Abstract
In the United States, racial differences in the prevalence and incidence of HIV infection and AIDS diagnoses are dramatic. These differences are large, have been recognized for nearly 20 years, and are as yet not well investigated. These disparities show no signs of diminishing, and in fact, are widening, particularly among drug users and women. Most observers of the racial disparities in prevalence and incidence of HIV infections and AIDS diagnoses in the United States have concluded that these disparities exist because prevention messages, supplies, and/or interventions do not effectively reach those at greatest risk of infection. In essence, such interpretations suggest that Blacks and Latinos continue to practice more risk behaviors than Whites. There is much data to suggest that this is, in fact, not true. Evidence from 232 “index” injection drug users and 465 of their drug and sexual network members participating in HPTN 037 is presented. These data describe lower utilization and/or access to drug treatment and needle exchange programs by Black injectors. In addition, data indicate the co-existence of increased prevalence of HIV in the networks of uninfected Black drug users and fewer associated risk behaviors in the networks of Black and Latino indices compared with networks of White indices. Understanding racial disparities in HIV is a critical challenge yet risk behaviors alone do not explain observed disparities in infection rates.
Keywords: HIV incidence and prevalence, HIV risk behaviors, racial disparities, IDUs
INTRODUCTION
In the United States, racial differences in the prevalence and incidence of HIV infection are a defining characteristic of the AIDS epidemic. Recent data from the CDC reveal that while Blacks account for 14% of the population within states reporting HIV infection, in 2009, Blacks accounted for 44% of all diagnosed HIV infections in the US, a rate almost eight times higher than that observed in Whites (CDC, 2011). CDC data further indicate that, in 2009, Black men were more than six times more likely than White men to be diagnosed with new HIV infection, nearly two and a half times that of Latino men and more than twice that of Black women. Among women, the estimated rate of new HIV infections for Blacks was 15 times the rate for Whites, and more than three times that of Latinas. Importantly, the rate of HIV infection among Blacks has nearly doubled since the prior decade when 25% of diagnosed HIV/AIDS cases were observed among Blacks.
Racial disparities in incident infections are seen among all risk groups (Kraut-Becher, Eisenberg, Voytek et al.,, 2008). Among heterosexuals, in 2009, approximately 60% of new HIV infections were observed among Blacks and about 18% among Latinos (CDC, 2011). Among MSM, in 2009, 38% of new HIV infections occurred among Blacks and 21% were observed among Latinos (CDC, 2011). Among IDUs, in 2009, 48% of diagnoses of new HIV infection were among Blacks and 21% were observed among Latinos (CDC, 2011).
Most of the understanding of racial disparities is derived from studies of men who have sex with men (MSM) and heterosexuals, the two largest risk groups in the US. While these populations dominate present discourse around HIV infection, injection-related transmissions remain an important mode of HIV transmission. In 2009, 9% of all HIV/AIDS cases were attributable to injection drug use (CDC, 2011). Importantly, there is significant regional variation in the prevalence and incidence of HIV infection among IDUs with the highest concentrations occurring in the northeast. For example, in Philadelphia, 30.7% (8,966) of all infections have occurred among heterosexual IDU and 3.7% (1087) among MSM/IDU (AACO, 2012). Although infections caused by IDU have decreased over the past ten years at higher rates than sexual transmissions, racial disparities persist. Among US IDUs living with HIV, 48% were Black, 23% were White and 26% Latino/a. Among IDUs living with an AIDS diagnosis, 52% were Black, 19% were White, and 26% were Latino/a (CDC, 2012). In Philadelphia, 63% (3,049) of the 4,842 IDUs living with HIV/AIDS are Black, 16% (764) are White, and 21% (1,029) Latino/a.
