Abstract
BACKGROUND
There are limited data on outcomes in transplant recipients with a history of pretransplant melanoma.
OBJECTIVE
To determine whether pretransplant melanoma is associated with differences in survival or posttransplant melanoma risk.
MATERIALS AND METHODS
We evaluated the outcomes of 185,039 US transplant recipients from the Transplant Cancer Match Study. We also evaluated the impact of transplantation on 141,441 patients with melanoma identified in cancer registries.
RESULTS
There were 336 transplant recipients (0.18%) with pretransplant melanoma; they had increased risk of melanoma-specific mortality (hazard ratio [HR], 27; 95% confidence interval [CI], 11–64, p < .0001), overall mortality (HR, 1.3; 95% CI, 1.0–1.5, p = .02), and incident melanoma (HR, 5.4; 95% CI, 2.9–9.8, p < .0001) after transplant, compared with recipients without pretransplant melanoma. The 10-year absolute risk difference was 2.97% for melanoma-specific mortality, 3.68% for incident melanoma, and 14.32% for overall mortality. Among the 141,441 patients with melanoma in the general population, 68 (0.05%) subsequently received a transplant. Transplantation increased melanoma-specific mortality, but not significantly (HR, 1.7; 95% CI, 0.61–4.5, p = .32).
CONCLUSION
Pretransplant melanoma is associated with increased melanoma-specific mortality, overall mortality, and incident melanoma after transplant. Nonetheless, the rarity of melanoma-related events supports the current practice for listing transplant candidates with a history of melanoma.
In 2014, more than 29,000 solid organ transplantations were performed in the United States; meanwhile, more than 134,000 candidates were waiting to receive a transplant (Organ Procurement and Transplantation Network data, March 13,2015). Solid organ transplant recipients are at increased risk for all forms of skin cancer because of the immunosuppressive medications required to protect the graft.1 The effect of transplantation is stronger for keratinocyte carcinomas (formerly referred to as nonmelanoma skin cancers) (relative risks of 65-fold for squamous cell carcinoma, and 10-fold for basal cell carcinoma) than for melanoma (relative risk of 2- to 3-fold).1,2 However, melanoma has higher mortality compared with keratinocyte carcinomas with melanoma accounting for approximately 75% of skin cancer deaths in the general population.3 Current research has focused on the risk of primary melanoma in the transplant population, and little is known about the outcomes of transplant recipients with a history of pretransplant melanoma, which is problematic in establishing guidelines for how transplant committees should consider these patients as potential candidates.
At present, there are no formal consensus guidelines for transplant committees or transplant dermatologists to follow with regard to the consideration of transplant candidates who have a history of melanoma. In the absence of guidelines, many clinicians refer to a 2005 paper by Otley and colleagues4 which presents recommendations for organ transplantation in candidates with a history of melanoma. Their recommendation is that patients with melanoma in situ are considered immediate candidates due to the theoretical disease-specific survival of 100%, whereas patients with invasive melanoma should rarely be considered for transplant without a disease-free period ranging from 3 to 10 years, depending on the stage of the tumor at diagnosis and other prognostic information also assessed for patients with melanoma in the general population. Although a waiting period may be feasible for patients with renal failure on dialysis, patients with an urgent need for liver, heart, or lung transplantation may not be able to survive a waiting period and may thus be excluded from transplantation on the basis of their melanoma diagnosis. Transplant boards make individual considerations to weigh the prognosis from melanoma against the prognosis of the patient without transplant.
The 2005 recommendations by Otley and colleagues4 were based on a review of the literature. This was an update from a 1996 recommendation for a 5-year wait time between melanoma diagnosis and transplant, based on data from the Cincinnati Transplant Tumor Registry. This was a small series of 31 patients with pretransplant melanoma and demonstrated a 19% recurrence rate after transplantation.5 Of the 6 patients who recurred, 3 had been treated for their melanoma within 2 years of transplant, 2 were treated 2 to 5 years before transplant, and 1 was treated 10 years before transplant.5 More recent literature suggests improved outcomes: in a Mayo Clinic retrospective review of 59 patients with 61 pretransplant melanomas, there were no local recurrences and only 2 patients with metastases after a median follow-up time of 10.5 years after transplantation.6 A case series of 9 pretransplant patients with melanoma with a mean follow-up of 5 years from the Skin Care in Organ Transplant Patients in Europe (SCOPE) network also demonstrated no recurrences.7 Another review describes minimal risk of recurrence in patients with thin melanomas (Breslow depth ≤2 mm) but does advise caution in interpreting results from other articles as often there is an overrepresentation of thin melanomas and a long interval between time of melanoma diagnosis and transplant.8
Not only are transplant patients at risk of having their pretransplant melanoma recur in a local, regional, or distant fashion, they are also at increased risk for a second primary incident melanoma posttransplant. Based on data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program, it is estimated that at 5 years less than 2% of melanoma survivors in the general population will develop a second melanoma.9 However, this risk of developing melanoma is 8.8-fold higher than observed in the general population with no history of melanoma.9 The risk of secondary melanoma may be higher in immunosuppressed organ transplant recipients, but no study has assessed this population.
