Abstract
From a high throughput screening of commercially available libraries against nontuberculous mycobacteria and Mycobacterium tuberculosis, numerous hits were identified with moderate activity. Extensive medicinal chemistry optimization has led to a series of potent benzothiazole amide antimycobacterial agents. Replacement of the adamantyl group with cyclohexyl derivatives and further development of this series resulted in an advanced lead compound, CRS400393, which demonstrated excellent potency and a mycobacteria-specific spectrum of activity. MIC values ranged from 0.03-0.12 μg/mL against Mycobacterium abscessus and other rapid-grower NTM, and 1-2 μg/mL against Mycobacterium avium complex. The preliminary mechanism of action studies suggested these agents may target MmpL3, a mycobacterial mycolic acid transporter. The series has demonstrated in vivo efficacy in a proof of concept mouse model of M. abscessus infection.
Keywords: mycobacteria, nontuberculous mycobacteria, MmpL3 inhibitor, tuberculosis, benzothiazole amide, antibiotics, Mycobacterium abscessus, Mycobacterium avium
GRAPHIC ABSTRACT:
Mycobacterial pathogens are intrinsically resistant to most antibiotics, and pose an enormous human health issue (1). While Mycobacterium tuberculosis (Mtb) has been the subject of extensive drug discovery efforts, the nontuberculous mycobacteria (NTM) pose a unique and under-resourced therapeutic challenge, associated with significant morbidity and mortality (2). There is growing epidemiologic evidence to suggest that NTM cause more infections in the United States than Mtb (3), yet there are virtually no antimicrobial drug discovery programs specifically targeting NTM.
Pulmonary disease caused by NTM is especially problematic in patients with underlying host susceptibility such as populations with immunosuppressive medications (4), cystic fibrosis (5, 6), ciliary dyskinesia, smoking-induced lung diseases such as chronic obstructive pulmonary disease COPD (7), HIV infection (8), bronchiectasis and malignancies (9) or underlying defects in IL-12, interferon gamma receptor or Nuclear factor κB essential modulator (NEMO) deficiency (10). Mycobacterium abscessus (Mabs) complex, in particular, is an emerging NTM infection for which effective therapy is often elusive, burdensome and costly (11, 12, 13, and 14). Chronic lung disease due to NTM (especially Mabs) can be particularly difficult to treat, even with multi-drug regimens (15). Recent evidence suggests that drug resistant Mabs subspecies massiliense can cause human-to-human transmission in cystic fibrosis patients (16, 17).
There have been several hospital acquired outbreaks of multi-drug and disinfectant resistant rapidly growing mycobacteria (RGM). One massive outbreak of skin and soft tissue infections occurred in Brazil by contaminated laparoscope with M. chelonae that was resistant to 2% glutaraldehyde (18). Due to the changing epidemiology and transmission dynamics of certain problematic NTM species such as Mabs, the threat of worldwide epidemics is escalating (16, 17). Given the emergence of NTM as a public health issue, finding new antimycobacterial agents is of paramount importance. Development of new agents specifically active against NTM is an under-explored area with no approved drug specifically treating NTM infections.
In addition, tuberculosis represents an ongoing public health threat as well, with over two billion people carrying the infection (19). Mtb can remain latent for extended periods of time; nonetheless, the large reservoir of latent infection fuels a growing population with active disease, amounting to 10.4 million of new cases per year with 1.7 million deaths in 2016 (20). Treatment regimens involve three to four drugs, administered for six months or longer. Emergence of multidrug resistant (MDR)-Mtb has added impetus to drug discovery efforts against this devastating pathogen. Historically, antimycobacterial drug discovery efforts have focused almost exclusively on Mtb, with virtually no concerted effort toward extended spectrum agents that cover NTM. In part, this reflects the intrinsic challenge of treating NTM, which are frequently refractive to anti-Mtb drugs, thus prompting our research to develop broad spectrum anti-mycobacterial agents. We are particularly excited to have discovered a lead series that exhibits broad antimycobacterial activity against NTM (including both rapid growing and slow growing species) and Mtb as well.