There is now an accumulation of research findings examining the factors associated with racial disparities that suggests that Blacks’ (and Latinos’) higher rates of infection relative to Whites may not be due to higher levels of risk behavior among them, but instead to “low” or “average” levels of risk behavior within networks that have a higher prevalence of HIV infection (Klovdahl, 1985; May and Anderson, 1987; Jolly et al., 2001; Ward, 2007; De, Cox, Bouvin, Platt, & Jolly, 2007; Perisse, Langeberg, Hungerford, Boulay, Charurat, Schechter, & Blattner, 2008; Sharpe, Harrison, & Dean, 2010; Leigh, Lycett, Weinert, Hughes, Fearnhill, & Dunn, 2011; Millett, Peterson, Flores, Hart, Jeffries, Wilson, et al, 2012). Hallfors and colleagues (2007) found that among young predominantly heterosexual adults, Whites had elevated risk for STI and HIV infection when engaging in high-risk drug and sex behaviors, but Blacks’ prevalence was high regardless of the level of engagement in risky behaviors. Thus, behavioral patterns alone did not predict STI and HIV risk.
Similar observations have been found for men who have sex with men (MSM). In a recent meta-analysis published in The Lancet, it was found that Black MSM engaged in fewer HIV risk behaviors than MSM of other races. Black MSM reported less unprotected anal intercourse with main male partners, fewer male sex partners, more condom use, less substance use, and less substance use during sex (Millet et al, 2012). Moreover, Black MSM were more likely than other MSM to report preventive behaviors against HIV infection, including using condoms, being tested regularly, and using post- or pre-exposure prophylaxis (Millet et al., 2012). Despite engaging in less risky behaviors and taking more preventive action, Black MSM continue to be disproportionately infected with HIV.
Studies of IDUs have made similar observations of risk, prevalence, and race. While Black injectors have higher HIV prevalence, rates of needle sharing are consistently lower (Amesty, Rivera, Fuller, 2011; Des Jarlais, Arasteh, Hagan et al., 2009; Kottiri, Friedman, Neigus et al., 2002; Friedman, Chapman, Perlis, et al., 1999). Other studies have reported that racial differences in HIV infections among IDUs have persisted despite increased availability of prevention services and overall reductions in HIV prevalence (Des Jarlais et al, 2009). Several studies that have identified racial/ethnic differences in HIV among IDUs have focused on social and/or structural factors that are likely fueling racial disparities. Kottiri, Friedman, Neigus et al. (2002) interviewed 662 IDUs in New York City about their sex and drug risk behaviors and risk behaviors of their risk network members. They found higher prevalence and lower risk behaviors among Blacks and that sex and drug risk networks were predominantly homogeneous with respect to race.
To date, no studies able to examine racial disparities have been conducted involving behavioral assessments and HIV testing on both IDUs and members of their risk networks (individuals with whom they have sex and use drugs). Data from the Philadelphia site of HPTN 037 provided the unique opportunity to examine relationships between risk behaviors, participation in drug treatment and needle exchange, HIV prevalence and race among both uninfected IDUs and their drug and sexual partners. HPTN 037 tested a peer education intervention promoting HIV risk reduction among injection drug users and members of their drug and sex networks (Latkin, Donnell, Metzger, et al., 2009). We examine some of the key factors that might help explain higher prevalence among Black injectors, including injection and sexual practices, use of preventive services such as syringe exchange and drug treatment, and composition of injectors’ risk networks.
METHODS
Injection drug users were recruited from community settings in Philadelphia, PA between December 2002 and November 2006 in an effort to identify HIV uninfected, active IDUs for participation in HPTN 037. Specific locations for recruitment of active injection drug users were identified by ethnographic methods conducted in neighborhoods with a high prevalence of HIV/AIDS and drug use. Ethnographers conducted observations and initiated contact with community members (IDU and non-IDU) and local gatekeepers in an effort to secure community support and access to locations where IDUs could be recruited and interviewed. Twenty-five distinct ‘risk pockets’ (places where drug use, drug sales, and sex exchange occur within close proximity) were identified by ethnographers (Perkins and Metzger, 2007). Mobile assessment units were then parked adjacent to these identified risk pockets. The use of these mobile units allowed for the convenient completion of confidential informed consent process followed by brief, interviewer administered pre-screening for behavioral eligibility. Of the 3,079 drug users who completed pre-screening interviews, 84% (n=2582/3079) reported injection drug use in the six months prior to assessment and 39% of this group (1002/2582) met eligibility criteria based upon self-reports (18 years of age or older, uninfected with HIV, injected drugs at least 12 times in the prior 3 months, not enrolled in methadone maintenance treatment for at least three months, willing to recruit drug and sex partners, and without plans to relocate). Of the 3079 individuals who initially presented at the mobile assessment unit, 2.1% (66/3079) self-identified as HIV positive.