Our study has 2 central aims. For the first aim, we examined transplant recipients to determine whether a history of pretransplant melanoma is associated with differences in survival or risk of developing a posttransplant melanoma. This information will assist in assessing transplant candidates with a history of melanoma and will contribute information to the development of consensus guidelines. For the second aim, we examined the melanoma-specific mortality of patients with melanoma who did and did not undergo transplantation. This assesses the impact that transplantation has on patients with melanoma following their diagnosis.
Materials and Methods
The Transplant Cancer Match (TCM) Study links the US Scientific Registry of Transplant Recipients (SRTR) with 15 state and regional cancer registries. The SRTR includes information on all US solid organ transplant recipients since 1987. Cancer data are available for all the recipients living in the geographic areas covered by the included cancer registries during years when the cancer registries collected data. The TCM Study thus includes cancer data on 47% of US transplants during 1987 to 2010.
Data on the cohort of transplant recipients were drawn from the SRTR database, including sex, age at transplant, race/ethnicity, transplanted organ, dates of transplant and of death, and cause of death. Race/ethnicity was dichotomized into non-Hispanic white versus other. Transplanted organ was dichotomized into abdominal (i.e., pancreas, kidney, liver) and thoracic organs (i.e., heart, lung) as a proxy for transplantation requiring relatively low versus high levels of immunosuppression.
Linked data from the cancer registries in the TCM Study were used to identify pretransplant melanomas and to capture incident melanoma after transplant. Characterization of these melanomas, based on information provided in the cancer registries, included behavior (in situ vs invasive) and SEER summary stage, which is defined as: in situ melanoma, localized melanoma, regional metastasis, distant metastasis, and unknown.10 Because most cases with invasive behavior were localized stage, and more than 10% of the subjects had unknown stage, we used the melanoma behavior variable (in situ vs invasive) rather than the stage variable in the Cox regression models (see Statistical Analysis for Aim 2, below). For pretransplant melanomas, the time from melanoma diagnosis to organ transplantation was dichotomized into less than 5 years and 5 or more years.
Cause of death was compiled from SRTR and cancer registry variables. Cause of death variables were screened using both numerical codes and text entries to identify melanoma-specific deaths. To address possible under-reporting of melanoma-specific death in the SRTR data set, we performed a search to identify additional skin cancer deaths, which we included in a sensitivity analysis.
Descriptive comparisons were performed with the Fisher exact test or Wilcoxon rank-sum test.
Aim 1
Impact of pretransplant melanoma on posttransplant survival and incidence of additional primary melanomas.
Subjects
For analyses of the impact of pretransplant melanoma on posttransplant survival and incidence of additional primary melanomas, subjects were drawn from the entire TCM cohort. Analysis was restricted to first transplant recipients (N = 224,153). We required cancer registry data to be available for 1 year before a subject’s date of transplant to ascertain any previous melanoma, which resulted in exclusion of 9,074 subjects. Another 15,020 subjects were excluded because they had no posttransplant follow-up. Finally 15,020 children less than 18 years were excluded, as they represent a clinically distinct population, and none had a pretransplant melanoma documented. The remaining 185,039 adult transplant recipients were included in our cohort (Figure 1A). We included all recipients with pretransplant melanomas, including those with in situ and metastatic disease.
Figure 1.