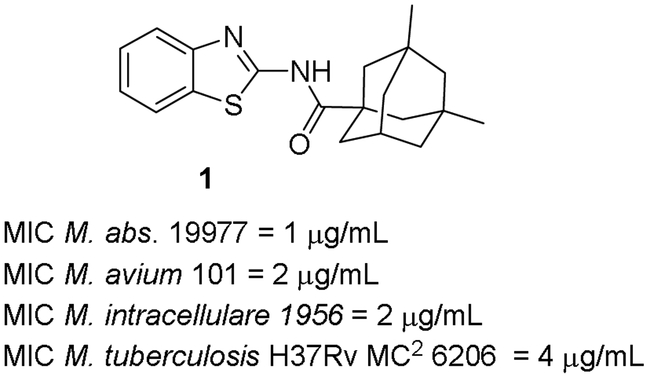
Compound 1 in Figure 1 was identified from the high throughput screening of over 350,000 small molecule compounds for Mabs whole cell activity (21). Parallel screening of the same compound collection against Mtb allowed us to identify screening hits with broad antimycobacterial spectrum. However, the adamantyl group in compound 1, although found in some commercial drugs, can be a liability in terms of its high lipophilicity and potential for nonspecific binding. Consequently, our initial strategy for this series involved trimming of the adamantyl group to identify the least amount of lipophilic structure that retains activity.
Figure 1.
Structure and activity of Compound 1
The general synthetic route of novel mycobacterial inhibitors is outlined in Scheme 1. The synthesis started from substituted 2-amino-benzothiazole intermediates and variably substituted cycloalkyl carboxylic acids under the amide coupling conditions using 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluoro-phosphate (HATU) in the presence of N,N-diisopropylethylamine (DIEA) in dichloroethane (DCE). The products were purified by column chromatography using ethyl acetate and hexanes as eluents and fully characterized by NMR and LC-MS.
Scheme 1.
Synthetic scheme of benzothiazole amide antimycobacterial agents
The compounds were first screened in a panel of RGM including M. abscessus ATCC 19977 (Mabs), M. abscessus 1, M. abscessus 21, M. abscessus 79, M. abscessus massiliense 119, M. fortuitum 41, M. chelonae 93, and M. peregrinum ATCC 700686. Since M. abscessus is a rapid emerging pathogen, for ease of discussion and limited space only activity for M. abscessus ATCC 19977 as a representative of RGM will be discussed, however, all MIC data for the panel of RGM is available in the supplemental materials. Compounds that showed good activity in the panel of RGM were then tested against two slow growing NTM species (SGM), M. avium 101 and M. intracellulare 1956. Select compounds were then tested against M. tuberculosis H37Rv mc2 6206.
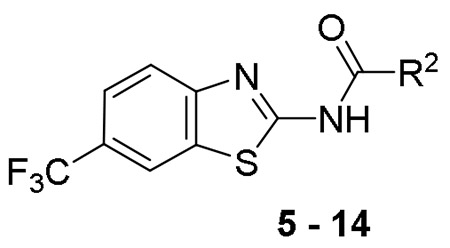
Table 1 summarizes the structure-activity relationship (SAR) of substituted cyclohexanes with 5-trifluomethyl substitution on the benzothiazole, which was found to be one of the early optimal aromatic RHS during previous SAR exploration (19). As one can see, anti-mycobacterial activity was affected by the position and degree of substitution about the cyclohexane ring. Generally, the MICs were elevated as the number of carbons was trimmed resulting in loss of antimycobacterial activity. However, substitution position matters (compound 12 vs 14 and compound 5 vs 6). The best substitution pattern was 3,3,5-trimethyl cyclohexane (compound 8). Although the MIC of compound 8 for the rapid grower such as Mabs was comparable to the MIC of compound 1 with adamantane as the right hand side (RHS), the activity against slow growers, such as M. avium 101 and M. intracellulare 1956 activity was diminished.
Table 1.