Those uninfected IDU who met behavioral eligibility criteria were invited to participate in the protocol-screening process which involved the completion of comprehensive assessments of drug use, HIV drug and sex risk behaviors, and HIV testing via Elisa with Western Blot confirmation. Information concerning the composition and characteristics of risk networks was gathered through the administration of the Social Network Inventory (Latkin, Mandell, & Vlahov, 1996). Participants were prompted to identify social network members with whom they share private and personal conversations, borrow money, socialize, ask advice, and/or discuss personal health issues. For each network member listed, information was collected for age, gender, race/ethnicity, HIV status, frequency of contact, and risk- relationship-type (drug risk, sex risk).
These assessments were completed at the project’s community based office and to be eligible index participants had to be HIV negative and able to recruit at least one of their drug using or sexual partners. If found to be negative for HIV, willing index IDU were given guidance on how to recruit members of their risk networks. The next and final step before enrollment was for the potential Index participant to successfully refer at least one of his/her network members to the study site to complete informed consent and assessments of drug and sex risk behaviors and HIV status. It is important to note that network members were able to be enrolled regardless of their HIV status. In all, 232 HIV-negative injection drug users (Index subjects) and 465 of their sex and/or drug using partners (network members) satisfied eligibility criteria and were formally enrolled in the study. Details of the study procedures and intervention are reported elsewhere (Latkin et al, 2009).
Analyses
Given the secondary nature of the descriptive analyses presented here we focus on comparing the prevalence of HIV infection, risk behaviors, and participation in HIV prevention services by race/ethnicity groups (Whites, Blacks and Latinos). The analyses reported here use only data from the pre-screening and baseline assessment of HPTN 037 at the Philadelphia site.
RESULTS
Prescreen Sample Characteristics
Of the 3,079 drug using individuals who completed prescreening assessments, 52% (N=1604) were Black, 35% (N=1083) White and 12% (N=409) Latino. The mean age was 39 years, ranging from 17 to 75. Sixty-four percent (N=1984) of the sample was male and approximately 65% (N=2014) reported having a high school education or equivalent.
Among those completing prescreens, 87.4% (N=2690) reported previous testing for HIV and of these, 2.5% (N=66) reported having been told that they had a positive result. The rates differed significantly by race (X2 = 10.2; p=0.017). The highest rate of infection was reported among Blacks (3.3%), followed by Latinos (2.5%) and Whites (1.3%).
Black participants engaged in fewer drug-related risk behaviors than did White and Latino participants (Table 1). Black participants were less likely than White and Latino participants to report injection drug use (71.2% vs. 98.8% and 94.1% respectively; χ2=390.24, p<0.001). For those who reported use of injection drugs, Black participants were also less likely than White and Latino participants to report sharing needles (21.5% vs. 46.0% and 34.8% respectively; χ2=145.9, p<0.001).
Table 1.
Risk behaviors and use of prevention services by race/ethnicity among those pre-screened for participation in HPTN 037, Philadelphia, PA.
| White (N= 1042) | Black (N=1576) | Latino (N= 409) | X2 | |
|---|---|---|---|---|
| Drug Risk | ||||
| Injected | 98.8 (n=1029) | 71.2 (n=1122) | 94.1 (n=385) | p<.001 |
| Shared needles | 45.4 (n=473) | 15.3 (n=241) | 32.8 (n=134) | p<.001 |
| Sex Risk | ||||
| Sexually Active | 81.9 (n=852) | 80.8 (n=1292) | 80.7 (n=330) | NS |
| Multiple Partners | 44.3 (n=446) | 43.5 (n=718) | 43.2 (n=174) | NS |
| Inconsistent Condom Use | 83.1 (n=708) | 77.6 (n=1003) | 77.6 (n=256) | p<.05 |
| MSM | 6.0 (n=45) | 2.8 (n=25) | 8.2 (n=26) | P<0.001 |
| Use of Prevention Service | ||||
| Drug Tx | 25.2 (n=263) | 13.7 (n=216) | 24.7 (n=101) | p <.001 |
| Syringe Exchange | 41.5 (n=432) | 24.4 (n=384) | 29.3 (n=120) | p <.001 |
Similar rates of sex activity were reported by each racial/ethnic group (χ2=5.665, p>0.1). Approximately 82% of the sample reported being sexually active in the past 3 months (Table 1). Reports of multiple partnerships were also similar between race/ethnic groups, with approximately 54% of each race group reporting having more than one partner. However, rates of other sex risk behaviors, such as condom use, varied by group. Whites were more likely than Blacks (83.1% vs. 77.6%) to use condoms inconsistently (χ2=10.7, p<0.05). Blacks and Latinos were similar in the degree to which they used condoms consistently. Black men (2.8%) were less likely than White (6.0%) and Latino (8.2%) men to report same sex partners (χ2=34.3, p<0.001).
