine design, the strategy to minimize escape mutations is to raise the genetic barrier for mutations by targeting multiple antigenic determinants. An immunogen that induces a broad and strong immunity against both B and T cell epitopes may be essential to avoid escape mutations and counter genetic diversity.
Mechanism of persistence.
As the host and HCV battle to control the outcome of the infection (Box 1), HCV is able to distort the activation of T cells, resulting in ineffective cell-mediated immunity19,65. HCV, through a yet-unknown mechanism, fails to properly activate the CD4+ T cell response and causes a rapid immune exhaustion phenotype of CD8+ T cells as the infection endures19,65. Once the infection progresses to the chronic phase, the host may no longer be capable of mounting an effective and vigorous immune response to eliminate the virus. Spontaneous viral clearance is rare in chronically infected individuals66. These immune evasion mechanisms may have important implications in vaccine development for HCV. An effective vaccine will need to prime the immune system in a sufficiently broad and highly reactive manner against the first sign of HCV infection before the virus has the chance to gain a foothold in the body and unleash its many immune escape mechanisms. In particular, rapid and accelerated activation of TH1 responses and neutralizing antibodies targeting conservative regions of the virus may be crucial in designing an HCV vaccine67,68.
Box 1 Mechanisms of HCV persistence.
Unlike HBV and HIV, HCV is not capable of persisting in infected cells as a dormant integrated form. Its infection persists despite a functional immune response (see accompanying Perspective by Gale et al.114). For this reason, HCV has to evolve multiple mechanisms to evade and counteract host immune response. In addition to passively mutating its genome in response to immune pressure, HCV commands several active strategies to achieve a high rate of chronic infection. At the virus level, it can hide from the antibody response and pass from cell to cell without being exposed to the circulating antibodies by complexing with lipoproteins115. At the cellular level, the virus can block intrinsic innate immunity by inhibiting interferon induction and action (see accompanying Perspective by Gale et al.114). The gpE2 envelope protein has been shown to attenuate NK, T and B cell activties by interacting with CD81 on these cells116–118. HCV can also exert an anti-apoptotic effect on infected cells to sustain viral replication and evade killing by the activated CD8+ T cell response119–121. HCV has been shown to interfere with antigen presentation of dendritic cells and antiviral functions of NK and NKT cells, although this effect remains controversial65.
Model systems.
An important barrier that has plagued the field of HCV research even before the virus was identified is the lack of convenient experimental model systems, ranging from cell culture to animal models. Not until 2005 was there a robust cell culture system (HCVcc) to propagate the virus69. Before 2005, many surrogate model systems, such as HCV replicons, pseudoviruses (HCVpp), recombinant viral proteins and virus-like particles were developed to study various aspects of HCV biology70 and to test the neutralizing potential of antibodies induced by various candidate vaccines71,72. HCVpp or HCVcc containing the structural proteins of various HCV genotypes are available for testing of cross-genotype neutralizing activities of antibodies73. Primary human hepatocyte culture has also been established as a valuable tool to study HCV infection48,74. Although these in vitro systems are useful in examining the humoral response generated by HCV vaccine candidates, they have not been extended successfully to studying the T cell response.
The lack of animal models remains a major obstacle to HCV vaccine development51. The only acceptable infectious animal model is the chimpanzee. However, the high cost and scarce supply of chimpanzees limits the statistical power of studies, precluding definitive conclusions. Although HCV vaccine testing is considered acceptable in chimpanzees by the US Institute of Medicine report on the use of chimpanzees for biomedical research, the likely increasing scrutiny of chimpanzee research will further dampen research efforts in HCV vaccines75. Other primate species, such as macaques and rhesus monkeys, are not susceptible to HCV76. Chimeric mouse models, in which mice are engrafted with human hepatocytes, support robust HCV infection and replication and can be used to study HCV- neutralizing antibodies, virus-receptor interactions and therapeutic interventions51. However, owing to their underlying immunodeficiency, these mice cannot be used to study active adaptive immunity or determinants of chronicity. Efforts to improve this model and to potentially create transgenic mice susceptible to HCV infection are ongoing77,78. A few other small-animal models have been reported, but their usefulness and reproducibility remain unclear51.
Animal models, although useful in vaccine development, may not accurately reflect vaccine response and efficacy in humans. Therefore, a logical strategy for vaccine development may be to transition expediently into phase 1 studies for evaluation of immunogenicity followed by phase 2 studies of the best candidates.
Approaches to HCV vaccine development
Strategies for HCV vaccine development have ranged from the traditional approach of producing recombinant envelope proteins to complex engineering of viral vectors directing the expression of multiple viral antigens (Table 1). The rationale for these varied approaches has been driven by the changing prevailing belief of what constitutes protective immunity in HCV infection and how best to achieve it. The success of the HBV vaccine, based on induction of neutralizing antibodies by purified or recombinant HBV surface antigen, had an early impact on the first generation of HCV vaccine in the form of recombinant HCV envelope proteins68. Initial testing in chimpanzees showed protection from HCV infection in some of the animals68; however, humoral immunity, although present in HCV-infected humans, has been questioned regarding its role in viral clearance and protective immunity. Despite this concern, the absence of a role for the antibody response in viral clearance during natural infection does not necessarily mean that antibody-associated protection should not be considered in vaccine-induced immunity. This logic extends to the possibility that HCV probably evolves to avoid or minimize humoral immunity for its survival. Thus, if this arm of immunity can be creatively activated for production of broadly neutralizing antibodies against conserved epitopes, as previously discussed, an Achilles’ heel of HCV may be exposed for vaccine-induced protective immunity. This paradigm is also being explored in HIV vaccine development, as the strategy of inducing T cell–mediated immunity fails to provide a clear answer62.