Structure Activity Relationship of Cyclohexane Analogs – RHS
 | |||||
|---|---|---|---|---|---|
| Cmpd# | R2 | MIC (μg/mL) | |||
|
M. abs. 19977 |
M. avium 101 |
M. intracellulare 1956 |
M. tuberculosis H37Rv |
||
| 5 | 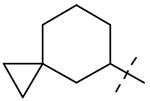 |
4 | > 64 | > 64 | NT * |
| 6 | 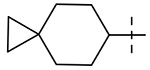 |
32 | 64 | > 64 | 32 |
| 7 | 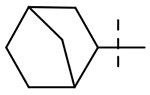 |
> 64 | 32 | 32 | NT |
| 8 | 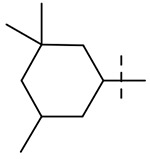 |
0.5 | > 64 | > 64 | 2 |
| 9 | 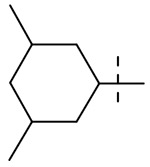 |
1 | > 64 | > 64 | 4 |
| 10 | 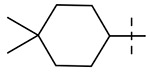 |
> 64 | 64 | 64 | NT |
| 11 | 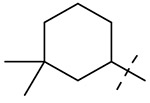 |
> 64 | > 64 | > 64 | NT |
| 12 | 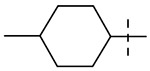 |
64 | > 64 | > 64 | NT |
| 13 | 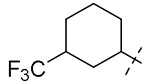 |
2 | 16 | 16 | NT |
| 14 | 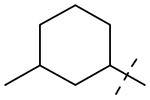 |
2 | 16 | 16 | 16 |
NT = not tested
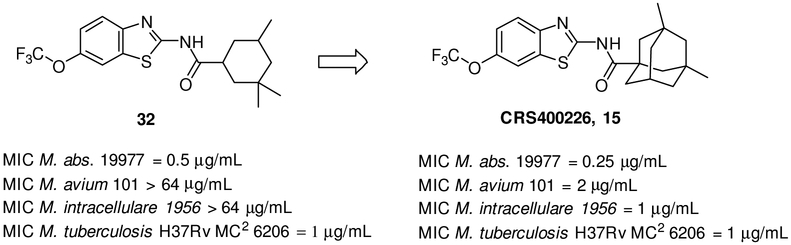
We kept the 3,3,5-trimethyl-cyclohexane RHS and explored the substitution on the benzothiazole ring to find the optimal substitution pattern (compounds 16 - 37). Table 2 summarizes the SAR of the left hand side (LHS). It is clear that introducing a single small halogen such as fluorine on the benzene ring at 4, 5, and 6-position resulted in improved spectrum activity (compounds 20, 27 and 30 vs compound 16). Di- or trifluoro- substituted analogs 17 and 18 had comparable activity against Mabs to the 6-fluoro analog 30, however the activity against the slow growers was reduced, indicating that position and degree of aromatic substitution has a substantial effect on the spectrum of activity. This effect is further exemplified in comparison of 5,7-difluoro compound 23 with the 5- and 7- mono-fluoro compounds (compounds 27 and 36) and with 4,6-difluoro compound 18. Larger halogens such as chloride and bromide provided less activity than the fluoride at 4 or 5-position of the benzothiazole (compounds 19, 25). However, at the 6-position, all halogen substitutions (compounds 28, 29 and 30) resulted in much improved activity against Mabs in comparison to unsubstituted compound 16. At the 6-position, trifluoromethyl (compound 8) and trifluoromethoxy (compound 32) analogs were also very active against Mabs while dimethylamine (compound 31), methoxy (compound 34), and trifluoromethylsulfanyl (compound 35) analogs lost activity against Mabs. Interestingly, the 5,7-disubstituted analogs 22, 23 and 24 were highly potent against Mabs and Mtb but with activity ranging from MIC 8 - >64 μg/ml against M. avium and M. intracellulare, respectively. In general, although most of the compounds had good activities against the RGM such as Mabs, the loss of activities against SGM is evident. We don’t have a good explanation yet for this observation, but we speculate that the elevated MIC for SGM may be due to the bovine albumin in the MIC testing media Middlebrook 7H9 broth since this series of compounds is highly protein bound ( > 99%). The best one in this subset of compounds was 26 with MIC = 0.25 μg/mL against Mabs and ≤ 0.12 μg/mL against Mtb, although it lacked activity against SGM as discussed above. For the sake of better understanding the SAR, when the adamantane was installed with the best left hand side (Figure 2, CRS400226, 15), the broad spectrum activity was recovered (22).