Black participants were less likely than White and Latino participants to report receipt of drug treatment (13.7% vs. 25.2% and 24.7%, respectively; χ2=65.9, p<0.001). They were also less likely to report use of needle exchange services (24.4% vs. 41.5% and 29.3% respectively; χ2=87.5, p<0.001).
An examination of the racial homophily between network indices and their network members revealed that Blacks have the most racially homophilous networks with 78% of Black indices having only Black network members; 60.5% of White indices had only White network members; and 46.2% of Latino indices had only Latino network members. These racial differences in the degree of homophily were significant (χ2=21.4, p<0.01).
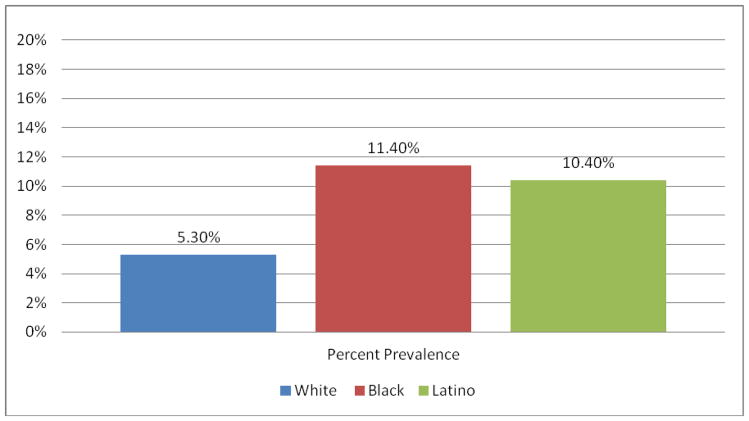
Among the 465 network members who were screened and enrolled in the study, an overall HIV prevalence rate of 9% was found. As shown in Figure 1., significantly higher HIV prevalence (p<.05) was found in network members of Black (11.4%) and Latino (10.4%) injectors than in the networks of White injectors (5.3%).
Figure 1.
Prevalence of HIV among network members by race of uninfected index IDU
DISCUSSION
Research has demonstrated that racial disparities in HIV cannot be explained by individual behavior patterns alone. Previous research has shown that Blacks’ and Latinos’ higher rates of infection as compared to Whites are not due to higher levels of risk behavior among them, but instead to “low” or “average” levels of risk behavior but within risk networks that have a higher baseline rate of HIV infection. Our study provides additional evidence supporting this notion. Further, our unique data show that HIV negative injectors who are Black and Latino are more likely than White injectors to have HIV in their risk networks.
Consistent with other studies, our data reveal that Black and Latino injectors engage in less risk behaviors than White injectors, but bear a disproportionate burden of HIV disease. Our results show that neither injection risk behaviors nor sex behaviors alone explain disparities. However, when we examine syringe exchange use and substance abuse treatment, we saw that Blacks were significantly less likely to access both services. Syringe exchange programs and substance abuse treatment are two effective HIV prevention services for injection drug users, so it might be concluded that lower utilization of these services among Black injectors partially explain the disparities. However, the reduced use of syringe exchange programs and drug treatment among Blacks has not translated into greater injection risk. Data from this study and others show that the prevalence of needle-sharing among Blacks is low and thus risk reduction messages and practices have penetrated Black injector networks. The results from this study indicate that continuing racial/ethnic disparities in HIV are related to the significantly higher prevalence of HIV in the networks of Black, and to a lesser degree Latino, HIV-negative injection drug users.