Table 1.
Prophylactic HCV vaccine studies in chimpanzees and humans
| Vaccine | Targeted immunity | Animal testing | Clinical trial | Ref. | |
|---|---|---|---|---|---|
| Recombinant protein |
gpE1 and gpE2 Adjuvant: MF59 and MF75 |
Humoral immunity | Immunogenic in small animals; protects chimpanzees from acute or chronic infection |
Phase 1a; neutralizing antibodies and CD4+ T cell responses |
68,100,101 |
| Core Adjuvant: Iscomatrix |
Cell-mediated immunity | Immunogenic in small animals and rhesus macaques |
Phase 1a; CD4+ and CD8+ T cell responses |
109,110 | |
| gpE1 Adjuvant: alum |
Humoral immunity | Immunogenic in small animals; protects chimpanzees from acute or chronic infection |
Phase 1a; antibodies and CD4+ T cell responses; no longer in development |
111 | |
| Core, NS3, NS4 and NS5 Adjuvant: Iscomatrix |
Cell-mediated immunity | Immunogenic in small animals; no protection from acute or chronic infection |
None | 101 | |
| HCV-like particles containing core, gpE1 and gpE2 Adjuvant: AS01B |
Humoral and cell-mediated immunity |
Immunogenic in small animals; protects chimpanzees from chronic infection |
None | 89 | |
| DNA and viral vector |
DNA prime and adeno expressing core and NS3–NS5 |
Cell-mediated immunity | Immunogenic in small animals; protects chimpanzees from chronic infection |
Phase 1/2 (NS3–NS5); CD4+ and CD8+ T cell responses |
67,102 |
| DNA prime and adeno virus expressing core, E1, E2 and NS3– NS5 Adjuvant: IL-12 plasmid |
Humoral and cell-mediated immunity |
Immunogenic in small animals; protected chimpanzees from chronic infection |
None | 86 | |
| DNA prime and MVA expressing core, E1, E2 and NS3, or NS3–NS5 |
Humoral and cell-mediated immunity |
Immunogenic in small animals; reduced peak viremia but did not protect chimpanzees from chronic infection |
Phase 1/2 as therapeutic vaccine; CD4+ and CD8+ T cell response |
81,83 | |
| VV expressing core, E1, E2, p7, NS2 and NS3 |
Humoral and cell-mediated immunity |
Immunogenic in small animals; protected chimpanzees from chronic infection |
None | 80 | |
| DNA expressing E2 | Humoral and cell-mediated immunity |
Immunogenic in small animals; protected chimpanzees from chronic infection |
None | 112 | |
| DNA/recombinant protein |
DNA prime and protein boost (core, E1, E2 and NS3) Adjuvant: alum |
Humoral and cell-mediated immunity |
Immunogenic in small animals; protected chimpanzees from chronic infection |
None | 113 |
Only chimpanzee studies with two or more chimpanzees are listed.
Candidate vaccines.
As the concept that T cell–mediated immunity is more crucial for protective immunity in HCV infection emerges, emphasis has shifted to the generation of T cell–based vaccines. This trend is further supported by the hypothesis that a vaccine against an insidious virus, such as HIV, HCV or herpes virus, needs to induce potent T cell immunity79. Viral vectors including adenovirus, vaccinia virus, modified vaccinia Ankara (MVA), fowl pox virus and other viruses, as well as novel yeast vectors, have been used to deliver various HCV structural and nonstructural antigens for induction of T cell–mediated immunity (Fig. 2)67,80–83. Adenoviral vectors seem to be the most promising delivery vehicle because they can induce robust CD4+ and CD8+ T cell responses with a predominant TH1 phenotype. They also have a long track record in HIV vaccine development84. Viral proteins such as core, NS3 and NS5 are commonly used in vaccine design, as they are the prime targets of T cell–mediated immunity during natural infection3. The two envelope proteins, gpE1 and gpE2, are often included as well, in the hope of inducing both neutralizing antibodies and T cell response. DNA immunization, although not as potent in humans as originally conceived, has been shown to be more immunogenic with alter- native delivery methods85. It is being used mainly as a prime-boost regimen to enhance immunogenicity67,81,86. Peptide-based vaccines targeting specific T cell epitopes have been developed for HCV therapy, but the induced immunity is often weak and unlikely to be effective in either therapy or prevention87.
Figure 2.
