Abstract
It is well established that older adults are less able to perform attentionally demanding motor tasks, placing them at greater risk of accident-related injury. The primary purpose of this study was to investigate whether the interplay between prefrontal and motor cortex activity could predict such age-related performance deficits. Using a dual-task (DT) paradigm, 15 younger and 15 older adults participated in experiment 1, where brain activity was simultaneously measured using functional near infrared spectroscopy (fNIRS) and transcranial magnetic stimulation (TMS). Experiment 1 demonstrated poorer performance for the older group across a range of DTs combining visuomotor arm tracking with a secondary cognitive or motor task. Interestingly however, older adults’ DT performance error was isolated to the motor component of DTs. TMS data revealed reduced motor cortex (M1) inhibition during DTs for older adults, and a trend for correlation with poorer performance. In contrast, poorer performing younger adults showed significantly higher M1 inhibition. Experiment 2 was conducted given a high amount of movement artifact in experiment 1 fNIRS data. Using fNIRS to measure prefrontal, premotor, and motor cortex activity in an additional 15 older adults, we found no evidence of an interplay between these regions predicting DT performance. Nevertheless, performance data replicated experiment 1 in showing that DT error was isolated to motor tasks in older adults, with no significant cognitive task error. Overall, this study shows that older adults seemed to adopt a ‘cognitive-first’ prioritisation strategy during the DTs involved in our study, and that deficits in DT performance may be related to the modulation of M1 inhibitory mechanisms. We propose that clinicians advise older adults to allocate greater attention to motor tasks during activities where they may be at risk of accident-related injury.
Keywords: dual-task, primary motor cortex, prefrontal cortex, prioritisation, inhibition
1. Introduction
Many studies have demonstrated that additional cognitive load is detrimental to our ability to perform a wide range of motor tasks, including walking (Al-Yahya et al., 2011), balancing (Li et al., 2010), and driving a motor vehicle (Blanco et al., 2006). Dual-task (DT) experiments, where participants are asked to perform two tasks simultaneously, have shown that these deficits in motor performance increase with advancing age (Verhaeghen et al., 2003), and demonstrated that DT performance can predict the future incidence of falls in older adults (Beauchet et al., 2007).
Prominent DT theories suggest that such deficits in motor performance are caused by spatial (Schumacher et al., 2003) and temporal interference (Pashler, 1992) in prefrontal cortex (PFC) information processing, leading to compromised motor response selection and execution. Age-related decreases in cortical volume are more pronounced in the PFC than other brain regions (Head et al., 2002; Salat et al., 2004), thus it has been suggested that motor performance deficits in older adults may be magnified because of a reduced capacity of PFC networks to activate the primary motor cortex (M1) structures required for task execution (Corp et al., 2013; Fujiyama et al., 2016).
In support of this hypothesis, DT experiments have shown reduced task-related brain activity in older adults in both the PFC (Heuninckx et al., 2008), and M1 (Fujiyama et al., 2012), and reduced disinhibition of PFC-M1 pathways during movement preparation (Fujiyama et al., 2016), that was related to poorer DT performance. In addition, better performing older adults have been shown to upregulate PFC activity during a motor DT (Goble et al., 2010), in line with prominent theories of age-related compensatory PFC activity to maintain task performance (Cabeza et al., 2002; Reuter-Lorenz and Cappell, 2008). These findings suggest a functional interplay between PFC and motor regions that is required for the successful performance of attentionally demanding motor tasks.
However, while DT experiments in older adults have shown task-related deficits in PFC and M1 activity separately, to the authors’ knowledge, it has yet to be demonstrated empirically whether changes in the functional interplay between these two brain regions during a DT can explain performance deficits in older adults. Thus, the primary aim of this study was to test the theory that DT performance in older adults was dependent on the concurrent upregulation of PFC and M1 activity (Corp et al., 2013). We hypothesised that multiple regression would show that a reduced ability to activate the PFC and M1 concurrently would predict poorer DT performance in older adults. While most studies involve only one DT condition, we included five different DT conditions to ascertain whether our findings were generalisable across tasks. Our initial experiment (experiment 1) used functional near-infrared spectroscopy (fNIRS) and TMS to concurrently measure PFC and M1 activity in 15 younger and 15 older adults. Unfortunately, much of the fNIRS data contained motion artifact, and we were thus unable to answer our primary question. Therefore we conducted a second experiment involving an additional 15 older adults (none of whom participated in experiment 1), to again address this aim.
2. Experiment 1 materials and methods
2.1. Participants
Fifteen younger (9 males; M=27.7; SD=3.1; range 21–35 years) and 15 older adults (9 males; M=65.2; SD=3.9; range 58–73 years) adults participated in experiment 1. Sample size was based on prior DT publications using TMS (Fujiyama et al., 2009; Fujiyama et al., 2012) and fNIRS (Beurskens et al., 2014; Holtzer et al., 2011), demonstrating significant group differences between younger and older adults. All participants were considered right handed as measured by the Edinburgh handedness questionnaire (Oldfield, 1971). All participants were above the Mini Mental State Examination (MMSE) cut-off for cognitive impairment (24) (Crum et al., 1993). There was no group difference in estimated IQ, based on the Weschler Test of Adult Reading (Wechsler, 2008) (younger mean=109.8; SD=8.2; older mean=110.6; SD=8.3; p>0.05). All participants provided informed consent and completed a health-screening questionnaire prior to participation. Exclusion criteria were: self-reported hearing or vision impairments; history of traumatic brain injury; a previous neurological condition; or other motor impairment affecting task performance. All forms and procedures were approved by the Deakin University Human Research Ethics Committee.
2.2. Tasks
Videos of the tasks can be viewed at <please provide a link to video 1 here> and <please provide a link to video 2 here>.
2.2.1. Arm tracking
Participants were seated in a custom-built chair seated approximately one metre from a computer screen (Figure 1C). An adjustable handle was held from below to ensure comfortable and consistent arm position (arm supination) for all participants. An electronic goniometer (Biometrics, Ltd., UK) measured the angle of the elbow joint, and communicated with a custom-built computer program (LabVIEW, National Instruments, U.S.A.), which showed the arm tracking task on the computer screen. Elbow angles were normalised for each participant, with full comfortable extension for each participant calibrated to be 0° in the computer program, and 90° being a true 90° angle as measured by a hand-held goniometer. Two markers were presented on the computer screen: one ‘target’ marker that moved at a sinusoidal rate of 0.08Hz, with an upper speed of 30° per second at the middle of the movement, and a lower speed of 0° at the top and bottom of the movement (where participants transitioned from flexion to extension, or vice versa); and a ‘participant’ marker, which moved with the elbow (elbow flexion=marker moved upward; elbow extension=downward). This variable arm tracking rate was used to ensure sustained vigilance. Participants were tasked with keeping their marker as close as possible to the ‘target’ marker throughout the one-minute trials.
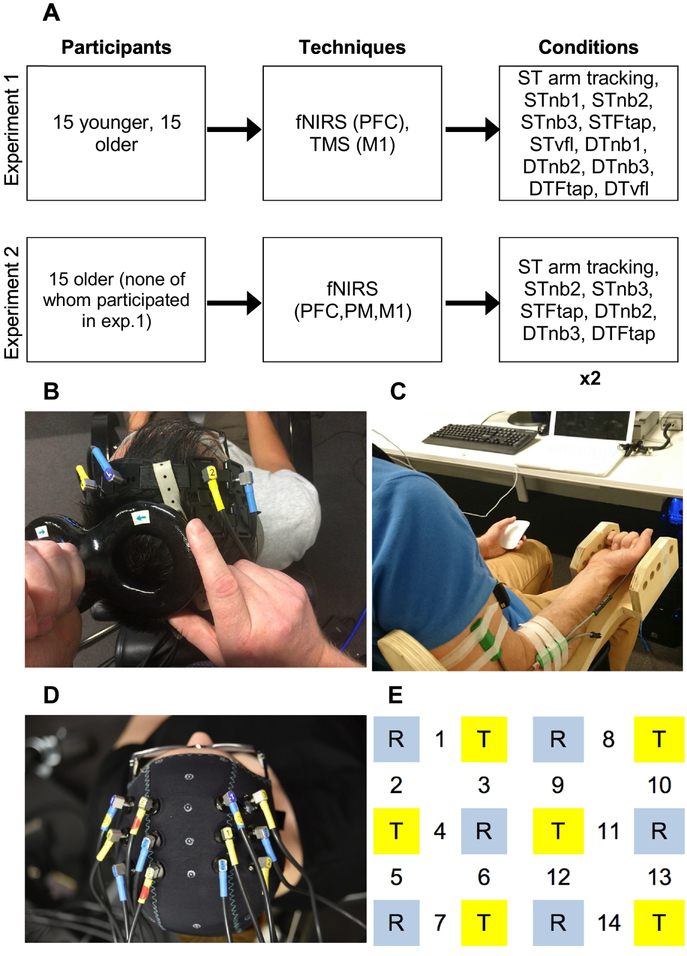
Figure 1. Study overview and experimental setup.
