Abstract
The pulmonary endothelial cell forms a critical semi-permeable barrier between the vascular and interstitial space. As part of the blood-gas barrier in the lung, the endothelium plays a key role in normal physiologic function and pathologic disease. Changes in endothelial cell shape, defined by its plasma membrane, determine barrier integrity. A number of key cytoskeletal regulatory and effector proteins including non-muscle myosin light chain kinase, cortactin, and Arp 2/3 mediate actin rearrangements to form cortical and membrane associated structures in response to barrier enhancing stimuli. These actin formations support and interact with junctional complexes and exert forces to protrude the lipid membrane to and close gaps between individual cells. The current knowledge of these cytoskeletal processes and regulatory proteins are the subject of this review. In addition, we explore novel advancements in cellular imaging that are poised to shed light on the complex nature of pulmonary endothelial permeability.
Keywords: Endothelial permeability, Lung injury, ARDS, Lamellipodia, Cortical actin, Cortactin, Arp 2/3, Non-muscle myosin light chain kinase, Super-resolution microscopy, Atomic force microscopy, Intravital microscopy
THE PULMONARY ENDOTHELIAL CELL MEMBRANE AND PHYSIOLOGY
The pulmonary blood-gas barrier is unique among all other microvascular beds in vertebrate animals including humans (West 1985, West 2013). Efficient gas exchange by simple diffusion demands exceptional thinness and vast surface area while maintaining separation of the vascular and airspace compartments. The architectural components of this barrier are remarkably conserved through evolution with a three layer design strategy composed of an epithelium and endothelium separated by extracellular matrix (Maina and West 2005). Despite a thickness of only ~0.3 μm (Low 1953), the blood-gas barrier is very strong and resistant to the increases in pulmonary capillary pressure necessary to accommodate increased cardiac output during strenuous exercise (West and Mathieu-Costello 1992). Eventually, disruptions in the pulmonary capillaries due to biomechanical forces were discovered during extreme physiologic conditions in normal lungs and as manifestations of cardiopulmonary diseases (West 1985). Lung and systemic vascular inflammation also disrupt the pulmonary blood-gas barrier with dysfunction in both the epithelial and endothelial cell layers contributing to a loss of functional integrity (Bhattacharya and Matthay 2013). A clinical consequence of these pathologic events is the Acute Respiratory Distress Syndrome (ARDS), when flooding of the alveolar airspaces with protein-rich edema fluid impairs gas exchange and causes life-threatening hypoxemia and end-organ failure (Fanelli and Ranieri 2015). Decades of research into the mechanisms underlying ARDS development have improved our understanding of this devastating syndrome. However, it remains a common and highly morbid condition in the intensive care unit (Bellani, Laffey et al. 2016).
The pulmonary endothelial cell (EC) forms a critical structural and biologically active component of the separation between vascular and alveolar spaces (Suresh and Shimoda 2016). Forming the vascular lumen itself, the endothelium is the first to experience and react to biomechanical forces in the form of pressure and shear stress (Fisher, Chien et al. 2001, Chatterjee, Nieman et al. 2014). During infection and systemic inflammation, the pulmonary EC is directly exposed to pathogens, endogenous signaling molecules and leukocytes present in the circulation (Goldenberg and Kuebler 2015). The EC also transduces mechanical forces from respiration or mechanical ventilation through connections to the basement membrane and extracellular matrix at focal adhesions (Infusino and Jacobson 2012, Zebda, Dubrovskyi et al. 2012). Finally, a functional pulmonary endothelial cell is essential to prevent the leakage of fluid and protein into the interstitial and alveolar spaces (Dudek and Garcia 2001). The cell membrane of the pulmonary endothelium is a nexus for these critical processes. It senses physical changes in the vascular lumen (Chatterjee and Fisher 2014, Tarbell, Simon et al. 2014, Chatterjee, Fujiwara et al. 2015), houses a multitude of receptors (Cioffi, Lowe et al. 2009, Mohan Rao, Esmon et al. 2014, Xiao, Liu et al. 2014), forms structures critical to cell signaling (Minshall, Sessa et al. 2003, Mineo and Shaul 2006, McVey, Tabuchi et al. 2012), and serves as substrate to generate important lipid signaling molecules (Anjum, Joshi et al. 2012, Zhang, Mao et al. 2012, Abbasi and Garcia 2013) (Figure 1A and B). Research over the last several years provides strong evidence that EC membranes are the structural basis and physical explanation for vascular barrier integrity (Lee, Ozaki et al. 2006, Ochoa and Stevens 2012, Breslin, Zhang et al. 2015, Choi, Camp et al. 2015, Belvitch, Brown et al. 2017); however, the endothelium cannot form an impenetrable wall. Tissues require the passage of oxygen, electrolytes, and nutrients, delivered by the circulation in exchange for carbon dioxide and other metabolic byproducts. The endothelium must carefully balance the opposing characteristics of permeability and barrier function. Importantly, endothelial barrier function is not constant throughout the pulmonary vasculature with the microvascular EC in capillaries demonstrating significantly reduced hydraulic conductance (Parker, Stevens et al. 2006) and increased transendothelial electrical resistance (TER) (Mehta and Malik 2006) compared to EC from conduit vessels. Fluid and solute can traverse the EC monolayer via the transcellular or paracellular pathway (Komarova and Malik 2010). Endothelial transcytosis plays an important role in the obligatory exchange of molecules between the interstitial and vascular spaces but may also contribute to pathologic disease (Predescu, Predescu et al. 2007). The paracellular route with associated loss of junctional integrity is considered the most critical during pathologic vascular leak observed during ARDS (Dudek and Garcia 2001, Stevens, Rosenberg et al. 2001, Ochoa and Stevens 2012, Kottke and Walters 2016). The cytoskeletal regulation of these essential changes in cell shape and membrane dynamics are the focus of this review.
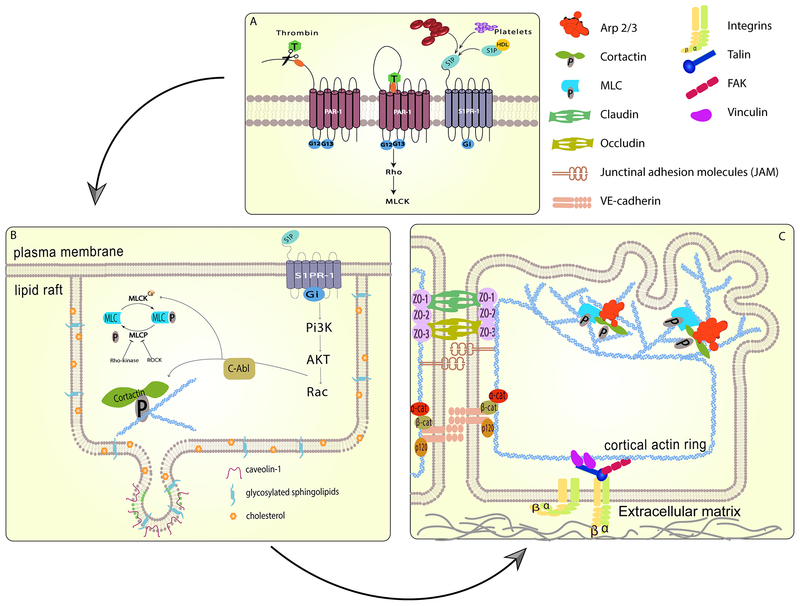
Figure 1. Barrier regulation by cytoskeletal and membrane structures.
Pulmonary endothelial barrier integrity is the result of coordinated cell processes involving receptors, signaling molecules, junctional complexes and protein-regulated cytoskeletal rearrangements leading to changes in membrane dynamics. (A) Activation of specific transmembrane receptors are critical to initiate barrier disruptive (PAR-1) or protective (S1PR1) signaling events. (B) Ligation of the S1PR1 receptor recruits signaling molecules and cytoskeletal effector proteins to lipid rafts. Phosphorylation of cortactin and myosin light chain kinase results in rapid cytoskeletal changes. (C) The formation of a cortical actin ring provides structural stability and anchoring for multiple membrane-bound junctional complexes which become activated, strengthening connections to neighboring cells and the extra cellular matrix. Branched actin polymerization, regulated by Arp 2/3 and cortactin, generates protrusive force on the plasma membrane, forming sheet-like projections or lamellipodia which close gaps between individual cells and further enhance membrane junctions.
ACTIN STRUCTURE AND FORCE GENERATION
The cytoskeleton consists of microtubules, intermediate filaments, and actin filaments and provides structure and strength to the EC membrane (Dudek and Garcia 2001, Ingber 2002, Revenu, Athman et al. 2004). Actin is a multi-functional protein found ubiquitously in eukaryotic cells. Vertebrates express three main isoforms: alpha-actin in skeletal, cardiac and smooth muscle cells, and beta- and gamma-actin in non-muscle cells (Dominguez and Holmes 2011). In particular, actin composes approximately 10% of total cellular protein in endothelial cells (Patterson and Lum 2001).
In physiological buffer conditions, monomeric globular G-actin binds ATP and can polymerize spontaneously in an alternating helical fashion to form double-stranded filaments or F-actin in the presence of stabilizing cations, in vitro with calcium and in vivo with magnesium (Stossel, Chaponnier et al. 1985, Prasain and Stevens 2009, Pollard 2016). Polymerization begins tenuously and slowly with the relatively unstable nucleation of dimers and trimers and the addition of a fourth subunit stabilizes the oligomer, upon which elongation commences (Stossel, Chaponnier et al. 1985, Pollard 2016). Elongation progresses at both ends of the nascent filament, albeit at faster rates on the barbed end than the pointed end (for kinetic details, please see Pollard, 2016). ATP hydrolysis occurs randomly on filamentous Mg-ATP-actin subunits and the intermediate Mg-ADP-Pi-actin exists for approximately six minutes (Pollard 2016). In this state, polymerization still progresses as nearly as rapidly as with Mg-ATP-actin. After release of the hydrolyzed phosphate, ADP-actin subunits dissociate from both ends of the filament (Lee and Gotlieb 2002, Lambrechts, Van Troys et al. 2004, Pollard 2016). These kinetics leave filamentous actin (F-actin) in a constant state of polymerization and depolymerization called treadmilling, a process that generates motility at the leading edge of the EC. Numerous, attendant actin-binding proteins associate with both monomers and actin filaments (Pollard 2016) and functionally regulate nucleation, polymerization, as well as filament capping, severing, and crosslinking (Stossel, Chaponnier et al. 1985, Pollard, Blanchoin et al. 2000, Pollard 2016). The delicate control of actin polymerization and its resulting structural formations build EC membrane architecture and barrier integrity (Prasain and Stevens 2009).
The three structural determinants of EC barrier integrity include (1) actin stress fibers, (2) the submembranous cytoskeleton, and (3) the cortical actin ring. Transcellular stress fibers and the membrane cytoskeleton, a 100-nm layer adjacent to the plasma membrane, are composed of short F-actin filaments (Brenner and Korn 1979, Brenner and Korn 1980, De Matteis and Morrow 2000, Rottner and Kage 2017), while, in contrast, the cortical actin ring consists of longer filaments bundled into thick cables (Heimann, Percival et al. 1999, De Matteis and Morrow 2000). The membrane cytoskeleton and cortical ring are distinct but cross-talk extensively via formation of large macromolecular complexes (Weed and Parsons 2001, Muller, Portwich et al. 2005, Brown, Adyshev et al. 2010, Wang, Bleher et al. 2015). This peripheral actin network plays important roles in cell-cell adhesion and cell-matrix tethering to construct membrane shape, cortex architecture, and ultimately EC barrier function (Dudek and Garcia 2001, Comerford, Lawrence et al. 2002, Muller, Portwich et al. 2005, Oldenburg and de Rooij 2014).
While the actin cytoskeleton generates piconewton forces at the barbed end of actin filaments from the free energy released by subunit association alone (Kovar and Pollard 2004), the chief mechanism for cytoskeletal force generation is the association of actin with myosin. The ATP-dependent ratcheting of myosin heads against actin filaments produces the “powerstroke” necessary to generate contractile tension. Activation of myosin requires diphosphorylation of Thr18 and Ser19 on its regulatory myosin light chain by non-muscle myosin light chain kinase (nmMLCK), a Ca2+/calmodulin-dependent kinase (Dudek and Garcia 2001, Shen, Rigor et al. 2010). Classically, the development of transcellular tension by stress fiber formation and contraction results in cell rounding and barrier disruption (Garcia, Verin et al. 1996, Bogatcheva, Garcia et al. 2002), but a growing body of literature purports that these same tensile forces, combined with differential membrane anchoring and precise directional control, also create membrane extensions, protrusions, and other complex compartmentalized structures (Carvalho, Tsai et al. 2013, Kapus and Janmey 2013, Rao and Mayor 2014, Arumugam and Bassereau 2015).
ENDOTHELIAL CELL MEMBRANE MORPHOLOGY AND BARRIER FUNCTION
In the 1960s, Manjo and Palade performed electron microscopy of rat vasculature after exposure to inflammatory mediators and discovered openings between individual endothelial cells at the sites of interendothelial junctions (Majno and Palade 1961). This study provided early evidence of the endothelial cell and EC membrane structure as the determinant physical barrier that maintains vascular integrity. Furthermore, these images highlighted the breakdown of this barrier as a primary pathologic mechanism resulting in inflammatory edema. The notion that cell shape and endothelial cell gap formation build functional barrier integrity was further explored by several investigators over the ensuing decades, which elucidated a more complete understanding of the cell membrane in vascular leak (Brett, Gerlach et al. 1989, Malik, Lynch et al. 1989, Meyrick, Hoover et al. 1989, Goldblum, Ding et al. 1994, Garcia and Schaphorst 1995). The intricate cellular mechanisms responsible for EC shape and movement of the lipid membrane remain an important topic of active investigation (Mehta, Ravindran et al. 2014, Kasa, Csortos et al. 2015).
By itself the outer EC lipid membrane exhibits complex biochemistry and changes its composition and physical properties in response to the local environment while relying on an underlying protein scaffolding and specific structural domains to define the cell boundary and connections to neighboring cells (Yamamoto and Ando 2015, Ayee and Levitan 2016, Zhang, Naik et al. 2018). Submembranous cytoskeletal dynamics with consequent force generation physically move the plasmalemma toward potential connection points which ultimately construct the EC barrier (Baldwin and Thurston 2001, Dudek and Garcia 2001, Birukova, Arce et al. 2009, Wang, Bleher et al. 2015). Quiescent endothelial cells in a confluent monolayer as well as those EC activated by the endogenous signaling molecule sphingosine-1-phosphate (S1P), arrange actomyosin filaments into a cortical ring in the cell periphery (Schaphorst, Chiang et al. 2003, McVerry and Garcia 2004, Birukova, Arce et al. 2009, Belvitch and Dudek 2012, Abbasi and Garcia 2013), wherein the cortical actin ring provides centrifugal force to support and stabilize the EC membrane outwardly to allow contact with neighboring cells and the basement membrane (Dudek and Garcia 2001, Viswanathan, Ephstein et al. 2016). These peripheral actin structures plus the submembranous cytoskeleton participate in constructing intercellular adherens junctions, tight junctions, and focal adhesions by binding directly to and stabilizing junctional proteins as well as recruiting additional signaling molecules (Belvitch and Dudek 2012, Vogel and Malik 2012, Sukriti, Tauseef et al. 2014). Furthermore, the cortical actin ring facilitates the formation of lamellipodia, sheet-like lateral protrusions of the cell membrane, induced by rapid, branched actin polymerization and filament network formation (Blanchoin, Amann et al. 2000, Brown, Adyshev et al. 2010, Doggett and Breslin 2011) (Figure 1C). Motile lamellipodia close intercellular gaps and initiate construction of cellular junctions, events which directly establish barrier integrity (Lee, Ozaki et al. 2006, Adderley, Lawrence et al. 2015, Breslin, Zhang et al. 2015, Choi, Camp et al. 2015, Belvitch, Brown et al. 2017). For a detailed review of lamellipodia in EC junctions and barrier integrity please see the chapter by Alves et al. in this volume (Alves, Motawe, et al. 2018).
Changes in plasma membrane shape, protrusion and retraction produced by the cytoskeleton require a physical connection between these cellular elements. This interaction is dynamic and complex (Bezanilla, Gladfelter et al. 2015, Koster and Mayor 2016). The concept of the lipid membrane as a loose sheet draped over a filamentous network of multiple components including actin, septin, spectrin and microtubules (Bezanilla, Gladfelter et al. 2015) is a simplified version of reality but these networks do provide a backbone of physical support to the outer lipid membrane. In addition, along with extracellular matrix, intracellular junctions, intramembrane proteins and cholesterol, cytoskeletal filaments function to prevent the free diffusion of membrane lipids and organize the membrane into distinct domains specialized for various cell processes (Trimble and Grinstein 2015). The complex and dynamic interplay between the membrane and cell cytoskeleton is a two-way street with extensive cross-talk and feedback between these components which necessarily involves many structural and regulatory proteins (Arumugam and Bassereau 2015, Koster and Mayor 2016). New high resolution imaging, molecular and biochemical techniques have begun to explore many of these phenomena (Arumugam and Bassereau 2015) and will also be highlighted at the end of this review.
The phospholipid composition of the cell membrane serves a mechanistic role for the cross-talk between membrane composition and cortical actin structures. Phosphatidylinositol and its phosphorylated products are critical regulatory and signaling molecules (Di Paolo and De Camilli 2006). Phosphatidylinositol-4,5-bisphosphate (PIP2) is an important precursor of intracellular second messengers generated by phospholipases (Berridge and Irvine 1989), but which also has direct signaling roles with actin related proteins as well (Lassing and Lindberg 1985, Yin and Janmey 2003). PIP2 is generated locally within cell membranes by the combined action of phosphoinositide kinases and phosphatases and its presence in the membrane alone produces changes in actin architecture (Legate, Takahashi et al. 2011, Ueno, Falkenburger et al. 2011). Furthermore, cytoskeletal responses to cellular mechanical strain are modulated by a PIP2-dependent process (Li and Russell 2013). Plasma membrane PIP2 is essential to the dynamic formation and growth of cortical actin networks by inhibiting the proteins gelsolin, cofilin, villin, and profilin that disrupt actin filaments through severing or depolarization (Yin and Janmey 2003).
Contractile and protrusive cytoskeletal forces are linked to the lipid membrane by a family of closely related proteins. Near the cell membrane the actin cytoskeleton forms a distinctive branched network formation stabilized by crosslinking of actin fibers to junctional proteins (Morone, Fujiwara et al. 2006, Prasain and Stevens 2009). Ezrin, radixin and moesin, collectively referred to as ERM proteins, are also important mediators of the cytoskeletal-membrane interaction. These highly conserved proteins connect the CD44 molecule in the lipid membrane directly with actin filaments (Yonemura, Hirao et al. 1998). Ezrin, the best studied ERM protein, is normally folded in an inactive state with the N-terminus bound to the membrane. Upon interaction with membrane PIP2 the protein opens allowing the C-terminus to interact with cortical actin (Bosk, Braunger et al. 2011). Additionally, the ERM proteins are able to bind transmembrane receptors and link their activation to downstream signaling events (Neisch and Fehon 2011). Recently, differential activation and regulation of these proteins has been implicated in pulmonary endothelial barrier function (Koss, Pfeiffer et al. 2006, Guo, Wang et al. 2009, Bogatcheva, Zemskova et al. 2011, Adyshev, Dudek et al. 2013, Zhang, Wu et al. 2014, Kovacs-Kasa, Gorshkov et al. 2016).