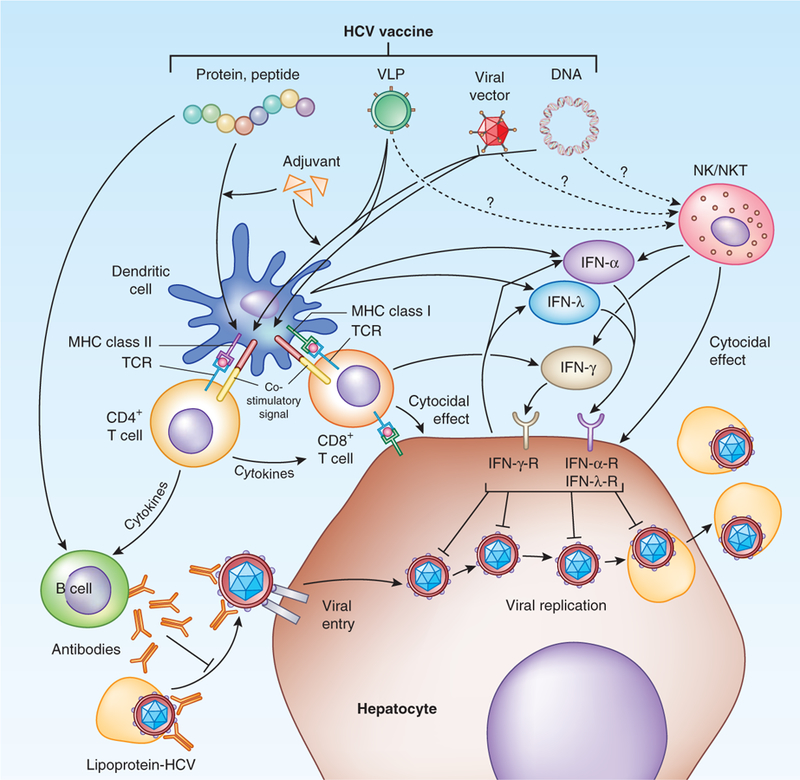
Targets and mechanisms of vaccine-induced immunity in prevention of HCV infection. Various vaccine candidates targeting different aspects of host immune responses are illustrated. Protein-based vaccine targets B cell immunity and, if produced either in unique form or with potent adjuvant, can cross-prime to the T cell pathway. Viral vectors or DNA plasmids induce predominantly T cell responses. They can also prime B cell immunity if the expressed antigens include HCV envelope proteins. VLPs can also target both B and T cell immune responses. B cell immunity, via neutralizing antibodies, can either neutralize circulating virus—usually in complex with lipoprotein—or virus at the entry step.T cell–mediated immunity can either directly eliminate viral-infected cells via cytocidal mechanisms or produce antiviral cytokines such as IFN-γ. IFN-α and IFN-λ can be produced by activated dendritic or other immune cells to inhibit viral replication. Innate immune cells, such as NK or NKT cells, may also be activated by vaccine to produce antiviral cytokines or exert direct cytotoxic effector function. MHC, major histocompatibility complex; TCR, T cell receptor.
Regarding cell-mediated immunity, vaccination to prime CD4+ T cells may be a simpler and more direct path, given that CD4+ T helper cells are necessary for the induction of both antibodies and cytotoxic CD8+ T cells, the two crucial arms of effector immune responses in antiviral immunity. The HCV-specific CD4+ T cell response can be induced by expressing viral gene products enriched in CD4+ epitopes, such as NS3, using DNA, viral vectors or other recombinant DNA technologies. A broad CD4+ T cell response primed by a candidate vaccine could react promptly to HCV infection and activate both antibody and cytotoxic T cell responses to eliminate the virus before it has a chance to unleash its various evasion mechanisms. Genotype-specific constructs might be generated and combined as a multivalent vaccine to provide broad protection against infection with diverse HCV strains, and such a vaccine could be used in various geographic regions. Mutational escape of HCV is less of a concern for the CD4+ T cell response than for antibody or CD8+ T cell responses88. Inclusion of multiple CD4+ T cell epitopes and sequences will also minimize the problem of mutational escape.
Generation of virus-like particles (VLPs) by expressing the structural proteins of HCV may be an alternative strategy to induce both the humoral and cellular arms of immune responses89. VLPs have been shown to cross-prime both major histocompatibility complex class I and II pathways because of the structural uniqueness of these particles90. Multivalent HCV-like particles can be generated to address the problem of high genetic diversity. VLP-based vaccines have been developed successfully for other viral infections91,92. The HBV vaccine is actually a VLP vaccine because the recombinant HBV surface antigen, which is produced in either yeast or mammalian cells, self-assembles to form HBV-like structures and is a potent inducer of antibody and T cell responses93. The VLP is also a unique platform for delivering other antigens of interest and is being explored as an alternative technology for vaccine development94.
Adjuvants.
Strategy to enhance the immunogenicity of vaccine by adjuvant has been an important addition to vaccine development (Fig. 2). Chemical adjuvants based on lipid formulations seem to be more potent than the classical adjuvant alum95 and, when combined with specific antigens, can induce strong humoral immunity as well as T cell immune responses, including the CD8+ T cell response64. New biological adjuvants targeting specific immunologic pathways, such as Toll-like receptors (TLR7 and TLR9), are potent inducers of immune response96 and have been tested in clinical trials97. Lipid- based adjuvants may also activate TLRs. Additionally, cytokines that are potent activators of specific immune cells have been tested and have shown promise in animal models98. Adjuvants may be crucial for protein- or peptide-based vaccines because of the weaker immunogenicity of recombinant HCV proteins, although they may not be necessary for viral vector–based vaccines because of the vectors’ inherent potency (Table 1).