Figure 1A shows the methods used for experiments 1&2. ×2 below indicates that each condition was performed twice in experiment 2. Figure 1B shows fNIRS channels 1&2 over the left and right PFC, and the TMS coil applying pulses to the BB representation of the left M1 (experiment 1). Figure 1C shows arm tracking and concurrent n-back task performance (responding with left hand mouse click). Figure 1D shows fNIRS montage in experiment 2, and Figure 1E zooms in on this optode montage. T (yellow squares) = transmitter; R (blue squares) = receiver. Channels 1–3 (left hemisphere) and 8–10 (right hemisphere) measured activity within PFC, channels 4–6 and 11–13 measured activity within PM, and channels 7 and 14 measured activity within M1. PFC, PM, and M1 appear in ‘techniques’ box to indicate the brain regions that were measured by each technique, in each experiment.
2.2.2. n-back task (nb)
Three levels of nb difficulty were included: nb1, nb2, and nb3, each performed separately for one-minute (Kirchner, 1958). The task was exclusively auditory (Inquisit, v4, Millisecond Software, USA). A separate laptop played the sequence of letters through earphones worn by participants (Figure 1C). Eight letters (c, h, k, l, q, r, s, t) were used. Twenty letter repetitions were read per trial with an inter-letter interval of three seconds. Thirty percent of letters were targets, which prompted participants to respond by clicking a wireless mouse held in their left hand (Figure 1C). Non-targets required no response; therefore responses to non-targets constituted an error.
2.2.3. Foot tapping task (Ftap)
Participants were instructed to tap their left foot for one-minute in time with a 0.5 Hz metronome played through earbuds, while keeping their heel in contact with the ground (video 2).
2.2.4. Verbal fluency task (vfl)
Participants were asked to recite as many words as possible starting with a target letter within the one-minute trial (Benton et al., 1994). Three target letters (either C, F, L or P, R, W - assigned randomly) were given consecutively (20 seconds each letter).
2.2.5. Task familiarisation
Familiarisation stages for all nb levels were pre-programmed, lasting 30 seconds. Arm tracking, foot-tapping and verbal fluency conditions were also practiced for approximately 30 seconds. Training was given under both single-task (ST) and DT conditions as recommended by Corp et al. (2014). Participants were instructed to attempt to perform both tasks equally well in DT conditions. We intended to limit any effects of practice; therefore this period was only so long as to allow understanding of the tasks. No feedback of performance was provided to participants during testing.
2.2.6. Single-task and dual-task conditions
In experiment 1, each of the aforementioned tasks were performed alone to form six ST conditions: 1) ST arm tracking; 2) STnb1; 3) STnb2; 4) STnb3; 5) STFtap; and 6) STvfl. These tasks were then combined with the ‘primary task’ of arm tracking to form five DT conditions: 1) DTnb1 (arm tracking + nb1); 2) DTnb2 (arm tracking + nb2); 3) DTnb3 (arm tracking + nb3); 4) DTFtap (arm tracking + Ftap); and 5) DTvfl (arm tracking + verbal fluency) (Figure 1A). Thus, each testing session comprised of 11 conditions, lasting for one-minute each. Conditions were performed in pseudo-random, counterbalanced order - with the presentation of ST and DT conditions alternated.
Given our interest in motor task performance during DTs, the arm tracking task is defined as the ‘primary task’, and other tasks (nb, Ftap, vfl) defined as ‘secondary tasks’.
2.3. Functional near-infrared spectroscopy
A fNIRS system (Oxymon MKIII, Artinis Medical Systems, Zetten, The Netherlands) measured cerebral oxygenated (HbO) and deoxygenated haemoglobin (HbR). This system used two wavelengths, 856 and 763nm, and a sampling rate of 10Hz. Measurement of HbO and HbR incorporated an equation for differential pathlength factor allowing for participant age (Duncan et al., 1996). Channels consisted of one source and one detector, with an interoptode distance of 3cm, placed either side of the target region (Koenraadt et al., 2014).
Two channels were used, with one channel over the right PFC, and one over the left PFC (Figure 1B). Based on previous DT experiments (Dux et al., 2009; Tombu et al., 2011), we placed our channels 1cm posterior to F3 and F4 (10–20 EEG system) (Jasper, 1958) located using an EEG cap (Easycap, Germany).
To establish baseline brain activity, participants were instructed to sit silently for approximately 30 seconds prior to task onset, keeping as still as possible, except for the vfl task, where participants were instructed to say “A, B, C, D” repeatedly until task onset.
2.4. Electromyography and transcranial magnetic stimulation
To improve methodological quality, a TMS checklist was referred to before data collection (Appendix A) (Chipchase et al., 2012).
EMG activity was recorded from wireless surface electrodes (Powerlab, USA) placed over the belly of the biceps brachii (BB) muscle of the participant’s dominant arm. EMG recordings (LabPro, USA) were amplified (×1000) with bandpass filtering between 10 Hz and 1000 Hz, and a sampling rate of 2000 Hz. A Magstim 2002 magnetic stimulator with a 70 mm figure-of-eight coil (Magstim, UK) was used, producing a monophasic pulse. The coil was heldby hand tangential to the skull inducing a posterior-anterior current in the cortex (Ziemann et al., 1996) (Figure 1B). The ‘optimal’ site of stimulation was marked on the scalp after finding the location at which the largest motor evoked potential (MEP) could be obtained in the participant’s right BB muscle.
The custom-built computer program (see section 2.2.1) triggered a single TMS pulse when the participants’ elbow angle reached 90° (flexion phase only) during the arm tracking task. The rate of one full arm tracking cycle was 0.08Hz, triggering a TMS pulse every 12.5 seconds, enabling us to collect five responses to TMS pulses per one-minute trial. While a greater number of TMS pulses would have increased reliability, this would have increased the duration of trials, possibly inducing fatigue. Given that intrasession reliability has previously been demonstrated using five TMS pulses (Christie et al., 2007; Doeltgen et al., 2009), this number represented a compromise between TMS data reliability and minimising possible bias of performance data due to fatigue. During all conditions, TMS pulses were applied at an intensity required to produce a 1mV response (Kujirai et al., 1993) in the BB muscle during the arm tracking task, which was determined prior to testing.
2.5. Data and statistical analysis
2.5.1. Performance data
For the arm tracking task, the position of the target and participant controlled markers was sampled at a rate of 1000Hz. Root mean square (RMS) error was used to evaluate performance, and was calculated by measuring the distance between the two markers across the trial. For nb tasks, percentage of correct responses were saved in the computer program and analysed offline. For the Ftap task, the sound recorder application within an iPhone (Apple, USA) was placed next to the participants left foot to capture tapping. This application shows sound waves visually so that the timing of the foot taps could be measured offline. Error was defined as the absolute time (ms) away from a foot tap every two seconds (given the 0.5Hz metronome): Σ((t2 – t1) − 2) / number of foot taps performed. For the vfl task, the number of correct word responses in one-minute was recorded. A sound recorder was used so that words could be checked offline.
Immediately after the performance of all conditions, participants were asked to provide a rating of difficulty on a five-point scale: 0 = no demand at all; 1 = small; 2 = moderate; 3 = high; 4 = very high; 5 = excessive demand.