MEMBRANE-RELATED SIGNALING AND BARRIER FUNCTION
Multitudes of membrane associated receptors (Breen 2007, Comellas and Briva 2009, Tauseef, Knezevic et al. 2012, Ni, Epshtein et al. 2014, Ebenezer, Fu et al. 2016), channels (Cioffi, Lowe et al. 2009, Earley and Brayden 2015), and adhesive contacts (Zebda, Dubrovskyi et al. 2012) are instrumental for the pulmonary EC ability to sense and react to the local environment with changes in permeability. Both barrier disruptive and stabilizing molecules initially signal through receptors bound in the plasma membrane (Dudek and Garcia 2001). Alterations in the extracellular matrix also modulate endothelial barrier function with stiffer substrates associated with decreased barrier integrity (Mambetsariev, Tian et al. 2014, Karki and Birukova 2018). Finally, the lung endothelium is unique in that it is exposed to both shear stress from the pulmonary circulation (Fisher, Chien et al. 2001) as well as cyclical mechanical stretch from inflation and deflation of alveolar units (Fisher, Chien et al. 2001, Birukov, Jacobson et al. 2003, Gulino-Debrac 2013). Both types of mechanical stimuli cause changes in EC permeability (Birukov, Jacobson et al. 2003, Gulino-Debrac 2013). Mediators of increased vascular permeability share common intracellular signaling cascades, effector proteins, and structural changes to produce centripetal forces, loss of junctional integrity and cell rounding. Similarly, barrier enhancing mediators share common machinery to generate cell spreading, enhanced junctional complexes and interendothelial gap closure. In the next section we will discuss barrier disruptive and protective agents with a focus on the role of cell membranes and membrane associated proteins.
Thrombin Induced Barrier Disruption
The endothelial responses induced by the serine protease thrombin, a central regulator in coagulation and inflammatory processes, serve as an ideal model to investigate barrier function. Multiple studies in vitro and in vivo implicate thrombin in vascular leak through EC rounding and contraction (Coughlin 2001, Finigan 2009). Thrombin initiates EC barrier disruption by binding to the membrane bound protease activated receptor (PAR-1), a peptide receptor that carries its own ligand. Thrombin induces proteolysis of the extracellular extension between Arg41 and Ser42 to generate PAR1 ligand which binds the receptor and initiates its downstream signaling events (Coughlin 2000). PAR-1 is a seven transmembrane G protein-coupled receptor with alpha subunits G12 and −13 responsible for cytoskeletal rearrangements through Rho activation and MLC phosphorylation (Vouret-Craviari, Boquet et al. 1998, Dudek and Garcia 2001) (Figure 1A). Activation of the downstream Rho kinase results in phosphorylation of MLC phosphatase, inactivating the enzyme (Amano, Chihara et al. 1997, Essler, Amano et al. 1998, Wojciak-Stothard and Ridley 2002). The combination of reduced MLC phosphatase activity and promotion of MLC phosphorylation tip the balance in favor of actin-myosin interaction and transcellular stress fiber formation (Amano, Chihara et al. 1997).
The role of intracellular calcium is critical to EC barrier disruption (Mehta, Ahmmed et al. 2003, Tauseef, Knezevic et al. 2012). Calcium serves as an early and master regulatory signal for a number of downstream events critical for cytoskeletal rearrangement (Cioffi, Barry et al. 2010). Thrombin activation of the PAR-1 receptor generates inositol triphosphate (IP3) to induce calcium release (Sun, Geyer et al. 2017). Exposure to an inflammatory stimulus increases intracellular calcium in two discrete phases. The first phase is transient and results from depletion of stores in the endoplasmic reticulum (ER). The next phase of calcium influx occurs through plasma membrane channels which result in restoration of the ER stores and sustains ongoing signaling events over a longer time period (Sundivakkam, Natarajan et al. 2013). The membrane associated transient receptor potential (TRP) family of channels are the most important regulators of endothelial calcium concentrations (Dietrich, Kalwa et al. 2010). The canonical or TRPC channels are the most studied in endothelial cells and have six transmembrane domains with a pore forming unit between domains 5 and 6 (Mehta, Ahmmed et al. 2003, Singh, Knezevic et al. 2007). TRP channels form as either homo- or heterotetramers. Of the various TRPC channels, TRPC1 and TRPC6 are highly expressed in the endothelium (Curry and Glass 2006). RhoA regulates influx of calcium through activation of TRPC1 (Mehta, Ahmmed et al. 2003). TRPC6 knock-out mice are protected from LPS-induced vascular leak and inflammation (Tauseef, Knezevic et al. 2012).
The rise of intracellular calcium concentration activates the calcium/calmodulin-dependent non-muscle myosin light chain kinase (nmMLCK), a critical regulator of actomyosin-mediated force generation (Dudek and Garcia 2001, Shen, Rigor et al. 2010). Similar to MLCK isolated from smooth muscle, the C-terminal half of the 1914 amino acid enzyme contains the catalytic and calmodulin-binding, KRP-binding, and myosin-binding domains (Garcia, Lazar et al. 1997). The endothelial specific nmMLCK also contains a unique N-terminal region with multiple regulatory sites including tyrosine phosphorylation sites for Src and c-Abl (Dudek, Chiang et al. 2010) (Garcia, Davis et al. 1995, Verin, Gilbert-McClain et al. 1998). Non-muscle MLCK diphosphorylation of MLC on Thr18/Ser19 stimulates the ATP-driven power stroke of myosin heads on actin filaments to generate tensile force along stress fibers (Garcia and Schaphorst 1995, Schnittler 2016).
S1P Barrier Enhancement: Role of Membrane Lipid Rafts
Sphingosine 1-phosphate (S1P) is an endogenous, angiogenic phospholipid with potent EC barrier enhancing effects (Wang and Dudek 2009). Numerous in vitro and in vivo studies have implicated S1P signaling as an important mediator of pulmonary endothelial permeability in the pathogenesis of lung injury (Garcia, Liu et al. 2001, Li, Uruno et al. 2004, Tauseef, Kini et al. 2008, Zhang, Xu et al. 2010) (McVerry, Peng et al. 2004, Peng, Hassoun et al. 2004, Camerer, Regard et al. 2009, Sammani, Moreno-Vinasco et al. 2010). Circulating S1P is typically bound to proteins such as HDL (Argraves, Gazzolo et al. 2008) and its physiologic concentration ranges from approximately 0.3–1.1 μM (Venkataraman, Thangada et al. 2006, Hammad, Pierce et al. 2010) (Figure 1A). Erythrocytes and platelets serve as repositories of S1P by differential expression of regulatory enzymes important in S1P synthesis (Ito, Anada et al. 2007). When activated by inflammatory stimuli, these cells release S1P into the circulation (Yatomi, Ruan et al. 1995, Camerer, Regard et al. 2009). The plasma membrane is crucial for S1P production. Breakdown of the structural membrane component sphingomyelin to ceramide, catalyzed by sphingomyelinases, is an early step in S1P synthesis. Ceramidase subsequently deacetylates ceramide to produce sphingosine which is then phosphorylated to generate S1P. This reversible phosphorylation by sphingosine kinases and the irreversible action of S1P lyase which degrades S1P to phosphoethanolamine and hexadecanal combine to closely regulate S1P levels (Hait, Oskeritzian et al. 2006, Tani, Ito et al. 2007).
S1P functions as both an intracellular and extracellular signaling molecule. A family of five G protein-coupled receptors (S1PR1–5) with differential expression patterns mediate S1P external signaling effects (Rosen, Gonzalez-Cabrera et al. 2009, Abbasi and Garcia 2013, Mahajan-Thakur, Bohm et al. 2015). S1P receptors 1–3 are highly expressed in the vascular endothelium and impact a number of EC processes (Blaho and Hla 2014). An expansive body of literature strongly implicates S1PR1 signaling in enhanced endothelial barrier function, particularly in the pulmonary vasculature (Camerer, Regard et al. 2009, Ephstein, Singleton et al. 2013, Ni, Epshtein et al. 2014, Li, Chen et al. 2015, Cai, Bolte et al. 2016, Camp, Chiang et al. 2016). S1PR1 is closely associated with Gi in a pertussis toxin-sensitive manner (Garcia, Liu et al. 2001, Dudek, Camp et al. 2007, Sammani, Moreno-Vinasco et al. 2010) (Figure 1A). In contrast, S1PR2 and S1PR3 are primarily barrier disruptive but have varied responses dependent on the S1P concentration (Singleton, Dudek et al. 2006, Lee, Gordon et al. 2009, Sammani, Moreno-Vinasco et al. 2010, Du, Zeng et al. 2012). Furthermore, S1PR3 has been identified as a novel biomarker in human lung injury patients (Sun, Singleton et al. 2012). Interestingly, this study found S1PR3 is released by activated endothelial cells via membrane-derived extracellular vesicles (EVs) and circulates in its nitrated form in plasma. For more information regarding the larger role of these EC-derived membrane structures in the lung vasculature, please see the EV review elsewhere in this volume (Bauer and Letsiou. 2018). In vitro studies confirmed that S1PR3-containing extracellular vesicles reduce endothelial barrier function (Sun, Singleton et al. 2012). Subsequent genetic studies revealed promoter variants with diminished S1PR3 expression were associated with protection from lung injury (Sun, Ma et al. 2013). Intracellular S1P is involved in many cell signaling processes as an important second messenger (Strub, Maceyka et al. 2010). Extracellular S1P can also induce its own intracellular generation through a sphingosine intermediary (Zhao, Kalari et al. 2007) and intracellular S1P can independently activate multiple pathways associated with EC barrier enhancement (Usatyuk, He et al. 2011).
The EC membrane plays a key role in several peripheral cell signaling and structural events initiated shortly after ligation of the S1P receptor, including the formation of membrane-associated lipid rafts or caveolin-enriched microdomains (CEM). Enriched in the regulatory protein caveolin-1, cholesterol, and glycosylated sphingolipids, these structures are distinct nanoscale environments within the plasma membrane (Schnitzer, Oh et al. 1995, Minshall, Sessa et al. 2003, Lingwood and Simons 2010). They can remain attached to the larger plasmalemma, communicate with the extracellular space through a variably sized neck structure, or detach completely to become either intracellular vesicles or extracellular signaling vesicles (Nabi and Le 2003, Quest, Leyton et al. 2004, Jin and Zhou 2009, Chidlow and Sessa 2010). In the S1P response, CEM closely approximate signaling molecules and cytoskeletal effector proteins to promote downstream functional changes in cytoskeletal dynamics that improve barrier function (Singleton, Dudek et al. 2005) (Figure 1B). Both S1PR1 and S1PR3 are recruited to lipid rafts 5–10 min following S1P treatment in addition to signaling molecules including PI3 kinase, the Rac GEF Tiam1, c-Abl tyrosine kinase, and focal adhesion kinase (FAK) (Singleton, Dudek et al. 2005). The cytoskeletal regulators α-actinin1/4 and filamin A/C as well as cytoskeletal effector proteins cortactin and nmMLCK are also found in CEM. Multiple tyrosine phosphorylation events occur in lipid rafts (Zhao, Singleton et al. 2009, Dudek, Chiang et al. 2010) and protein-specific knockdown of FAK, nmMLCK, cortactin, filamin A, or filamin C attenuate the S1P response (Singleton, Dudek et al. 2005, Zhao, Singleton et al. 2009), highlighting the importance of these specialized membrane structures.
Pulmonary EC barrier enhancement by S1P depends strongly on actin dynamics. This is exemplified by the ability of cytochalasin B, the actin depolymerizing agent, or latrunculin, an inhibitor of actin polymerization, to completely abolish S1P-induced barrier enhancement (Garcia, Liu et al. 2001). The disruptor of microtubules, nocodazole decreases baseline EC barrier function as measured by TER but does not inhibit the increase in resistance seen after S1P stimulation (Garcia, Liu et al. 2001). Actin polymerization following S1P is focused in the cell periphery and actin fibers are found in close association with phosphorylated MLC, an indication that mechanical force generation plays an important role in barrier protection as it does following barrier disruption by thrombin (Garcia, Liu et al. 2001, Dudek, Jacobson et al. 2004, Brown, Adyshev et al. 2010). This arrangement of actin translates into measurable biophysical changes in the cell membrane that can be quantified as an elastic modulus by atomic force microscopy (AFM). This technique and its implications for investigation of EC barrier function are discussed later in this review. Following S1P, the elastic modulus significantly increases at the periphery of the cell, indicating a stiffening of the membrane and its underlying structures to promote EC barrier function (Arce, Whitlock et al. 2008, Wang, Bleher et al. 2015).
S1P receptor ligation activates the Rho family of small GTPases including RhoA, Rac, ROCK, and Cdc42 and provides a link from membrane signaling to dynamic actin rearrangement. Rho family proteins are involved in both barrier enhancing and disruptive processes with Rac signaling most closely associated with cortical ring formation and lamellipodial development (Garcia, Liu et al. 2001, Wojciak-Stothard and Ridley 2002, Gorshkova, He et al. 2008). Activation of Rac in a G protein-dependent manner results in downstream actin rearrangement through the p21-associated Ser/Thr kinase (PAK) (Garcia, Liu et al. 2001). While traditionally associated with barrier disruption (Dudek and Garcia 2001, Minshall and Malik 2006), Rho kinase activity is also required for maximal S1P barrier enhancement as chemical inhibition results in a 30% attenuation of the S1P response (Garcia, Liu et al. 2001, Xu, Waters et al. 2007).
PROTEIN REGULATION OF CORTICAL ACTIN
A number of cytoskeletal regulatory and effector proteins are associated with the formation of the cortical actin ring and the generation of lamellipodia (Dudek, Jacobson et al. 2004, Garcia-Ponce, Citalan-Madrid et al. 2015) (Figure 1C). The principal driver of cytoskeletal force generation is the ATP-dependent ratcheting of actin and myosin, a process catalyzed by the calcium/calmodulin-dependent enzyme non-muscle myosin light chain kinase (nmMLCK) (nmMLCK) (Dudek and Garcia 2001, Shen, Rigor et al. 2010). Non-muscle MLCK function is most closely associated with transcellular actin stress fibers, centripetal tension, and the formation of interendothelial gaps (Garcia and Schaphorst 1995, Stevens, Garcia et al. 2000, Vandenbroucke, Mehta et al. 2008, Vogel and Malik 2012). However, numerous studies also implicate nmMLCK in S1P-induced barrier protection (Dudek, Jacobson et al. 2004) and its location in peripheral membrane structures leading to strengthened barrier integrity (Chiang, Camp et al. 2009, Zhao, Singleton et al. 2009, Brown, Adyshev et al. 2010). These seemingly contradictory roles may relate to changes in S1P concentration targeting alternate S1P receptors (Singleton, Dudek et al. 2006, Lee, Gordon et al. 2009, Sammani, Moreno-Vinasco et al. 2010, Du, Zeng et al. 2012) but also highlight the importance of the subcellular localization of cytoskeletal effector proteins in regulation of barrier function.
Cortactin (CTTN) is another actin-binding protein with a long-established role in regulating cytoskeletal dynamics to maintain the EC barrier (Dudek, Jacobson et al. 2004, Jacobson, Dudek et al. 2006, Arce, Whitlock et al. 2008, Schnoor, Lai et al. 2011, Choi, Camp et al. 2015, Garcia Ponce, Citalan Madrid et al. 2016). CTTN function is critical to many cellular processes including motility, host-pathogen interactions, endocytosis, and junctional assembly by linking signaling cascades with dynamic cytoskeletal rearrangement (Weed and Parsons 2001, Cosen-Binker and Kapus 2006). A modular regulatory molecule, CTTN contains a unique actin-binding domain composed of a series of unstructured tandem repeats which induce architectural changes of the intact actin filament (Shvetsov, Berkane et al. 2009). Additionally, a proline-rich α-helix domain contains numerous serine and tyrosine phosphorylation sites important for differential activation of CTTN (Zhao, Singleton et al. 2009, Oser, Mader et al. 2010). The CTTN C-terminus consists of a Src homology 3 domain (SH3), a site of interaction with numerous other effector molecules (Zarrinpar, Bhattacharyya et al. 2003, Cosen-Binker and Kapus 2006). CTTN rapidly locates to the cell periphery and associates with cortical actin following a barrier enhancing stimulus such as S1P (Dudek, Chiang et al. 2010, Garcia-Ponce, Citalan-Madrid et al. 2015). Barrier enhancing conditions are also associated with CTTN translocation to lamellipodia where it plays a significant role in the development and protrusion of these critical structures (Brown, Adyshev et al. 2010, Choi, Camp et al. 2015). Specifically, CTTN serves to stabilize the polarized array of actin filaments which form a distinct cross-linked actin network and serves as a nucleation promotion factor (NPF) for the branched actin polymerization that occurs in lamellipodia (Ammer and Weed 2008). Impaired CTTN expression or functional inhibition of the SH3 domain significantly attenuates S1P-induced barrier protection (Dudek, Jacobson et al. 2004, Lee, Ozaki et al. 2006).
CTTN and nmMLCK directly interact through the SH3 domain of CTTN (Dudek, Birukov et al. 2002, Dudek, Jacobson et al. 2004). The maximal S1P barrier enhancement is attenuated when this interaction is inhibited (Dudek, Jacobson et al. 2004). Proline-rich regions between amino acids #972–979 and #1019–1025 in nmMLCK conform well to consensus SH3 domain recognition motifs (Sparks, Rider et al. 1996) and were identified as the putative sites of the CTTN-nmMLCK interaction by using blocking antibodies and interfering peptides (Dudek, Birukov et al. 2002). These studies demonstrated a reduction in nmMLCK binding to F-actin in the absence of CTTN but no loss of enzyme activity (Dudek, Birukov et al. 2002). Subsequent work by our group employed nmMLCK constructs in which two key proline residues were mutated to alanine at each of the putative CTTN binding sites (Belvitch, Adyshev et al. 2014). Proline deficiency resulted in an increased association with CTTN in nmMLCK fragments and increased kinase activity in the full-length constructs. Fluorescent imaging studies identified more robust stress fiber formation and EC membrane retractions after treatment with thrombin in cells transfected with a mutant GFP-nmMLCK construct as compared to wild-type nmMLCK. These studies provide strong evidence that the nmMLCK-CTTN interaction is a critical regulator of both cytoskeletal and membrane events associated with barrier function.
The protrusive force necessary to extend the membrane edge into interendothelial space depends on branched actin polymerization (Blanchoin, Amann et al. 2000, Pollard and Borisy 2003). The actin-related protein complex 2/3 (Arp 2/3) initiates the formation of new actin filaments through nucleation on the side of existing filaments at a characteristic seventy degree angle from the mother strand (Pollard 2007). The structure of Arp 2/3 mimics that of actin itself with subunits 2 and 3 being homologous to actin monomers (Nolen, Littlefield et al. 2004). After binding to the mother strand, crystallization studies indicate Arp2 and Arp3 move from an inactive end-to-end or “splayed” conformation to an in-line or “short-pitch” arrangement. This active conformation closely approximates the structure of an actin dimer and initiates the polymerization of a daughter strand (Rouiller, Xu et al. 2008). Transition of Arp 2/3 from the inactive to active state requires the participation of additional proteins termed nucleation promotion factors or NPFs. These regulators are necessary to bring actin monomers, existing filaments, and the Arp 2/3 complex itself into close approximation allowing polymerization to proceed (Higgs and Pollard 2001, Uruno, Liu et al. 2001). The proteins WASP and Scar/WAVE (WASP family veroprolin homologue) are well-studied, potent NPFs. Interestingly, the interaction of these proteins with the membrane-embedded PIP2 itself (Higgs and Pollard 2000), or as a consequence of the second messenger function of PIP2 (Ho, Rohatgi et al. 2004, Koronakis, Hume et al. 2011), activates the proteins and induces Arp2/3 polymerization. As expected, a number of NPFs, including the aforementioned CTTN, are observed in membrane lipid rafts closely associated with EC lamellipodia during barrier enhancing conditions (Cosen-Binker and Kapus 2006, Zhao, Singleton et al. 2009). CTTN interacts with Arp 2/3 through its N-terminal acidic domain and is required for the endothelial barrier enhancement produced by S1P (Uruno, Liu et al. 2001, Li, Uruno et al. 2004). Arp 2/3 function is also implicated in the maintenance and repair of junctional complexes via its role in membrane protrusions (DeMali, Barlow et al. 2002, Abu Taha and Schnittler 2014, Abu Taha, Taha et al. 2014).