Current landscape of HCV vaccines
Chimpanzee studies.
The protective effects of several antibody- and T cell–based vaccines have been tested in chimpanzees (Table 1). Whereas some showed promise in preventing acute infection and chronicity, the number of chimpanzees per study was too small to make any definitive conclusions. A recent meta-analysis of the chimpanzee studies comparing various parameters among all the naive, vaccinated and re-challenged animals revealed that many of the vaccines, either antibody- or T cell–based, induced strong immune responses that seemed to rapidly control HCV replication; however, the levels of induced T cell response did not correlate with vaccine potency and effectiveness99. Interestingly, vaccines that contained HCV structural proteins were more effective than those containing nonstructural proteins, the inclusion of which might actually be detrimental in inducing protective immune response99. The antibody vaccine proved better in preventing persistence. These unexpected observations are tempered by the caveat of divergent experimental conditions and small sample sizes in this meta-analysis. In a few studies, sterilizing immunity was observed in some vaccinated animals, but only against challenge with a homologous or closely related HCV strain68,99. Although cross-protection has been reported in convalescent chimpanzees re-exposed to HCV of another genotype20, it was not addressed in these studies. Whether broad protection induced by either antibody- or T cell–based vaccines is attain- able remains an unanswered question and an important challenge. Considering the genetic diversity of HCV, broadly targeting HCV vaccines are necessary.
Human trials.
Despite the uncertainty in what constitutes the best vaccine-induced protective immunity, two candidate HCV vaccines that showed promise in chimpanzee studies have advanced to clinical trials. One of the vaccine candidates contains recombinant envelope glycoproteins E1 and E2 of HCV-1 and is formulated with a potent adjuvant, MF59. In a phase 1 study of human volunteers (ClinicalTrials.gov NCT00500747), it induced strong antibody responses that neutralized in vitro infectivity by HCV-1 as well as a genotype 2 virus, although to a lesser extent72,100,101. Further development of this candidate vaccine is currently on hold. The other candidate is a T cell–based vaccine (ClinicalTrials.gov NCT01070407) based on two serologically distinct adenoviral vectors, one a rare human adenovirus, Ad6, and the other a chimpanzee adenovirus, Ad3Ch3 (refs. 67,102), both of which have low seroprevalence rates in the human population. HCV nonstructural genes spanning the NS3-NS5B sequences were incorporated into the viral vectors. Both vaccines induced strong TH1 cell responses that targeted multiple regions and that were sustained up to 1 year. Neutralizing antibodies and T cell responses against the adenovirus were also detected after the priming and probably limited the boosting effect of a second immunization. To overcome this problem, a different viral vector, MVA, is currently being tested in a prime-boost regimen with the AdCh3 vector (ClinicalTrials.gov NCT01436357) in a phase 1/2 study to assess the safety, efficacy and immunogenicity of the AdCh3-MVA regimen as a prophylactic vaccine in about 300 intravenous drug users.
Clinical trial design and implementation
The success of developing a preventive HCV vaccine rests not only on identifying the optimal immunization regimen but also on devising the best strategy to test vaccine efficacy in clinical trials (Fig. 3). Much of the current work on HCV vaccines has been done with HCV genotype 1, but a large proportion of HCV-infected people in the world are infected with other genotypes. Without knowing enough about the cross-reactive protective immunity induced by a vaccine of one genotype, it will be difficult to select the best candidate. Designing a preventive HCV vaccine trial is also a challenge. In a general population with a HCV prevalence of 1–2%, as is the case in the US and other Western countries, a trial would have to enroll an enormous number (>100,000 people) of subjects to show efficacy. An alternative trial design would be to target a high-risk population with a high exposure rate, such as intravenous drug users, sex workers, health- care workers and public servants, or people with sexual contacts with HCV-infected persons. A high-endemic area, such as Egypt, would be an appropriate place to conduct a vaccine trial9; unfortunately, the most prevalent HCV genotype there is specific for this region (genotype 4 in Egypt), and the study result may not be applicable to other countries. In addition, developing countries such as Egypt often lack infrastructural support and may have intrinsic ethical and regulatory concerns.
Figure 3.