2.5.2. fNIRS data
The fNIRS processing stream was as follows: raw fNIRS data were exported to the Homer2 fNIRS processing package (v2.0) (Huppert et al., 2009), where data were converted into changes in optical density data, and motion correction applied using the spline interpolation method (Scholkmann et al., 2010) with the following settings: p = 0.99; tMotion = 1.5 s; tMask = 1.5 s; STDEVthresh = 20; AMPthresh = 0.08. A low pass filter of 0.5Hz was then employed, and data were converted into concentration changes in HbO and HbR. A high-pass filter was not considered necessary because trials were relatively short (one-minute) and therefore there was not a significant amount of signal drift. Experiment 1 data contained motion artifacts mostly due to contact between the optodes and the TMS coil, therefore at this point all data were screened for excessive artifact by one author (DC). Data containing multiple motion artifacts that were judged to significantly distort HbO and HbR responses, even after motion correction, were removed. Although these judgments were somewhat subjective, data were deidentified to avoid bias. Task related (based on one-minute duration of each of ST and DT condition) HbO and HbR concentrations of remaining data were then calculated using the equation: (10 to 60 second average Hb concentration post task onset) – (− 10 to 0 seconds average Hb concentration prior to task onset). Lastly, the haemoglobin differential (HbDiff) was calculated using the equation (HbO – HbR), and used as the dependent variable to estimate brain activity (Ayaz et al., 2012; Lu et al., 2015). The typical response to cortical activation as revealed by fNIRS is an increase in HbO and a concomitant decrease in HbR, in order to facilitate tissue oxygenation (Ferrari and Quaresima, 2012). Thus, we use HbDiff because the consideration of both HbO and HbR is suggested to be a more reliable estimate of brain activity in comparison to the analysis of HbO in isolation (Obrig and Villringer, 2003).
Due to motion artifact, as an additional quality control we checked whether fNIRS data were reproducible after applying a range of other artifact removal methods (see settings of each method in Appendix B). These analyses generally demonstrated high Pearson correlation coefficients between the results of each method (Appendix C).
2.5.3. TMS data
MEP amplitude, reflecting corticospinal excitability (Hallett, 1996), was quantified off-line using Labchart 7 (AD Instruments, USA) by measuring the peak-to-peak amplitude of the waveform. Silent period (SP) duration, reflecting M1 inhibition (Inghilleri et al., 1993), was measured by visual inspection, and defined as the time from the onset of the MEP until the return of the subsequent EMG signal (Christie and Kamen, 2014; Latella et al., 2012). Pre-stimulus muscle activity was measured by calculating the RMS of EMG activity −100 ms to −50 ms prior to the TMS pulse.
2.5.4. DT normalisation (DTchange)
Performance, TMS, and fNIRS data from DT conditions were normalised to corresponding ST conditions to isolate the influence of the additional task on each of these variables. For all data, this normalisation procedure is presented as ‘DTchange’, which was then used as the dependent variable for statistical analyses. The equations used to derive the DTchange for each variable were as follows (for performance data, DTchange was calculated for both primary [arm tracking] and secondary tasks [nb, Ftap, and vfl): primary task DTchange = DT performance error of primary task / ST error of primary task × 100; secondary task DTchange = DT performance error of secondary task / ST error of secondary task × 100. For TMS data, both MEP amplitude and SP duration were normalised to a DTchange using the equation: DT/ST × 100. For example, the DTchange of MEP amplitude in DTnb1 condition = (MEP amplitude DTnb1 condition) / (MEP amplitude ST arm tracking condition) × 100. Thus, for performance and TMS data, the normalised ST value was 100. For experiment 1 fNIRS data, 38.3% of ST arm tracking trials had to be removed due to motion artifact (see section 3.1.2), which restricted our ability to perform DT normalisation based on ST condition data. Thus, we present fNIRS activity (dependent variable = HbDiff) (without DT normalisation) in each of the ST and DT conditions.
2.5.5. Statistical analyses
For performance data, to test for an effect of an additional task within groups, one-sample t-tests compared DTchange to 100 for each DT condition. Repeated measures ANOVA tested for main effect of age group on DTchange, and an independent samples t-test compared DTchange between groups, within each DT condition.
For fNIRS data, a linear mixed effects regression model was used within each hemisphere separately to examine the main effect of age group on HbDiff across the five DT conditions (regression performed in Stata 13, StataCorp, USA; all other statistical analyses were conducted using JMP Pro 13, SAS Institute, USA). Regression was preferred over repeated-measures ANOVA given missing data (due to motion artifact). A mixed-effects model was used to account for the repeated measures data (i.e., the five DT conditions nested within individuals) and thus models included a random intercept clustered by the individual, and used a full information maximum likelihood (FIML) approach to allow us to retain all available data (Graham, 2009). FIML contrasts with listwise deletion used by repeated measures ANOVA (Roth, 1994). Post-hoc comparisons compared HbDiff in the ST arm tracking condition to each DT condition, within groups, and were performed by examining the marginal mean differences using a Wald test.
For TMS data, to test the reliability of visual inspection of SP durations, we quantified intraclass correlation coefficients (ICC) between original SPs measured by the first author (DC), and a second investigator blinded to these results (JR). ICCs were high in all conditions according to conventional thresholds (Portney and Watkins, 2009). Thus, original SP durations assessed by the first author were subsequently used for all statistical analyses. ICCs values are presented in Appendix D. To test for the effect an additional task within groups, one-sample t-tests compared MEP amplitude DTchange to 100, and compared SP duration DTchange to 100, for each DT condition. Repeated-measures ANOVA tested the main effect of age group on the dependent variables of MEP amplitude DTchange, and SP duration DTchange. An independent samples t-test compared DTchange between groups, within each DT condition. Repeated measures ANOVA tested muscle excitability prior to the TMS pulse (RMS of EMG activity −100 ms to −50 ms prior to TMS pulse).
As per the primary aim of this study, we planned to use multiple regression to investigate whether the combined activity of the PFC and M1 could predict DT performance in older adults. However, due to missing fNIRS data (see section 3.1.2), multiple regression on the small number of data points remaining may have yielded biased parameter estimates (Graham, 2009). Therefore, we instead used correlation to investigate possible relationships between fNIRS, TMS, and performance data. For correlations between fNIRS and performance data, we used Spearman’s rank correlation coefficient (rho) given small sample size and missing data (Mukaka, 2012), while Pearson’s correlation coefficient was used to investigate relationships between TMS and performance data.
Correlations were made between DTchange variables within corresponding DT conditions (e.g. MEP amplitude DTchange in DTnb1 condition correlated with DTchange in arm tracking error for DTnb1 condition). However, as noted, normalisation was not performed for fNIRS data, thus raw HbDiff data was correlated with DTchange in performance data. Significance was set at p<0.05 for all comparisons.
3. Experiment 1 results
3.1. DT performance
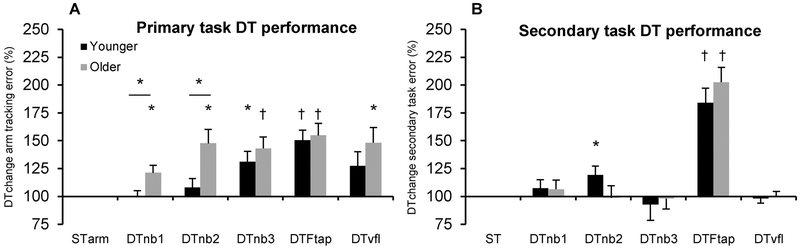
For the primary task, with DTchange collapsed across DT conditions, ANOVA showed that older adults had significantly greater DTchange in performance error (poorer DT performance) (F=4.68; df=1,28; p=0.039). More specifically, independent samples t-test showed that older adults had significantly higher DTchange in primary task error in the DTnb1 and DTnb2 conditions, compared to the younger group (Figure 2A). Figure 2A also shows conditions where there was significant within group DTchange in primary task performance error, compared to ST arm tracking.
Figure 2. DTchange in primary and secondary task performance error for younger and older adults in experiment 1.
Figures 2A&B show M±SE DTchange in performance error with additional tasks. Higher values represent worse DT performance. Asterisks/crosses show significant within group difference compared to normalised single-task conditions, and also between group differences in DTchange in the DTnb1 and DTnb2 conditions (Figure A). * = p<0.025; † = p<0.001. STarm = ST arm tracking. ‘ST’ in Figure 2B denotes normalised secondary task ST performance, e.g. STnb1, STnb2 etc.
