Abstract
At the turn of the millennium, a neuropeptide with pronounced inhibitory actions on avian pituitary gonadotropin secretion was identified and named gonadotropin-inhibitory hormone (GnIH). Across bird species, GnIH acts at the level of the pituitary and the gonadotropin-releasing hormone (GnRH) neuronal system to inhibit reproduction. Since this initial discovery, orthologs of GnIH have been identified and characterized across a broad range of species. In many vertebrates, the actions of GnIH and its orthologs serve functional roles analogous to those seen in birds. In other cases, GnIH and its orthologs exhibit more diverse actions dependent on sex, species, season and reproductive condition. The present overview highlights the discovery and functional implications of GnIH across species, focusing on research domains in which the significance of this neuropeptide has been most explored.
Introduction
Since the discovery of gonadotropin-releasing hormone (GnRH) as a central regulator of the reproductive axis in the early 1970’s (1-4), substantial progress has been made by the neuroendocrine community in advancing our understanding of the neurochemical circuits underlying reproductive function. It is generally accepted that the GnRH system acts as a point of convergence for the integration of intrinsic and extrinsic factors that guide reproductive effort based on appropriate developmental and metabolic states, time of day or year, current environmental conditions, hormonal status, and other variables relevant to reproductive success. In most cases, these signals are not deciphered directly by the GnRH system, but through signaling via upstream systems directly responsive to reproductively-relevant cues.
Prior to the recent advent of retrograde, viral tract tracing techniques permitting the identification of cell-phenotype-specific afferents, it was challenging to identify upstream regulators of the widely distributed GnRH network. As new reproductively-relevant candidate systems were identified, each was examined, in turn, for its influence on the GnRH neuronal system. Despite being less efficient than is now possible, this approach, combined with broad genomic and proteomic screening, led to the identification of a number of novel neuropeptides critical for reproductive function. In fact, quite unexpectedly, the 21st century saw the discovery of pronounced positive and negative regulators of the hypothalamo-pituitary-gonadal (HPG) axis (e.g., (5-7)), kisspeptin and gonadotropin-inhibitory hormone (GnIH), respectively, that caused neuroendocrinologists to question long-held beliefs about the neural control of reproduction. The present overview focuses on the discovery and functional significance of one of these neuropeptides, GnIH, and considers future directions aimed at further clarifying the neural targets, mechanisms of action, and commonalities/disparities among species for this neurochemical.
Discovery of GnIH in Birds
In the late 1970’s, a cardioexcitatory peptide with a Phe-Met-Arg-Phe-NH2 (FMRFamide) C-terminal motif was identified in the ganglia of the sunray venus clam (Macrocallista nimbosa). Over the next two decades, RFamide (Arg-Phe-NH2) peptides serving central roles in neurochemical and hormonal communication were discovered across species. Given the significant role of this class of peptides across taxa, at the turn of the millennium Tsutsui and colleagues undertook a search for novel RFamide peptides in the central nervous system and identified a new hypothalamic RFamide peptide whose axons terminated at the level of the median eminence, pointing to a potential role in hypophyseal regulation. By applying this neuropeptide to cultured quail pituitary, Tsutsui and his group found that this peptide rapidly and dose-dependently inhibits gonadotropin release, thereby leading them to designate this peptide GnIH (5). These findings pointed to a novel mechanism of gonadotropin control independent of inhibitory actions on the GnRH system.
Following this initial discovery, the cDNA encoding the precursor polypeptide for GnIH was identified in quail (8) and other avian species (9-11), with the GnIH precursor encoding one GnIH and two GnIH-related peptides (GnIH-RP-1 and GnIH-RP-2) that possess a common C-terminal LPXRFamide (X = L or Q) motif (reviewed in (9, 11, 12)). Subsequently, GnIH was isolated as a mature peptide in starlings (13) and zebra finches (14). In birds, GnIH is localized to the PVN with projections to the median eminence and to GnRH neurons (15). In birds, it is now well established that GnIH regulates reproduction by decreasing gonadotropin synthesis and release from anterior pituitary gonadotropes and/or by acting via the GnIH receptor (GnIH-R; GPR147, also called neuropeptide FF receptor 1 (NPFF1)) on GnRH neurons (reviewed in (9, 11, 12)).