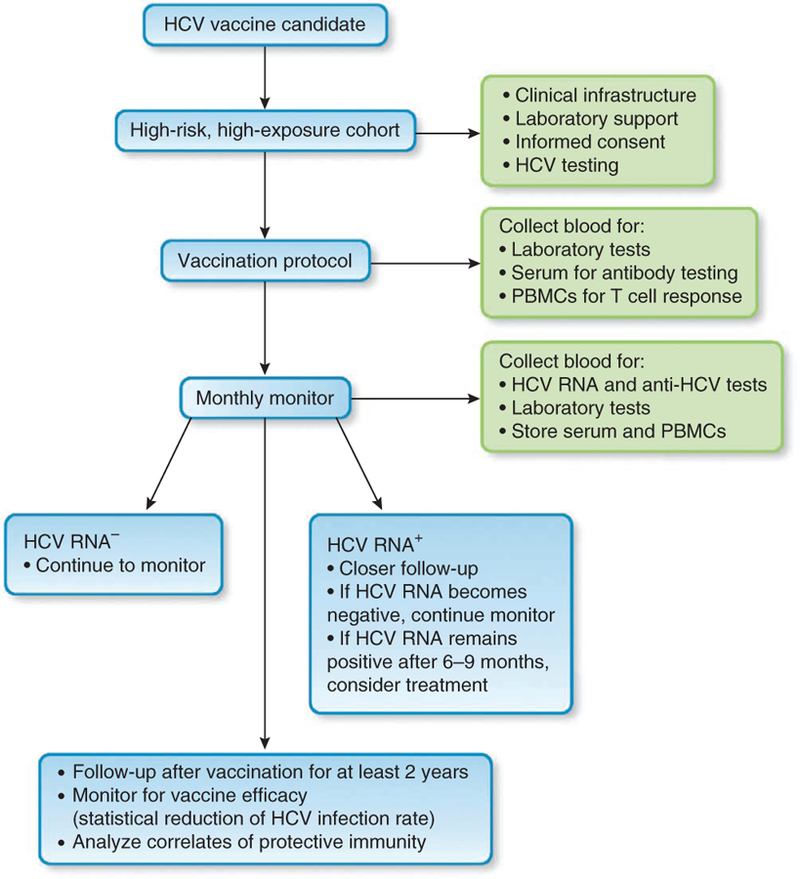
Diagram of steps for vaccine trial design and important parameters to monitor during study. PBMCs, peripheral blood mononuclear cells.
Several factors for specific populations are crucial in designing a vaccine trial103, such as HCV prevalence, exposure frequency (how often the subjects are exposed), infectivity (the risk of infection with each exposure) and chronicity (the percentage of infected individuals that progresses to persistent infection). Determination of a sample size would also depend on the acceptable vaccine efficacy, which should be at least 50% reduction of infection rate. Depending on the value assigned to each of the above parameters, Strictland et al.103 calculated that about 500–10,000 people are needed for a vaccine trial in a high-risk population. This number is not insurmountable but certainly poses a challenge for testing a large number of candidate vaccines. Therefore, selection of a candidate vaccine for clinical trial will require considerable prudence.
A realistic goal for vaccine efficacy should be prevention of chronic infection. Thus, chronicity as an endpoint needs to be clearly defined. Chronic HCV infection cannot be simply defined according to the classical definition of chronic HBV infection: persistence of active viral replication for more than 6 months. During acute HCV infection, the viremic level can fluctuate markedly from nondetectability to a high level (>106 IU ml−1)104, and viral clearance may not occur until 1 year later, indicating that a longer trial duration—at least 2 years—is necessary. More frequent blood sampling of the study cohort are required to capture those with transient viremia without antibody seroconversion, which has been reported105. In the same vein, correlates of protective immunity need to be clearly determined and monitored in any clinical trials of prophylactic HCV vaccines. Surrogate immune markers have to be developed for efficacy evaluation and validation in enrolled subjects. Standardization of methodology should be applied to all trials. Extensive immunological studies of both humoral and cellular immune response will be crucial to advance the development of effective vaccines.
Another issue relates to the ethic of withholding therapy, which has a high success rate in acute HCV infection (up to 90% with interferon- based therapy). The commonly accepted practice is to treat acutely infected individuals after a close follow-up of 6–9 months106. Thus, a vaccine trial design will have to address this issue and may need to include a larger number of subjects. Finally, how vaccination strategy should be implemented once an effective vaccine becomes available is a complicated issue that will require open discussions among public health officers, clinicians, researchers and patient groups, and it will also depend on the country where the vaccine will be deployed. Careful cost-benefit analyses107 need to be scrutinized extensively before public policies can be issued. Targeting high-risk groups as discussed above will probably have the most impact, but universal vaccination may be appropriate in countries with a high HCV prevalence. Implementation of a mass vaccination program in developing countries will be a major challenge, as it continues to be for HBV.
Conclusions
Although the burden of hepatitis C might be slowly conquered by steadily improving therapies, the endgame would not be complete without the successful development and implementation of an effective preventive vaccine. To achieve such a goal, the intricate interplays and mechanisms of action between the virus and host at the molecular, cellular and immunologic levels early during infection need to be elucidated. What triggers or prevents a successful immune response, leading to viral clearance or persistence, respectively, holds the key to designing a successful vaccine with induced immunity that is broadly targeted, cross-protective and long lasting. Robust animal models are urgently needed. Advances in vaccinology will provide a technology platform upon which effective preventive HCV vaccines can be built. Currently, adenovirus-based vector vaccines seem to be the most potent vaccines capable of inducing a broad, robust, lasting and multifunctional immune response that mimics natural protective immunity. Accumulating knowledge in developing preventive vaccines can also be translated to potentially new therapies based on immunologic strategies. Finally, funding and commitment on the part of public and private sectors are sorely needed to galvanize this effort. Technology and scientific innovation will not be the limiting factors. It will require the collective will and wisdom of the scientific community, together with public health agencies and pharmaceutical industry, to bring this once daunting medical challenge to a proper ending.