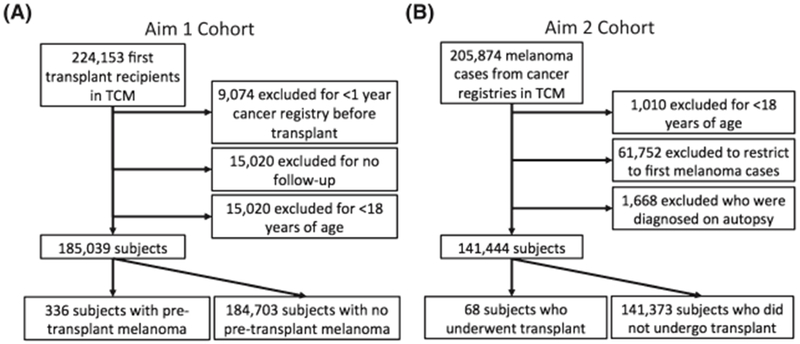
Study design. Study design for Aim 1: Impact of pretransplant melanoma on posttransplant survival and incidence of additional primary melanomas (A). Study design for Aim 2: Impact of organ transplantation on melanoma-specific mortality in patients with a history of melanoma before transplant (B).
Statistical Analysis
We used Cox regression models to analyze risk for all-cause mortality, melanoma-specific mortality, and incident posttransplant melanoma among recipients of a first transplant. We followed subjects from their first transplant until the earliest of death, loss to follow-up by SRTR, or end of cancer registry coverage. The primary predictor in the models was pretransplant melanoma, and the models were adjusted for sex, age at transplant, race/ethnicity, year of transplant, and organ transplanted. For the melanoma outcome analyses, we ended the follow-up at melanoma diagnosis. The proportional hazards assumption was tested and confirmed with the Schoenfeld test.
Competing risks regression methods were used for the outcome of melanoma-specific death and incident melanoma, taking other deaths as a competing risk. Cumulative incidence measures were derived from the hazard function, standardizing estimates for recipients with or without a pretransplant melanoma so that they were representative of the population with pretransplant melanoma.11 We also display a Kaplan–Meier plot to graphically depict the unadjusted all-cause mortality in recipients with and without a pretransplant melanoma.
Sensitivity analyses were performed for the melanoma outcome analyses in which subjects with regional, distant, or unknown stage of melanoma were excluded. As melanoma deaths may be misclassified as deaths from other types of skin cancer, we also performed a sensitivity analysis in which associations were estimated with death from any skin cancer.
Aim 2
Impact of organ transplantation on melanoma-specific mortality in patients with a history of melanoma before transplant.
Subjects
For analysis of the impact of organ transplantation on melanoma-specific mortality in patients with a history of melanoma before transplant, we selected patients with melanoma from 6 cancer registries in the TCM Study that provided data on postcancer mortality (Colorado, Connecticut, Iowa, Michigan, New Jersey, Texas). We required that these general population patients with melanoma had not previously had an organ transplant, although some of these patients later received a transplant. Starting with 205,874 melanoma cases diagnosed in or after 1987, we excluded 1,010 cases among children less than 18 years of age. Restricting to first melanoma cases left 143,112 patients with melanoma diagnosed in 1987 to 2010. Of these, we excluded 1,668 who were diagnosed at the time of death. This resulted in 141,441 subjects included in this analysis (Figure 1B).
Statistical Analysis
We used Cox regression to model the outcome of melanoma-specific mortality. We followed patients from their first melanoma diagnosis until death or end of cancer registry outcome ascertainment. The primary predictor was organ transplantation, which we treated as a time-dependent covariate. The model was adjusted for sex, age at melanoma diagnosis, non-Hispanic white versus other race/ethnicity, and melanoma behavior (in situ vs invasive).
Results
Aim 1
Impact of pretransplant melanoma on posttransplant survival and incidence of additional primary melanomas.
We identified 185,039 solid organ transplant recipients older than 18 years from the TCM Study (Table 1). Men and non-Hispanic whites accounted for 62% and 61%, respectively, of the population. The mean age at transplant for the entire cohort was 49 years (range, 18–89 years).
TABLE 1.
Demographic Characteristics of US Transplant Recipients
| N = 185,039* | |
|---|---|
| Female | 70,284 (37.98) |
| Male | 114,755 (62.02) |
| Age at transplant (yrs) | 49 ± 13 (18–89) |
| Non-Hispanic white | 112,590 (60.85) |
| Other | 72,449 (39.15) |
| Year of transplant | 2001 ± 5 (1987–2010) |
| Abdominal organ | 158,173 (85.48) |
| Thoracic organ | 26,866 (14.52) |
| No history of pretransplant melanoma | 184,703 (99.82) |
| History of pretransplant melanoma | 336 (0.18) |
Data are expressed as n (%) or mean ± SD and range.