We recently investigated the effects of the Arp 2/3 complex on functional measures of pulmonary EC barrier integrity. The small molecule inhibitor CK-666 binds between Arp2 and Arp3 subunits and blocks the complex’s transition from the inactive to active conformation (Nolen, Tomasevic et al. 2009, Hetrick, Han et al. 2013). Pulmonary EC treated with this inhibitor demonstrate significantly decreased barrier function as measured by transendothelial electrical resistance (TER) at baseline, a reduced S1P response, and delayed recovery of barrier function after thrombin (Belvitch, Brown et al. 2017). These functional changes were correlated with increased interendothelial gaps and significant differences in the depth of individual lamellipodia, as measured by immunofluorescence and high-resolution confocal microscopy respectively, providing direct evidence of the critical role of endothelial cell shape and membrane dynamics in barrier integrity.
JUNCTIONAL COMPLEXES LINK MEMBRANE TO CYTOSKELETON
Centripetal force generation by the actin cytoskeleton is linked to the cell membrane through cell-cell and cell-matrix adhesion molecules (Figure 1C). Intracellular connections are primarily formed by two junctional complexes, adherens junctions (AJ), and tight junctions (TJ), which provide both mechanical stability and transduction of extracellular signals into the cell (Vestweber 2000). Focal adhesions (FA) are protein complexes that regulate contact between the basal cell membrane and extracellular matrix (Romer, Birukov et al. 2006, Belvitch and Dudek 2012, Zebda, Dubrovskyi et al. 2012). Adherens junctions (AJ) are arguably the most critical cell-cell adhesion molecules forming the endothelial barrier (Dudek and Garcia 2001, Vandenbroucke, Mehta et al. 2008, Komarova and Malik 2010, Vogel and Malik 2012, Gulino-Debrac 2013, Sukriti, Tauseef et al. 2014). Vascular endothelial (VE)-cadherin is the primary structural molecule of the AJ complex located where membranes from two individual cells are closely apposed (Dejana, Corada et al. 1995, Dejana, Bazzoni et al. 1999, Bazzoni and Dejana 2004). These endothelial-specific membrane proteins are composed of extracellular, juxtamembrane, and C-terminal domains. The extracellular domain mediates the adhesive properties through homologous binding with VE-cadherin extending from neighboring cells in a calcium-dependent manner (Lampugnani, Resnati et al. 1992). Blocking antibodies directed at the extracellular domain increase paracellular permeability and alter vascular development (Corada, Liao et al. 2001). The intracellular domain links AJs to the actin cytoskeleton through the family of anchoring proteins known as catenins (Bazzoni and Dejana 2004). Beta and γ-catenin (i.e. plakoglobin) competitively bind α-catenin which in turn binds F-actin (Ben-Ze’ev and Geiger 1998). Conditional inactivation of the gene encoding β-catenin results in changes to actin structural organization, decreased intracellular adhesion and vascular fluid leakage (Cattelino, Liebner et al. 2003). The actin-binding proteins vinculin and actinin also bind α-catenin and strengthen the AJ-cytoskeletal structural complex (Knudsen, Soler et al. 1995, Nieset, Redfield et al. 1997, Watabe-Uchida, Uchida et al. 1998). The VE-cadherin juxtamembrane domain binds to a fourth catenin, p120, which is a target of Src kinase and plays an important role in regulation of AJ stability (Anastasiadis and Reynolds 2000, Thoreson, Anastasiadis et al. 2000). P120 is a negative regulator of the contractile RhoA GTPase (Noren, Liu et al. 2000). Binding of non-phosphorylated p120 to the juxtamembrane domain protects VE-cadherin from degradation (Iyer, Ferreri et al. 2004). Interestingly, both increased and decreased expression of p120 result in endothelial barrier disruption (Iyer, Ferreri et al. 2004). Intracellular tension is an important factor for stable binding of VE-cadherin to the actin cytoskeleton, when tensile force is applied to the junctional complex, the α-catenin association to β-catenin and F-actin increases and thus stabilizes cell-cell adhesion (Drees, Pokutta et al. 2005, Yamada, Pokutta et al. 2005, Buckley, Tan et al. 2014, Leckband and de Rooij 2014).
EC barrier disruption is closely tied to a loss of AJ stability mediated through VE-cadherin/catenin signaling. Tyrosine phosphorylation of VE-cadherin, β-catenin, plakoglobin, and p120 results in disorganization of AJ proteins (Dejana, Orsenigo et al. 2008) and detachment of VE-cadherin from the actin cytoskeleton (Komarova, Kruse et al. 2017). Multiple studies have demonstrated phosphorylation of VE-cadherin followed by internalization as a key step in increased endothelial permeability (Mukherjee, Tessema et al. 2006, Dejana, Orsenigo et al. 2008, Yang, Yao et al. 2015). Specific residues on the cytoplasmic tail of VE-cadherin are implicated in EC permeability changes induced by inflammatory mediators (Orsenigo, Giampietro et al. 2012) and lymphocyte migration (Turowski, Martinelli et al. 2008). In response to the reactive oxygen H2O2 which is commonly released during inflammation, the heterotrimeric G protein Gα13 binds to VE-cadherin and promotes Src phosphorylation on Tyr658 (Gong, Gao et al. 2014). This phosphorylation event prevents VE-cadherin-p120 binding and triggers subsequent AJ disassembly followed by internalization (Nanes, Chiasson-MacKenzie et al. 2012). Overexpression of p120-catenin also prevents VE-cadherin internalization by antagonizing Src-dependent phosphorylation of the juxtamembrane domain (Alcaide, Martinelli et al. 2012). This VE-cadherin-p120 interaction and related phosphorylation events leading to VE-cadherin internalization may serve as a master regulatory signal from diverse upstream signaling processes to ultimately regulate cell-cell adhesion and endothelial permeability (Dejana, Orsenigo et al. 2008, Vandenbroucke St Amant, Tauseef et al. 2012, Haines, Beard et al. 2015, Zhang, Feng et al. 2016).
Tight junctions (TJs) make up about 20% of endothelial junctional proteins and are located in apical regions or localized with AJ. They are more developed in the arterial or capillary vessels as opposed to venules (Kevil, Okayama et al. 1998). The protein components of TJs include the transmembrane adhesion molecules, occludin, claudin, and junctional adhesion molecules (JAMs) (Bazzoni 2006). Occludin is specific to TJs of the epithelium and endothelium (Furuse, Hirase et al. 1993) with adhesive properties activated after association with the cytoplasmic structural protein zonula occludens-1 (ZO-1) (Furuse, Itoh et al. 1994). Interestingly, occludin-null mice form normal TJs and do not exhibit intestinal barrier defects but exhibit inflammatory and other histologic abnormalities in several tissues, suggesting a complex role in signaling (Saitou, Furuse et al. 2000). Claudins are a group of 24 membrane-spanning adhesion molecules with claudin-5 being endothelial-specific (Tsukita and Furuse 2000). In an example of cross-talk between endothelial junctional complexes, VE-cadherin assembly in AJs upregulates expression of claudin-5 through sequestration of the transcriptional repressor β-catenin in the cell membrane, thereby preventing β-catenin action in the nucleus (Bazzoni and Dejana 2004, Taddei, Giampietro et al. 2008). Claudins are important determinants of paracellular transit in both epithelium and endothelium and claudin knockout mice are more susceptible to inflammatory lung injury (Kage, Flodby et al. 2014, Tamura and Tsukita 2014). Simvastatin is known to increase claudin-5 protein expression and reduce vascular leak in a murine lung injury model (Chen, Sharma et al. 2014). JAMs support TJ assembly, regulate endothelial permeability, and leukocyte transendothelial migration (Luissint, Nusrat et al. 2014, Reglero-Real, Colom et al. 2016). Both occludin and claudins are anchored to the actin cytoskeleton via ZO-1 which binds with α-catenin and the actin scaffolding protein spectrin (Muller, Portwich et al. 2005). Interruption of the TJ-actin interaction through ZO-1 depletion in ECs induces reorganization of actomyosin structures, tight junction disruption, and a loss of cell-cell mechanotransduction (Tornavaca, Chia et al. 2015), processes critical to barrier function. The TJ protein ZO-1 also binds cadherins and thus provides a structural connection between TJ, AJ, and the cytoskeleton (Hartsock and Nelson 2008).
The anchoring of microvascular ECs to the underlying matrix is an important determinant of permeability and is mediated by multiprotein transmembrane structures known as focal adhesions (FAs) (Wu 2005). Integrins are the primary structural proteins in focal adhesions. These transmembrane proteins span the gap between the cytoskeleton and extra cellular matrix by binding directly to various matrix proteins, such as fibronectin and collagen, and indirectly to cytoskeletal elements via cytoplasmic linker proteins (Wu 2007, Kuo 2014) (Figure 1C). Structurally, FAs balance barrier-disruptive contractile forces and barrier-protective tethering forces through connections to the actin cytoskeleton (Birukova, Shah et al. 2016). Functionally, FAs facilitate two-way signaling between the intracellular space and extracellular matrix (Dejana 2004, Wang and Dudek 2009, Komarova and Malik 2010, Wang, Bittman et al. 2015). Extracellular integrin domains have heterodimeric receptors containing α and β subunits and, to date, 24 different subtypes have been identified (Lu and Wang 2014). One hundred eighty proteins have been identified as components of integrin mediated cell-ECM adhesion (Zaidel-Bar, Itzkovitz et al. 2007, Zaidel-Bar and Geiger 2010). Disruption of integrin-mediated cell-ECM adhesion causes cell detachment from the matrix (Dejana, Lampugnani et al. 1990, Dejana, Bazzoni et al. 1999) and inhibition of integrin binding to fibronectin, leading to increases in endothelial permeability (Wu, Ustinova et al. 2001).
The cytoplasmic integrin domains interact with the cytoskeleton either directly or indirectly through intracellular linker proteins including paxillin, talin, vinculin, and α-actinin (Petit and Thiery 2000, Hodivala-Dilke, Reynolds et al. 2003). Paxillin is a focal adhesion scaffolding protein that functions to recruit multiple structural and signaling molecules (Turner 2000). Paxillin differentially regulates endothelial barrier responses to growth factors via modulation of Rac-Rho signaling (Birukova, Cokic et al. 2009). Tyrosine phosphorylation of paxillin by focal adhesion kinase (FAK) activates the Rho family of GTPases leading to the formation of filopodia, lamellipodia, and stress fibers (Birukova, Alekseeva et al. 2008). FAK, a cytoplasmic tyrosine kinase, is a central regulator in integrin-mediated singal transduction, and will be discussed in more detail below (Parsons, Slack-Davis et al. 2008). LPS induces paxillin phosphorylation at Y31 and Y118 via c-Abl tyrosine kinase to increase EC permeability (Fu, Usatyuk et al. 2015). Vinculin is a globular protein with five helical head domains (D1–D5) connected to the vinculin tail domain (Vt) by a flexible linker region (Bakolitsa, Cohen et al. 2004). The tail domain has binding sites for F-actin, paxillin, and PIP2, and the head domain, D1, has binding sites for talin, α-actinin, and α-catenin. Vinculin plays an important role in transmitting mechanical forces and orchestrating mechanical signaling events (Birukova, Shah et al. 2016). Vinculin’s association with talin and the actin cytoskeleton is important for the force-induced strengthening of FA, recruitment of additional FA proteins such as paxillin, and anchoring contractile stress fibers (Goldmann, Auernheimer et al. 2013)
On the cytoplasmic side of the cell membrane, integrins also act as signaling molecules and recruit FAK to FAs upon activation by vascular endothelial growth factor (Avraham, Lee et al. 2003), neutrophils (Guo, Wu et al. 2005), transforming growth factor β1 (Lee, Kayyali et al. 2007) and hepatocyte growth factor (Chen, Chan et al. 1998). FAK is a non-receptor tyrosine kinase which has N- and C-terminal domains. The C-terminal focal-adhesion targeting (FAT) domain serves as a signaling and protein binding site for other cytoplasmic proteins (Schaller 2001, Parsons 2003, Schlaepfer, Mitra et al. 2004, Wu 2005). Six tyrosine residues (Y397, Y407, Y576, Y577, Y861, Y925) are observed as critical phosphorylation sites, which result in differential responses to varying stimuli (Schlaepfer, Hauck et al. 1999). Autophosphorylation of FAK on Y397 creates a high-affinity binding site for the Src family kinases, which further phosphorylate FAK on other tyrosine residues and play an important role in integrin signaling (Xing, Chen et al. 1994, Cary, Klinghoffer et al. 2002). Phosphorylation of Y925 induces conformational changes to reveal Grb2-SH2 domain binding sites, which in turn mediate the Ras pathway and activate ERK1/2 (Schlaepfer, Hanks et al. 1994). SH2 domain-containing protein tyrosine phosphatase 2 (SHP2) increases pulmonary endothelial integrity by FAK phosphorylation at Y397 and Y925 (Chichger, Braza et al. 2015). Integrins signal through FAK to regulate small GTPases Rho and Rac. These cytoskeletal signaling proteins along with PAK play a role in modulating cell adhesion, migration, actin polymerization, and MAP kinase signaling (Parsons 2003). The group of N-terminal four-point-one, ezrin, radixin, moesin binding (FERM) domain proteins inhibit FAK activity by directly binding the catalytic domain (Frame, Patel et al. 2010) and blocking multiple FAK phosphorylation sites including the Src target, Y397. FAK-deficient cells migrate poorly in response to chemotactic signals (Sieg, Hauck et al. 2000) while overexpression of FAK enhances cell migration (Cary, Chang et al. 1996). Lipopolysaccharide (LPS) increases FAK expression in neonates and increases FAK phosphorylation in adult mice (Ying, Alvira et al. 2018). FAK-deficient EC have enhanced cell-cell attachment and enhanced endothelial barrier function (Arnold, Goeckeler et al. 2013). Inhibition of FAK by the pharmacologic FAK inhibitor PF-228 attenuates endothelial barrier disruption and is currently being studied in clinical trials as a potential therapeutic agent for non-small cell lung cancers (Howe, Xiao et al. 2016, Lederer, Zhou et al. 2018). S1P-dependent phosphorylation of FAK on Y576 leading to endothelial barrier enhancement depends upon Gi signaling, phospholipase C activity, and intracellular calcium levels. FAK phosphorylation leads to Gi protein dissociation from α and βγ subunits to further stimulate phospholipase C. This S1P-dependent increase in intracellular calcium sustains this crucial signaling pathway (Lee, Lee et al. 2000). EC treated with the S1P analog tysiponate significantly increased focal adhesion formation and phosphorylation of FAK, while pharmacologic inhibition of FAK significantly attenuated EC barrier enhancement induced by tysiponate (Wang, Bittman et al. 2015).
Recently, the importance of crosstalk between junctional complexes has been revealed. In particular, mechanotransduction events by both integrins and cadherins regulate their spatial relationships, signaling, and intracellular forces connected through the actin cytoskeleton (Mui, Chen et al. 2016). Furthermore, differential phosphorylation of vinculin has been shown to regulate force transduction from AJs through increasing its association with cadherins (Bays, Peng et al. 2014) while not affecting its role in FAs. Finally, mechanotransduction through VE-cadherin complexes triggers local actin and vinculin recruitment as well as cytoskeletal remodeling (Barry, Wang et al. 2015). This crosstalk between junctional proteins and cytoskeletal force generation serves as an important link in the EC’s ability to both sense and then respond to its local environment.
The complex cellular processes that determine EC barrier function are both diverse and interconnected. The cytoskeleton, plasma membrane, and associated proteins coordinate their activity at the right time and right place within the cell to effect physiologic changes in barrier integrity. Historically, our knowledge of this intricate process is the result of countless biochemical assays combined with cellular imaging techniques. While highly informative, these methods require many inferences on the part of the investigator and have left several gaps unfilled. The ideal investigation of endothelial barrier integrity would allow the observer to precisely localize protein activity while simultaneously characterizing changes in actin structure and membrane dynamics in an individual living cell. The last decade has seen tremendous advancement in the acquisition, processing and analysis of protein and membrane images. In this last section, we will explore new imaging techniques that hold significant promise for advancing our understanding of how cortical actin dynamics intersect with membrane structure to regulate endothelial permeability.
NEW INSIGHTS INTO CYTOSKELETAL AND MEMBRANE IMAGING
Since Antonie Philips van Leeuwenhoek first revealed “animalcules” to the world, scientists have continued to probe the mysteries of the cell with microscopes. Today we have a wealth of fluorescent molecules and microscopy techniques to probe the function of individual molecules within the comparatively vast cell. These techniques measuring changes in the spatial localization of or emitted fluorescence intensity from fluorophores at last enable us to comprehend biochemistry as it happens in live cells. Despite the complexity of current advanced microscopes, they obey the same principles of optics physics as do basic fluorescence microscopes [for detailed reviews, please see (Lichtman and Conchello 2005, Sanderson, Smith et al. 2014)]. A light source beam is focused via an objective lens onto a specimen stained with or expressing a fluorescent molecule. The focused beam excites the fluorophore, which then emits a photon at a slightly lower energy level as the fluorophore relaxes to its ground state. The emitted photon is captured and focused by the objective lens and travels to a detection source, be it our eyes, a digital camera, photomultiplier tube, or other advanced detector. The limit to what objects can be resolved by a basic fluorescence microscope is governed by Abbe’s Equation, which states that the lateral resolvable distance between two minute objects equals the excitation wavelength divided by twice the numerical aperture of the objective lens (d = λex/2NA). The resolvable axial distance between two small objects is d = 2λex/NA2. Depending on the objectives and wavelengths used, lateral resolution averages to ~200 nm and axial to ~ 600–700 nm (Liu, Toussaint et al. 2018). Today these restrictions are overcome via super-resolution microscopy techniques, detailed in the succeeding section.
Super-Resolution Microscopy
The benefits of super-resolution microscopy on the pulmonary endothelial cytoskeleton have yet to be fully realized but its potential is tremendous. In other cell types, super-resolution microscopy has uncovered subdiffraction details of focal adhesions, actin cortex density, myosin filament organization, and intercellular adherens junctions (Shelden, Colburn et al. 2016). Exposing these structures in the pulmonary endothelium will shed light on the exact structural biology and formation of cortical actin bands or stress fibers, how these structures generate tension, and sustain barrier integrity by revealing the intricate connections of the submembranous cytoskeleton to adherens junctions. For example, many disease-associated single nucleotide polymorphisms have been found in key cytoskeletal regulatory proteins such as nmMLCK and cortactin (Choi, Camp et al. 2015, Wang, Brown et al. 2018). Super-resolution microscopy can uncover their structural defects and mechanistic cause of pathology.
The primary modes of super-resolution microscopy are stimulated emission depletion microscopy (STED), single molecule localization techniques [ground-state depletion microscopy (GSD), stochastic optical reconstruction microscopy (STORM), photo-activated localization microscopy (PALM)], and structured illumination microscopy (SIM). Figure 2 illustrates the fundamental mechanistic principles for these modalities.