The creation of social networks occurs not by chance, but according to predictable patterns of behavior, with racial preferences being of particular importance. In particular, risk behaviors occur as a constellation of behaviors where the presence of one risk behavior generally indicates the presence of others as well (Friedman, Neaigus, Curtis et al., 2007). There is not a great deal of research concerning the formation of IDU risk networks but normative social network formation demonstrates that individuals are more likely to include people in their network if they are of the same race (Latkin C, Mandell W, Vlahov D, et al., 1996), social circumstances (Suh, Mandell, Latkin et al., 1997), and life stage (Latkin, Mandell, Vlahov et al., 1995). These social networks tend to have consistent membership over time, with a solid core membership that often lasts over a decade (Suh, Mandell, Latkin et al., 1997). Ties that are cross-gender or cross-race are more likely to be dropped than are ties among demographically similar friends (Neaigus, Friedman, Jose et al., 1996).
Racial homogeneity within networks has implications for sex partnering and disease transmission. Mixing patterns between individuals in different sex activity classes (i.e., different rates of partner change) vary according to race/ethnicity (Turner, Garnett, Ghani, et al., 2004). In the United States, individuals tend to select sex partners within their own race/ethnic group, but this has adverse consequences for Blacks given higher background prevalence of infectious diseases within Black communities. Laumann and Youm (1999) demonstrated this with a nationally representative sample, showing that same race partnering results in lower risk “peripheral” Blacks partnering with higher risk “core” Blacks; this dissortative mixing facilitates spread of infection through the Black population.
The results of this study indicate the need for more research and interventions that target individuals and their networks. Without including sex and drug partners in HIV research and interventions, preventing HIV among all races will be greatly stalled. Further, although the data show that there is a high degree of assortative racial mixing within networks, there is still a fair degree of mixing: 20–40 percent among Blacks and Whites and over 50% among Latinos. This suggests the possibility of infection spread between races. Latinos, in particular, may operate as a bridging group. More research is needed on Latino injectors and their network and behavioral norms.
Conclusions
Observers of the racial disparities in prevalence and incidence of HIV infections and AIDS diagnoses in the United States have concluded that these disparities exist because prevention messages, supplies, and/or interventions do not effectively reach those at greatest risk of infection. In essence, such interpretations suggest that Blacks and Latinos continue to practice more risk behaviors than Whites. Results reported here suggest that this is not the case, but instead that Black and Latino injection drug users who are HIV negative have a higher prevalence of HIV in their social and risk networks and thus a higher probability of encountering the virus even with relatively low rates of risk behaviors. Interventions with greater impact among Blacks will be required to reduce transmission. This conceptualization has implications for both research and prevention. For Black and Latino injectors, minimal engagement in risky behaviors brings with it a higher likelihood of infection. Risk reduction messages, supplies, and interventions targeted toward HIV negative injection drug users remain important prevention activities, as well as linkage to care efforts for HIV positive injectors (DesJarlais, Cooper, Bramson et al., 2012).
Acknowledgments
Financial Support:
The HPTN 037 study was conducted by the HIV Prevention Trials Network and sponsored by the National Institute of Allergy and Infectious Diseases through a grant (U01-AI-48014) to the University of Pennsylvania. Work on this paper was supported by the Penn Center for AIDS Research (P30 AI 045008) and the HPTN Scholars Program (UM1 AI068610) 2012–2013 funded through a supplement from National Institute of Allergy and Infectious Diseases (NIAID) and National Institute for Mental Health (NIMH).