Table 2.
Structure Activity Relationship of Cyclohexane Analogs – Left Hand Side (LHS)
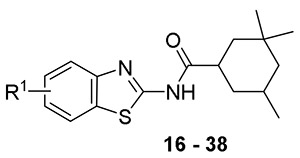 | |||||
|---|---|---|---|---|---|
| Cmpd# | R1 | MIC (μg/mL) | |||
|
M. abs. 19977 |
M. avium 101 |
M. intracellulare 1956 |
M. tuberculosis H37Rv |
||
| 16 | H | 2 | 8 | 8 | 8 |
| 17 | 4,5,6-triF | 0.5 | > 64 | > 64 | 1 |
| 18 | 4,6-diF | 0.5 | 64 | 4 | 1 |
| 19 | 4-Cl | 16 | > 64 | 16 | NT* |
| 20 | 4-F | 2 | 8 | 8 | 4 |
| 21 | 4-F-6-Br | 1 | > 64 | 16 | 1 |
| 22 | 5,7-diCl | 0.25 | 8 | 16 | 1 |
| 23 | 5,7-diF | 0.25 | 8 | 8 | 0.25 |
| 24 | 5,7-diMe | 0.5 | > 64 | > 64 | 0.25 |
| 25 | 5-Br | 32 | > 64 | > 64 | NT |
| 26 | 5-CF3 | 0.25 | > 64 | > 64 | ≤ 0.12 |
| 27 | 5-F | 1 | 16 | 16 | 4 |
| 28 | 6-Br | 0.5 | > 64 | > 64 | 2 |
| 8 | 6-CF3 | 0.5 | > 64 | > 64 | 2 |
| 29 | 6-Cl | 0.5 | 16 | 4 | 4 |
| 30 | 6-F | 0.5 | 8 | 8 | 4 |
| 31 | 6-NMe2 | 32 | > 64 | > 64 | NT |
| 32 | 6-OCF3 | 0.5 | > 64 | > 64 | 1 |
| 33 | 6-OCHF2 | 1 | > 64 | 8 | 2 |
| 34 | 6-OMe | 1 | 16 | 8 | 8 |
| 35 | 6-SCF3 | 16 | > 64 | > 64 | 2 |
| 36 | 7-F | 0.5 | 8 | 16 | 1 |
| 37 | 7-OH | 16 | 16 | 4 | NT |
NT = not tested
Figure 2.
Structure of Compound 32 and CRS400226
We hypothesized that loss of activity against SGM in Table 1 may be due in part to the lack of fourth substitution on the 1-position of the cyclohexane ring. To examine this hypothesis, we synthesized two pairs of analogs with the cycloalkyl ring RHS methylated at 1-position as seen in Table 3. Importantly, the methylation at 1-position on both the cyclohexane and cycloheptane (compounds 39 and 41) recovered the SGM activity and also resulted in improvement of anti-Mtb activity while the non-methylated counterparts (compounds 38 and 40) were ≥ 16-fold less active than the methylated compounds against SGM.
Table 3.