A total of 336 subjects (0.18%) had a pretransplant melanoma diagnosis (Table 2). Of these, 323 had only 1 pretransplant melanoma, whereas 12 had a second pretransplant melanoma and 1 had a third pretransplant melanoma. We did not consider the second and third melanomas further in our analysis, and recipients were categorized according to the characteristics of their first pretransplant melanoma. There were 112 (33.3%) in situ and 224 (66.7%) invasive pretransplant melanomas. SEER summary stage information was available for 296 of the 336 melanomas. Of these, 5 patients had regional metastases and 2 had distant metastases. There was a correlation between invasive melanoma and a longer time between melanoma diagnosis and transplant, with a median time of 5.2 years for invasive melanoma (interquartile range [IQR], 2.7–9.5) compared with 3.8 years for in situ melanoma (IQR, 1.7–6.4).
TABLE 2.
Characteristics of Pretransplant Melanomas
| N = 336 | |
|---|---|
| SEER behavior | |
| In situ | 112 (33.33) |
| Invasive | 224 (66.67) |
| SEER summary stage | |
| In situ melanoma | 112 (33.33)* |
| Localized melanoma | 177 (52.68) |
| Regional metastasis | 5 (1.49) |
| Distant metastasis | 2 (0.60) |
| Unknown | 40 (11.90) |
| Histology | |
| Melanoma NOS | 201 (59.82) |
| Superficial spreading melanoma | 75 (22.32) |
| Lentigo maligna melanoma | 43 (12.80) |
| Other specified | 17 (5.06) |
Data are expressed as n (%).
This category includes 9 cases listed as “unknown” in the SEER summary stage but with behavior listed as in situ.
A total of 58,015 subjects (31.4%) died during follow-up (Table 3). Of these, 85 (0.05%) died from melanoma, and 143 (0.08%) died from any type of skin cancer. A total of 798 patients (0.43%) developed an incident melanoma posttransplant.
TABLE 3.
Associations of Pretransplant Melanoma With Outcomes After Organ Transplantation
| Number of Events (%) | Person-years | Incidence Rate per 100,000 Person-years | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| No pretransplant melanoma (n = 184,703) | 57,908 (31) | 1,156,958 | 5,005 | 1.0 | 1.0 |
| Pretransplant melanoma (n = 336) | 107 (32) | 1,351 | 7,920 | 1.6 (1.3–1.9) | 1.3 (1.0–1.5) |
| Melanoma-specific mortality | |||||
| No pretransplant melanoma (n = 184,703) | 79 (0.04) | 1,156,958 | 6.8 | 1.0 | 1.0 |
| Pretransplant melanoma (n = 336) | 6 (1.8) | 1,351 | 444 | 59 (26–136) | 27 (11–64) |
| Incident posttransplant melanoma | |||||
| No pretransplant melanoma (n = 184,703) | 787 (0.4) | 1,153,994 | 68 | 1.0 | 1.0 |
| Pretransplant melanoma (n = 336) | 11 (3.3) | 1,330 | 827 | 10 (5.6–19) | 5.4 (2.9–9.8) |
Melanoma-specific death and incident melanoma models incorporate other death as a competing risk. Models are adjusted for sex, age at transplantation, non-Hispanic white versus other race/ethnicity, thoracic versus abdominal transplant, and year of transplantation.
As shown in Table 3, transplant recipients with pretransplant melanoma had a 27-fold increased risk of death due to melanoma (adjusted hazard ratio [HR], 27; 95% confidence interval [CI], 11–64; p < .0001). Of note, 2 of the 6 subjects with pretransplant melanoma who died due to melanoma had developed another posttransplant incident melanoma. Transplant recipients with pretransplant melanoma had a 30% increase in overall mortality (adjusted HR, 1.3; 95% CI, 1.0–1.5; p = .02) (Figure 2) and had a 5.4-fold increased risk of incident melanoma after transplant (adjusted HR, 5.4; 95% CI, 2.9–9.8; p < .0001). A sensitivity analysis in which recipients with regional, distant, or unknown stage melanoma before transplant were excluded showed that strong associations with localized melanoma were still present for overall mortality (adjusted HR, 1.2; 95% CI, 1.0–1.5, p = .05), melanoma-specific mortality (adjusted HR, 11; 95% CI, 2.6–46; p = .001), and incident melanoma after transplant (adjusted HR, 6.2; 95% CI, 3.3–12; p < .001).