ACKNOWLEDGMENTS
This work is supported by the Intramural Research Program of the US National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Choo QL et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244, 359–362 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Liang TJ, Rehermann B, Seeff LB & Hoofnagle JH Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann. Intern. Med 132, 296–305 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Bowen DG & Walker CM Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436, 946–952 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Lindenbach BD & Rice CM Unravelling hepatitis C virus replication from genome to function. Nature 436, 933–938 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Hoofnagle JH & Seeff LB Peginterferon and ribavirin for chronic hepatitis C. N. Engl. J. Med 355, 2444–2451 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Schaefer EA & Chung RT Anti–hepatitis C virus drugs in development. Gastroenterology 142, 1340–1350. e1 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Liang TJ & Ghany MG Current and future therapies for hepatitis C virus infection. N. Engl. J. Med 368, 1907–1917 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang MH et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med 336, 1855–1859 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Talaat M et al. Evolution of infection control in Egypt: achievements and challenges. Am. J. Infect. Control 34, 193–200 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Strickland GT Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology 43, 915–922 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Armstrong GL et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann. Intern. Med 144, 705–714 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Southern WN et al. Hepatitis C testing practices and prevalence in a high-risk urban ambulatory care setting. J. Viral Hepat 18, 474–481 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volk ML, Tocco R, Saini S & Lok AS Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology 50, 1750–1755 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Mesquita PM et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J. Antimicrob. Chemother 67, 1730–1738 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen MS, Muessig KE, Smith MK, Powers K & Kashuba AD Antiviral agents and HIV prevention: controversies, conflicts and consensus. AIDS 26, 1585–1598 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corey L, McElrath MJ & Kublin JG Post-step modifications for research on HIV vaccines. AIDS 23, 3–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray G, Buchbinder S & Duerr A Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr. Opin. HIV AIDS 5, 357–361 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stramer SL et al. Nucleic acid testing to detect HBV infection in blood donors. N. Engl. J. Med 364, 236–247 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Rehermann B Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J. Clin. Invest 119, 1745–1754 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanford RE et al. Cross-genotype immunity to hepatitis C virus. J. Virol 78, 1575–1581 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osburn WO et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 138, 315–324 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas DL et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. J. Am. Med. Assoc 284, 450–456 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Ferrari C et al. T-cell response to structural and nonstructural hepatitis C virus antigens in persistent and self-limited hepatitis C virus infections. Hepatology 19, 286–295 (1994). [PubMed] [Google Scholar]
- 24.Missale G et al. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Invest 98, 706–714 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoukry NH et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med 197, 1645–1655 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grakoui A et al. HCV persistence and immune evasion in the absence of memory T cell help. Science 302, 659–662 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Yee LJ Host genetic determinants in hepatitis C virus infection. Genes Immun 5, 237–245 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Barth H et al. Both innate and adaptive immunity mediate protective immunity against hepatitis C virus infection in chimpanzees. Hepatology 54, 1135–1148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Lorenzo C, Angus AG & Patel AH Hepatitis C virus evasion mechanisms from neutralizing antibodies. Viruses 3, 2280–2300 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavillette D et al. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J. Virol 79, 6023–6034 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pestka JM et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc. Natl. Acad. Sci. USA 104, 6025–6030 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dowd KA, Netski DM, Wang XH, Cox AL & Ray SC Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology 136, 2377–2386 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logvinoff C et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 101, 10149–10154 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P et al. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc. Natl. Acad. Sci. USA 106, 7537–7541 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu YK et al. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J. Virol 68, 1494–1500 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner AJ et al. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc. Natl. Acad. Sci. USA 89, 3468–3472 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helle F et al. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J. Virol 81, 8101–8111 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.André P et al. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J. Virol 76, 6919–6928 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christie JM et al. Clinical outcome of hypogammaglobulinaemic patients following outbreak of acute hepatitis C: 2 year follow up. Clin. Exp. Immunol 110, 4–8 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Post JJ et al. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J. Infect. Dis 189, 1846–1855 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Cooper S et al. Analysis of a successful immune response against hepatitis C virus. Immunity 10, 439–449 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Meunier JC et al. Neutralizing antibodies to hepatitis C virus in perinatally infected children followed up prospectively. J. Infect. Dis 204, 1741–1745 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun JC, Beilke JN & Lanier LL Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge D et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461, 399–401 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Thomas DL et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461, 798–801 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka Y et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet 41, 1105–1109 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Suppiah V et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat. Genet 41, 1100–1104 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Thomas E et al. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology 142, 978–988 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey J Lessons from chimpanzee-based research on human disease: the implications of genetic differences. Altern. Lab. Anim 39, 527–540 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Heeney JL et al. Transmission of simian immunodeficiency virus SIVcpz and the evolution of infection in the presence and absence of concurrent human immunodeficiency virus type 1 infection in chimpanzees. J. Virol 80, 7208–7218 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bukh J Animal models for the study of hepatitis C virus infection and related liver disease. Gastroenterology 142, 1279–1287. e3 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Simmonds P et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 42, 962–973 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Duffy S, Shackelton LA & Holmes EC Rates of evolutionary change in viruses: patterns and determinants. Nat. Rev. Genet 9, 267–276 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Bowen DG & Walker CM Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J. Exp. Med 201, 1709–1714 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Hahn T et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology 132, 667–678 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Timm J et al. Human leukocyte antigen-associated sequence polymorphisms in hepatitis C virus reveal reproducible immune responses and constraints on viral evolution. Hepatology 46, 339–349 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Neumann-Haefelin C et al. Protective effect of human leukocyte antigen B27 in hepatitis C virus infection requires the presence of a genotype-specific immunodominant CD8+ T-cell epitope. Hepatology 51, 54–62 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim Y, Sette A & Peters B Applications for T-cell epitope queries and tools in the Immune Epitope Database and Analysis Resource. J. Immunol. Methods 374, 62–69 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vidovic D & Matzinger P Unresponsiveness to a foreign antigen can be caused by self-tolerance. Nature 336, 222–225 (1988). [DOI] [PubMed] [Google Scholar]
- 60.Wölfl M et al. Hepatitis C virus immune escape via exploitation of a hole in the T cell repertoire. J. Immunol 181, 6435–6446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haberstroh A et al. Neutralizing host responses in hepatitis C virus infection target viral entry at postbinding steps and membrane fusion. Gastroenterology 135, 1719–1728. e1 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Walker LM & Burton DR Rational antibody-based HIV-1 vaccine design: current approaches and future directions. Curr. Opin. Immunol 22, 358–366 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong L et al. Structural basis of hepatitis C virus neutralization by broadly neutralizing antibody HCV1. Proc. Natl. Acad. Sci. USA 109, 9499–9504 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schijns VE & Lavelle EC Trends in vaccine adjuvants. Expert Rev. Vaccines 10, 539–550 (2011). [DOI] [PubMed] [Google Scholar]
- 65.Rehermann B Chronic infections with hepatotropic viruses: mechanisms of impairment of cellular immune responses. Semin. Liver Dis 27, 152–160 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Raghuraman S et al. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J. Infect. Dis 205, 763–771 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Folgori A et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat. Med 12, 190–197 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Choo QL et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. USA 91, 1294–1298 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wakita T et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med 11, 791–796 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barth H, Robinet E, Liang TJ & Baumert TF Mouse models for the study of HCV infection and virus-host interactions. J. Hepatol 49, 134–142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meunier JC et al. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J. Infect. Dis 204, 1186–1190 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stamataki Z, Coates S, Abrignani S, Houghton M & McKeating JA Immunization of human volunteers with hepatitis C virus envelope glycoproteins elicits antibodies that cross-neutralize heterologous virus strains. J. Infect. Dis 204, 811–813 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gottwein JM et al. Development and characterization of hepatitis C virus genotype 1–7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology 49, 364–377 (2009). [DOI] [PubMed] [Google Scholar]
- 74.Podevin P et al. Production of infectious hepatitis C virus in primary cultures of human adult hepatocytes. Gastroenterology 139, 1355–1364 (2010). [DOI] [PubMed] [Google Scholar]
- 75.Institute of Medicine. Chimpanzees in biomedical and behavior research: assessing the necessity (National Academy Press, 2011). [PubMed] [Google Scholar]
- 76.Bukh J, Apgar CL, Govindarajan S, Emerson SU & Purcell RH Failure to infect rhesus monkeys with hepatitis C virus strains of genotypes 1a, 2a or 3a. J. Viral Hepat 8, 228–231 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Washburn ML et al. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology 140, 1334–1344 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dorner M et al. A genetically humanized mouse model for hepatitis C virus infection. Nature 474, 208–211 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilbert SC T-cell–inducing vaccines—what’s the future. Immunology 135, 19–26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Youn JW et al. Evidence for protection against chronic hepatitis C virus infection in chimpanzees by immunization with replicating recombinant vaccinia virus. J. Virol 82, 10896–10905 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rollier CS et al. Vaccine-induced early control of hepatitis C virus infection in chimpanzees fails to impact on hepatic PD-1 and chronicity. Hepatology 45, 602–613 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Haller AA et al. Whole recombinant yeast-based immunotherapy induces potent T cell responses targeting HCV NS3 and Core proteins. Vaccine 25, 1452–1463 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Habersetzer F et al. A poxvirus vaccine is safe, induces T-cell responses, and decreases viral load in patients with chronic hepatitis C. Gastroenterology 141, 890–899. e1–e4 (2011). [DOI] [PubMed] [Google Scholar]