Structure Activity Relationship of Cyclohexane Analogs – Alpha-methylation
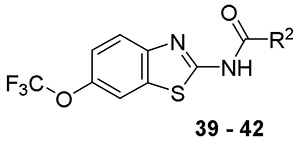 | |||||
|---|---|---|---|---|---|
| Cmpd# | R2 | MIC (μg/mL) | |||
|
M. abs. 19977 |
M. avium 101 |
M. intracellulare 1956 |
M. tuberculosis H37Rv |
||
| 38 | 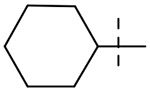 |
32 | > 64 | > 64 | 64 |
| 39 | 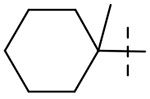 |
0.25 | 8 | 4 | 4 |
| 40 | 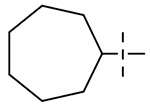 |
> 64 | > 64 | > 64 | 64 |
| 41 | 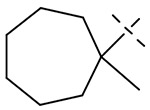 |
0.12 | 8 | 4 | 0.5 |
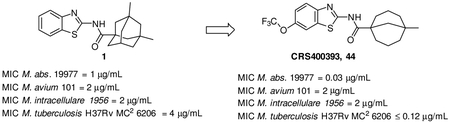
Encouraged by these results, we identified additional non-adamantane, tetra-substituted cycloalkyl carboxylic acids at the 1-position as building blocks to synthesize the final compounds. One analog stood out as a major breakthrough in improving the antimycobacterial activity, a compound derived from 5-methylbicyclo[3.3.1]nonane-1-carboxylic acid (compound 52, CRS400359) with MIC as low as 0.06 μg/mL against Mabs and MIC = 2 μg/mL against the slow growers M. avium 101 and M. intracellulare 1956. CRS400359 was also potent against Mtb with MIC = 1 μg/mL. Follow-up analogs (compounds 42 - 51) with the best substitution patterns from earlier SAR were synthesized based on the new, exciting RHS as shown in Table 4 to find the optimal LHS substitution. Most of the compounds demonstrated great antimycobacterial activity, not only against the RGM, but also the SGM and Mtb. One of the best compounds was compound 43 (CRS400393), demonstrating MIC = 0.03, 2, and ≤ 0.12 μg/mL against Mabs, Mycobacterium avium complex (MAC), and Mtb respectively. This was a significant development in activity against mycobacteria and also indicated that the adamantane moiety was not necessary to maintain the broad spectrum antimycobacterial activity. Although our initial plan was to reduce the lipophilicity of the series, the loss of activity against mycobacteria by trimming the adamantane group indicated that lipophilicity may be needed for binding to the target mmpL3. The level of improvement in potency of CRS400359 and CRS400393 from the initial hit compound 1 warranted further characterization of these compounds in ADME, PK, efficacy and toxicity.
Table 4.
Structure Activity Relationship of Cyclohexane Analogs – With the Best RHS
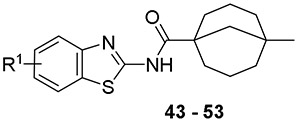 | |||||
|---|---|---|---|---|---|
| Cmpd# | R1 | MIC (μg/mL) | |||
|
M. abs. 19977 |
M. avium 101 |
M. intracellulare 1956 |
M. tuberculosis H37Rv |
||
| 42 | 5-Br | 0.06 | 4 | 1 | ≤ 0.12 |
| 43 | 5,7-diCl | 0.03 | 2 | 2 | ≤ 0.12 |
| 44 | 5,7-diMe | 0.12 | 8 | 8 | 0.25 |
| 45 | 5,7-diF | 0.06 | 4 | 4 | 0.25 |
| 46 | 4,5,6-triF | 0.12 | > 8 | 8 | ≤ 0.12 |
| 47 | 4,6-diF | 0.12 | 4 | 4 | 0.25 |
| 48 | 6-CF3 | 0.25 | > 8 | 4 | 0.25 |
| 49 | 6-Br | 0.12 | 8 | 4 | 0.5 |
| 50 | 6-Cl | 0.12 | 8 | 4 | 0.5 |
| 51 | 6-OCHF2 | 0.12 | 8 | 4 | 1 |
| 52 | 6-OCF3 | 0.06 | 2 | 2 | 1 |
Although the primary focus of the project was to improve the antimycobacterial activity of the benzothiazole amide hit series to identify potent antimycobacteral agents, understanding the mechanism of action of the compound series was also part of this effort. Due to the structural similarity of the initial hit compound 1 to the known inhibitors of mmpL3 (the trehalose monomycolate transporter protein large), a mycolic acid transporter that plays important role in mycobacterial outer membrane formation, we believed the benzothiazole amide series also targeted mmpL3. We selected compounds 18 and 32 to confirm the mechanism of action. The data suggested that these compounds affect the transfer of mycolic acids, the mycobacterial outer membrane building blocks, to their cell envelope acceptors in both Mabs and Mtb, most likely through the inhibition of mmpL3 (23).