SBSRN JAIDS SUBMISSION/TOC
| AUTHORS | TITLE |
|---|---|
| Michael Blank PhD David Metzger PhD, Gina M. Wingood, ScD, MPH, Ralph J. DiClemente, PhD, |
The Social and Behavioral Sciences Research Network: Translational Research to Reduce Disparities in HIV |
| James W. Curran, MD James A. Hoxie, MD |
Translating Social and Behavioral Science Research to the AIDS Epidemic: A CFAR Perspective |
| Dianne M. Rausch, Cynthia I. Grossman, Emily J. Erbelding |
Integrating Behavioral and Biomedical Research in HIV Interventions: Challenges and Opportunities |
| Sten H. Vermund, MD, PhD José A. Tique, MD Holly M. Cassell, MPH Megan E. Johnson, MS Philip J. Ciampa, MD, MPH Carolyn M. Audet, PhD |
Translation of biomedical prevention strategies for HIV: Prospects and pitfalls |
| Russell E. Glasgow, PhD Erin T. Eckstein, MSW M Khair ElZarrad PhD, MPH |
Implementation Science Perspectives and Opportunities for HIV/AIDS Research: Integrating Science, Practice and Policy |
| Scott D. Rhodes, Ph.D., MPH; Stacy Duck, BA; Jorge Alonzo, JD; Jason Daniel, Ph.D., MPH; Robert E. Aronson, Dr.P.H. |
Using Community-Based Participatory Research to Prevent HIV Disparities: Assumptions and Opportunities Identified by The Latino Partnership |
| Gina M. Wingood, ScD, MPH Ralph J. DiClemente, PhD LaShun Robinson-Simpson, PhD Delia L. Lang, PhD, MPH Angela Caliendo, MD, PhD James W. Hardin, PhD |
Efficacy of an HIV Intervention in Reducing High-Risk HPV, Non-viral STIs, and Concurrency among African-American Women: A Randomized Controlled Trial |
| Michael Blank, PhD Marlene Eisenberg, PhD |
Tailored Treatment for HIV+ Persons with Mental Illness: The Intervention Cascade |
| Josiah D. Rich, MD, MPH Ralph DiClemente, PhD Judith Levy, PhD Karen Lyda, LCSW, MS, NP Monica Ruiz, PhD, MPH David L. Rosen, PhD, MD Dora Dumont, PhD, MPH |
Correctional Facilities as Partners in Reducing HIV Disparities |
| Carl A. Latkin, Melissa A. Davey-Rothwell, Amy R. Knowlton, Kamila A. Alexander Chyvette T. Williams ???? Boodram |
Social network approaches to recruitment, HIV prevention, medical care, and medication adherence |
| Gina M. Wingood, ScD, MPH; Priscilla Reddy, PhD; Delia L. Lang, PhD, MPH; Dorina Saleh-Onoya, PhD; Nikia Braxton, MPH; Sibusiso Sifunda, PhD; Ralph J. DiClemente, PhD |
Efficacy of SISTA South Africa on sexual behavior, HIV stigma and relationship control among isiXhosa women in the Western Cape Province, South Africa: Results of a Randomized Controlled Trial |
| Ralph J. DiClemente, Erin Bradley, Teaniese L. Davis, Jennifer L. Brown, Mary Ukuku, Jessica M. Sales, Eve S. Rose Gina M. Wingood, |
Adoption and Implementation of a Computer-delivered HIV/STD Risk-Reduction Intervention for African American Adolescent Females Seeking Services at County Health Departments: Implementation Optimization is Urgently Needed |
|
C Hendricks Brown, PhD David C. Mohr, PhD Carlos G. Gallo, PhD Christopher Mader, BS Lawrence Palinkas, PhD Gina Wingood, PhD Guillermo Prado, PhD Sheppard G. Kellam MD Hilda Pantin, PhD Jeanne Poduska, PhD Robert Gibbons, PhD John McManus, PhD Mitsunori Ogihara, PhD Thomas Valente, PhD Fred Wulczyn, PhD Sara Czaja, PhD Geoff Sutcliffe, PhD Juan Villamar MS Ed Christopher Jacobs |
A Computational Future for Preventing HIV in Minority Communities: How Advanced Technology Can Improve Implementation of Effective Programs |
| Ralph J. DICLEMENTE, Jennifer L. BROWN, Jessica M. SALES, & Eve S. ROSE |
Rate of Decay in Proportion of Condom Protected Sex Acts among Adolescents Following Participation in an HIV Risk-Reduction Intervention |
| Chyvette Williams, Marlene M. Eisenberg, Julie Becher, Annet Davis-Vogel, Danielle Fiore, David S. Metzger |
“Racial disparities in HIV and associated risk behaviors among injection drug users and their network |
| Gina M. Wingood, ScD, MPH; Kristin Dunkle, PhD, Christina Camp; PhD; Shilpa Patel, MPH; Julia E. Painter, PhD, MPH, Ralph J. DiClemente, PhD |