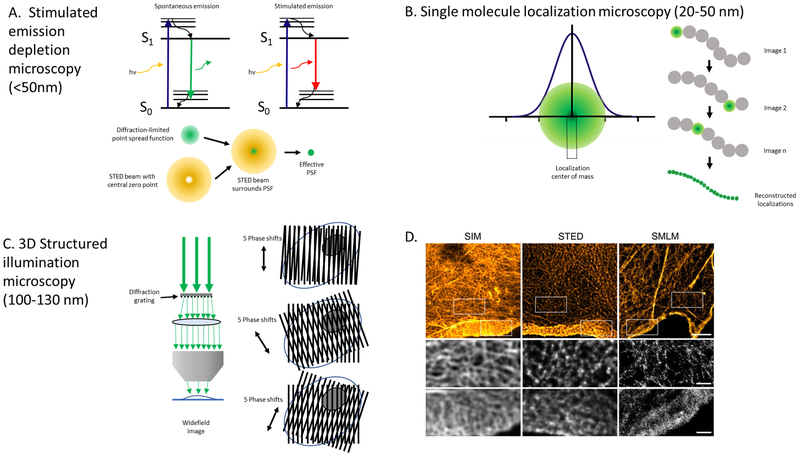
Figure 2. Super-resolution microscopy techniques.
The fluorescence and optical principles for three super-resolution microscopy techniques are shown with demonstrated lateral resolution noted in parentheses. (A) Stimulated emission depletion microscopy (STED): Effectively reduces the volume of the point spread function (PSF) of an objective lens, which is the three-dimensional diffraction volume of emitted light transmitted by an objective lens from an infinitesimally small point source of light in the specimen. A red-shifted laser with hollow beam (depletion laser) quenches fluorophores on the periphery of the PSF, leaving a small central subdiffraction volume of excited fluorophores that emit photons. Adapted from Blom and Widegren, 2017. (B) Single molecule localization microscopy (SMLM): Encompasses several related techniques that obtain many thousands of images of a given sample in which only a very small number of fluorophores are fluorescing at a given time. Precise localization of the geometric centers of individual fluorophores within the thousands of collected images can then be used to reconstruct a super-resolution image. (C) Structured illumination microscopy (SIM): A moveable diffraction grating is placed in the illumination aperture of the excitation beam path of a laser-illuminated wide-field microscopy set-up to generate a sinusoidal pattern of light with resulting Moiré fringes. The high frequency illumination stripes and high frequency object organization create even higher spatial frequencies below the diffraction limit. Multiple raw images must be collected at different diffraction orientations to reconstruct the final super-resolved image. (D) Actin cytoskeleton of COS-7 cells labeled with phalloidin-AlexaFluor 488 using SIM and STED and phalloidin-AlexaFluor647 using SMLM. Boxed areas note higher resolution areas of the lamella and lamellipodium in each optical section. Reprinted with permission from Wegel et al., 2016.
In STED, two lasers illuminate—a central, Gaussian, diffraction-limited excitation laser surrounded by a red-shifted excitatory laser, made hollow (doughnut-shaped) by a small phase plate placed at its center, raised to sufficient power to quench outlying fluorophores by stimulated emission (Figure 2A), leaving only a central, sub-diffraction area of fluorophores emitting photons and transitioning normally and reversibly between their ground and excited states (Hein, Willig et al. 2008, Blom and Widengren 2017). The diameter of the central focal area and thus resolution depends upon the power of the depletion laser, but lateral resolution can reach <50 nm (Hein, Willig et al. 2008, Wegel, Gohler et al. 2016, Blom and Widengren 2017, Vicidomini, Bianchini et al. 2018). A recent advancement in STED is the 3D time-gated STED (gSTED), which utilizes pulsed excitation combined with continuous wave laser depletion and time-gated detection (Vicidomini, Bianchini et al. 2018). Another hollow depletion laser in the z dimension improves axial resolution to 100–150 nm (Hein, Willig et al. 2008, Wildanger, Medda et al. 2009). Examples of STED application to the endothelial cytoskeleton include the detailed structure of podosomes consisting of a polygonal arrangement of vinculin around cortactin and actin foci ~900 nm in diameter linked by interconnecting F-actin cables, as well as the simultaneous incorporation of non-muscle myosin II A and B into heteropolymers in nascent filaments and segregation later during maturation of the cellular contractile system in human umbilical vein EC (Shutova, Spessott et al. 2014, Daubon, Spuul et al. 2016, Spuul, Daubon et al. 2016). At endothelial intercellular junctions, STED microscopy demonstrated differential VE-cadherin clustering under varying degrees of shear-flow stress (Seebach, Donnert et al. 2007). VE-cadherin was observed to cluster roundly at 63 nm in diameter, distributed randomly along intercellular junctions, and formed progressively denser linear clusters with concomitant increases in diameter to 80 nm under low flow conditions. These clusters are hypothesized to provide the mechanical resistance to sustain barrier integrity under conditions of shear-flow stress.
STORM, PALM, and GSD are single molecule localization microscopy (SMLM) techniques. All rely on the stochastic switching of fluorescent molecules between a fluorescing state and a dark state (Figure 2B); for specifics regarding each technique, please see (Nollmann and Georgieva 2015, Shelden, Colburn et al. 2016, Sahl, Hell et al. 2017). At any time, only a very small minority of molecules fluoresce with the distance between fluorescing molecules greater than the resolution limit of the microscope. In this case, every detected fluorescent spot comes from a single molecule. Thousands of diffraction-limited (wide-field) images are captured, each image with a different population of fluorescing molecules, and the center of mass position and intensity of each single fluorescence spot is fitted at subdiffraction precision for each image acquired (Sauer and Heilemann 2017). All of these images are reassembled into one final super-resolved image that can achieve lateral resolutions of ~20–50 nm (Wegel, Gohler et al. 2016) and axial resolutions of 50–100 nm (Sahl, Hell et al. 2017). 3D-STORM has imaged submembranous actin rings around fenestrations in rat liver sinusoidal EC (Monkemoller, Oie et al. 2015) and showed that α-actinin-4 governed the clustering of CD147 along membrane protrusions surrounding adherent Neisseria meningitides bacteria on human bone marrow EC (Maissa, Covarelli et al. 2017). Interferometric PALM studies discovered a stratified architecture of adhesions, wherein vinculin extends ~30 nm to bridge the cadherin-catenin compartment to the F-actin compartment with its attendant regulators zyxin and VASP (Bertocchi, Wang et al. 2017). Wang et al. employed interferometric PALM to resolve HUVEC F-actin filaments to a remarkable 15 nm in the Z dimension (Wang and Kanchanawong 2016). Recently, GSD revealed that syndecan-4 knock-down in aortic EC reduced actin filament branching and resulted in much longer and thicker filopodia than in wild-type EC while also uncoupling vinculin from actin filaments, which led to reduced adhesion to fibronectin and laminin substrates (Cavalheiro, Lima et al. 2017).
The technique commonly used on the cytoskeleton has been SIM, very likely because any fluorophore can be used and it depicts the very fine, continuous cytoskeletal network with generally higher signal-to-noise ratio than STED or SMLM (Figure 2C); for a thorough comparison, see (Wegel, Gohler et al. 2016). SIM can be employed with a diffraction-limited microscope either in wide-field or total internal reflection fluorescence microscopy mode. The technique achieves subdiffraction resolution by placing an interference grating in an illumination aperture and, in 2D SIM three, or in the case of 3D SIM, five sinusoidal high-frequency interference images with Moiré fringes are captured, each at a different phase step (Lu-Walther, Kielhorn et al. 2015). The gratings are rotated 60° (2D SIM) or 36° (3D SIM) and more images are captured (Demmerle, Innocent et al. 2017). Finally a subdiffraction image is calculated in Fourier space from the combined rotation series, usually 9–15 images depending on whether two or three illumination beams are used (Lu-Walther, Kielhorn et al. 2015). Resolution depends heavily on the grid frequency, number of rotation angles, and prominent specimen features (Heintzmann and Huser 2017). Generally, a final lateral resolution of ~100–150 nm and ~280–350 nm axially is achieved, although this has recently been enhanced to 88 nm laterally with a Hessian deconvolution algorithm (Demmerle, Innocent et al. 2017, Huang, Fan et al. 2018). Recent faster implementations of SIM have made it an excellent technique for live cells (Lu-Walther, Kielhorn et al. 2015). In particular, Huang et al. captured Lifeact-EGFP dynamics in human umbilical vein EC in 6,800 images at sub-millisecond exposures at a maximum speed of 188 Hz with significantly fewer artifacts than the reconstructed images by the conventional Wiener algorithm (Huang, Fan et al. 2018). Structural features that were previously unresolved in live cells include individual non-muscle myosin II isoforms A and B co-assembling in heterotypic filaments featuring two puncta spaced 300 nm apart along transverse arcs, ventral stress fibers, and subnuclear stress fibers (Beach, Shao et al. 2014) Additionally, focal adhesions composed of 300 nm linear subarrays within which paxillin, vinculin, and zyxin colocalize have been described (Hu, Tee et al. 2015). More recently, SIM revealed that claudin forms dynamic strand patches that can break and re-anneal between fibroblasts and ZO-1 can stabilize these claudin strands (Van Itallie, Tietgens et al. 2017). Interestingly, claudin associates with actin through ZO-1, although claudin binding to actin is intermittent and highly dynamic rather than continuous (Van Itallie, Tietgens et al. 2017). The authors speculated that intermittent binding between claudin and actin allowed for flexibility in coupling between claudins (Van Itallie, Tietgens et al. 2017).
Correlative Super-Resolution Microscopy and Atomic Force Microscopy
Much detail on the mechanobiology of the pulmonary endothelial cytoskeleton has recently been revealed by atomic force microscopy (AFM) (Arce, Whitlock et al. 2008, Dudek, Chiang et al. 2010, Wang, Bleher et al. 2015, Wang, Bleher et al. 2017, Wang, Wang et al. 2018). Now this technique paired with super-resolution microscopy can correlatively probe the spatiotemporal mechanodynamics of the cytoskeleton in EC with nanometer resolution (Hauser, Wojcik et al. 2017). AFM provides a detailed topography of a cell at atomic resolution and detects changes in elasticity or rigidity at the cell surface by deflection of an exquisitely sensitive, scanning cantilever, as depicted in Figure 3A. Its deflections are detected by shifts in laser beam light reflected from the back of the cantilever to a photodiode detector. AFM paired with super-resolution microscopy techniques have been applied correlatively to the cytoskeleton of various epithelial and fibroblast cells but not yet to pulmonary EC as of this writing.
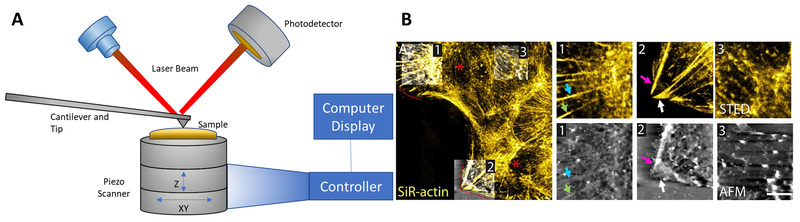
Figure 3. Combined super-resolution and atomic force microscopy.
(A) Components of a typical atomic force microscope (AFM) are depicted. AFM employs an exquisitely sensitive cantilever upon which a laser beam is reflected and a photodetector senses laser beam deflections to map the cell surface at atomic resolution of 0.1 nm in both lateral and axial dimensions. Adapted from Shan and Wang, 2015. (B) Representative image of a live murine astrocyte obtained by sequential, correlated STED-AFM microscopy. Super-resolution STED images (top inset) reveal polarized F-actin in the lamella and near the leading edge. Actin labelled with SiR-actin can be seen with corresponding thick filaments in AFM images (bottom panel) associated with focal adhesions (arrows). Reprinted with permission from Curry, Ghezali et al. 2017.
In correlative AFM-super-resolution microscopy, AFM and optical microscopy are commonly performed sequentially. Harke employed STED to generate an image map to target AFM to a particular region of interest and successfully correlated the local topology and elasticity with the positions of fluorescent tubulin filaments in fixed COS-7 cells (Harke 2012). In a subsequent study, Chacko et al. correlated STED and stochastic optical resolution microscopy (STORM) with AFM and detected a partial correlation between fixed HeLa cell topology height and cellular elasticity with microtubule filaments, admitting some interference by other unlabeled cytoskeletal filaments (Chacko, Canale et al. 2013). Odermatt et al. performed AFM and PALM sequentially on live CHO-K1 cells. First, high-speed AFM was performed to track the topology of the cell’s leading edge over time (recordings < 1 min), followed by PALM (recording ~ 4 min) of paxillin-mEos2 at the leading edge which captured the nanoscale evolution of focal adhesions at an active lamellipodial protrusion of known topography (Odermatt, Shivanandan et al. 2015). In a study with direct clinical relevance, Sharma et al. used correlative AFM followed by STED microscopy to determine that cisplatin induces a dose-dependent increase in stiffness coupled with strong peripheral banding of actin in lamellae adjacent to lamellipodia in an ovarian cancer cell line. Such properties are not present in cisplatin-resistant ovarian cancer cells, which instead exhibit a lower cell stiffness similar to untreated cells and exhibit radially aligned actin filaments from the nucleus to the lamella (Sharma, Santiskulvong et al. 2012). More recently, the polarization of actin and migration of live murine astrocytes was interrogated with STED immediately followed by AFM (Figure 3B). In the same live astrocyte, the presence of polarized actin filaments near the migrating edge strongly correlated with AFM topological images and increased membrane stiffness (Curry, Ghezali et al. 2017).
Simultaneous AFM and super-resolution microscopy has been achieved by two groups thus far. The Ando group combined HS-AFM with total internal reflection fluorescence microscopy (TIRFM) with single molecule capability (Fukuda, Uchihashi et al. 2013). Precise positional correlation was verified between TIRFM and HS-AFM images of rhodamine-labelled actin filaments in live astrocytes. Video recordings between the two types of microscopy were synchronized and the hand-over-hand movement of tail-truncated myosin V-Cy3 was tracked on a single actin filament in vitro. Concurrent but independently, Yu and colleagues (2013) constructed their own AFM-STED system equipped with a super-continuum pulsed laser to supply both fluorescence excitatory and depletion beam illumination with a picoseconds delay between both excitatory and depletion pulses. AFM topological and STED images of actin filaments in filopodia of fixed CaSki cells were acquired virtually simultaneously pixel-by-pixel (Yu, Yuan et al. 2013). Application of these two techniques simultaneously to live cells has not been reported as of this writing, but faster positional scanners that correlate AFM tips and illumination lasers are being constructed and will significantly advance simultaneous imaging of mechanotransduction by HS-AFM with super-resolved fluorescent molecular dynamics (Fukuda, Uchihashi et al. 2013, Qin, Li et al. 2017). Application of these techniques to pulmonary EC carrying mutations or disease-associated single nucleotide polymorphisms may elucidate the cellular pathobiology of asthma and acute lung injury (Gao, Grant et al. 2007, Xiong, Wang et al. 2017, Wang, Brown et al. 2018).
Intravital Microscopy
The advent of intravital lung microscopy now makes possible visualization of pulmonary pathobiology in a physiological context with consequences affecting the whole tissue (Looney and Bhattacharya 2014). The development of transgenic mice expressing Lifeact and fluorescence resonance energy transfer (FRET) biosensors enables imaging of intact tissue and realizes the concept of in vivo cell biology, viewing biochemical signaling in its native physiological environment (Riedl, Flynn et al. 2010, Direnberger, Mues et al. 2012, Johnsson, Dai et al. 2014, Nobis, Herrmann et al. 2017). In the pulmonary endothelium, these tools have the potential to reveal real-time physiological signaling and cytoskeletal perturbations in asthma, infection or sepsis, and acute lung injury.
Intact lung tissue scatters confocal laser illumination and makes imaging beyond the surface impossible (Follain, Mercier et al. 2017). The utilization of pulsed lasers at infrared wavelengths that focused laser light only at the focal plane led to the development of two-photon (2P) microscopy and made imaging inside tissue feasible. In conventional confocal microscopy, single excitation photon absorption produces a single red-shifted photon emission whereas in 2P microscopy, the simultaneous absorption of two photons with half the energy each can excite the same fluorophore. Hence, the photon density must be concentrated in both time and space by pulsed lasers and objective lenses with very high numerical aperture. Tissue penetration by 2P lasers occurs via several properties: (1) the nonlinear properties of 2P absorption confine the excitation to a small volume; (2) infrared light is poorly absorbed and scattered by biological tissue; (3) excitation occurs exclusively at the focal plane, obviating the need for pinholes. These properties result in tissue penetration by several hundred micrometers, strong signal-to-noise ratio, and reduced phototoxicity (Follain, Mercier et al. 2017, Sewald 2018). The development of the thoracic vacuum window made intravital 2P microscopy in mouse lung feasible (Looney, Thornton et al. 2011). Its utilization realized 2P imaging of mouse lung and revealed spatiotemporal cellular dynamics of lung immunity, mechanics, and microcirculation (Looney and Bhattacharya 2014).
Most of the 2P intravital microscopy (2P-IVM) in lung generally has focused on immunology and tumor invasion (Lelkes, Headley et al. 2014, Looney and Bhattacharya 2014, Entenberg, Rodriguez-Tirado et al. 2015, Kamioka, Takakura et al. 2017). In the last decade, most of the pulmonary endothelial imaging has focused on the glycocalyx, apoptosis, and endothelial interactions with platelets, leukocytes, and neutrophils (Kuebler 2011, Yang, Yang et al. 2013, Brown, Hunt et al. 2014, Lelkes, Headley et al. 2014, Looney and Bhattacharya 2014, Gill, Rohan et al. 2015, Park, Choe et al. 2018). Surprisingly very little 2P-IVM has been employed to examine the pulmonary endothelial cytoskeleton specifically, regardless of potential revelations in acute lung injury, infection, and asthma/disease progression (Gao, Grant et al. 2007, Wang, Brown et al. 2018). Transgenic mice expressing Lifeact-mRuby or –EGFP with verified expression in lung have been available since 2010 and 2P imaging of Lifeact-GFP in neutrophils and acinar cells in transgenic mice has been reported (Riedl, Flynn et al. 2010, Weigert, Sramkova et al. 2010, Lammermann, Afonso et al. 2013); however, 2P-IVM studies of the pulmonary endothelium have not yet been applied to Lifeact-expressing transgenic mice. The cytoskeletal dynamics of actin in EC activated by thrombin or S1P are very well established in vitro; now utilization of Lifeact-expressing transgenic mice to interrogate endothelial cytoskeletal dynamics via these signaling pathways is warranted. Subcellular resolution image analysis in live lung has been documented and, therefore, imaging subcellular cytoskeletal structures is feasible (Entenberg, Rodriguez-Tirado et al. 2015). Moreover, disease application with these transgenic mice can reveal cytoskeletal perturbations via insult by inflammation and/or infection.
Several signaling FRET biosensors for Rac1 (Johnsson, Dai et al. 2014), RhoA (Nobis, Herrmann et al. 2017), PKA (Yamauchi, Kamioka et al. 2016), cGMP (Thunemann, Schmidt et al. 2014), and a FRET Ca2+ indicator (Direnberger, Mues et al. 2012) transgenic mouse lines now exist that can be used to interrogate signaling in the submembranous cytoskeleton of the pulmonary endothelium. Two-photon IVM FRET in lung has been accomplished by Kamioka et al. (2017) wherein Erk activity was detected by phosphorylation of the EKAREV Erk biosensor, which induces a conformational change that brings ECFP and YPet into FRET-generating proximity (Kamioka, Sumiyama et al. 2012, Kamioka, Takakura et al. 2017). Two-photon IVM FRET imaging and expression of the Erk biosensor in leukocytes and other polymorphonuclear cells was employed to localize Erk activity in the vasculature around alveoli. ECFP was excited at 840 nm and both ECFP and YPet fluorescence emission were captured and ratioed. Erk was activated strongly in polymorphonuclear cells around tumor emboli in mouse lung (Kamioka, Takakura et al. 2017). The in vivo application of these transgenic mice to investigate of RhoA-mediated endothelial barrier disruption, Rac1-mediated cortical actin ring formation, regulation of vascular permeability by PKA, and vasodilation by cGMP, and calcium activation of nmMLCK, will begin to reveal the native physiological regulation of pulmonary endothelial cytoskeletal architecture. The forces generated in this architecture can be imaged once mechanotransduction FRET biosensors, such as in vinculin, α-catenin, and VE-cadherin, are finally incorporated into transgenic mice in the near future (Hirata and Kiyokawa 2016, Dorland and Huveneers 2017).