Figure 2.
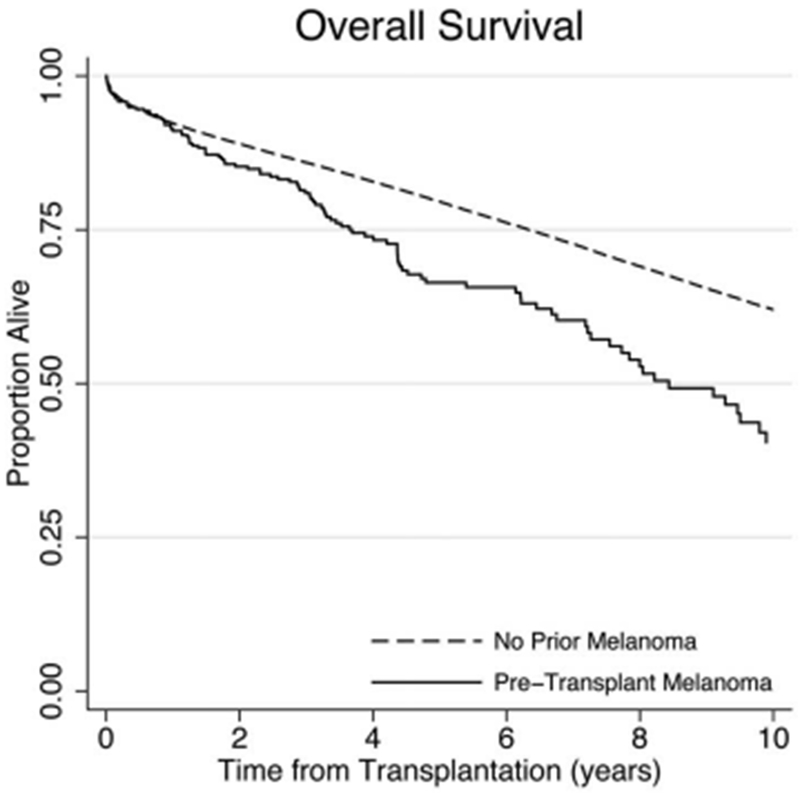
Overall survival for transplant recipients. Kaplan–Meier plot showing overall survival for Aim 1 cohort, transplant recipients with and without pretransplant melanoma.
To address potential underreporting of melanoma deaths, we performed a sensitivity analysis using any skin cancer death as an outcome. Fifty-eight additional deaths were captured with this method, including 1 in a patient with pretransplant melanoma. The association between pretransplant melanoma and skin cancer death (HR, 19.5; 95% CI, 8.78–43.1; p < .0001) was similar to our result for melanoma-specific death.
Despite these elevated relative risks, melanoma-related events were rare after transplantation (Table 4). The 5 and 10-year cumulative incidence of melanoma-specific death was 1.22% and 3.09% in subjects with pretransplant melanoma compared with 0.05% and 0.12% in subjects without pretransplant melanoma, an absolute risk difference of 1.17% and 2.97%. Similarly, the 5- and 10-year cumulative incidence of incident posttransplant primary melanoma was 2.51% and 4.54% in subjects with pretransplant melanoma compared with 0.47% and 0.86% in subjects without pretransplant melanoma, an absolute risk difference of 2.04% and 3.68%.
TABLE 4.
Cumulative Incidence of Melanoma Outcomes After Organ Transplantation
| 5 yrs (%) | 10 yrs (%) | |
|---|---|---|
| All-cause mortality | ||
| No pretransplant melanoma (n = 184,703) | 27.38 | 56.58 |
| Pretransplant melanoma (n = 336) | 34.32 | 70.90 |
| Absolute difference | 6.94 | 14.32 |
| Melanoma-specific mortality | ||
| No pretransplant melanoma (n = 184,703) | 0.05 | 0.12 |
| Pretransplant melanoma (n = 336) | 1.22 | 3.09 |
| Absolute difference | 1.17 | 2.97 |
| Incident posttransplant melanoma | ||
| No pretransplant melanoma (n = 184,703) | 0.47 | 0.86 |
| Pretransplant melanoma (n = 336) | 2.51 | 4.54 |
| Absolute difference | 2.04 | 3.68 |
Results are derived from adjusted survival models.