- 84.Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K & Hill AV Viral vectors as vaccine platforms: deployment in sight. Curr. Opin. Immunol 23, 377–382 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Chiarella P, Fazio VM & Signori E Application of electroporation in DNA vaccination protocols. Curr. Gene Ther 10, 281–286 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Youn JW et al. Sustained E2 antibody response correlates with reduced peak viremia after hepatitis C virus infection in the chimpanzee. Hepatology 42, 1429–1436 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Klade CS et al. Therapeutic vaccination of chronic hepatitis C nonresponder patients with the peptide vaccine IC41. Gastroenterology 134, 1385–1395 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Walker CM Adaptive immunity to the hepatitis C virus. Adv. Virus Res 78, 43–86 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elmowalid GA et al. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc. Natl. Acad. Sci. USA 104, 8427–8432 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yewdell JW, Norbury CC & Bennink JR Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv. Immunol 73, 1–77 (1999). [DOI] [PubMed] [Google Scholar]
- 91.Jennings GT & Bachmann MF The coming of age of virus-like particle vaccines. Biol. Chem 389, 521–536 (2008). [DOI] [PubMed] [Google Scholar]
- 92.Buonaguro L, Tagliamonte M, Tornesello ML & Buonaguro FM Developments in virus-like particle-based vaccines for infectious diseases and cancer. Expert Rev. Vaccines 10, 1569–1583 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Barnaba V, Franco A, Alberti A, Benvenuto R & Balsano F Selective killing of hepatitis B envelope antigen-specific B cells by class I–restricted, exogenous antigen–specific T lymphocytes. Nature 345, 258–260 (1990). [DOI] [PubMed] [Google Scholar]
- 94.Caldeira JC et al. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine 28, 4384–4393 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kool M, Fierens K & Lambrecht BN Alum adjuvant: Some of the Tricks of the oldest adjuvant. J. Med. Microbiol 61, 927–934 (2012). [DOI] [PubMed] [Google Scholar]
- 96.De Gregorio E, D’Oro U & Wack A Immunology of TLR-independent vaccine adjuvants. Curr. Opin. Immunol 21, 339–345 (2009). [DOI] [PubMed] [Google Scholar]
- 97.Nicholls EF, Madera L & Hancock RE Immunomodulators as adjuvants for vaccines and antimicrobial therapy. Ann. NY Acad. Sci 1213, 46–61 (2010). [DOI] [PubMed] [Google Scholar]
- 98.Tovey MG & Lallemand C Adjuvant activity of cytokines. Methods Mol. Biol 626, 287–309 (2010). [DOI] [PubMed] [Google Scholar]
- 99.Dahari H, Feinstone SM & Major ME Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology 139, 965–974 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frey SE et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 28, 6367–6373 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Houghton M Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunol. Rev 239, 99–108 (2011). [DOI] [PubMed] [Google Scholar]
- 102.Barnes E et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med 4, 115ra111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Strickland GT, El-Kamary SS, Klenerman P & Nicosia A Hepatitis C vaccine: supply and demand. Lancet Infect. Dis 8, 379–386 (2008). [DOI] [PubMed] [Google Scholar]
- 104.Heller T & Rehermann B Acute hepatitis C: a multifaceted disease. Semin. Liver Dis 25, 7–17 (2005). [DOI] [PubMed] [Google Scholar]
- 105.Zou S, Dodd RY, Stramer SL & Strong DM Probability of viremia with HBV, HCV, HIV, and HTLV among tissue donors in the United States. N. Engl. J. Med 351, 751–759 (2004). [DOI] [PubMed] [Google Scholar]
- 106.Ghany MG, Strader DB, Thomas DL & Seeff LB Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 49, 1335–1374 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krahn MD et al. Potential cost-effectiveness of a preventive hepatitis C vaccine in high risk and average risk populations in Canada. Vaccine 23, 1549–1558 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Burton DR Antibodies, viruses and vaccines. Nat. Rev. Immunol 2, 706–713 (2002). [DOI] [PubMed] [Google Scholar]
- 109.Drane D et al. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: a phase I study in healthy volunteers. Hum. Vaccin 5, 151–157 (2009). [DOI] [PubMed] [Google Scholar]
- 110.Polakos NK et al. Characterization of hepatitis C virus core–specific immune responses primed in rhesus macaques by a nonclassical ISCOM vaccine. J. Immunol 166, 3589–3598 (2001). [DOI] [PubMed] [Google Scholar]
- 111.Leroux-Roels G et al. A candidate vaccine based on the hepatitis C E1 protein: tolerability and immunogenicity in healthy volunteers. Vaccine 22, 3080–3086 (2004). [DOI] [PubMed] [Google Scholar]
- 112.Forns X et al. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology 32, 618–625 (2000). [DOI] [PubMed] [Google Scholar]
- 113.Rollier C et al. Control of heterologous hepatitis C virus infection in chimpanzees is associated with the quality of vaccine-induced peripheral T-helper immune response. J. Virol 78, 187–196 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Horner SM & Gale M Jr. Nat. Med 19, aaa–bbb (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Timpe JM et al. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology 47, 17–24 (2008). [DOI] [PubMed] [Google Scholar]
- 116.Crotta S et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med 195, 35–41 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rosa D et al. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus–associated B lymphocyte disorders. Proc. Natl. Acad. Sci. USA 102, 18544–18549 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Serra A et al. Coligation of the hepatitis C virus receptor CD81 with CD28 primes naive T lymphocytes to acquire type 2 effector function. J. Immunol 181, 174–185 (2008). [DOI] [PubMed] [Google Scholar]
- 119.Nanda SK, Herion D & Liang TJ The SH3 binding motif of HCV [corrected] NS5A protein interacts with Bin1 and is important for apoptosis and infectivity. Gastroenterology 130, 794–809 (2006). [DOI] [PubMed] [Google Scholar]
- 120.Gale M Jr., Kwieciszewski B, Dossett M, Nakao H & Katze MG Antiapoptotic and oncogenic potentials of hepatitis C virus are linked to interferon resistance by viral repression of the PKR protein kinase. J. Virol 73, 6506–6516 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simonin Y et al. Calpain activation by hepatitis C virus proteins inhibits the extrinsic apoptotic signaling pathway. Hepatology 50, 1370–1379 (2009). [DOI] [PubMed] [Google Scholar]