Most recently, a permanent window for IVM of murine lung has been constructed (Entenberg, Voiculescu et al. 2018). The lung adheres to the window without any reported inflammation or tissue damage over a two-week period. The window can be immobilized on an inverted microscope stage and imaged with a 2P laser and 20×/0.95 NA objective lens. Fiduciary markers on the window facilitate registration and visualization of the same vasculature day-to-day. Motion of subcellular organelles and VE-cadherin-labelling of endothelial cells has been captured, as has transendothelial migration of tumor cells (Entenberg, Voiculescu et al. 2018). Now the tools to probe signaling and cytoskeletal visualization exist for in vivo subcellular biology of the pulmonary endothelium and their utilization and revelations await.
CONCLUSION
Pulmonary endothelial permeability is integral to normal physiologic function but is also responsible for devastating pathologic disease. The fine-tuned regulation of the blood-gas barrier depends on the EC membrane to form a physical partition between the vascular and interstitial spaces. The concepts outlined in this review highlight the importance of cortical actin and dynamic cytoskeletal rearrangements, directed by numerous regulatory proteins, in determination of barrier integrity. Continued work on actin-related proteins, such as cortactin, nmMLCK, and Arp 2/3, should improve our understanding of these complex processes and may help identify therapeutic targets to reduce vascular leak and inflammatory lung injury. Exploration of the connection between cytoskeletal force generation and movement of the lipid membrane is in many ways just beginning. These studies demand precise spatiotemporal characterization of protein activity and subsequent structural changes within the cell. The recent advancements in super-resolution, atomic force, and intravital microscopy techniques are beginning to facilitate these investigations and complement the wealth of biochemistry that has already been described. A focus on the endothelial cell membrane, its dynamic structure, and mechanisms of regulation are sure to bring new and exciting discoveries to the world of vascular biology in the years to come.
ACKNOWLEDGEMENTS
This work was supported by funding from the National Heart Lung and Blood Institute NIH grants R01 HL133059 (SD) and K08 HL135318.
REFERENCES
- Abbasi T and Garcia JG (2013). “Sphingolipids in lung endothelial biology and regulation of vascular integrity.” Handb Exp Pharmacol(216): 201–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Taha A and Schnittler HJ (2014). “Dynamics between actin and the VE-cadherin/catenin complex: novel aspects of the ARP2/3 complex in regulation of endothelial junctions.” Cell Adh Migr 8(2): 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Taha A, Taha M, Seebach J and Schnittler HJ (2014). “ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity.” Mol Biol Cell 25(2): 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adderley SP, Lawrence C, Madonia E, Olubadewo JO and Breslin JW (2015). “Histamine activates p38 MAP kinase and alters local lamellipodia dynamics, reducing endothelial barrier integrity and eliciting central movement of actin fibers.” Am J Physiol Cell Physiol 309(1): C51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adyshev DM, Dudek SM, Moldobaeva N, Kim KM, Ma SF, Kasa A, Garcia JG and Verin AD (2013). “Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin.” Am J Physiol Lung Cell Mol Physiol 305(3): L240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaide P, Martinelli R, Newton G, Williams MR, Adam A, Vincent PA and Luscinskas FW (2012). “p120-Catenin prevents neutrophil transmigration independently of RhoA inhibition by impairing Src dependent VE-cadherin phosphorylation.” Am J Physiol Cell Physiol 303(4): C385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y and Kaibuchi K (1997). “Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase.” Science 275(5304): 1308–1311. [DOI] [PubMed] [Google Scholar]
- Ammer AG and Weed SA (2008). “Cortactin branches out: roles in regulating protrusive actin dynamics.” Cell Motil Cytoskeleton 65(9): 687–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadis PZ and Reynolds AB (2000). “The p120 catenin family: complex roles in adhesion, signaling and cancer.” J Cell Sci 113 (Pt 8): 1319–1334. [DOI] [PubMed] [Google Scholar]
- Anjum F, Joshi K, Grinkina N, Gowda S, Cutaia M and Wadgaonkar R (2012). “Role of sphingomyelin synthesis in pulmonary endothelial cell cytoskeletal activation and endotoxin-induced lung injury.” Am J Respir Cell Mol Biol 47(1): 94–103. [DOI] [PubMed] [Google Scholar]
- Arce FT, Whitlock JL, Birukova AA, Birukov KG, Arnsdorf MF, Lal R, Garcia JG and Dudek SM (2008). “Regulation of the micromechanical properties of pulmonary endothelium by S1P and thrombin: role of cortactin.” Biophys J 95(2): 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argraves KM, Gazzolo PJ, Groh EM, Wilkerson BA, Matsuura BS, Twal WO, Hammad SM and Argraves WS (2008). “High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function.” J Biol Chem 283(36): 25074–25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold KM, Goeckeler ZM and Wysolmerski RB (2013). “Loss of focal adhesion kinase enhances endothelial barrier function and increases focal adhesions.” Microcirculation 20(7): 637–649. [DOI] [PubMed] [Google Scholar]
- Arumugam S and Bassereau P (2015). “Membrane nanodomains: contribution of curvature and interaction with proteins and cytoskeleton.” Essays Biochem 57: 109–119. [DOI] [PubMed] [Google Scholar]
- Avraham HK, Lee TH, Koh Y, Kim TA, Jiang S, Sussman M, Samarel AM and Avraham S (2003). “Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase.” J Biol Chem 278(38): 36661–36668. [DOI] [PubMed] [Google Scholar]
- Ayee MA and Levitan I (2016). “Paradoxical impact of cholesterol on lipid packing and cell stiffness.” Front Biosci (Landmark Ed) 21: 1245–1259. [DOI] [PubMed] [Google Scholar]
- Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, Critchley DR, Craig SW and Liddington RC (2004). “Structural basis for vinculin activation at sites of cell adhesion.” Nature 430(6999): 583–586. [DOI] [PubMed] [Google Scholar]
- Baldwin AL and Thurston G (2001). “Mechanics of endothelial cell architecture and vascular permeability.” Crit Rev Biomed Eng 29(2): 247–278. [DOI] [PubMed] [Google Scholar]
- Barry AK, Wang N and Leckband DE (2015). “Local VE-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers.” J Cell Sci 128(7): 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays JL, Peng X, Tolbert CE, Guilluy C, Angell AE, Pan Y, Superfine R, Burridge K and DeMali KA (2014). “Vinculin phosphorylation differentially regulates mechanotransduction at cell-cell and cell-matrix adhesions.” J Cell Biol 205(2): 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G (2006). “Endothelial tight junctions: permeable barriers of the vessel wall.” Thromb Haemost 95(1): 36–42. [PubMed] [Google Scholar]
- Bazzoni G and Dejana E (2004). “Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis.” Physiol Rev 84(3): 869–901. [DOI] [PubMed] [Google Scholar]
- Beach JR, Shao L, Remmert K, Li D, Betzig E and Hammer JA 3rd (2014). “Nonmuscle myosin II isoforms coassemble in living cells.” Curr Biol 24(10): 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS and Pesenti A (2016). “Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries.” Jama 315(8): 788–800. [DOI] [PubMed] [Google Scholar]
- Belvitch P, Adyshev D, Elangovan VR, Brown ME, Naureckas C, Rizzo AN, Siegler JH, Garcia JG and Dudek SM (2014). “Proline-rich region of non-muscle myosin light chain kinase modulates kinase activity and endothelial cytoskeletal dynamics.” Microvasc Res 95: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvitch P, Brown ME, Brinley BN, Letsiou E, Rizzo AN, Garcia JGN and Dudek SM (2017). “The ARP 2/3 complex mediates endothelial barrier function and recovery.” Pulm Circ 7(1): 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvitch P and Dudek SM (2012). “Role of FAK in S1P-regulated endothelial permeability.” Microvasc Res 83(1): 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze’ev A and Geiger B (1998). “Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer.” Curr Opin Cell Biol 10(5): 629–639. [DOI] [PubMed] [Google Scholar]
- Berridge MJ and Irvine RF (1989). “Inositol phosphates and cell signalling.” Nature 341(6239): 197–205. [DOI] [PubMed] [Google Scholar]
- Bertocchi C, Wang Y, Ravasio A, Hara Y, Wu Y, Sailov T, Baird MA, Davidson MW, Zaidel-Bar R, Toyama Y, Ladoux B, Mege RM and Kanchanawong P (2017). “Nanoscale architecture of cadherin-based cell adhesions.” Nat Cell Biol 19(1): 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M, Gladfelter AS, Kovar DR and Lee WL (2015). “Cytoskeletal dynamics: a view from the membrane.” J Cell Biol 209(3): 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya J and Matthay MA (2013). “Regulation and repair of the alveolar-capillary barrier in acute lung injury.” Annu Rev Physiol 75: 593–615. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD and Garcia JG (2003). “Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch.” Am J Physiol Lung Cell Mol Physiol 285(4): L785–797. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Alekseeva E, Cokic I, Turner CE and Birukov KG (2008). “Cross talk between paxillin and Rac is critical for mediation of barrier-protective effects by oxidized phospholipids.” Am J Physiol Lung Cell Mol Physiol 295(4): L593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Arce FT, Moldobaeva N, Dudek SM, Garcia JG, Lal R and Birukov KG (2009). “Endothelial permeability is controlled by spatially defined cytoskeletal mechanics: atomic force microscopy force mapping of pulmonary endothelial monolayer.” Nanomedicine 5(1): 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Cokic I, Moldobaeva N and Birukov KG (2009). “Paxillin is involved in the differential regulation of endothelial barrier by HGF and VEGF.” Am J Respir Cell Mol Biol 40(1): 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Shah AS, Tian YF, Moldobaeva N and Birukov KG (2016). “Dual role of vinculin in barrier-disruptive and barrier-enhancing endothelial cell responses.” Cellular Signalling 28(6): 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho VA and Hla T (2014). “An update on the biology of sphingosine 1-phosphate receptors.” J Lipid Res 55(8): 1596–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L, Amann KJ, Higgs HN, Marchand JB, Kaiser DA and Pollard TD (2000). “Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins.” Nature 404(6781): 1007–1011. [DOI] [PubMed] [Google Scholar]
- Blom H and Widengren J (2017). “Stimulated Emission Depletion Microscopy.” Chem Rev 117(11): 7377–7427. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Garcia JG and Verin AD (2002). “Molecular mechanisms of thrombin-induced endothelial cell permeability.” Biochemistry (Mosc) 67(1): 75–84. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Zemskova MA, Gorshkov BA, Kim KM, Daglis GA, Poirier C and Verin AD (2011). “Ezrin, radixin, and moesin are phosphorylated in response to 2-methoxyestradiol and modulate endothelial hyperpermeability.” Am J Respir Cell Mol Biol 45(6): 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosk S, Braunger JA, Gerke V and Steinem C (2011). “Activation of F-actin binding capacity of ezrin: synergism of PIP(2) interaction and phosphorylation.” Biophys J 100(7): 1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen EC (2007). “VEGF in biological control.” J Cell Biochem 102(6): 1358–1367. [DOI] [PubMed] [Google Scholar]
- Brenner SL and Korn ED (1979). “Spectrin-actin interaction. Phosphorylated and dephosphorylated spectrin tetramer cross-link F-actin.” J Biol Chem 254(17): 8620–8627. [PubMed] [Google Scholar]
- Brenner SL and Korn ED (1980). “Spectrin/actin complex isolated from sheep erythrocytes accelerates actin polymerization by simple nucleation. Evidence for oligomeric actin in the erythrocyte cytoskeleton.” J Biol Chem 255(4): 1670–1676. [PubMed] [Google Scholar]
- Breslin JW, Zhang XE, Worthylake RA and Souza-Smith FM (2015). “Involvement of local lamellipodia in endothelial barrier function.” PLoS One 10(2): e0117970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett J, Gerlach H, Nawroth P, Steinberg S, Godman G and Stern D (1989). “Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins.” J Exp Med 169(6): 1977–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Adyshev D, Bindokas V, Moitra J, Garcia JG and Dudek SM (2010). “Quantitative distribution and colocalization of non-muscle myosin light chain kinase isoforms and cortactin in human lung endothelium.” Microvasc Res 80(1): 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MB, Hunt WR, Noe JE, Rush NI, Schweitzer KS, Leece TC, Moldobaeva A, Wagner EM, Dudek SM, Poirier C, Presson RG Jr., Gulbins E and Petrache I (2014). “Loss of cystic fibrosis transmembrane conductance regulator impairs lung endothelial cell barrier function and increases susceptibility to microvascular damage from cigarette smoke.” Pulm Circ 4(2): 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ and Dunn AR (2014). “Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force.” Science 346(6209): 1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Bolte C, Le T, Goda C, Xu Y, Kalin TV and Kalinichenko VV (2016). “FOXF1 maintains endothelial barrier function and prevents edema after lung injury.” Sci Signal 9(424): ra40. [DOI] [PubMed] [Google Scholar]
- Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R and Coughlin SR (2009). “Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice.” The Journal of clinical investigation 119(7): 1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp SM, Chiang ET, Sun C, Usatyuk PV, Bittman R, Natarajan V, Garcia JG and Dudek SM (2016). ““Pulmonary Endothelial Cell Barrier Enhancement by Novel FTY720 Analogs: Methoxy-FTY720, Fluoro-FTY720, and beta-Glucuronide-FTY720”.” Chem Phys Lipids 194: 85–93. [DOI] [PubMed] [Google Scholar]
- Carvalho K, Tsai FC, Lees E, Voituriez R, Koenderink GH and Sykes C (2013). “Cell-sized liposomes reveal how actomyosin cortical tension drives shape change.” Proc Natl Acad Sci U S A 110(41): 16456–16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary LA, Chang JF and Guan JL (1996). “Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn.” J Cell Sci 109 (Pt 7): 1787–1794. [DOI] [PubMed] [Google Scholar]
- Cary LA, Klinghoffer RA, Sachsenmaier C and Cooper JA (2002). “SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading.” Mol Cell Biol 22(8): 2427–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R and Dejana E (2003). “The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility.” J Cell Biol 162(6): 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro RP, Lima MA, Jarrouge-Boucas TR, Viana GM, Lopes CC, Coulson-Thomas VJ, Dreyfuss JL, Yates EA, Tersariol ILS and Nader HB (2017). “Coupling of vinculin to F-actin demands Syndecan-4 proteoglycan.” Matrix Biol 63: 23–37. [DOI] [PubMed] [Google Scholar]
- Chacko JV, Canale C, Harke B and Diaspro A (2013). “Sub-diffraction nano manipulation using STED AFM.” PLoS One 8(6): e66608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S and Fisher AB (2014). “Mechanotransduction in the endothelium: role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow.” Antioxid Redox Signal 20(6): 899–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Fujiwara K, Perez NG, Ushio-Fukai M and Fisher AB (2015). “Mechanosignaling in the vasculature: emerging concepts in sensing, transduction and physiological responses.” Am J Physiol Heart Circ Physiol 308(12): H1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Nieman GF, Christie JD and Fisher AB (2014). “Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation.” Am J Physiol Lung Cell Mol Physiol 307(9): L668–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Chan PC, Tang MJ, Cheng CH and Chang TJ (1998). “Tyrosine phosphorylation of focal adhesion kinase stimulated by hepatocyte growth factor leads to mitogen-activated protein kinase activation.” J Biol Chem 273(40): 25777–25782. [DOI] [PubMed] [Google Scholar]
- Chen W, Sharma R, Rizzo AN, Siegler JH, Garcia JG and Jacobson JR (2014). “Role of claudin-5 in the attenuation of murine acute lung injury by simvastatin.” Am J Respir Cell Mol Biol 50(2): 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang ET, Camp SM, Dudek SM, Brown ME, Usatyuk PV, Zaborina O, Alverdy JC and Garcia JG (2009). “Protective effects of high-molecular weight polyethylene glycol (PEG) in human lung endothelial cell barrier regulation: role of actin cytoskeletal rearrangement.” Microvascular research 77(2): 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichger H, Braza J, Duong H and Harrington EO (2015). “SH2 domain-containing protein tyrosine phosphatase 2 and focal adhesion kinase protein interactions regulate pulmonary endothelium barrier function.” Am J Respir Cell Mol Biol 52(6): 695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow JH Jr. and Sessa WC (2010). “Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation.” Cardiovasc Res 86(2): 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Camp SM, Dan A, Garcia JG, Dudek SM and Leckband DE (2015). “A genetic variant of cortactin linked to acute lung injury impairs lamellipodia dynamics and endothelial wound healing.” Am J Physiol Lung Cell Mol Physiol 309(9): L983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi DL, Barry C and Stevens T (2010). “Store-operated calcium entry channels in pulmonary endothelium: the emerging story of TRPCS and Orai1.” Adv Exp Med Biol 661: 137–154. [DOI] [PubMed] [Google Scholar]
- Cioffi DL, Lowe K, Alvarez DF, Barry C and Stevens T (2009). “TRPing on the lung endothelium: calcium channels that regulate barrier function.” Antioxid Redox Signal 11(4): 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comellas AP and Briva A (2009). “Role of endothelin-1 in acute lung injury.” Transl Res 153(6): 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford KM, Lawrence DW, Synnestvedt K, Levi BP and Colgan SP (2002). “Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability.” FASEB J 16(6): 583–585. [DOI] [PubMed] [Google Scholar]
- Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P and Dejana E (2001). “Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability.” Blood 97(6): 1679–1684. [DOI] [PubMed] [Google Scholar]
- Cosen-Binker LI and Kapus A (2006). “Cortactin: the gray eminence of the cytoskeleton.” Physiology (Bethesda) 21: 352–361. [DOI] [PubMed] [Google Scholar]
- Coughlin SR (2000). “Thrombin signalling and protease-activated receptors.” Nature 407(6801): 258–264. [DOI] [PubMed] [Google Scholar]
- Coughlin SR (2001). “Protease-activated receptors in vascular biology.” Thromb Haemost 86(1): 298–307. [PubMed] [Google Scholar]
- Curry FR and Glass CA (2006). “TRP channels and the regulation of vascular permeability: new insights from the lung microvasculature.” Circ Res 99(9): 915–917. [DOI] [PubMed] [Google Scholar]
- Curry N, Ghezali G, Kaminski Schierle GS, Rouach N and Kaminski CF (2017). “Correlative STED and Atomic Force Microscopy on Live Astrocytes Reveals Plasticity of Cytoskeletal Structure and Membrane Physical Properties during Polarized Migration.” Front Cell Neurosci 11: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubon T, Spuul P, Alonso F, Fremaux I and Genot E (2016). “VEGF-A stimulates podosome-mediated collagen-IV proteolysis in microvascular endothelial cells.” J Cell Sci 129(13): 2586–2598. [DOI] [PubMed] [Google Scholar]
- De Matteis MA and Morrow JS (2000). “Spectrin tethers and mesh in the biosynthetic pathway.” J Cell Sci 113 (Pt 13): 2331–2343. [DOI] [PubMed] [Google Scholar]
- Dejana E (2004). “Endothelial cell-cell junctions: happy together.” Nat Rev Mol Cell Biol 5(4): 261–270. [DOI] [PubMed] [Google Scholar]
- Dejana E, Bazzoni G and Lampugnani MG (1999). “Vascular endothelial (VE)-cadherin: only an intercellular glue?” Exp Cell Res 252(1): 13–19. [DOI] [PubMed] [Google Scholar]