In contrast, the 5 and 10-year cumulative all-cause mortality was 34.32% and 70.90% in subjects with pretransplant melanoma, compared with 27.38% and 56.58% in subjects without pretransplant melanoma, an absolute risk difference of 6.94% and 14.32%.
Aim 2
Impact of organ transplantation on melanoma-specific mortality in patients with a history of melanoma before transplant.
To investigate the impact of organ transplantation on melanoma-specific death after a diagnosis of melanoma, we identified 141,441 adults older than 18 years with an invasive melanoma after 1987 from the TCM Study (Table 5). Of these, 68 subjects (0.05%) received an organ transplant during the follow-up period.
TABLE 5.
Association of Transplantation with Melanoma-specific Mortality Following an Invasive Melanoma Diagnosis
| Melanoma Cases | Melanoma-specific Deaths, n (%) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|---|
| No transplant | 141,373 | 15,500 (11.0) | 1.0 | 1.0 |
| Transplant | 68 | 4 (5.9) | 1.7 (0.6–4.5) | 1.7 (0.6–4.5) |
Hazard ratios are estimated from multivariate Cox regression. Models are adjusted for sex, age at melanoma diagnosis, non-Hispanic white versus other race/ethnicity.
A total of 15,504 subjects (11.0%) died of melanoma during the follow-up period. Organ transplantation was associated with a 1.7-fold increased risk of melanoma death, but this effect did not achieve statistical significance (adjusted HR, 1.7; 95% CI, 0.6–4.5; p = .32) (Table 5).
Discussion
This study aimed to investigate outcomes of transplant recipients with a history of pretransplant melanoma. Our data demonstrate that these individuals have increased all-cause mortality, melanoma-specific mortality, and incidence of new melanoma diagnoses after transplantation.
The strongest effect size observed was the 27-fold increase in melanoma-specific death in transplant recipients with a history of pretransplant melanoma. This might initially sound alarming to transplant listing committees, but it is important to take the absolute risk into consideration to provide clinical relevance. Because melanoma death after transplantation is rare, this large relative risk translates to a very small absolute difference in melanoma-specific mortality between those with and without pretransplant melanoma, that is, 1.17% and 2.97% at 5 and 10 years, respectively. As these tumors were staged using SEER methods, we were limited by the inability to risk stratify localized tumors by the features dermatologists use for risk stratification, such as American Joint Committee on Cancer (AJCC) T stage.
It is logical to expect increased melanoma-specific mortality in a population with pretransplant melanoma compared with the overall transplant population, as the control cohort would only be at risk for this outcome after developing a posttransplant incident melanoma. Therefore, we also investigated the impact of organ transplantation on melanoma-specific death among patients with melanoma (Aim 2). We did this to determine if transplant affects risk of poor outcome, which would point to immunity as an important factor in controlling any subclinically remaining tumor. The relationship of immunity and melanoma is of great interest, particularly given the new immunotherapies for melanoma including anti-CTLA4, anti-PD1, and anti-PDL1 antibodies.12 Transplantation increased the risk of death from melanoma, although this finding did not achieve statistical significance in the small sample size (adjusted HR, 1.7; 95% CI, 0.61–4.5, p = .32).
New melanomas are also rare events after transplantation, so that the 5.4-fold increased risk in patients with pretransplant melanoma translates to only a 2.04% and 3.68% absolute risk difference at 5 and 10 years. Given the increased risk of an incident melanoma, it is important to establish recommendations for appropriate monitoring and patient follow-up. Several of the risk factors for multiple primary melanomas are also associated with increased risk of keratinocyte carcinomas, which are also commonly seen in the transplant population. This highlights the need for close follow-up monitoring and the involvement of a transplant dermatologist in the care of transplant patients. Indeed, 2 of the 6 melanoma deaths associated with a pretransplant melanoma were in people who developed an incident melanoma following transplant, and if those melanomas could have been prevented or detected earlier, then the deaths might have been prevented. Current practice guidelines for following immunocompetent patients after a melanoma diagnosis recommend evaluation by a dermatologist every 3 months during the first 3 years, and every 6 to 12 months thereafter.13 These guidelines should be applied to transplant recipients with a history of melanoma.