Compounds with good antimycobacterial activities were further profiled in a secondary panel of Gram-positive and Gram-negative bacteria to test for selectivity of spectrum. All compounds tested showed no activity below 32 μg/mL. CRS400393 was also characterized in time kill kinetic studies (23) and it was bacteriostatic against Mabs, for 3 days, followed by a drop of 2 Log10 CFU after 5 days. Importantly, no regrowth was observed following a single addition of compound due to emergence of resistance (23).
The long term goal of the project is to identify compounds that have the drug like properties. Thus, one question that we needed to answer was whether these compounds would affect metabolic enzymes such as cytochrome P450 (CYP) since the patients infected with NTM will likely take more than one drug due to the potential for bacterial resistance. Select mycobacterial inhibitors 9, 28, 29 and 32 were screened in an in vitro panel of six common isoforms of CYP enzymes CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 (Table 5). Compound 32 appeared to show the least propensity for CYP inhibition, with IC50 for all isoforms > 20 μM. Compound 9 had IC50 below 20 μM only for CYP2D6 isoform (1.4 μM). Compound 28 and 29 hit three of the six isoforms with IC50 less than 20 μM. Compounds CRS400359 and CRS400393 were also tested against CYP2B6 and CYP3A4, the two more relevant CYP enzymes for pulmonary delivery, at 10 μM concentration, and neither of the compounds inhibited the enzymes more than 50% at such concentration (data not shown).
Table 5.
CYP Inhibition of Benzothiazole Amides
| Cmpd# | CYP IC50(μM) | ||||||
|---|---|---|---|---|---|---|---|
| CYP 2B6 |
CYP 2C8 |
CYP 2C9 |
CYP 2C19 |
CYP 2D6 |
CYP3A4- Midazolam |
CYP3A4- Testosterone |
|
| 9 | > 20.0 | > 20.0 | > 20.0 | > 20.0 | 1.40 | > 20.0 | > 20.0 |
| 28 | 12.8 | > 20.0 | > 20.0 | 12.7 | 1.60 | > 20.0 | > 20.0 |
| 29 | 14.9 | > 20.0 | > 20.0 | 15.5 | 0.20 | > 20.0 | > 20.0 |
| 32 | > 20.0 | > 20.0 | > 20.0 | > 20.0 | > 20.0 | > 20.0 | > 20.0 |
The compounds were also screened in a hemolysis assay for potential toxicity. None of the compounds tested showed hemolysis at the highest concertation of 128 μg/ml. The compounds generally had low solubility (data not shown) and high protein binding in human serum (> 99%). Select compounds were also screened for cytotoxicity towards the HepG2 cell line and compound 32 had IC50 > 100 μM.
Early in the program, representative compounds 9 and 28 were selected for pharmacokinetic studies in mice. Oral bioavailability of these compounds was 53% and 75%, respectively, when dosed at 100 mg/kg. The plasma clearance (CL) after intravenous (IV, 10 mg/kg) administration was 1.7 L/hr/kg and 1.5 L/hr/kg for compounds 9 and 28, respectively.
Compound 18 was further tested orally in a single dose toxicity study at 100 mg/kg and 300 mg/kg. All mice appeared normal with no abnormal behavior observed through the course of the study.
Although the compounds were well tolerated with reasonable bioavailability, the poor solubility and lipophilicity present challenges for oral pharmaceutic development. Instead, intrapulmonary delivery methods for treatment of lung infections such as NTM and Mtb were explored. An earlier lead compound, CRS400226 (Figure 2), was further developed for this delivery pathway and demonstrated in vivo efficacy as proof of concept for the approach (23). With the significant advances made in broad spectrum anti-mycobacterial activity, along with the supporting data demonstrating tolerability, we are looking forward to advancing lead compounds such as CRS400393 (compound 43) into in vivo efficacy and tolerability studies using the intra-pulmonary delivery approach previously validated for this project.