- Dejana E, Corada M and Lampugnani MG (1995). “Endothelial cell-to-cell junctions.” Faseb j 9(10): 910–918. [PubMed] [Google Scholar]
- Dejana E, Lampugnani MG, Giorgi M, Gaboli M and Marchisio PC (1990). “Fibrinogen induces endothelial cell adhesion and spreading via the release of endogenous matrix proteins and the recruitment of more than one integrin receptor.” Blood 75(7): 1509–1517. [PubMed] [Google Scholar]
- Dejana E, Orsenigo F and Lampugnani MG (2008). “The role of adherens junctions and VE-cadherin in the control of vascular permeability.” J Cell Sci 121(Pt 13): 2115–2122. [DOI] [PubMed] [Google Scholar]
- DeMali KA, Barlow CA and Burridge K (2002). “Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion.” J Cell Biol 159(5): 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmerle J, Innocent C, North AJ, Ball G, Muller M, Miron E, Matsuda A, Dobbie IM, Markaki Y and Schermelleh L (2017). “Strategic and practical guidelines for successful structured illumination microscopy.” Nat Protoc 12(5): 988–1010. [DOI] [PubMed] [Google Scholar]
- Di Paolo G and De Camilli P (2006). “Phosphoinositides in cell regulation and membrane dynamics.” Nature 443(7112): 651–657. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H and Gudermann T (2010). “TRPC channels in vascular cell function.” Thromb Haemost 103(2): 262–270. [DOI] [PubMed] [Google Scholar]
- Direnberger S, Mues M, Micale V, Wotjak CT, Dietzel S, Schubert M, Scharr A, Hassan S, Wahl-Schott C, Biel M, Krishnamoorthy G and Griesbeck O (2012). “Biocompatibility of a genetically encoded calcium indicator in a transgenic mouse model.” Nat Commun 3: 1031. [DOI] [PubMed] [Google Scholar]
- Doggett TM and Breslin JW (2011). “Study of the actin cytoskeleton in live endothelial cells expressing GFP-actin.” J Vis Exp(57). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R and Holmes KC (2011). “Actin structure and function.” Annu Rev Biophys 40: 169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorland YL and Huveneers S (2017). “Cell-cell junctional mechanotransduction in endothelial remodeling.” Cell Mol Life Sci 74(2): 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ and Weis WI (2005). “Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly.” Cell 123(5): 903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zeng C, Li Q, Chen B, Liu H, Huang X and Huang Q (2012). “LPS and TNF-alpha induce expression of sphingosine-1-phosphate receptor-2 in human microvascular endothelial cells.” Pathol Res Pract 208(2): 82–88. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Birukov KG, Zhan X and Garcia JG (2002). “Novel interaction of cortactin with endothelial cell myosin light chain kinase.” Biochem Biophys Res Commun 298(4): 511–519. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V and Garcia JG (2007). “Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor.” Cellular signalling 19(8): 1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Chiang ET, Camp SM, Guo Y, Zhao J, Brown ME, Singleton PA, Wang L, Desai A, Arce FT, Lal R, Van Eyk JE, Imam SZ and Garcia JG (2010). “Abl tyrosine kinase phosphorylates nonmuscle Myosin light chain kinase to regulate endothelial barrier function.” Mol Biol Cell 21(22): 4042–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM and Garcia JG (2001). “Cytoskeletal regulation of pulmonary vascular permeability.” J Appl Physiol (1985) 91(4): 1487–1500. [DOI] [PubMed] [Google Scholar]
- Dudek SM and Garcia JG (2001). “Cytoskeletal regulation of pulmonary vascular permeability.” J Appl Physiol 91(4): 1487–1500. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X and Garcia JG (2004). “Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase.” The Journal of biological chemistry 279(23): 24692–24700. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X and Garcia JG (2004). “Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase.” J Biol Chem 279(23): 24692–24700. [DOI] [PubMed] [Google Scholar]
- Earley S and Brayden JE (2015). “Transient receptor potential channels in the vasculature.” Physiol Rev 95(2): 645–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenezer DL, Fu P and Natarajan V (2016). “Targeting sphingosine-1-phosphate signaling in lung diseases.” Pharmacol Ther 168: 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entenberg D, Rodriguez-Tirado C, Kato Y, Kitamura T, Pollard JW and Condeelis J (2015). “In vivo subcellular resolution optical imaging in the lung reveals early metastatic proliferation and motility.” Intravital 4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entenberg D, Voiculescu S, Guo P, Borriello L, Wang Y, Karagiannis GS, Jones J, Baccay F, Oktay M and Condeelis J (2018). “A permanent window for the murine lung enables high-resolution imaging of cancer metastasis.” Nat Methods 15(1): 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephstein Y, Singleton PA, Chen W, Wang L, Salgia R, Kanteti P, Dudek SM, Garcia JG and Jacobson JR (2013). “Critical role of S1PR1 and integrin beta4 in HGF/c-Met-mediated increases in vascular integrity.” J Biol Chem 288(4): 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC and Aepfelbacher M (1998). “Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells.” J Biol Chem 273(34): 21867–21874. [DOI] [PubMed] [Google Scholar]
- Fanelli V and Ranieri VM (2015). “Mechanisms and clinical consequences of acute lung injury.” Ann Am Thorac Soc 12 Suppl 1: S3–8. [DOI] [PubMed] [Google Scholar]
- Finigan JH (2009). “The coagulation system and pulmonary endothelial function in acute lung injury.” Microvasc Res 77(1): 35–38. [DOI] [PubMed] [Google Scholar]
- Fisher AB, Chien S, Barakat AI and Nerem RM (2001). “Endothelial cellular response to altered shear stress.” Am J Physiol Lung Cell Mol Physiol 281(3): L529–533. [DOI] [PubMed] [Google Scholar]
- Follain G, Mercier L, Osmani N, Harlepp S and Goetz JG (2017). “Seeing is believing - multi-scale spatio-temporal imaging towards in vivo cell biology.” J Cell Sci 130(1): 23–38. [DOI] [PubMed] [Google Scholar]
- Frame MC, Patel H, Serrels B, Lietha D and Eck MJ (2010). “The FERM domain: organizing the structure and function of FAK.” Nat Rev Mol Cell Biol 11(11): 802–814. [DOI] [PubMed] [Google Scholar]
- Fu P, Usatyuk PV, Lele A, Harijith A, Gregorio CC, Garcia JG, Salgia R and Natarajan V (2015). “c-Abl mediated tyrosine phosphorylation of paxillin regulates LPS-induced endothelial dysfunction and lung injury.” Am J Physiol Lung Cell Mol Physiol 308(10): L1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Uchihashi T, Iino R, Okazaki Y, Yoshida M, Igarashi K and Ando T (2013). “High-speed atomic force microscope combined with single-molecule fluorescence microscope.” Rev Sci Instrum 84(7): 073706. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S and Tsukita S (1993). “Occludin: a novel integral membrane protein localizing at tight junctions.” J Cell Biol 123(6 Pt 2): 1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S and Tsukita S (1994). “Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions.” J Cell Biol 127(6 Pt 1): 1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Grant AV, Rafaels N, Stockton-Porter M, Watkins T, Gao P, Chi P, Munoz M, Watson H, Dunston G, Togias A, Hansel N, Sevransky J, Maloney JP, Moss M, Shanholtz C, Brower R, Garcia JG, Grigoryev DN, Cheadle C, Beaty TH, Mathias RA and Barnes KC (2007). “Polymorphisms in the myosin light chain kinase gene that confer risk of severe sepsis are associated with a lower risk of asthma.” J Allergy Clin Immunol 119(5): 1111–1118. [DOI] [PubMed] [Google Scholar]
- Garcia-Ponce A, Citalan-Madrid AF, Velazquez-Avila M, Vargas-Robles H and Schnoor M (2015). “The role of actin-binding proteins in the control of endothelial barrier integrity.” Thromb Haemost 113(1): 20–36. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Davis HW and Patterson CE (1995). “Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation.” J Cell Physiol 163(3): 510–522. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ and Verin AD (1997). “Myosin light chain kinase in endothelium: molecular cloning and regulation.” Am J Respir Cell Mol Biol 16(5): 489–494. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR and English D (2001). “Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement.” The Journal of clinical investigation 108(5): 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG and Schaphorst KL (1995). “Regulation of endothelial cell gap formation and paracellular permeability.” J Investig Med 43(2): 117–126. [PubMed] [Google Scholar]
- Garcia JG, Verin AD and Schaphorst KL (1996). “Regulation of thrombin-mediated endothelial cell contraction and permeability.” Semin Thromb Hemost 22(4): 309–315. [DOI] [PubMed] [Google Scholar]
- Garcia Ponce A, Citalan Madrid AF, Vargas Robles H, Chanez Paredes S, Nava P, Betanzos A, Zarbock A, Rottner K, Vestweber D and Schnoor M (2016). “Loss of cortactin causes endothelial barrier dysfunction via disturbed adrenomedullin secretion and actomyosin contractility.” Sci Rep 6: 29003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SE, Rohan M and Mehta S (2015). “Role of pulmonary microvascular endothelial cell apoptosis in murine sepsis-induced lung injury in vivo.” Respir Res 16: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum SE, Ding X, Funk SE and Sage EH (1994). “SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function.” Proc Natl Acad Sci U S A 91(8): 3448–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg NM and Kuebler WM (2015). “Endothelial cell regulation of pulmonary vascular tone, inflammation, and coagulation.” Compr Physiol 5(2): 531–559. [DOI] [PubMed] [Google Scholar]
- Goldmann WH, Auernheimer V, Thievessen I and Fabry B (2013). “Vinculin, cell mechanics and tumour cell invasion.” Cell Biol Int 37(5): 397–405. [DOI] [PubMed] [Google Scholar]
- Gong H, Gao X, Feng S, Siddiqui MR, Garcia A, Bonini MG, Komarova Y, Vogel SM, Mehta D and Malik AB (2014). “Evidence of a common mechanism of disassembly of adherens junctions through Galpha13 targeting of VE-cadherin.” J Exp Med 211(3): 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorshkova I, He D, Berdyshev E, Usatuyk P, Burns M, Kalari S, Zhao Y, Pendyala S, Garcia JG, Pyne NJ, Brindley DN and Natarajan V (2008). “Protein kinase C-epsilon regulates sphingosine 1-phosphate-mediated migration of human lung endothelial cells through activation of phospholipase D2, protein kinase C-zeta, and Rac1.” J Biol Chem 283(17): 11794–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulino-Debrac D (2013). “Mechanotransduction at the basis of endothelial barrier function.” Tissue Barriers 1(2): e24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Wu MH, Granger HJ and Yuan SY (2005). “Focal adhesion kinase in neutrophil-induced microvascular hyperpermeability.” Microcirculation 12(2): 223–232. [DOI] [PubMed] [Google Scholar]
- Guo X, Wang L, Chen B, Li Q, Wang J, Zhao M, Wu W, Zhu P, Huang X and Huang Q (2009). “ERM protein moesin is phosphorylated by advanced glycation end products and modulates endothelial permeability.” Am J Physiol Heart Circ Physiol 297(1): H238–246. [DOI] [PubMed] [Google Scholar]
- Haines RJ, Beard RS Jr. and Wu MH (2015). “Protein tyrosine kinase 6 mediates TNFalpha-induced endothelial barrier dysfunction.” Biochem Biophys Res Commun 456(1): 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Oskeritzian CA, Paugh SW, Milstien S and Spiegel S (2006). “Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases.” Biochimica et biophysica acta 1758(12): 2016–2026. [DOI] [PubMed] [Google Scholar]
- Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, Klein RL, Hannun YA, Bielawski J and Bielawska A (2010). “Blood sphingolipidomics in healthy humans: impact of sample collection methodology.” J Lipid Res 51(10): 3074–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harke B (2012). “A nanoscopic tool by combining AFM with STED micrscopy.” Opt Nanosc 1(3). [Google Scholar]
- Hartsock A and Nelson WJ (2008). “Adherens and tight junctions: structure, function and connections to the actin cytoskeleton.” Biochim Biophys Acta 1778(3): 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M, Wojcik M, Kim D, Mahmoudi M, Li W and Xu K (2017). “Correlative Super-Resolution Microscopy: New Dimensions and New Opportunities.” Chem Rev 117(11): 7428–7456. [DOI] [PubMed] [Google Scholar]
- Heimann K, Percival JM, Weinberger R, Gunning P and Stow JL (1999). “Specific isoforms of actin-binding proteins on distinct populations of Golgi-derived vesicles.” J Biol Chem 274(16): 10743–10750. [DOI] [PubMed] [Google Scholar]
- Hein B, Willig KI and Hell SW (2008). “Stimulated emission depletion (STED) nanoscopy of a fluorescent protein-labeled organelle inside a living cell.” Proc Natl Acad Sci U S A 105(38): 14271–14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzmann R and Huser T (2017). “Super-Resolution Structured Illumination Microscopy.” Chem Rev 117(23): 13890–13908. [DOI] [PubMed] [Google Scholar]
- Hetrick B, Han MS, Helgeson LA and Nolen BJ (2013). “Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change.” Chem Biol 20(5): 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs HN and Pollard TD (2000). “Activation by Cdc42 and PIP(2) of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex.” J Cell Biol 150(6): 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs HN and Pollard TD (2001). “Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins.” Annu Rev Biochem 70: 649–676. [DOI] [PubMed] [Google Scholar]
- Hirata E and Kiyokawa E (2016). “Future Perspective of Single-Molecule FRET Biosensors and Intravital FRET Microscopy.” Biophys J 111(6): 1103–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HY, Rohatgi R, Lebensohn AM, Le M, Li J, Gygi SP and Kirschner MW (2004). “Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex.” Cell 118(2): 203–216. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, Reynolds AR and Reynolds LE (2003). “Integrins in angiogenesis: multitalented molecules in a balancing act.” Cell Tissue Res 314(1): 131–144. [DOI] [PubMed] [Google Scholar]
- Howe GA, Xiao B, Zhao HJ, Al-Zahrani KN, Hasim MS, Villeneuve J, Sekhon HS, Goss GD, Sabourin LA, Dimitroulakos J and Addison CL (2016). “Focal Adhesion Kinase Inhibitors in Combination with Erlotinib Demonstrate Enhanced Anti-Tumor Activity in Non-Small Cell Lung Cancer.” Plos One 11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Tee YH, Kabla A, Zaidel-Bar R, Bershadsky A and Hersen P (2015). “Structured illumination microscopy reveals focal adhesions are composed of linear subunits.” Cytoskeleton (Hoboken) 72(5): 235–245. [DOI] [PubMed] [Google Scholar]
- Huang X, Fan J, Li L, Liu H, Wu R, Wu Y, Wei L, Mao H, Lal A, Xi P, Tang L, Zhang Y, Liu Y, Tan S and Chen L (2018). “Fast, long-term, super-resolution imaging with Hessian structured illumination microscopy.” Nat Biotechnol 36(5): 451–459. [DOI] [PubMed] [Google Scholar]
- Infusino GA and Jacobson JR (2012). “Endothelial FAK as a therapeutic target in disease.” Microvasc Res 83(1): 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE (2002). “Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology.” Circ Res 91(10): 877–887. [DOI] [PubMed] [Google Scholar]
- Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A and Igarashi Y (2007). “Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes.” Biochemical and biophysical research communications 357(1): 212–217. [DOI] [PubMed] [Google Scholar]
- Iyer S, Ferreri DM, DeCocco NC, Minnear FL and Vincent PA (2004). “VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function.” Am J Physiol Lung Cell Mol Physiol 286(6): L1143–1153. [DOI] [PubMed] [Google Scholar]
- Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD and Garcia JG (2006). “Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin.” Am J Physiol Lung Cell Mol Physiol 291(2): L289–295. [DOI] [PubMed] [Google Scholar]
- Jin S and Zhou F (2009). “Lipid raft redox signaling platforms in vascular dysfunction: features and mechanisms.” Curr Atheroscler Rep 11(3): 220–226. [DOI] [PubMed] [Google Scholar]
- Johnsson AE, Dai Y, Nobis M, Baker MJ, McGhee EJ, Walker S, Schwarz JP, Kadir S, Morton JP, Myant KB, Huels DJ, Segonds-Pichon A, Sansom OJ, Anderson KI, Timpson P and Welch HCE (2014). “The Rac-FRET mouse reveals tight spatiotemporal control of Rac activity in primary cells and tissues.” Cell Rep 6(6): 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kage H, Flodby P, Gao D, Kim YH, Marconett CN, DeMaio L, Kim KJ, Crandall ED and Borok Z (2014). “Claudin 4 knockout mice: normal physiological phenotype with increased susceptibility to lung injury.” Am J Physiol Lung Cell Mol Physiol 307(7): L524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamioka Y, Sumiyama K, Mizuno R, Sakai Y, Hirata E, Kiyokawa E and Matsuda M (2012). “Live imaging of protein kinase activities in transgenic mice expressing FRET biosensors.” Cell Struct Funct 37(1): 65–73. [DOI] [PubMed] [Google Scholar]
- Kamioka Y, Takakura K, Sumiyama K and Matsuda M (2017). “Intravital Forster resonance energy transfer imaging reveals osteopontin-mediated polymorphonuclear leukocyte activation by tumor cell emboli.” Cancer Sci 108(2): 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapus A and Janmey P (2013). “Plasma membrane--cortical cytoskeleton interactions: a cell biology approach with biophysical considerations.” Compr Physiol 3(3): 1231–1281. [DOI] [PubMed] [Google Scholar]
- Karki P and Birukova AA (2018). “Substrate stiffness-dependent exacerbation of endothelial permeability and inflammation: mechanisms and potential implications in ALI and PH (2017 Grover Conference Series).” Pulm Circ 8(2): 2045894018773044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasa A, Csortos C and Verin AD (2015). “Cytoskeletal mechanisms regulating vascular endothelial barrier function in response to acute lung injury.” Tissue Barriers 3(1–2): e974448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevil CG, Okayama N, Trocha SD, Kalogeris TJ, Coe LL, Specian RD, Davis CP and Alexander JS (1998). “Expression of zonula occludens and adherens junctional proteins in human venous and arterial endothelial cells: role of occludin in endothelial solute barriers.” Microcirculation 5(2–3): 197–210. [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR and Wheelock MJ (1995). “Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin.” J Cell Biol 130(1): 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova Y and Malik AB (2010). “Regulation of endothelial permeability via paracellular and transcellular transport pathways.” Annu Rev Physiol 72: 463–493. [DOI] [PubMed] [Google Scholar]
- Komarova YA, Kruse K, Mehta D and Malik AB (2017). “Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability.” Circ Res 120(1): 179–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V, Hume PJ, Humphreys D, Liu T, Horning O, Jensen ON and McGhie EJ (2011). “WAVE regulatory complex activation by cooperating GTPases Arf and Rac1.” Proc Natl Acad Sci U S A 108(35): 14449–14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss M, Pfeiffer GR 2nd, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, Doerschuk CM and Wang Q (2006). “Ezrin/radixin/moesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells.” J Immunol 176(2): 1218–1227. [DOI] [PubMed] [Google Scholar]
- Koster DV and Mayor S (2016). “Cortical actin and the plasma membrane: inextricably intertwined.” Curr Opin Cell Biol 38: 81–89. [DOI] [PubMed] [Google Scholar]
- Kottke MA and Walters TJ (2016). “Where’s the Leak in Vascular Barriers? A Review.” Shock 46(3 Suppl 1): 20–36. [DOI] [PubMed] [Google Scholar]
- Kovacs-Kasa A, Gorshkov BA, Kim KM, Kumar S, Black SM, Fulton DJ, Dimitropoulou C, Catravas JD and Verin AD (2016). “The protective role of MLCP-mediated ERM dephosphorylation in endotoxin-induced lung injury in vitro and in vivo.” Sci Rep 6: 39018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR and Pollard TD (2004). “Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces.” Proceedings of the National Academy of Sciences of the United States of America 101(41): 14725–14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler WM (2011). “Real-time imaging assessment of pulmonary vascular responses.” Proc Am Thorac Soc 8(6): 458–465. [DOI] [PubMed] [Google Scholar]