For DTchange in secondary task error, ANOVA showed no significant effect of age group (F=0.22; df=1,28; p=0.884). Figure 2B shows significant DTchange in secondary task error in the DTFtap condition for both groups, and significant DTchange in secondary task error in the DTnb2 condition for the younger group. Thus, the additional task did not produce any increases in performance error in the older group for any of the cognitive tasks (nb1, nb2, nb3, vfl), suggesting that older adults prioritised cognitive over motor tasks.
Interestingly, both groups showed the greatest (primary and secondary) DTchange in performance error for the motor-motor DT (DTFtap) (Figure 2), despite it being rated the second easiest DT by both groups (Table 1).
Table 1.
Mean ± SE ratings of difficulty for each condition in experiment 1&2. Arm tr. = ST arm tracking.
| ST | DT | |||||
|---|---|---|---|---|---|---|
| Task | Younger (Exp. 1) |
Older (Exp. 1) |
Older (Exp. 2) |
Younger (Exp. 1) |
Older (Exp. 1) |
Older (Exp. 2) |
| Arm tr. | 1.1 ± 0.2 | 1.6 ± 0.2 | 1.9 ± 0.2 | - | - | - |
| nb1 | 1.0 ± 0.2 | 1.4 ± 0.2 | - | 1.6 ± 0.2 | 2.4 ± 0.2 | - |
| nb2 | 1.6 ± 0.2 | 2.9 ± 0.4 | 2.8 ± 0.3 | 2.5 ± 0.2 | 3.1 ± 0.1 | 3.5 ± 0.3 |
| nb3 | 3.1 ± 0.3 | 4.1 ± 0.3 | 4.0 ± 0.3 | 3.5 ± 0.3 | 4.1 ± 0.2 | 4.5 ± 0.2 |
| Ftap | 0.9 ± 0.1 | 1.5 ± 0.3 | 1.2 ± 0.2 | 2.3 ± 0.2 | 2.7 ± 0.3 | 3.0 ± 0.3 |
| vfl | 2.5 ± 0.3 | 3.1 ± 0.2 | - | 3.0 ± 0.3 | 3.4 ± 0.2 | - |
3.2. fNIRS data
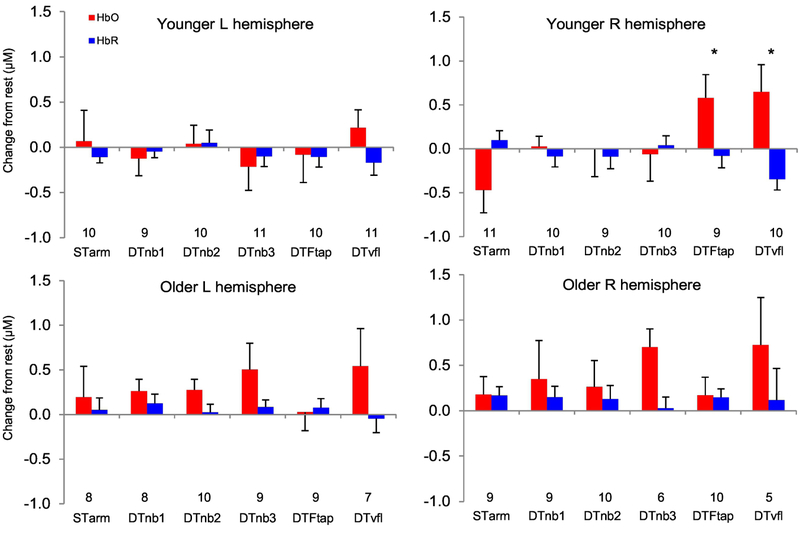
Unfortunately, in experiment 1, 37.5% of fNIRS data from all ST and DT conditions had to be removed due to motion artifact. Numbers along x-axes of Figure 3 shows the data points (of the original 15 participants) remaining within each group/condition.
Figure 3. Prefrontal cortex activity during dual-tasking in experiment 1.
M±SE Oxygenated (HbO) and deoxygenated (HbR) haemoglobin responses during STarm tracking (‘STarm’) and dual-task conditions. Although HbO and HbR are presented, the dependent variable used for statistical analyses was ‘HbDiff’ (HbO-HbR). The number of remaining data points (due to fNIRS data exclusion) per condition are included above the x-axis headings. μM = micromolar. Asterisks show a significant increase for the younger group in HbDiff in the DTFtap (p=0.003) and DTvfl condition (p<0.001), compared to HbDiff in ST arm tracking condition.
There was no main effect of age group for HbDiff collapsed across DT conditions - left PFC HbDiff: b=0.24; SE=0.36; p=0.51; right PFC HbDiff: b=0.15; SE=0.39; p=0.70.
Within groups, younger adults showed an increase in right PFC HbDiff compared to ST arm tracking, for both the DTFtap and DTvfl condition (Figure 3). There were no other increases in HbDiff due to the additional task for any other DT conditions.
3.3. TMS data
There was no difference in pre-stimulus EMG between conditions for either younger (F=0.11; df=5,89; p=0.99) or older groups (F=0.04; df=5,89; p=0.99).
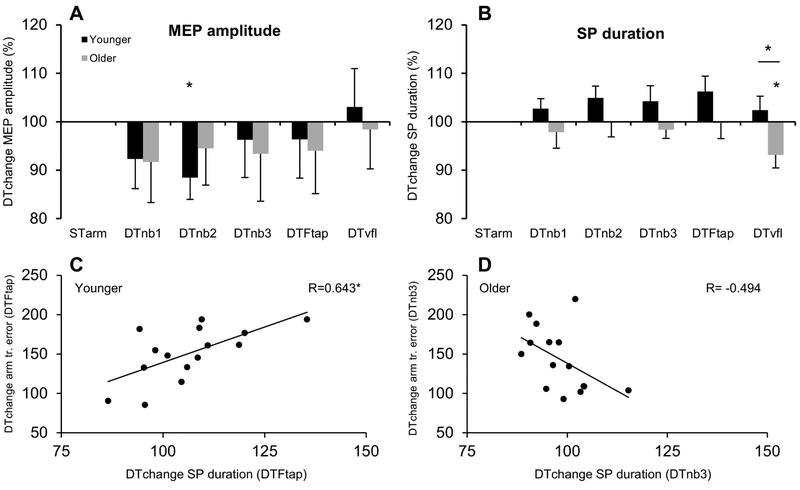
ANOVA showed no effect of age group (F=0.02; df=1,28; p=0.90), on MEP amplitude. Within groups, there was a significant reduction in MEP amplitude DTchange in the DTnb2 condition for the younger group (t=2.56; p=0.023) (Figure 4A).
Figure 4. Corticospinal activity during dual-tasking in experiment 1.
Figures A&B show M±SE MEP amplitude & SP duration DTchange (DT/STarm tracking ×100) Asterisks denote significant (p<0.05) DTchange within group compared to ST arm tracking, and also a between-groups difference in SP duration DTchange for the DTvfl condition. With DTs collapsed across conditions, ANOVA showed significantly reduced SP duration for older adults compared to the younger group (see text). Figures 4C&D show correlations between SP duration and DT performance. Figure 4A shows that poorer performing younger adults in the DTFtap condition had significantly greater SP duration DTchange (p=0.0098). Conversely, figure 4D shows that poorer performing older adults in the DTnb3 condition had a trend for reduced SP duration DTchange (p=0.061). Note that all 15 participants’ data are present in Figure D, but that one data point is concealed behind another at x,y: 104,109. (STarm=ST arm tracking condition).
ANOVA showed significantly reduced DTchange in SP duration across conditions for older, compared to younger, adults (shorter normalised SP duration in DT conditions) (F=5.16; df=1,28; p=0.031). Specifically, independent t-test showed reduced DTchange in SP duration for the older group in the DTvfl condition, compared to the younger group (t=2.34, p=0.027). Within groups, compared to ST tracking, older adults showed significant reduction in SP duration in the DTvfl condition (t=2.55; p=0.023). Increases in SP duration for DTnb2 and DTFtap conditions (compared to ST tracking) in the younger group did not reach significance (both p=0.07).