Association of Demographic, Sexual Behaviors, and Social Influences with Potential Adoption of Pre-exposure Prophylaxis (PrEP): Results of a National Survey on Women |
| Patrick S Sullivan, DVM, PhD Jeremy A Grey, MA B R Simon Rosser, PhD, MPH |
Emerging technologies for HIV prevention for MSM: What we’ve learned, and ways forward |
| Gina M. Wingood, ScD, MPH, Anna Rubtsova, PhD, Ralph J. DiClemente, PhD, David Metzger PhD, Michael Blank PhD |
A New Paradigm for Optimizing HIV Intervention Synergy The Role of Interdependence in Integrating HIV Prevention Interventions |
Footnotes
Conflicts of Interest: None Reported
References
- 1.Amesty S, Rivera AV, Fuller CM. Overview of HIV among injection drug users in New York City: Critical next steps to eliminate racial/ethnic disparities. Substance Use and Misuse. 2001;46:285–294. doi: 10.3109/10826084.2011.523287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Updated Slide Set: HIV Surveillance in Injection Drug Users through 2010. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV/AIDS fact sheet: Estimates of New HIV Infections in the United States, 2006–2009. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 4.De P, Cox J, Boivin J-F, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007;102(11):1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 5.DesJarlais DC, Cooper HLF, Bramson H, Deren S, Hatzakis A, Hagan H. Racial and ethnic disparities and implications for the prevention of HIV among persons who inject drugs. Current Opinion in HIV & AIDS. 2012;7(4):354–61. doi: 10.1097/COH.0b013e328353d990. [DOI] [PubMed] [Google Scholar]
- 6.DesJarlais DC, Arasteh K, Hagan H, McKnight C, Perlman DC, Friedman SR. Persistence and change in disparities in HIV infection among injection drug users in New York City after large-scale syringe exchange programs. American Journal of Public Health. 2009;99:S445–S451. doi: 10.2105/AJPH.2008.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman S, Neaigus A, Jose B, Curtis R, Goldstein M, Ildefonso G, Rothenberg R, Des Jarlais D. Sociometric risk networks and risk for HIV infection. Am J Public Health. 1997 Aug;87(8):1289–96. doi: 10.2105/ajph.87.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman SR, Chapman TF, Perlis TE, Rockwell R, Paone D, Sotheran JL, DesJarlais DC. Similarities and differences by race/ethnicity in changes of HIC seroprevalence and related behaviors among drug injectors in New York City, 1991–1996. JAIDS. 1999;22:83–91. doi: 10.1097/00042560-199909010-00011. [DOI] [PubMed] [Google Scholar]
- 9.Hallfors DD, Iritani BJ, Miller WC, Bauer DJ. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. American Journal of Public Health. 2007;J97:125–32. doi: 10.2105/AJPH.2005.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jolly AM, Muth SQ, Wylie JL, Potterat JJ. Sexual networks and sexually transmitted infections: a tale of two cities. Journal of Urban Health. 2001;78:433–445. doi: 10.1093/jurban/78.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klovdahl AS. Social networks and the spread of infectious diseases: the AIDS example. Social Science Medicine. 1985;21:1203–16. doi: 10.1016/0277-9536(85)90269-2. [DOI] [PubMed] [Google Scholar]
- 12.Kottiri BJ, Friedman SR, Neaigus A, Curtis R, DesJarlais DC. Risk networks and racial/ethnic differences in the prevalence of HIV infection among injection drug users. JAIDS. 2002;30:95–104. doi: 10.1097/00042560-200205010-00013. [DOI] [PubMed] [Google Scholar]
- 13.Kraut-Becher J, Eisenberg M, Voytek C, Brown T, Metzger DS, Aral SO. Examining Racial Disparities in HIV: Lessons From Sexually Transmitted Infections Research. J Acquir Immune Defic Syndr. 2008;47:S20–S27. doi: 10.1097/QAI.0b013e3181605b95. [DOI] [PubMed] [Google Scholar]