In summary, starting from a high throughput screening hit, we applied an iterative process of lead optimization and made substantial progress toward the development of compounds as antimycobacterial agents. NTM are a diverse group of pathogens, and most drugs show a wide range of potencies, even between different strains within the same species. By testing for both Mtb and NTM activity in the medicinal chemistry optimization process, we sought to maintain broad antimycobacterial spectrum of activity. Over the course of several hundred compounds, this process resulted in progressive improvement of MIC potency from the initial lead compound 1 to much more advanced compounds such as CRS400393, generating increasingly potent compounds against RGM while maintaining activity against SGM and Mtb. Further compound optimization to improve the antimycobacterial activities and physicochemical properties and evaluation of pulmonary delivery methods is needed. Development and optimization of inhaled formulation vehicles and in vivo screening of efficacy, tolerability and pharmacokinetics will be important advances toward the development of a drug candidate such as compound CRS400393 for the treatment of mycobacterial lung infections. The benzothiazole amide series holds promise for development of a novel therapeutic agent with broad antimycobacterial activity.
Supplementary Material
A series of potent benzothiazole amide antimycobacterial agents identified.
Compound CRS400393 demonstrated excellent potency and mycobacteria-specific activity.
The series may target MmpL3, a mycobacterial mycolic acid transporter.
The series demonstrated in vivo efficacy in a mouse model of M. abscessus infection.
ACKNOWLEDGEMENTS:
The authors are grateful to Dr. Gonzalez-Juarrero and her team at Colorado State University for the animal efficacy model support. We also thank Dr. Mary Jackson and her colleagues at Colorado State University, for the characterization of the mechanism of action of our novel antimycobacterial compounds. We thank Scott Franzblau (Univ. Illinois at Chicago) for providing screening hits. This work was supported by NIH-NIGMS via SBIR Phase I and II grants GM110848 to Crestone, Inc. and by the NIAID preclinical services contract HHSN272201100009I.
ABBREVIATIONS:
- NTM
nontuberculous mycobacteria
- Mtb
Mycobacterium tuberculosis
- SAR
structure activity relationship
- Mbs
Mycobacterium abscessus ATCC 19977
- MIC
minimum inhibitory concentration
- RHS
right hand side
- LHS
left hand side
- ADME
absorption, distribution, metabolism, and excretion
- PK
pharmacokinetics
- CYP
cytochrome P450
- RGM
rapid growing mycobacteria
- SGM
slowly growing mycobacteria
Footnotes
SUPPORTING INFORMATION: Supplementary data (Experimental details and characterization of selected compounds) associated with this article can be found, in the online version, at
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Nguyen L, Thompson CJ. Foundations of antibiotic resistance in bacterial physiology: the mycobacterial paradigm. Trends Microbiol 2006; 14 (7):304–312. [DOI] [PubMed] [Google Scholar]
- 2.Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis 2016; 45:123–134. [DOI] [PubMed] [Google Scholar]
- 3.Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med 2013; 34 (1):87–94. [DOI] [PubMed] [Google Scholar]
- 4.Winthrop KL, Baxter R, Liu L, Varley CD, Curtis JR, Baddley JW, McFarland B, Austin D, Radcliffe L, Suhler E, Choi D, Rosenbaum JT, Herrinton LJ. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis 2013; 72:37–42. [DOI] [PubMed] [Google Scholar]
- 5.Leung JM, Olivier KN. Nontuberculous mycobacteria in patients with cystic fibrosis. Semin Respir Crit Care Med 2013; 34 (1): 124–134. [DOI] [PubMed] [Google Scholar]