3.4. Relationships between brain activity and dual-task performance
Younger adults had a significant positive correlation between DTchange in SP duration and DTchange in arm tracking error in the DTFtap condition (Figure 4C). In other words, younger adults with higher DT arm tracking error showed higher M1 inhibition. Conversely, in the DTnb3 condition, older adults with greater DTchange in arm tracking error had lower DTchange in SP duration (Figure 4D), although this trend did not reach significance (p=0.061). There were no correlations between DT performance and HbDiff.
4. Experiment 2 materials and methods
4.1. Overview and rationale
Experiment 1 data contained motion artifact mostly caused by contact between the TMS coil and the fNIRS optodes. Therefore we could not use regression to answer our primary research question of whether the combined activity of PFC and M1 could predict DT performance in older adults. Thus, we recruited an additional 15 older adults (none of whom participated in experiment 1) to again attempt to answer this question. A younger group was not recruited in experiment 2 because the sole aim was to test whether brain activity could predict DT performance in older adults. Methods in experiment 2 were mostly identical to those used in experiment 1, with some exceptions (Figure 1A). Firstly, only fNIRS was used (no TMS) in order to reduce motion artifact. This also allowed us to measure responses of fNIRS channels over the premotor cortex (PM), valuable given that this brain region has been suggested to act as an intermediary between the PFC and M1 in coordinating DT performance (Corp et al., 2013; Fujiyama et al., 2016). Secondly, fewer conditions were included, but participants performed each condition twice (Figure 1A, and see methods below). This was done to ensure that our aforementioned performance results were not due to chance, given that each condition was only performed once in experiment 1.
4.2. Participants
An additional 15 older adults (7 males; M=66.3; SD=4.6; range 60–76 years), none of whom participated in experiment 1, participated in experiment 2. Estimated IQ did not differ to scores of either of the experiment 1 groups: mean=112.5; SD=7.9) (p>0.05). Consent, exclusion criteria, and ethics procedures were identical to those described in experiment 1.
4.3. Tasks
All tasks and experimental procedures, including familiarisation, were identical to those described in experiment 1. However, experiment two involved only four STs: 1) STarm tracking; 2) STnb2; 3) STnb3; and 4) STFtap. These tasks were then combined with the primary task of arm tracking to form three DT conditions: 1) DTnb2 (arm tracking + nb2); 2) DTnb3 (arm tracking + nb3); and 3) DTFtap (arm tracking + Ftap) (Figure 1A). Each of these ST and DT conditions were performed twice, with results averaged between trials.
4.4. Functional near-infrared spectroscopy
The fNIRS system used was the same as described in experiment 1, however two machines were used to enable the use of 14 channels. The channels spanned the PFC, PM, and M1 (Figure 1D&E). The anterior-most channels (1&8) were located over F3 and F4, and the posterior-most channels (7&14) were located 4.5cm lateral and 1cm anterior of Cz, over our best estimation of the M1 representation for the BB (Brasil-Neto et al., 1992; Fuhr et al., 1991). The middle channels (4&11) were located 3cm anterior of the M1 channels, approximately corresponding to the dorsal PM cortex (Boros et al., 2008; Picard and Strick, 2001). Caps were custom made so that optodes could be placed in these particular positions, and two different sized caps were made to allow for variable head size.
4.5. Data and statistical analysis
Performance and fNIRS data were processed using the same methods as described in experiment 1. However, given that we now had fNIRS data from 14 channels (as opposed to 2 channels in experiment 1), we grouped channels into three brain regions: PFC; PM; and M1 (Figure 1C&D). The use of multiple fNIRS channels within brain region reduces data variance, and is useful given the low spatial resolution and weaker signal to noise ratio of fNIRS in comparison to other imaging modalities (Cui et al., 2011; Lu et al., 2010), The data from channels within these regions was averaged to give a mean HbDiff value for left and right hemisphere PFC, PM, and M1.
4.5.1. DT normalisation (DTchange)
We used the same normalisation method for performance data as in experiment 1, to create a DTchange for primary and secondary task error. For fNIRS data, DTchange was calculated using the equation: DT HbDiff – ST HbDiff (Szameitat et al., 2011). As with performance data, DTchange was calculated for primary (e.g. HbDiff DTnb2 condition – HbDiff ST arm tracking condition), and secondary tasks (e.g. HbDiff DTnb2 condition – HbDiff STnb2 condition).
4.5.2. Statistical analyses
For performance data, to test for the effect of an additional task, a one-sample t-test compared DTchange to 100 (normalised ST value), in each DT condition.
For fNIRS data, within each brain region (PFC, PM, M1), to test whether there was an effect of the additional task, a one-sample t-test compared HbDiff DTchange of each DT condition to 0 (normalised ST value), for primary and secondary tasks.
As per the primary aim of the paper, we then performed a number of multiple regression analyses to assess whether the combined HbDiff within the PFC, PM, and M1 could predict DT performance. Here, we used all possible combinations of left and right hemisphere PFC, PM, and M1 brain regions as independent variables in attempting to predict DT performance in each condition (16 possible combinations per DT condition; 3 DT conditions = 48 multiple regression analyses in total). All independent and dependent variables for multiple regression analyses are listed in the supplementary methods file (Appendix B).
5. Experiment 2 results
5.1. Performance data
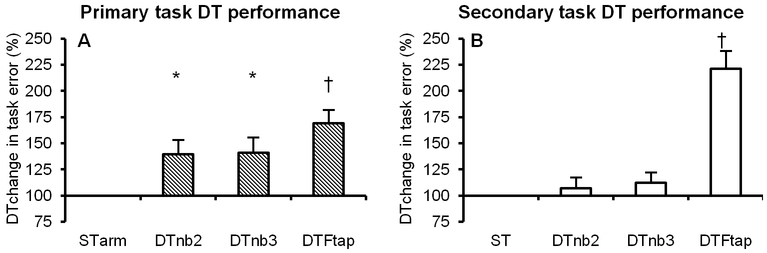
Experiment 2 performance data replicated findings of experiment 1. Results suggested that older adults again prioritised cognitive tasks at the expense of motor tasks (Figure 5), with significant DTchange in arm tracking performance in all conditions, but no DTchange in error for cognitive tasks (nb2 or nb3). In addition, the motor-motor DT again showed the greatest DTchange in performance error despite being rated the easiest DT (Table 1).
Figure 5. DT performance of older adults in experiment 2.
Figure 5A shows the influence of an additional task (M±SE DTchange) on primary task performance error (e.g. DTnb2 arm tracking error / STarm tracking error × 100). Figure 5B shows the influence of an additional task on secondary task performance error (e.g. DTnb2 response error % / STnb2 response error % ×100). * = p<0.025; † = p<0.001 compared to ST. As in experiment 1, the additional task resulted in significant error in older adults’ performance of motor, but not cognitive tasks.
5.2. fNIRS data
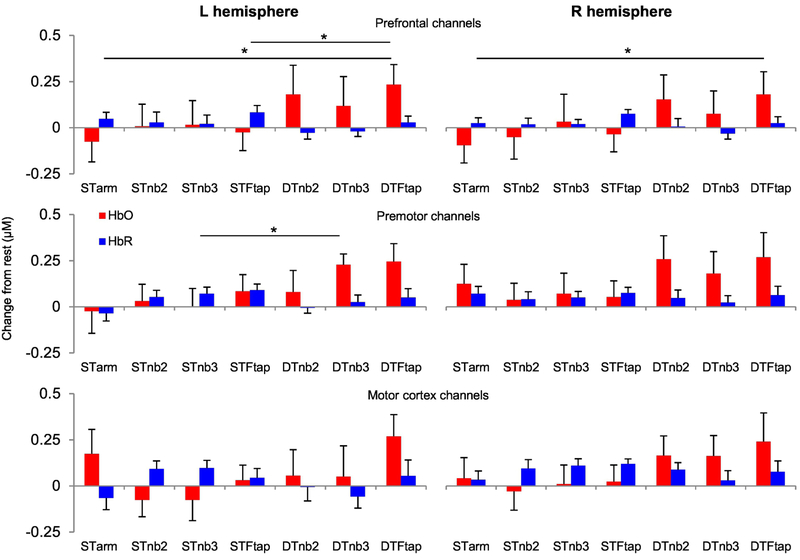
Using the same motion artifact removal methods as in experiment 1, we removed one participants’ data from three conditions (STtracking, STFtap, DTFtap).