- 14.Laumann EO, Youm MA. Racial/ethnic differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sexually Transmitted Disease. 1999;26:250–261. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Latkin CA, Donnell D, Metzger D, Sherman S, Aramrattna A, Davis-Vogel A, Quan VM, Gandham S, Vongchak T, Perdue T, Celentano DD. The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia, USA. Social Science & Medicine. 2009;68(4):740–8. doi: 10.1016/j.socscimed.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latkin CA, Mandell W, Vlahov D. The relationship between risk networks’ patterns of crack cocaine and alcohol consumption and HIV-related sexual behaviors among adult injection drug users: a prospective study. Drug and Alcohol Dependence. 1996;42:175–181. doi: 10.1016/s0376-8716(96)01279-3. [DOI] [PubMed] [Google Scholar]
- 17.Latkin C, Mandell W, Vlahov D, Oziemkowska M, Celentano D. People and places: behavioral settings and personal network characteristics as correlates of needle sharing. J Acquir Immune Defic Syndr Hum Retrovirol. 1996 Nov;13(3):273–80. doi: 10.1097/00042560-199611010-00010. [DOI] [PubMed] [Google Scholar]
- 18.Latkin C, Mandell W, Vlahov D, Knowlton A, Oziemkowska M, Celentano D. Personal network characteristics as antecedents to needle-sharing and shoting gallery attendance. Social Networks. 1995;17(3–4):219–28. [Google Scholar]
- 19.Leigh Brown A, Lycett S, Weinert L, Hughes G, Fearnhill E, Dunn D. Transmission Network Parameters Estimated From HIV Sequences for a Nationwide Epidemic. Journal of Infectious Diseases. 2011;204(9):1463–1469. doi: 10.1093/infdis/jir550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May R, Anderson R. Transmission dynamics of HIV infection. Nature. 1987;326:137–42. doi: 10.1038/326137a0. [DOI] [PubMed] [Google Scholar]
- 21.Millett GA, Peterson JL, Flores SA, Hart TA, Jeffries WL, Wilson PA, Rourke SB, Heilig CM, Elford J, Fenton KA, Remis RS. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. The Lancet. 2012;380:341–348. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 22.Neaigus A, Friedman S, Jose B, Goldstein M, Curtis R, Ildefonso G, Des Jarlais D. High-risk personal networks and syringe sharing as risk factors for HIV infection among new drug injectors. J Acquir Immune Defic Syndr Hum Retrovirol. 1996 Apr;11(5):499–509. doi: 10.1097/00042560-199604150-00011. [DOI] [PubMed] [Google Scholar]
- 23.Perisse ARS, Langenberg P, Hungerford L, Boulay M, Charurat M, Schechter M, Blattner W. The Use of Supplementary Techniques to Increase Recall of Sex Partners in a Network-Based Research Study in Rio de Janeiro, Brazil. Sexually Transmitted Diseases. 2008;35(7):674–678. doi: 10.1097/OLQ.0b013e31816b323d. [DOI] [PubMed] [Google Scholar]
- 24.Perkins WE, Metzger D. AIDS in Philadelphia: Emerging from the Shadow of Crack. In: Bowser Benjamin, Quimby Ernest, Singer Merrill., editors. When Communities Assess their AIDS Epidemics. Lexington Books; 2007. [Google Scholar]
- 25.Sharpe TT, Harrison K, Dean H. Summary of CDC Consultation to Address Social Determinants of Health for Prevention of Disparities in HIV/AIDS, Viral Hepatitis, Sexually Transmitted Diseases, and Tuberculosis. Public Health Reports. 2010;125(Sup 4):11–15. doi: 10.1177/00333549101250S404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suh T, Mandell W, Latkin C, Kim J. Social network characteristics and injecting HIV-risk behaviors among street injection drug users. Drug Alcohol Depend. 1997 Aug;47(2):137–43. doi: 10.1016/s0376-8716(97)00082-3. [DOI] [PubMed] [Google Scholar]
- 27.Turner KM, Garnett GP, Ghani AC, Sterne JA, Low N. Investigating ethnic \inequalities in the incidence of sexually transmitted infections: mathematical modeling study. Sexually Transmitted Infections. 2004;80:379–85. doi: 10.1136/sti.2003.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward H. Prevention strategies for sexually transmitted infections: importance of sexual network structure and epidemic phase. Sexually Transmitted Infections. 2007 Aug;83(Supplement 1):i43–i49. doi: 10.1136/sti.2006.023598. [DOI] [PubMed] [Google Scholar]