All-cause mortality was also significantly increased in subjects with pretransplant melanoma (adjusted HR, 1.3). As death is not a rare event after transplantation, this small increase in risk translates to a 6.94% and 14.32% absolute risk difference at 5 and 10 years, respectively. We propose 3 possible explanations for this elevated mortality.
First, it is possible that we underestimated melanoma-specific deaths, and that the increase in all-cause mortality is entirely attributed to such deaths. SRTR data rely on transplant center reporting of cause of death, and this information may be missing or inaccurate. To address this possibility, we performed a sensitivity analysis with an outcome of death from any skin cancer, and the result was similar to our main analysis. Furthermore, the additional skin cancer deaths that we identified were still too few to account for the increased overall mortality in recipients with a pretransplant melanoma.
Second, subjects with pretransplant melanoma may die from other cancers. Melanoma is genetically linked to breast, ovarian, and pancreatic cancers.14 However, population-based evaluation of patients with melanoma has revealed only modest increased risk of developing a few malignancies (e.g., breast, prostate, and thyroid cancers, and non-Hodgkin lymphoma),9 arguing that the excess overall mortality seen in the transplant population is probably not due to the occurrence of nonmelanoma cancers.
Third, it may be that subjects with pretransplant melanoma were transplanted despite their history of melanoma due to greater urgency in their underlying end-stage organ disease, which might then translate into higher posttransplant mortality. Unfortunately, the acuity for transplant is not measurable with the current data. The increased risk for all-cause mortality in subjects with pretransplant melanoma deserves further study, ideally in a cohort with detailed data on comorbidities and other transplant-related factors that may be confounding our results.
This study is the largest investigation of pretransplant melanoma to date and is based on population-based data from transplant and cancer registries. Nonetheless, these findings are limited by the small number of subjects with pretransplant melanoma and the number of outcomes in this group. We note that there was a trend toward increased melanoma-specific and all-cause mortality in subjects with a history of invasive versus in situ melanoma, but because of the small numbers, the results were too imprecise to be reliable (data not shown).
A second limitation of the available data is that most cases recorded by the cancer registries did not have information on AJCC tumor stage or Breslow depth. For example, of the 177 pretransplant melanomas that were localized by SEER staging in the TCM database, 123 (69.5%) had missing Breslow depth, 51 (28.8%) were thin (<2 mm) Breslow depth, and only 3 (1.7%) were thick (≥2 mm). Thus, we were unable to include these variables in our analysis. Future studies may incorporate these and additional tumor characteristics such as ulceration and mitotic index.
Another limitation is that there are no specific markers for level of immunosuppression. To partially address this limitation, we adjusted analyses for transplanted organ, as thoracic recipients require higher levels of immunosuppression than abdominal recipients. Furthermore, preferred immunosuppressant medications have changed over time. We accounted for this era effect by adjusting for the year of transplantation as a proxy for practice pattern changes in immunosuppression.
The issue of transplant candidacy and organ allocation depends on multiple factors. A history of cancer in a transplant candidate raises the concern for poor patient outcomes and reduced graft survival in settings of limited organ availability for allocation. Our data reflect the current transplant practices in the absence of formal consensus guidelines. We observed that patients with invasive melanoma were transplanted at a median time of 5.2 years after diagnosis, whereas patients with melanoma in situ were transplanted at a median time of 3.8 years. These “real-world” data reflect the Otley and colleagues4 recommendation for immediate consultation with a transplant dermatologist for patients with localized melanoma and a waiting period of 3 to 10 years before transplantation for candidates with localized melanoma in whom the transplant is initially denied, with a longer wait time with increased stage. We do not know whether the waiting periods we observed were imposed by the transplant center or merely reflect the time elapsed between melanoma diagnosis and the organ failure leading to transplantation.
Although precise tumor-stratified quantification of the increased risks for overall mortality, melanoma-specific mortality, and incident melanoma was not possible because of limitations in the available data, our study suggests that current practices have resulted in acceptable outcomes. The absolute incidence of posttransplant melanoma and melanoma-specific death were small, but significantly increased, supporting a role for close dermatologic screening and follow-up after transplantation. Because our observation of an elevated overall mortality for transplant recipients with a history of melanoma is unexplained, listing committees should use a candidate’s history of melanoma as an indication that other factors may be present that might predispose the patient to a poor transplant outcome, and incorporate this information into case-by-case decisions.