For the primary task, there was a significant DTchange (DTFtap – ST arm tracking) in HbDiff in both the left (t=2.90; df=13, p=0.013) and right PFC (t=2.59; df=13; p=0.023) for the DTFtap condition.
For the secondary task, there was a significant DTchange (DTFtap – STFtap) in HbDiff in the left PFC for the DTFtap condition (t=2.77; df=13, p=0.016). In addition, there was a significant DTchange in HbDiff in the left PM (t=3.01; df=14; p=0.010) for the DTnb3 condition.
A number of tests approached significance for DTchange in HbDiff: primary task increase in right PFC for DTnb2 condition (p=0.051); secondary task increase in right PFC for DTFtap condition (p=0.063); secondary task increase in right M1 for DTnb3 condition (p=0.051); secondary task increase in left M1 for DTFtap condition (p=0.069).
5.3. Relationships between brain activity and dual-task performance
Multiple regression analyses did not reveal any significant results for the independent variables of PFC, PM, and M1 activity in predicting DT performance.
6. Discussion
The primary aim of this study was to investigate whether the combined activity of prefrontal and motor brain regions could predict DT performance in older adults. We found no evidence of such a phenomenon; yet suggest that this was largely due to high interindividual variability in fNIRS response, discussed further in section 6.1. Despite this, there were a number of noteworthy findings. First, M1 inhibition was reduced in older, compared to younger adults during dual-tasking. Second, M1 inhibition was correlated with DT performance, and the direction of this relationship differed between younger and older adults. Lastly, despite our instruction to attempt to perform both tasks equally well when dual-tasking, results suggest that older adults prioritised the performance of cognitive tasks at the expense of motor tasks.
6.1. Inverse task-related fNIRS responses
Experiment 1&2 fNIRS data contained numerous inverse task-related responses (i.e. decrease in HbO, increase in HbR) across multiple conditions, leading to high interindividual variability. For example, in experiment 1, six of the 10 younger participants’ data (five of original 15 data points excluded due to motion artifact) showed an inverse response in the left PFC during the DTFtap condition, and in experiment 2, eight of the 15 older participants’ data showed an inverse response over the left PFC (channel 1) in the STnb3 condition (Appendix E). Inverse task-related responses have previously occurred in response to a number of tasks, such as motor imagery (Holper et al., 2011), memory encoding and retrieval (Jahani et al., 2017), and other DT experiments (Koenraadt et al., 2014; Mandrick et al., 2013), yet the exact physiological explanation and cognitive interpretation of this response remains unclear (Issard and Gervain, 2018). Inverse responses in the present study increased interindividual variability and therefore limited our ability to detect relationships between brain activity and DT performance.
6.2. fNIRS findings
The most noteworthy finding from fNIRS data was that PFC, PM, and M1 activity was highest in the DTFtap (motor-motor) condition in experiment 2. In agreement, Mochizuki et al. (2007) demonstrated higher brain activity in the PM for a motor-motor vs motor-cognitive DT, which correlated with better performance. We interpret these findings as support of a ‘multiprocessor’ theory of attention (Allport et al., 1972; Schumacher et al., 2003), whereby the motor-motor DT in our study required the concurrent use of more similar attentional processing mechanisms than motor-cognitive DTs, resulting in both higher spatial interference (and therefore the highest DT performance error - Figures 2&5) and fNIRS signal intensity. In support of this hypothesis, DT cost has shown to be higher when there is greater topological overlap between the regions of the brain representing the STs (Alavash et al., 2015). Interestingly, PFC HbDiff was relatively low in older adults in the DTFtap condition in experiment 1. This suggests that attentional processing structures for this DT are located more anteriorly, closer to the dorsolateral PFC (optode location in experiment 2), than the inferior frontal junction (PFC optode location in experiment 1).
6.3. ‘Cognitive-first’ prioritisation strategy for older adults
Data demonstrated that DT performance error in older adults was isolated to motor tasks. This was surprising given the high incidence of falls and motor vehicle accidents in older populations (Cooper et al., 1993; Gill et al., 2005). Nevertheless, prior research seems to align with our findings. A number of authors (e.g. Bernard-Demanze et al., 2009; Verghase et al., 2007) have cited Shumway-Cook et al. (1997) to suggest that older adults have an innate preference for adopting a ‘posture-first’ prioritisation strategy to avoid falling. However, in that study, Shumway-Cook et al. (1997) showed higher error for motor tasks in the elderly, and acknowledged that their results did not support their original ‘posture-first’ hypothesis. Many studies have shown reduced motor performance during additional cognitive tasks in older adults (for review, see (Al-Yahya et al., 2011). Unfortunately, not all of these studies measured both motor and cognitive outcomes to compare prioritisation strategy. However, a number of studies to do so have also demonstrated greater motor task error for older adults (Shumway-Cook et al., 1997; Yang et al., 2017; Yogev-Seligmann et al., 2010), as in the present study. A ‘Cognitive-first’ prioritisation strategy may also apply to stroke (Plummer et al., 2013) and Parkinson’s disease (PD) patients (Bloem et al., 2006). For example, Heinzel et al. (2016) recently showed that walking error during dual-tasking significantly predicted subsequent falls in PD patients, while cognitive error did not. However, Plummer et al. (2013) showed inconsistent patterns of prioritisation for balance tasks, suggesting that prioritisation strategy for older adults might be task-specific in certain cases.
6.4. TMS findings
For MEP amplitude, although only one DT condition showed significant difference compared to ST arm tracking, there was a general trend of lower MEP amplitude during dual-tasking (Figure 4A). MEP amplitude responses due to an additional task have been inconsistent in prior DT studies (Corp et al., 2014), which may be due to the different nature of tasks; DTs where attention is maintained continuously to the motor task seem to show unchanged or increased MEP amplitudes (Corp et al., 2015; Holste et al., 2015), while those involving a transient diversion from the motor task seem to show reduced MEP amplitude (Master and Tremblay, 2009; Poston et al., 2012). Given higher error in DT performance for arm tracking (than cognitive tasks) in the present study, reduced MEP amplitude could also be the result of motor task distraction. However, to the author’s knowledge, no study has yet shown a relationship between MEP amplitude and DT performance. Thus, it’s difficult to speculate further as to the biological mechanism of MEP amplitude change during dual-tasking.
In contrast, changes in SP duration during DTs appear to be linked to behaviour. We showed the opposite relationship between SP duration and DT performance for younger and older adults; with higher SP duration correlated with better performance in younger adults, but poorer performance in older adults. Two other DT studies have shown reduced SP duration for older, compared to younger adults (Fujiyama et al., 2009; Fujiyama et al., 2012), with Fujiyama et al. (2012) demonstrating a correlation between shorter SP duration and poorer performance in the most difficult DT condition. These authors suggested that older adults could have a reduced ability to regulate M1 inhibition, required for the timing and coordination of movements.
However, in comparison to prefrontal regions, the motor cortices are relatively resistant to age-related degeneration (Barrick et al., 2010; Head et al., 2002). Thus, changes in M1 inhibition may also reflect a reduced capacity of older adults to activate motor structures via long-range connections (Corp et al., 2013). Top-down control of motor performance is dependent on the activation of the M1 via long-range inhibitory connections from prefrontal, and secondary motor regions (Buch et al., 2010; Neubert et al., 2010). Using dual-site paired-pulse TMS, Fujiyama et al. (2016) demonstrated that bimanual performance in older adults was correlated with the inhibitory activity of PFC to M1 pathways, and also microstructural organisation of these pathways as confirmed by diffusion tensor imaging.
The present study also showed that higher M1 inhibition for younger adults was significantly correlated with worse DTFtap performance. Thus, instead of upregulation of cortical pathways in response to age-related degeneration, it may be beneficial for younger adults to ‘disinhibit’ M1 structures to facilitate movement (Byblow et al., 2007; Fujiyama et al., 2016). Given that the DTFtap condition required the concurrent use of both motor cortices, disinhibition may also have been mediated via interhemispheric connections (Fling and Seidler, 2012; Hinder et al., 2012).