- 6.Seddon P, Fidler K, Raman S, Wyatt H, Ruiz G, Elston C, Perrin F, Gyi K, Bilton D, Drobniewski F, Newport M. Prevalence of Nontuberculous Mycobacteria in Cystic Fibrosis Clinics, United Kingdom, 2009. Emerg Infect Dis 2013; 19 (7): 1128–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Clinical symptoms and survival in non-smoking and smoking HIV-negative patients with non-tuberculous mycobacterial isolation. Scand J Infect Dis 2011; 43 (3):188–196. [DOI] [PubMed] [Google Scholar]
- 8.Tan CK, Lai CC, Liao CH, Chou CH, Hsu HL, Huang YT, Hsueh PR. Mycobacterial bacteraemia in patients infected and not infected with human immunodeficiency virus, Taiwan. Clin Microbiol Infect 2010; 16 (6):627–630. [DOI] [PubMed] [Google Scholar]
- 9.Shachor-Meyouhas Y, Sprecher H, Eluk O, Ben-Barak A, Kassis I. An outbreak of Mycobacterium mucogenicum bacteremia in pediatric hematology-oncology patients. Pediatr Infect Dis J. 2011; 30 (1):30–32. [DOI] [PubMed] [Google Scholar]
- 10.Niehues T, Reichenbach J, Neubert J, Gudowius S, Puel A, Horneff G, Lainka E, Dirksen U, Schroten H, Döffinger R, Casanova JL, Wahn V. Nuclear factor kappaB essential modulator-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. J Allergy Clin Immunol 2004; 114 (6):1456–1462. [DOI] [PubMed] [Google Scholar]
- 11.Ballarino GJ, Olivier KN, Claypool RJ, Holland SM, Prevots DR. Pulmonary nontuberculous mycobacterial infections: antibiotic treatment and associated costs. Respir Med 2009;103:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith DE. Therapy of nontuberculous mycobacterial disease. Curr Opin Infect Dis 2007; 20:198–213. [DOI] [PubMed] [Google Scholar]
- 13.Griffith DE. Nontuberculous mycobacterial lung disease. Curr Opin Infect Dis 2010; 23:185–190. [DOI] [PubMed] [Google Scholar]
- 14.Leber A, Marras TK. The cost of medical management of pulmonary nontuberculous mycobacterial disease in Ontario, Canada. Canada Eur Respir J. 2011; 37:1158–1165. [DOI] [PubMed] [Google Scholar]
- 15.De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis 2006; 42:1756–1763. [DOI] [PubMed] [Google Scholar]
- 16.Bryant JM, Harris SR, Parkhill J, Dawson R, Diacon AH, van Helden P, Pym A, Mahayiddin AA, Chuchottaworn C, Sanne IM, Louw C, Boeree MJ, Hoelscher M, McHugh TD, Bateson AL, Hunt RD, Mwaigwisya S, Wright L, Gillespie SH, Bentley SD. Whole-genome sequencing to establish relapse or re-infection with Mycobacterium tuberculosis: a retrospective observational study. Lancet Respir Med 2013; 1(10):786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aitken ML, Limaye A, Pottinger P, Whimbey E, Goss CH, Tonelli MR, Cangelosi GA, Dirac MA, Olivier KN, Brown-Elliott BA, McNulty S, Wallace RJ Jr. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 2012; 185:231–232. [DOI] [PubMed] [Google Scholar]
- 18.Lorena NS, Duarte RS, Pitombo MB. Rapidly growing mycobacteria infection after videosurgical procedures - the glutaraldehyde hypothesis. Rev Col Bras Cir 2009; 36:266–267. [DOI] [PubMed] [Google Scholar]
- 19.Fox GJ, Dobler CC, Marais BJ, Denholm JT. Preventive therapy for latent tuberculosis infection - the promise and the challenges. Int J Infect Dis 2017; 56:68–76. [DOI] [PubMed] [Google Scholar]
- 20.WHO (2017). Global Tuberculosis Report (Geneva: World Health Organization; ). [Google Scholar]
- 21.Franzblau SG, DeGroote MA, Cho SH, Andries K, Nuermberger E, Orme IM, Mdluli K, Angulo-Barturen I, Dick T, Dartois V, Lenaerts AJ. Comprehensive analysis of methods used for the evaluation of compounds against Mycobacterium tuberculosis. Tuberculosis (Edinb), 2012; 92 (6):453–488. [DOI] [PubMed] [Google Scholar]
- 22.Wong C, Graham J, Day J, McFaddin E, Ochsner UA, Hoang T, Young CL, Ribble W, De Groote MA, Jarvis TC, Sun X. Discovery of a new class of adamantane amides as potent antimycobacterial agents. Unpublished data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Groote MA, Jarvis TC, Wong C, Graham J, Hoang T, Young CL, Ribble W, Day J, Li W, Jackson M, Gonzalez-Juarrero M, Sun X, Ochsner UA. Front Microbiol. Manuscript accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.