- Kuo JC (2014). “Focal adhesions function as a mechanosensor.” Prog Mol Biol Transl Sci 126: 55–73. [DOI] [PubMed] [Google Scholar]
- Lambrechts A, Van Troys M and Ampe C (2004). “The actin cytoskeleton in normal and pathological cell motility.” Int J Biochem Cell Biol 36(10): 1890–1909. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA and Germain RN (2013). “Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo.” Nature 498(7454): 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP and Dejana E (1992). “A novel endothelial-specific membrane protein is a marker of cell-cell contacts.” J Cell Biol 118(6): 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassing I and Lindberg U (1985). “Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin.” Nature 314(6010): 472–474. [DOI] [PubMed] [Google Scholar]
- Leckband DE and de Rooij J (2014). “Cadherin adhesion and mechanotransduction.” Annu Rev Cell Dev Biol 30: 291–315. [DOI] [PubMed] [Google Scholar]
- Lederer P, Zhou T, Chen W, Epshtein Y, Wang H, Mathew B and Jacobson JR (2018). “Attenuation of murine acute lung injury by PF-573,228, an inhibitor of focal adhesion kinase.” Vascul Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, Lominadze D and Lee MJ (2009). “Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature.” Am J Physiol Heart Circ Physiol 296(1): H33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JF, Ozaki H, Zhan X, Wang E, Hla T and Lee MJ (2006). “Sphingosine-1-phosphate signaling regulates lamellipodia localization of cortactin complexes in endothelial cells.” Histochem Cell Biol 126(3): 297–304. [DOI] [PubMed] [Google Scholar]
- Lee JS and Gotlieb AI (2002). “Microtubule-actin interactions may regulate endothelial integrity and repair.” Cardiovasc Pathol 11(3): 135–140. [DOI] [PubMed] [Google Scholar]
- Lee OH, Lee DJ, Kim YM, Kim YS, Kwon HJ, Kim KW and Kwon YG (2000). “Sphingosine 1-phosphate stimulates tyrosine phosphorylation of focal adhesion kinase and chemotactic motility of endothelial cells via the G(i) protein-linked phospholipase C pathway.” Biochem Biophys Res Commun 268(1): 47–53. [DOI] [PubMed] [Google Scholar]
- Lee YH, Kayyali US, Sousa AM, Rajan T, Lechleider RJ and Day RM (2007). “Transforming growth factor-beta1 effects on endothelial monolayer permeability involve focal adhesion kinase/Src.” Am J Respir Cell Mol Biol 37(4): 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate KR, Takahashi S, Bonakdar N, Fabry B, Boettiger D, Zent R and Fassler R (2011). “Integrin adhesion and force coupling are independently regulated by localized PtdIns(4,5)2 synthesis.” Embo j 30(22): 4539–4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelkes E, Headley MB, Thornton EE, Looney MR and Krummel MF (2014). “The spatiotemporal cellular dynamics of lung immunity.” Trends Immunol 35(8): 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J and Russell B (2013). “Phosphatidylinositol 4,5-bisphosphate regulates CapZbeta1 and actin dynamics in response to mechanical strain.” Am J Physiol Heart Circ Physiol 305(11): H1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Chen B, Zeng C, Fan A, Yuan Y, Guo X, Huang X and Huang Q (2015). “Differential activation of receptors and signal pathways upon stimulation by different doses of sphingosine-1-phosphate in endothelial cells.” Exp Physiol 100(1): 95–107. [DOI] [PubMed] [Google Scholar]
- Li Y, Uruno T, Haudenschild C, Dudek SM, Garcia JG and Zhan X (2004). “Interaction of cortactin and Arp2/3 complex is required for sphingosine-1-phosphate-induced endothelial cell remodeling.” Exp Cell Res 298(1): 107–121. [DOI] [PubMed] [Google Scholar]
- Lichtman JW and Conchello JA (2005). “Fluorescence microscopy.” Nat Methods 2(12): 910–919. [DOI] [PubMed] [Google Scholar]
- Lingwood D and Simons K (2010). “Lipid rafts as a membrane-organizing principle.” Science 327(5961): 46–50. [DOI] [PubMed] [Google Scholar]
- Liu W, Toussaint KC, Okoro C, Zhu D, Chen Y, Kuang C and Liu X (2018). “Breaking the Axial Diffraction Limit: A Guide to Axial Super-Resolution Fluorescence Microscopy.” Laser & Photonics Reviews 12(8). [Google Scholar]
- Looney MR and Bhattacharya J (2014). “Live imaging of the lung.” Annu Rev Physiol 76: 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW and Krummel MF (2011). “Stabilized imaging of immune surveillance in the mouse lung.” Nat Methods 8(1): 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low FN (1953). “The pulmonary alveolar epithelium of laboratory mammals and man.” Anat Rec 117(2): 241–263. [DOI] [PubMed] [Google Scholar]
- Lu-Walther HW, Kielhorn M, Forster R, Jost A, Wicker K and Heintzmann R (2015). “fastSIM: a practical implementation of fast structured illumination microscopy.” Methods Appl Fluoresc 3(1): 014001. [DOI] [PubMed] [Google Scholar]
- Lu S and Wang Y (2014). “Single-cell imaging of mechanotransduction in endothelial cells.” Prog Mol Biol Transl Sci 126: 25–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luissint AC, Nusrat A and Parkos CA (2014). “JAM-related proteins in mucosal homeostasis and inflammation.” Semin Immunopathol 36(2): 211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan-Thakur S, Bohm A, Jedlitschky G, Schror K and Rauch BH (2015). “Sphingosine-1-Phosphate and Its Receptors: A Mutual Link between Blood Coagulation and Inflammation.” Mediators Inflamm 2015: 831059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina JN and West JB (2005). “Thin and strong! The bioengineering dilemma in the structural and functional design of the blood-gas barrier.” Physiol Rev 85(3): 811–844. [DOI] [PubMed] [Google Scholar]
- Maissa N, Covarelli V, Janel S, Durel B, Simpson N, Bernard SC, Pardo-Lopez L, Bouzinba-Segard H, Faure C, Scott MGH, Coureuil M, Morand PC, Lafont F, Nassif X, Marullo S and Bourdoulous S (2017). “Strength of Neisseria meningitidis binding to endothelial cells requires highly-ordered CD147/beta2-adrenoceptor clusters assembled by alpha-actinin-4.” Nat Commun 8: 15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G and Palade GE (1961). “Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study.” J Biophys Biochem Cytol 11: 571–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AB, Lynch JJ and Cooper JA (1989). “Endothelial barrier function.” J Invest Dermatol 93(2 Suppl): 62s–67s. [DOI] [PubMed] [Google Scholar]
- Mambetsariev I, Tian Y, Wu T, Lavoie T, Solway J, Birukov KG and Birukova AA (2014). “Stiffness-activated GEF-H1 expression exacerbates LPS-induced lung inflammation.” PLoS One 9(4): e92670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVerry BJ and Garcia JG (2004). “Endothelial cell barrier regulation by sphingosine 1-phosphate.” J Cell Biochem 92(6): 1075–1085. [DOI] [PubMed] [Google Scholar]
- McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA and Garcia JG (2004). “Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury.” Am J Respir Crit Care Med 170(9): 987–993. [DOI] [PubMed] [Google Scholar]
- McVey M, Tabuchi A and Kuebler WM (2012). “Microparticles and acute lung injury.” Am J Physiol Lung Cell Mol Physiol 303(5): L364–381. [DOI] [PubMed] [Google Scholar]
- Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD and Malik AB (2003). “RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability.” J Biol Chem 278(35): 33492–33500. [DOI] [PubMed] [Google Scholar]
- Mehta D and Malik AB (2006). “Signaling mechanisms regulating endothelial permeability.” Physiol Rev 86(1): 279–367. [DOI] [PubMed] [Google Scholar]
- Mehta D, Ravindran K and Kuebler WM (2014). “Novel regulators of endothelial barrier function.” Am J Physiol Lung Cell Mol Physiol 307(12): L924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B, Hoover R, Jones MR, Berry LC Jr. and Brigham KL (1989). “In vitro effects of endotoxin on bovine and sheep lung microvascular and pulmonary artery endothelial cells.” J Cell Physiol 138(1): 165–174. [DOI] [PubMed] [Google Scholar]
- Mineo C and Shaul PW (2006). “Circulating cardiovascular disease risk factors and signaling in endothelial cell caveolae.” Cardiovasc Res 70(1): 31–41. [DOI] [PubMed] [Google Scholar]
- Minshall RD and Malik AB (2006). “Transport across the endothelium: regulation of endothelial permeability.” Handb Exp Pharmacol(176 Pt 1): 107–144. [DOI] [PubMed] [Google Scholar]
- Minshall RD, Sessa WC, Stan RV, Anderson RG and Malik AB (2003). “Caveolin regulation of endothelial function.” Am J Physiol Lung Cell Mol Physiol 285(6): L1179–1183. [DOI] [PubMed] [Google Scholar]
- Mohan Rao LV, Esmon CT and Pendurthi UR (2014). “Endothelial cell protein C receptor: a multiliganded and multifunctional receptor.” Blood 124(10): 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkemoller V, Oie C, Hubner W, Huser T and McCourt P (2015). “Multimodal super-resolution optical microscopy visualizes the close connection between membrane and the cytoskeleton in liver sinusoidal endothelial cell fenestrations.” Sci Rep 5: 16279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morone N, Fujiwara T, Murase K, Kasai RS, Ike H, Yuasa S, Usukura J and Kusumi A (2006). “Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography.” J Cell Biol 174(6): 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui KL, Chen CS and Assoian RK (2016). “The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces.” Journal of Cell Science 129(6): 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Tessema M and Wandinger-Ness A (2006). “Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function.” Circ Res 98(6): 743–756. [DOI] [PubMed] [Google Scholar]
- Muller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE and Krause G (2005). “The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1.” J Biol Chem 280(5): 3747–3756. [DOI] [PubMed] [Google Scholar]
- Nabi IR and Le PU (2003). “Caveolae/raft-dependent endocytosis.” J Cell Biol 161(4): 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA and Kowalczyk AP (2012). “p120-catenin binding masks an endocytic signal conserved in classical cadherins.” J Cell Biol 199(2): 365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisch AL and Fehon RG (2011). “Ezrin, Radixin and Moesin: key regulators of membrane-cortex interactions and signaling.” Curr Opin Cell Biol 23(4): 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Epshtein Y, Chen W, Zhou T, Xie L, Garcia JG and Jacobson JR (2014). “Interaction of integrin beta4 with S1P receptors in S1P- and HGF-induced endothelial barrier enhancement.” J Cell Biochem 115(6): 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR and Wheelock MJ (1997). “Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin.” J Cell Sci 110 (Pt 8): 1013–1022. [DOI] [PubMed] [Google Scholar]
- Nobis M, Herrmann D, Warren SC, Kadir S, Leung W, Killen M, Magenau A, Stevenson D, Lucas MC, Reischmann N, Vennin C, Conway JRW, Boulghourjian A, Zaratzian A, Law AM, Gallego-Ortega D, Ormandy CJ, Walters SN, Grey ST, Bailey J, Chtanova T, Quinn JMW, Baldock PA, Croucher PI, Schwarz JP, Mrowinska A, Zhang L, Herzog H, Masedunskas A, Hardeman EC, Gunning PW, Del Monte-Nieto G, Harvey RP, Samuel MS, Pajic M, McGhee EJ, Johnsson AE, Sansom OJ, Welch HCE, Morton JP, Strathdee D, Anderson KI and Timpson P (2017). “A RhoA-FRET Biosensor Mouse for Intravital Imaging in Normal Tissue Homeostasis and Disease Contexts.” Cell Rep 21(1): 274–288. [DOI] [PubMed] [Google Scholar]
- Nolen BJ, Littlefield RS and Pollard TD (2004). “Crystal structures of actin-related protein 2/3 complex with bound ATP or ADP.” Proc Natl Acad Sci U S A 101(44): 15627–15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen BJ, Tomasevic N, Russell A, Pierce DW, Jia Z, McCormick CD, Hartman J, Sakowicz R and Pollard TD (2009). “Characterization of two classes of small molecule inhibitors of Arp2/3 complex.” Nature 460(7258): 1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollmann M and Georgieva M (2015). “Superresolution microscopy for bioimaging at the nanoscale: from concepts to applications in the nucleus.” Research and Reports in Biology. [Google Scholar]
- Noren NK, Liu BP, Burridge K and Kreft B (2000). “p120 catenin regulates the actin cytoskeleton via Rho family GTPases.” J Cell Biol 150(3): 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa CD and Stevens T (2012). “Studies on the cell biology of interendothelial cell gaps.” Am J Physiol Lung Cell Mol Physiol 302(3): L275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt PD, Shivanandan A, Deschout H, Jankele R, Nievergelt AP, Feletti L, Davidson MW, Radenovic A and Fantner GE (2015). “High-Resolution Correlative Microscopy: Bridging the Gap between Single Molecule Localization Microscopy and Atomic Force Microscopy.” Nano Lett 15(8): 4896–4904. [DOI] [PubMed] [Google Scholar]
- Oldenburg J and de Rooij J (2014). “Mechanical control of the endothelial barrier.” Cell Tissue Res 355(3): 545–555. [DOI] [PubMed] [Google Scholar]
- Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, Kurtcuoglu V, Poulikakos D, Baluk P, McDonald D, Grazia Lampugnani M and Dejana E (2012). “Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo.” Nat Commun 3: 1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ and Condeelis J (2010). “Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia.” J Cell Sci 123(Pt 21): 3662–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I, Choe K, Seo H, Hwang Y, Song E, Ahn J, Hwan Jo Y and Kim P (2018). “Intravital imaging of a pulmonary endothelial surface layer in a murine sepsis model.” Biomed Opt Express 9(5): 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JC, Stevens T, Randall J, Weber DS and King JA (2006). “Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers.” Am J Physiol Lung Cell Mol Physiol 291(1): L30–37. [DOI] [PubMed] [Google Scholar]
- Parsons JT (2003). “Focal adhesion kinase: the first ten years.” J Cell Sci 116(Pt 8): 1409–1416. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Slack-Davis J, Tilghman R and Roberts WG (2008). “Focal adhesion kinase: targeting adhesion signaling pathways for therapeutic intervention.” Clin Cancer Res 14(3): 627–632. [DOI] [PubMed] [Google Scholar]
- Patterson CE and Lum H (2001). “Update on pulmonary edema: the role and regulation of endothelial barrier function.” Endothelium 8(2): 75–105. [DOI] [PubMed] [Google Scholar]
- Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM and Garcia JG (2004). “Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury.” Am J Respir Crit Care Med 169(11): 1245–1251. [DOI] [PubMed] [Google Scholar]
- Petit V and Thiery JP (2000). “Focal adhesions: structure and dynamics.” Biol Cell 92(7): 477–494. [DOI] [PubMed] [Google Scholar]
- Pollard TD (2007). “Regulation of actin filament assembly by Arp2/3 complex and formins.” Annu Rev Biophys Biomol Struct 36: 451–477. [DOI] [PubMed] [Google Scholar]
- Pollard TD (2016). “Actin and Actin-Binding Proteins.” Cold Spring Harb Perspect Biol 8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Blanchoin L and Mullins RD (2000). “Molecular mechanisms controlling actin filament dynamics in nonmuscle cells.” Annu Rev Biophys Biomol Struct 29: 545–576. [DOI] [PubMed] [Google Scholar]
- Pollard TD and Borisy GG (2003). “Cellular motility driven by assembly and disassembly of actin filaments.” Cell 112(4): 453–465. [DOI] [PubMed] [Google Scholar]
- Prasain N and Stevens T (2009). “The actin cytoskeleton in endothelial cell phenotypes.” Microvasc Res 77(1): 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predescu SA, Predescu DN and Malik AB (2007). “Molecular determinants of endothelial transcytosis and their role in endothelial permeability.” Am J Physiol Lung Cell Mol Physiol 293(4): L823–842. [DOI] [PubMed] [Google Scholar]
- Qin G-G, Li W-H, Xu J-C, Kou X-L, Zhao R, Luo F and Fang X-H (2017). “Development of Integrated Atomic Force Microscopy and Fluorescence Microscopy for Single-Molecule Analysis in Living Cells.” Chinese Journal of Analytical Chemistry 45(12): 1813–1823. [Google Scholar]
- Quest AF, Leyton L and Parraga M (2004). “Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease.” Biochem Cell Biol 82(1): 129–144. [DOI] [PubMed] [Google Scholar]
- Rao M and Mayor S (2014). “Active organization of membrane constituents in living cells.” Curr Opin Cell Biol 29: 126–132. [DOI] [PubMed] [Google Scholar]
- Reglero-Real N, Colom B, Bodkin JV and Nourshargh S (2016). “Endothelial Cell Junctional Adhesion Molecules: Role and Regulation of Expression in Inflammation.” Arterioscler Thromb Vasc Biol 36(10): 2048–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C, Athman R, Robine S and Louvard D (2004). “The co-workers of actin filaments: from cell structures to signals.” Nat Rev Mol Cell Biol 5(8): 635–646. [DOI] [PubMed] [Google Scholar]
- Riedl J, Flynn KC, Raducanu A, Gartner F, Beck G, Bosl M, Bradke F, Massberg S, Aszodi A, Sixt M and Wedlich-Soldner R (2010). “Lifeact mice for studying F-actin dynamics.” Nat Methods 7(3): 168–169. [DOI] [PubMed] [Google Scholar]
- Romer LH, Birukov KG and Garcia JG (2006). “Focal adhesions: paradigm for a signaling nexus.” Circulation research 98(5): 606–616. [DOI] [PubMed] [Google Scholar]
- Rosen H, Gonzalez-Cabrera PJ, Sanna MG and Brown S (2009). “Sphingosine 1-phosphate receptor signaling.” Annu Rev Biochem 78: 743–768. [DOI] [PubMed] [Google Scholar]
- Rottner K and Kage F (2017). “Actin Networks: Adapting to Load through Geometry.” Curr Biol 27(23): R1274–r1277. [DOI] [PubMed] [Google Scholar]
- Rouiller I, Xu XP, Amann KJ, Egile C, Nickell S, Nicastro D, Li R, Pollard TD, Volkmann N and Hanein D (2008). “The structural basis of actin filament branching by the Arp2/3 complex.” J Cell Biol 180(5): 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl SJ, Hell SW and Jakobs S (2017). “Fluorescence nanoscopy in cell biology.” Nat Rev Mol Cell Biol 18(11): 685–701. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T and Tsukita S (2000). “Complex phenotype of mice lacking occludin, a component of tight junction strands.” Mol Biol Cell 11(12): 4131–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, Wang T, Mathew B, Husain A, Moitra J, Sun X, Nunez L, Jacobson JR, Dudek SM, Natarajan V and Garcia JG (2010). “Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung.” Am J Respir Cell Mol Biol 43(4): 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson MJ, Smith I, Parker I and Bootman MD (2014). “Fluorescence microscopy.” Cold Spring Harb Protoc 2014(10): pdb top071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M and Heilemann M (2017). “Single-Molecule Localization Microscopy in Eukaryotes.” Chem Rev 117(11): 7478–7509. [DOI] [PubMed] [Google Scholar]
- Schaller MD (2001). “Biochemical signals and biological responses elicited by the focal adhesion kinase.” Biochim Biophys Acta 1540(1): 1–21. [DOI] [PubMed] [Google Scholar]
- Schaphorst KL, Chiang E, Jacobs KN, Zaiman A, Natarajan V, Wigley F and Garcia JG (2003). “Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products.” Am J Physiol Lung Cell Mol Physiol 285(1): L258–267. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T and van der Geer P (1994). “Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase.” Nature 372(6508): 786–791. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR and Sieg DJ (1999). “Signaling through focal adhesion kinase.” Prog Biophys Mol Biol 71(3–4): 435–478. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK and Ilic D (2004). “Control of motile and invasive cell phenotypes by focal adhesion kinase.” Biochim Biophys Acta 1692(2–3): 77–102. [DOI] [PubMed] [Google Scholar]