These data will inform the development of consensus guidelines. Future studies in this area may benefit from more detailed information on tumor data, such as molecular markers and AJCC tumor stage, as well as additional detailed follow-up to ascertain the clinical course after transplantation.
Acknowledgments
The authors acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California, Colorado (Jack Finch), Connecticut (Lou Gonsalves), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa (Charles Lynch), Illinois (Lori Koch), Michigan (Glenn Copeland), New Jersey, New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Melanie Williams), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). The authors also thank analysts at Information Management Services for programming support (Matthew Chaloux, Michael Curry, Ruth Parsons) and Ryutaro Hirose for critical feedback on this manuscript. Supported in part by the Intramural Research Program of the National Cancer Institute. During the initial period when registry linkages were performed, the SRTR was managed by Arbor Research Collaborative for Health in Ann Arbor, MI (contract HHSH234200537009C); beginning in September 2010, the SRTR was managed by Minneapolis Medical Research Foundation in Minneapolis, MN (HHSH250201000018C). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5658DP000805-04), Michigan (5U58DP000812-03), New Jersey (5U58/DP003931-02), New York (U58DP003879), North Carolina (U58DP000832), and Texas (5U58DP000824-04). The following cancer registries were supported by the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201300021I, N01-PC-2013-00021), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN261201000026C). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, New Jersey, New York (Cancer Surveillance Improvement Initiative 14-2491), Texas, and Washington, as well as the Fred Hutchinson Cancer Research Center in Seattle, WA. A. K. Raymond is funded by the American Society for Dermatologic Surgery Cutting Edge Research Grant Program.
S. T. Arron and A. K. Raymond contributed equally to this work. The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors.
Footnotes
The authors have indicated no significant interest with commercial supporters.
References
- 1.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. Epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol 2011; 65:253–61; quiz 262. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011; 306:1891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts and Figures 2014. Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/index. Accessed November 2, 2014.
- 4.Otley CC, Hirose R, Salasche SJ. Skin cancer as a contraindication to organ transplantation. Am J Transplant 2005;5:2079–84. [DOI] [PubMed] [Google Scholar]
- 5.Penn I Malignant melanoma in organ allograft recipients. Transplantation 1996;61:274–8. [DOI] [PubMed] [Google Scholar]
- 6.Brewer JD, Christenson LJ, Weaver AL, Dapprich DC, et al. Malignant melanoma in solid transplant recipients: collection of database cases and comparison with surveillance, epidemiology, and end results data for outcome analysis. Arch Dermatol 2011;147:790–6. [DOI] [PubMed] [Google Scholar]
- 7.Matin RN, Mesher D, Proby CM, McGregor JM, et al. Melanoma in organ transplant recipients: clinicopathological features and outcome in 100 cases. Am J Transplant 2008;8:1891–900. [DOI] [PubMed] [Google Scholar]
- 8.Russo I, Piaserico S, Belloni-Fortina A, Alaibac M. Cutaneous melanoma in solid organ transplant patients. G Ital Dermatol Venereol 2014;149:389–94. [PubMed] [Google Scholar]
- 9.Freedman D, Miller B, Tucker M. Chapter 13 new malignancies following melanoma of the skin, Eye melanoma, and non-melanoma Eye cancer In: Curtis R, Freedman D, Ron E, Ries L, Hacker D, Edwards B, et al. editors. New malignancies among Cancer Survivors: SEER Cancer registries, 1973-2000 National Cancer Institute; Bethesda, MD: NIH Publ. No. 05-5302; 2006. [Google Scholar]
- 10.Young JJ, Roffers S, Ries L, Fritz A, Hurlbut A, editors. SEER summary staging Manual-2000: codes and coding Instructions NIH Pub. No. 01-4969. Bethesda, MD: National Cancer Institute; 2001. [Google Scholar]
- 11.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 12.Sanlorenzo M, Vujic I, Posch C, Dajee A, et al. Melanoma immunotherapy. Cancer Biol Ther 2014;15:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dummer R, Hauschild A, Guggenheim M, Keilholz U, Pentheroudakis G, ESMO Guidelines Working Group. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23(Suppl 7): vii86–91. [DOI] [PubMed] [Google Scholar]
- 14.Leachman SA, Carucci J, Kohlmann W, Banks KC, et al. Selection criteria for genetic assessment of patients with familial melanoma. J Am Acad Dermatol 2009;61:677.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