7. Limitations
A number of limitations should be acknowledged. First, the collection of more than five responses to TMS pulses would have increased data reliability. Second, fNIRS stimulus artifact resulted in data removal and smaller sample sizes for comparisons in experiment 1. This also prevented us from normalising fNIRS data to a DTchange variable in experiment 1, as it was for TMS and performance data. Lastly, we did not use short separation channels for fNIRS, meaning that extrabrain blood flow could’ve accounted for some of our ‘brain’ activity.
8. Conclusions and clinical implications
This study has shown reduced M1 inhibition for older, compared to younger, adults during dual-tasking, and a trend for lower M1 inhibition for poorer performing older adults. In contrast, poorer performing younger adults showed significantly higher M1 inhibition. Performance data suggested a ‘cognitive-first’ prioritisation strategy for older adults, who had marked DT error for motor, but not cognitive tasks. We propose that clinicians advise older adults to allocate greater attention to motor tasks for activities where they may be at risk of accident-related injury.
Supplementary Material
Figure 6. Cortical activity during dual-tasking in older adults in Experiment 2.
M±SE oxygenated (HbO) and deoxygenated (HbR) haemoglobin responses from 14 channels were grouped into three brain regions: PFC, PM, and M1 (see Figure 1D&E). Asterisks show DT conditions in which there was a significant DTchange (p<0.05) in HbDiff (HbO-HbR), compared to either primary (ST arm tracking) or secondary (STnb2, STnb3, or STFtap) ST condition. μM = micromolar.
Highlights.
Older adults’ performance error isolated to motor component of dual-tasks
Lower M1 inhibition for older adults during dual-tasking
Higher M1 inhibition for poorer performing younger adults
Trend for lower M1 inhibition for poorer performing older adults
We suggest a ‘cognitive-first’ prioritisation strategy for older adults
Acknowledgements
Authors thank Dr. Emily Kothe for technical assistance and Luke Barisic for help with producing videos.
Funding sources
DC was supported by a Victoria Fellowship awarded by the Veski Foundation. RAC is supported by a National Health and Medical Research Council R.D. Wright Biomedical Fellowship (#1090415). APL was partly supported by the Sidney R. Baer Jr. Foundation, the NIH (R01MH100186, R01HD069776, R01NS073601, R21 NS082870, R21 MH099196, R21 NS085491, R21 HD07616), the Football Players Health Study at Harvard University, and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758).
Abbreviations:
- ST
single-task
- DT
dual-task
- nb
n-back task
- Ftap
foot-tapping task
- vfl
verbal fluency task
- HbO
oxygentated haemoglobin
- HbR
deoxygentated haemoglobin
- HbDiff
(HbO – HbR)
- DTchange
(DT/ST) × 100 (performance and TMS data); or DT-ST (fNIRS data)
- PFC
prefrontal cortex
- PM
premotor cortex
- M1
primary motor cortex
- BB
biceps brachii
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: a systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews 2011;35:715–728. [DOI] [PubMed] [Google Scholar]
- Alavash M, Hilgetag CC, Thiel CM, Gießing C. Persistency and flexibility of complex brain networks underlie dual-task interference. Human brain mapping 2015;36:3542–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport DA, Antonis B, Reynolds P. On the division of attention: A disproof of the single channel hypothesis. The Quarterly journal of experimental psychology 1972;24:225–235. [DOI] [PubMed] [Google Scholar]
- Ayaz H, Shewokis PA, Bunce S, Izzetoglu K, Willems B, Onaral B. Optical brain monitoring for operator training and mental workload assessment. Neuroimage 2012;59:36–47. [DOI] [PubMed] [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage 2010;51:565–577. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Dubost V, Allali G, Gonthier R, Hermann FR, Kressig RW. ‘Faster counting while walking’ as a predictor of falls in older adults. Age and ageing 2007;36:418. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KD, Sivan AB. Multilingual aphasia examination: manual of instructions. Iowa City: University of Iowa: AJA Associates. [Google Scholar]
- Bernard-Demanze L, Dumitrescu M, Jimeno P, Borel L, Lacour M. Age-related changes in posture control are differentially affected by postural and cognitive task complexity. Current aging science 2009;2:135–149. [PubMed] [Google Scholar]
- Beurskens R, Helmich I, Rein R, Bock O. Age-related changes in prefrontal activity during walking in dual-task situations: a fNIRS study. International Journal of Psychophysiology 2014;92:122–128. [DOI] [PubMed] [Google Scholar]
- Blanco M, Biever WJ, Gallagher JP, Dingus TA. The impact of secondary task cognitive processing demand on driving performance. Accident Analysis & Prevention 2006;38:895–906. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson’s disease. Journal of the neurological sciences 2006;248:196–204. [DOI] [PubMed] [Google Scholar]
- Boros K, Poreisz C, Münchau A, Paulus W, Nitsche MA. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. European Journal of Neuroscience 2008;27:1292–1300. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. Journal of clinical neurophysiology 1992;9:132–136. [PubMed] [Google Scholar]
- Buch ER, Mars RB, Boorman ED, Rushworth MF. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. Journal of Neuroscience 2010;30:1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byblow WD, Coxon JP, Stinear CM, Fleming MK, Williams G, Müller JFM, Ziemann U. Functional connectivity between secondary and primary motor areas underlying hand–foot coordination. Journal of neurophysiology 2007;98:414–422. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 2002;17:1394–1402. [DOI] [PubMed] [Google Scholar]
- Chipchase L, Schabrun S, Cohen L, Hodges P, Ridding M, Rothwell JC, Taylor J, Ziemann U. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: An international consensus study. Clinical Neurophysiology 2012;123:1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A, Fling B, Crews RT, Mulwitz LA, Kamen G. Reliability of motor-evoked potentials in the ADM muscle of older adults. Journal of neuroscience methods 2007;164:320–324. [DOI] [PubMed] [Google Scholar]
- Christie A, Kamen G. Cortical inhibition is reduced following short-term training in young and older adults. Age 2014;36:749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PJ, Tallman K, Tuokko H, Beattie BL. Vehicle crash involvement and cognitive deficit in older drivers. Journal of Safety Research 1993;24:9–17. [Google Scholar]
- Corp DT, Drury HGK, Young K, Do M, Perkins T, Pearce AJ. Corticomotor responses to attentionally demanding motor performance: a mini-review. Frontiers in Psychology 2013;4:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corp DT, Lum JAG, Tooley GA, Pearce AJ. Corticospinal activity during dual tasking: A systematic review and meta-analysis of TMS literature from 1995–2013. Neuroscience & Biobehavioral Reviews 2014;43:74–87. [DOI] [PubMed] [Google Scholar]
- Corp DT, Rogers MA, Youssef GJ, Pearce AJ. The effect of dual-task difficulty on the inhibition of the motor cortex. Experimental Brain Research 2015;234:443–452. [DOI] [PubMed] [Google Scholar]
- Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage 2011;54:2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeltgen SH, Ridding MC, O’Beirne GA, Dalrymple-Alford J, Huckabee M-L. Test–retest reliability of motor evoked potentials (MEPs) at the submental muscle group during volitional swallowing. Journal of neuroscience methods 2009;178:134–137. [DOI] [PubMed] [Google Scholar]
- Duncan A, Meek JH, Clemence M, Elwell CE, Fallon P, Tyszczuk L, Cope M, Delpy DT. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatric research 1996;39:889. [DOI] [PubMed] [Google Scholar]
- Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron 2009;63:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage 2012;63:921–935. [DOI] [PubMed] [Google Scholar]
- Fling BW, Seidler RD. Task-dependent effects of interhemispheric inhibition on motor control. Behavioural brain research 2012;226:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr P, Cohen LG, Roth BJ, Hallett M. Latency of motor evoked potentials to focal transcranial stimulation varies as a function of scalp positions stimulated. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section 1991;81:81–89. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Garry M, Levin O, Swinnen SP, Summers JJ. Age-related differences in inhibitory processes during interlimb coordination. Brain research 2009;1262:38–47. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Hinder MR, Schmidt MW, Garry MI, Summers JJ. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiology of Aging 2012;33:1–14. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Van Soom J, Rens G, Gooijers J, Leunissen I, Levin O, Swinnen SP. Age-Related Changes in Frontal Network Structural and Functional Connectivity in Relation to Bimanual Movement Control. The Journal of Neuroscience 2016;36:1808–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T, Taylor AW, Pengelly A. A population-based survey of factors relating to the prevalence of falls in older people. Gerontology 2005;51:340–345. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, De Vos J, Wenderoth N, Swinnen SP. The neural control of bimanual movements in the elderly: Brain regions exhibiting age-related increases in activity, frequency-induced neural modulation, and task-specific compensatory recruitment. Human brain mapping 2010;31:1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: Making it work in the real world. Annual review of psychology 2009;60:549–576. [DOI] [PubMed] [Google Scholar]