- Schnittler H (2016). “Contraction of endothelial cells: 40 years of research, but the debate still lives.” Histochem Cell Biol 146(6): 651–656. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Jacobson BS and Dvorak AM (1995). “Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca(2+)-ATPase, and inositol trisphosphate receptor.” Proc Natl Acad Sci U S A 92(5): 1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor M, Lai FP, Zarbock A, Klaver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K and Vestweber D (2011). “Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo.” J Exp Med 208(8): 1721–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebach J, Donnert G, Kronstein R, Werth S, Wojciak-Stothard B, Falzarano D, Mrowietz C, Hell SW and Schnittler HJ (2007). “Regulation of endothelial barrier function during flow-induced conversion to an arterial phenotype.” Cardiovasc Res 75(3): 596–607. [DOI] [PubMed] [Google Scholar]
- Sewald X (2018). “Visualizing Viral Infection In Vivo by Multi-Photon Intravital Microscopy.” Viruses 10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Santiskulvong C, Bentolila LA, Rao J, Dorigo O and Gimzewski JK (2012). “Correlative nanomechanical profiling with super-resolution F-actin imaging reveals novel insights into mechanisms of cisplatin resistance in ovarian cancer cells.” Nanomedicine 8(5): 757–766. [DOI] [PubMed] [Google Scholar]
- Shelden EA, Colburn ZT and Jones JC (2016). “Focusing super resolution on the cytoskeleton.” F1000Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Rigor RR, Pivetti CD, Wu MH and Yuan SY (2010). “Myosin light chain kinase in microvascular endothelial barrier function.” Cardiovasc Res 87(2): 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutova MS, Spessott WA, Giraudo CG and Svitkina T (2014). “Endogenous species of mammalian nonmuscle myosin IIA and IIB include activated monomers and heteropolymers.” Curr Biol 24(17): 1958–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvetsov A, Berkane E, Chereau D, Dominguez R and Reisler E (2009). “The actin-binding domain of cortactin is dynamic and unstructured and affects lateral and longitudinal contacts in F-actin.” Cell Motil Cytoskeleton 66(2): 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH and Schlaepfer DD (2000). “FAK integrates growth-factor and integrin signals to promote cell migration.” Nat Cell Biol 2(5): 249–256. [DOI] [PubMed] [Google Scholar]
- Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB and Mehta D (2007). “Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin.” J Biol Chem 282(11): 7833–7843. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Dudek SM, Chiang ET and Garcia JG (2005). “Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin.” Faseb J 19(12): 1646–1656. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Dudek SM, Ma SF and Garcia JG (2006). “Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family.” J Biol Chem 281(45): 34381–34393. [DOI] [PubMed] [Google Scholar]
- Sparks AB, Rider JE, Hoffman NG, Fowlkes DM, Quillam LA and Kay BK (1996). “Distinct ligand preferences of Src homology 3 domains from Src, Yes, Abl, Cortactin, p53bp2, PLCgamma, Crk, and Grb2.” Proc Natl Acad Sci U S A 93(4): 1540–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spuul P, Daubon T, Pitter B, Alonso F, Fremaux I, Kramer I, Montanez E and Genot E (2016). “VEGF-A/Notch-Induced Podosomes Proteolyse Basement Membrane Collagen-IV during Retinal Sprouting Angiogenesis.” Cell Rep 17(2): 484–500. [DOI] [PubMed] [Google Scholar]
- Stevens T, Garcia JG, Shasby DM, Bhattacharya J and Malik AB (2000). “Mechanisms regulating endothelial cell barrier function.” Am J Physiol Lung Cell Mol Physiol 279(3): L419–422. [DOI] [PubMed] [Google Scholar]
- Stevens T, Rosenberg R, Aird W, Quertermous T, Johnson FL, Garcia JG, Hebbel RP, Tuder RM and Garfinkel S (2001). “NHLBI workshop report: endothelial cell phenotypes in heart, lung, and blood diseases.” Am J Physiol Cell Physiol 281(5): C1422–1433. [DOI] [PubMed] [Google Scholar]
- Stossel TP, Chaponnier C, Ezzell RM, Hartwig JH, Janmey PA, Kwiatkowski DJ, Lind SE, Smith DB, Southwick FS, Yin HL and et al. (1985). “Nonmuscle actin-binding proteins.” Annu Rev Cell Biol 1: 353–402. [DOI] [PubMed] [Google Scholar]
- Strub GM, Maceyka M, Hait NC, Milstien S and Spiegel S (2010). “Extracellular and intracellular actions of sphingosine-1-phosphate.” Adv Exp Med Biol 688: 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukriti S, Tauseef M, Yazbeck P and Mehta D (2014). “Mechanisms regulating endothelial permeability.” Pulm Circ 4(4): 535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MY, Geyer M and Komarova YA (2017). “IP3 receptor signaling and endothelial barrier function.” Cell Mol Life Sci 74(22): 4189–4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Ma SF, Wade MS, Acosta-Herrera M, Villar J, Pino-Yanes M, Zhou T, Liu B, Belvitch P, Moitra J, Han YJ, Machado R, Noth I, Natarajan V, Dudek SM, Jacobson JR, Flores C and Garcia JG (2013). “Functional promoter variants in sphingosine 1-phosphate receptor 3 associate with susceptibility to sepsis-associated acute respiratory distress syndrome.” Am J Physiol Lung Cell Mol Physiol 305(7): L467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Singleton PA, Letsiou E, Zhao J, Belvitch P, Sammani S, Chiang ET, Moreno-Vinasco L, Wade MS, Zhou T, Liu B, Parastatidis I, Thomson L, Ischiropoulos H, Natarajan V, Jacobson JR, Machado RF, Dudek SM and Garcia JG (2012). “Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury.” Am J Respir Cell Mol Biol 47(5): 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundivakkam PC, Natarajan V, Malik AB and Tiruppathi C (2013). “Store-operated Ca2+ entry (SOCE) induced by protease-activated receptor-1 mediates STIM1 protein phosphorylation to inhibit SOCE in endothelial cells through AMP-activated protein kinase and p38beta mitogen-activated protein kinase.” J Biol Chem 288(23): 17030–17041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh K and Shimoda LA (2016). “Lung Circulation.” Compr Physiol 6(2): 897–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S and Dejana E (2008). “Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5.” Nat Cell Biol 10(8): 923–934. [DOI] [PubMed] [Google Scholar]
- Tamura A and Tsukita S (2014). “Paracellular barrier and channel functions of TJ claudins in organizing biological systems: advances in the field of barriology revealed in knockout mice.” Semin Cell Dev Biol 36: 177–185. [DOI] [PubMed] [Google Scholar]
- Tani M, Ito M and Igarashi Y (2007). “Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space.” Cellular signalling 19(2): 229–237. [DOI] [PubMed] [Google Scholar]
- Tarbell JM, Simon SI and Curry FR (2014). “Mechanosensing at the vascular interface.” Annu Rev Biomed Eng 16: 505–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB and Mehta D (2008). “Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells.” Circ Res 103(10): 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, Birnbaumer L, Malik AB and Mehta D (2012). “TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation.” J Exp Med 209(11): 1953–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK and Reynolds AB (2000). “Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion.” J Cell Biol 148(1): 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunemann M, Schmidt K, de Wit C, Han X, Jain RK, Fukumura D and Feil R (2014). “Correlative intravital imaging of cGMP signals and vasodilation in mice.” Front Physiol 5: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K and Balda MS (2015). “ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation.” J Cell Biol 208(6): 821–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble WS and Grinstein S (2015). “Barriers to the free diffusion of proteins and lipids in the plasma membrane.” J Cell Biol 208(3): 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S and Furuse M (2000). “The structure and function of claudins, cell adhesion molecules at tight junctions.” Ann N Y Acad Sci 915: 129–135. [DOI] [PubMed] [Google Scholar]
- Turner CE (2000). “Paxillin interactions.” J Cell Sci 113 Pt 23: 4139–4140. [DOI] [PubMed] [Google Scholar]
- Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E and Greenwood J (2008). “Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration.” J Cell Sci 121(Pt 1): 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Falkenburger BH, Pohlmeyer C and Inoue T (2011). “Triggering actin comets versus membrane ruffles: distinctive effects of phosphoinositides on actin reorganization.” Sci Signal 4(203): ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, Mueller SC and Zhan X (2001). “Activation of Arp2/3 complex-mediated actin polymerization by cortactin.” Nat Cell Biol 3(3): 259–266. [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, He D, Bindokas V, Gorshkova IA, Berdyshev EV, Garcia JG and Natarajan V (2011). “Photolysis of caged sphingosine-1-phosphate induces barrier enhancement and intracellular activation of lung endothelial cell signaling pathways.” Am J Physiol Lung Cell Mol Physiol 300(6): L840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Tietgens AJ and Anderson JM (2017). “Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1.” Mol Biol Cell 28(4): 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke E, Mehta D, Minshall R and Malik AB (2008). “Regulation of endothelial junctional permeability.” Ann N Y Acad Sci 1123: 134–145. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA and Malik AB (2012). “PKCalpha activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity.” Circ Res 111(6): 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL and Hla T (2006). “Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient.” The Biochemical journal 397(3): 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verin AD, Gilbert-McClain LI, Patterson CE and Garcia JG (1998). “Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium.” Am J Respir Cell Mol Biol 19(5): 767–776. [DOI] [PubMed] [Google Scholar]
- Vestweber D (2000). “Molecular mechanisms that control endothelial cell contacts.” J Pathol 190(3): 281–291. [DOI] [PubMed] [Google Scholar]
- Vicidomini G, Bianchini P and Diaspro A (2018). “STED super-resolved microscopy.” Nat Methods 15(3): 173–182. [DOI] [PubMed] [Google Scholar]
- Viswanathan P, Ephstein Y, Garcia JG, Cho M and Dudek SM (2016). “Differential elastic responses to barrier-altering agonists in two types of human lung endothelium.” Biochem Biophys Res Commun 478(2): 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel SM and Malik AB (2012). “Cytoskeletal dynamics and lung fluid balance.” Compr Physiol 2(1): 449–478. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V, Boquet P, Pouyssegur J and Van Obberghen-Schilling E (1998). “Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function.” Mol Biol Cell 9(9): 2639–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Bittman R, Garcia JG and Dudek SM (2015). “Junctional complex and focal adhesion rearrangement mediates pulmonary endothelial barrier enhancement by FTY720 S-phosphonate.” Microvasc Res 99: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L and Dudek SM (2009). “Regulation of vascular permeability by sphingosine 1-phosphate.” Microvascular research 77(1): 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L and Dudek SM (2009). “Regulation of vascular permeability by sphingosine 1-phosphate.” Microvasc Res 77(1): 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Brown ME, Kelly GT, Camp SM, Mascarenhas JB, Sun X, Dudek SM and Garcia JGN (2018). “Myosin light chain kinase (MYLK) coding polymorphisms modulate human lung endothelial cell barrier responses via altered tyrosine phosphorylation, spatial localization, and lamellipodial protrusions.” Pulm Circ 8(2): 2045894018764171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bleher R, Brown ME, Garcia JG, Dudek SM, Shekhawat GS and Dravid VP (2015). “Nano-Biomechanical Study of Spatio-Temporal Cytoskeleton Rearrangements that Determine Subcellular Mechanical Properties and Endothelial Permeability.” Sci Rep 5: 11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bleher R, Wang L, Garcia JGN, Dudek SM, Shekhawat GS and Dravid VP (2017). “Imatinib Alters Agonists-mediated Cytoskeletal Biomechanics in Lung Endothelium.” Sci Rep 7(1): 14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang L, Garcia JGN, Dudek SM, Shekhawat GS and Dravid VP (2018). “The Significant Role of c-Abl Kinase in Barrier Altering Agonists-mediated Cytoskeletal Biomechanics.” Sci Rep 8(1): 1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y and Kanchanawong P (2016). “Three-dimensional Super Resolution Microscopy of F-actin Filaments by Interferometric PhotoActivated Localization Microscopy (iPALM).” J Vis Exp(118). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED and Takeichi M (1998). “alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells.” J Cell Biol 142(3): 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed SA and Parsons JT (2001). “Cortactin: coupling membrane dynamics to cortical actin assembly.” Oncogene 20(44): 6418–6434. [DOI] [PubMed] [Google Scholar]
- Wegel E, Gohler A, Lagerholm BC, Wainman A, Uphoff S, Kaufmann R and Dobbie IM (2016). “Imaging cellular structures in super-resolution with SIM, STED and Localisation Microscopy: A practical comparison.” Sci Rep 6: 27290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert R, Sramkova M, Parente L, Amornphimoltham P and Masedunskas A (2010). “Intravital microscopy: a novel tool to study cell biology in living animals.” Histochem Cell Biol 133(5): 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB (1985). “Fragility of pulmonary capillaries.” J Appl Physiol (1985) 115(1): 1–15. [DOI] [PubMed] [Google Scholar]
- West JB (2013). “Role of the fragility of the pulmonary blood-gas barrier in the evolution of the pulmonary circulation.” Am J Physiol Regul Integr Comp Physiol 304(3): R171–176. [DOI] [PubMed] [Google Scholar]
- West JB and Mathieu-Costello O (1992). “Strength of the pulmonary blood-gas barrier.” Respir Physiol 88(1–2): 141–148. [DOI] [PubMed] [Google Scholar]
- Wildanger D, Medda R, Kastrup L and Hell SW (2009). “A compact STED microscope providing 3D nanoscale resolution.” J Microsc 236(1): 35–43. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B and Ridley AJ (2002). “Rho GTPases and the regulation of endothelial permeability.” Vascul Pharmacol 39(4–5): 187–199. [DOI] [PubMed] [Google Scholar]
- Wu C (2007). “Focal adhesion: a focal point in current cell biology and molecular medicine.” Cell Adh Migr 1(1): 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH (2005). “Endothelial focal adhesions and barrier function.” J Physiol 569(Pt 2): 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, Ustinova E and Granger HJ (2001). “Integrin binding to fibronectin and vitronectin maintains the barrier function of isolated porcine coronary venules.” J Physiol 532(Pt 3): 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Liu Y and Wang N (2014). “New paradigms in inflammatory signaling in vascular endothelial cells.” Am J Physiol Heart Circ Physiol 306(3): H317–325. [DOI] [PubMed] [Google Scholar]
- Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D and Guan JL (1994). “Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain.” Mol Biol Cell 5(4): 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Wang C, Shi L, Wang L, Zhou Z, Chen D, Wang J and Guo H (2017). “Myosin Light Chain Kinase: A Potential Target for Treatment of Inflammatory Diseases.” Front Pharmacol 8: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Waters CL, Hu C, Wysolmerski RB, Vincent PA and Minnear FL (2007). “Sphingosine 1-phosphate rapidly increases endothelial barrier function independently of VE-cadherin but requires cell spreading and Rho kinase.” Am J Physiol Cell Physiol 293(4): C1309–1318. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI and Nelson WJ (2005). “Deconstructing the cadherin-catenin-actin complex.” Cell 123(5): 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K and Ando J (2015). “Vascular endothelial cell membranes differentiate between stretch and shear stress through transitions in their lipid phases.” Am J Physiol Heart Circ Physiol 309(7): H1178–1185. [DOI] [PubMed] [Google Scholar]
- Yamauchi F, Kamioka Y, Yano T and Matsuda M (2016). “In Vivo FRET Imaging of Tumor Endothelial Cells Highlights a Role of Low PKA Activity in Vascular Hyperpermeability.” Cancer Res 76(18): 5266–5276. [DOI] [PubMed] [Google Scholar]
- Yang J, Yao W, Qian G, Wei Z, Wu G and Wang G (2015). “Rab5-mediated VE-cadherin internalization regulates the barrier function of the lung microvascular endothelium.” Cell Mol Life Sci 72(24): 4849–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang G and Schmidt EP (2013). “In vivo measurement of the mouse pulmonary endothelial surface layer.” J Vis Exp(72): e50322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Hakomori S and Igarashi Y (1995). “Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets.” Blood 86(1): 193–202. [PubMed] [Google Scholar]
- Yin HL and Janmey PA (2003). “Phosphoinositide regulation of the actin cytoskeleton.” Annu Rev Physiol 65: 761–789. [DOI] [PubMed] [Google Scholar]
- Ying L, Alvira CM and Cornfield DN (2018). “Developmental differences in focal adhesion kinase expression modulate pulmonary endothelial barrier function in response to inflammation.” Am J Physiol Lung Cell Mol Physiol 315(1): L66–L77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S and Tsukita S (1998). “Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2.” J Cell Biol 140(4): 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Yuan J, Zhang X, Liu J and Fang X (2013). “Nanoscale imaging with an integrated system combining stimulated emission depletion microscope and atomic force microscope.” Chinese Science Bulletin 58(33): 4045–4050. [Google Scholar]
- Zaidel-Bar R and Geiger B (2010). “The switchable integrin adhesome.” J Cell Sci 123(Pt 9): 1385–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R and Geiger B (2007). “Functional atlas of the integrin adhesome.” Nat Cell Biol 9(8): 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A, Bhattacharyya RP and Lim WA (2003). “The structure and function of proline recognition domains.” Sci STKE 2003(179): RE8. [DOI] [PubMed] [Google Scholar]
- Zebda N, Dubrovskyi O and Birukov KG (2012). “Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions.” Microvasc Res 83(1): 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Naik JS, Jernigan NL, Walker BR and Resta TC (2018). “Reduced membrane cholesterol after chronic hypoxia limits Orai1-mediated pulmonary endothelial Ca(2+) entry.” Am J Physiol Heart Circ Physiol 314(2): H359–h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu Y, Xuan Z, Zhang S, Wang X, Hao Y, Wu J and Zhang S (2014). “p38MAPK, Rho/ROCK and PKC pathways are involved in influenza-induced cytoskeletal rearrangement and hyperpermeability in PMVEC via phosphorylating ERM.” Virus Res 192: 6–15. [DOI] [PubMed] [Google Scholar]
- Zhang G, Xu S, Qian Y and He P (2010). “Sphingosine-1-phosphate prevents permeability increases via activation of endothelial sphingosine-1-phosphate receptor 1 in rat venules.” Am J Physiol Heart Circ Physiol 299(5): H1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Mao YS, Janmey PA and Yin HL (2012). “Phosphatidylinositol 4, 5 bisphosphate and the actin cytoskeleton.” Subcell Biochem 59: 177–215. [DOI] [PubMed] [Google Scholar]
- Zhang P, Feng S, Liu G, Wang H, Zhu H, Ren Q, Bai H, Fu C and Dong C (2016). “Mutant B-Raf(V600E) Promotes Melanoma Paracellular Transmigration by Inducing Thrombin-mediated Endothelial Junction Breakdown.” J Biol Chem 291(5): 2087–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Singleton PA, Brown ME, Dudek SM and Garcia JG (2009). “Phosphotyrosine protein dynamics in cell membrane rafts of sphingosine-1-phosphate-stimulated human endothelium: role in barrier enhancement.” Cell Signal 21(12): 1945–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Singleton PA, Brown ME, Dudek SM and Garcia JG (2009). “Phosphotyrosine protein dynamics in cell membrane rafts of sphingosine-1-phosphate-stimulated human endothelium: role in barrier enhancement.” Cellular signalling 21(12): 1945–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, Berdyshev EV and Natarajan V (2007). “Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1.” The Journal of biological chemistry 282(19): 14165–14177. [DOI] [PMC free article] [PubMed] [Google Scholar]