- Hallett M Transcranial magnetic stimulation: a useful tool for clinical neurophysiology. Annals of neurology 1996;40:344–345. [DOI] [PubMed] [Google Scholar]
- Head D, Raz N, Gunning-Dixon F, Williamson A, Acker JD. Age-related differences in the course of cognitive skill acquisition: The role of regional cortical shrinkage and cognitive resources. Psychology and Aging 2002;17:72. [DOI] [PubMed] [Google Scholar]
- Heinzel S, Maechtel M, Hasmann SE, Hobert MA, Heger T, Berg D, Maetzler W. Motor dual-tasking deficits predict falls in Parkinson’s disease: A prospective study. Parkinsonism & Related Disorders 2016;26:73–77. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. The Journal of Neuroscience 2008;28:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder MR, Fujiyama H, Summers JJ. Premotor-motor interhemispheric inhibition is released during movement initiation in older but not young adults. PloS one 2012;7:e52573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holper L, Shalóm DE, Wolf M, Sigman M. Understanding inverse oxygenation responses during motor imagery: a functional near-infrared spectroscopy study. European Journal of Neuroscience 2011;33:2318–2328. [DOI] [PubMed] [Google Scholar]
- Holste KG, Yasen AL, Hill MJ, Christie AD. Motor Cortex Inhibition is Increased During a Secondary Cognitive Task. Motor control 2015;20:380–394. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2011;66A:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Applied optics 2009;48:D280–D298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. The Journal of Physiology 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Issard C, Gervain J. Variability of the hemodynamic response in infants: Influence of experimental design and stimulus complexity. Developmental cognitive neuroscience 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahani S, Fantana AL, Harper D, Ellison JM, Boas DA, Forester BP, Yücel MA. fNIRS can robustly measure brain activity during memory encoding and retrieval in healthy subjects. Scientific reports 2017;7:9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH. The ten twenty electrode system of the international federation. Electroencephalography and clinical neurophysiology 1958;10:371–375. [PubMed] [Google Scholar]
- Kirchner WK. Age differences in short-term retention of rapidly changing information. Journal of experimental psychology 1958;55:352. [DOI] [PubMed] [Google Scholar]
- Koenraadt KLM, Roelofsen EGJ, Duysens J, Keijsers NLW. Cortical control of normal gait and precision stepping: An fNIRS study. NeuroImage 2014;85, Part 1:415–422. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia M, Rothwell JC, Day B, Thompson P, Ferbert A, Wroe S, Asselman P, Marsden C. Corticocortical inhibition in human motor cortex. The Journal of Physiology 1993;471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latella C, Kidgell DJ, Pearce AJ. Reduction in corticospinal inhibition in the trained and untrained limb following unilateral leg strength training. European Journal of Applied Physiology 2012;1–11. [DOI] [PubMed] [Google Scholar]
- Li KZH, Roudaia E, Lussier M, Bherer L, Leroux A, McKinley P. Benefits of cognitive dual-task training on balance performance in healthy older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2010;65:1344. [DOI] [PubMed] [Google Scholar]
- Lu C-M, Zhang Y-J, Biswal BB, Zang Y-F, Peng D-L, Zhu C-Z. Use of fNIRS to assess resting state functional connectivity. Journal of neuroscience methods 2010;186:242–249. [DOI] [PubMed] [Google Scholar]
- Lu CF, Liu YC, Yang YR, Wu YT, Wang RY. Maintaining Gait Performance by Cortical Activation during Dual-Task Interference: A Functional Near-Infrared Spectroscopy Study. PLoS One 2015;10:e0129390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrick K, Derosiere G, Dray G, Coulon D, Micallef J-P, Perrey S. Prefrontal cortex activity during motor tasks with additional mental load requiring attentional demand: a near-infrared spectroscopy study. Neuroscience research 2013; [DOI] [PubMed] [Google Scholar]
- Master S, Tremblay F. Task-specific increase in corticomotor excitability during tactile discrimination. Experimental brain research 2009;194:163–172. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Tashiro M, Gyoba J, Suzuki M, Okamura N, Itoh M, Yanai K. Brain activity associated with dual-task management differs depending on the combinations of response modalities. Brain research 2007;1172:82–92. [DOI] [PubMed] [Google Scholar]
- Mukaka M A guide to appropriate use of Correlation coefficient in medical research. Malawi Medical Journal 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- Neubert F-X, Mars RB, Buch ER, Olivier E, Rushworth MF. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proceedings of the National Academy of Sciences 2010;107:13240–13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig H, Villringer A. Beyond the visible—imaging the human brain with light. Journal of Cerebral Blood Flow & Metabolism 2003;23:1–18. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- Pashler H Attentional limitations in doing two tasks at the same time. Current Directions in Psychological Science 1992;44–48. [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Current opinion in neurobiology 2001;11:663–672. [DOI] [PubMed] [Google Scholar]
- Plummer P, Eskes G, Wallace S, Giuffrida C, Fraas M, Campbell G, Clifton K, Skidmore ER. Cognitive-motor interference during functional mobility after stroke: state of the science and implications for future research. Archives of physical medicine and rehabilitation 2013;94:2565–2574.e2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portney LG, Watkins MP. Foundations of clinical research: applications to practice. Pearson/Prentice Hall. [Google Scholar]
- Poston B, Kukke SN, Paine RW, Francis S, Hallett M. Cortical silent period duration and its implications for surround inhibition of a hand muscle. European Journal of Neuroscience 2012;36:2964–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current directions in psychological science 2008;17:177–182. [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cerebral cortex 2004;14:721–730. [DOI] [PubMed] [Google Scholar]
- Scholkmann F, Spichtig S, Muehlemann T, Wolf M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiological measurement 2010;31:649. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D’esposito M. Neural evidence for representation-specific response selection. Journal of Cognitive Neuroscience 2003;15:1111–1121. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M, Kerns KA, Baldwin M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 1997;52:M232–M240. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Schubert T, Müller HJ. How to test for dual-task-specific effects in brain imaging studies—an evaluation of potential analysis methods. NeuroImage 2011;54:1765–1773. [DOI] [PubMed] [Google Scholar]
- Tombu MN, Asplund CL, Dux PE, Godwin D, Martin JW, Marois R. A unified attentional bottleneck in the human brain. Proceedings of the National Academy of Sciences 2011;108:13426–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Kuslansky G, Holtzer R, Katz M, Xue X, Buschke H, Pahor M. Walking while talking: effect of task prioritization in the elderly. Archives of physical medicine and rehabilitation 2007;88:50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J. Aging and dual-task performance: a meta-analysis. Psychology and Aging 2003;18:443. [DOI] [PubMed] [Google Scholar]
- Wechsler D Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson; 2008;22:498. [Google Scholar]
- Yang L, Lam F, Huang M, He C, Pang M. Dual-task mobility among individuals with chronic stroke: changes in cognitive-motor interference patterns and relationship to difficulty level of mobility and cognitive tasks. European journal of physical and rehabilitation medicine 2017; [DOI] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Rotem-Galili Y, Mirelman A, Dickstein R, Giladi N, Hausdorff JM. How does explicit prioritization alter walking during dual-task performance? Effects of age and sex on gait speed and variability. Physical Therapy 2010;90:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. The Journal of Physiology 1996;496:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.