The significance of GnIH in the control of reproduction in birds stimulated further research aimed at whether or not this neuropeptide was common across vertebrate species in terms of its expression and function (16, 17). Indeed, peptides possessing the C-terminal LPXRFamide (X = L or Q) motif have been identified in a number of vertebrates, including mammals (for reviews, see (12, 16, 18-20)). In mammals, this neuropeptide precursor cDNA encodes three peptides [also known as RFamide-related peptides (RFRPs)], RFRP-1, -2, and -3, in bovine and human (for reviews, see (9-11). RFRP-1 and -3 are LPXRFamide peptides, but RFRP-2 is not. RFRP-3 is now considered to be the mammalian ortholog of avian GnIH, although effects of RFRP-1 on central and peripheral reproductive functioning have been reported, requiring further investigation to clarify the specific role of neuropeptide (e.g., (21-25)
GnIH as a regulator of mammalian reproductive function
Across mammals, including humans, RFRP-3, generally inhibits gonadotropin synthesis and/or release (e.g., (26-36)). However, RFRP-3 stimulates gonadotropin secretion in some contexts, dependent on sex, season, and/or reproductive status, (33, 37-39). These disparate effects of RFRP-3 are likely due to the fact that GPR147 can be coupled to either Gαi, Gαs, or Gαq proteins, and this coupling may differ by sex and/or reproductive status in some species (10, 40). Across mammals, RFRP-3 acts directly on GnRH cells; RFRP-3 cell terminal fibers are found in close apposition to GnRH cells, around 1/3rd of GnRH cells express GPR147 and application of RFRP-3 inhibits GnRH cellular activity (33, 41-45). RFRP-3 may also act through suppression of the positive stimulator of the reproductive axis, kisspeptin, as a subset of kisspeptin cells in the anteroventral periventricular and the arcuate nuclei express the RFRP-3 receptor (GPR147) (43, 46). Additionally, as discussed in greater detail below, RFRP-3 may also act directly on pituitary gonadotropes in some species.
Commonalities and Diversity of GnIH/RFRP-3
Although many commonalities exist, the locations and mode(s) of action of GnIH/RFRP-3 are not universal across vertebrate classes, findings not unexpected given the variability in reproductive physiology and strategies across species. The present overview explores these commonalities and discrepancies to common teleost models in GnIH research.
GnIH:GnRH interaction in the brain
As indicated previously, the first evidence of direct interaction of GnIH and GnRH in the brain came from a neuroanatomical study on birds (15). Putative contacts by GnIH/RFRP-3 cells onto GnRH cells in the hypothalamus have since been demonstrated in all vertebrates studied, including fish, frogs, hamsters, mice, rats, sheep, monkeys, naked mole-rats, and humans (30, 36, 44, 45, 47-49). Although there is putative contact between GnIH/RFRP-3 and GnRH cells in all vertebrates studied, a functional interaction remains to be demonstrated in some instances. However, in birds and mammals, GnRH neurons express GPR147 (13, 33). Thus, GnIH/RFRP-3 has the potential to influence HPG axis activity via action on GnRH neurons.
Examination of GnIH:GnRH actions in the fish brain have been particularly informative. In zebrafish (Danio rerio), an elegant study on the GnIH ortholog (LPXRFamide, or LPXRFa) demonstrated that in the preoptic area, LPXRFa fibers interact with gonadotropin-releasing hormone 3 (GnRH3) cell bodies, and LPXRFa-3 reduced GnRH3 expression in brain slices (50). Further, zebrafish LPXRFa-2 and LPXRFa-3 antagonizes kisspeptin-2 (Kiss2) activation of kisspeptin receptor-1a (Kiss1ra) and kiss1ra-expressing neurons in the preoptic area are innervated by LPXRFa-immunoreactive (ir) fibers (see (51) for an overview of teleost kisspeptin). Thus, LPXRFa may act as a reproductive inhibitory neuropeptide in zebrafish via action uniquely on GnRH3 neurons in the brain (and not on the other GnRHs) while also potentially affecting the Kiss2/Kiss1ra pathway. As described further below, GnIH also acts on gonadotropes in the pituitary in zebrafish. In another teleost species, Nile tilapia (Oreochromis niloticus), although LPXRFa-R-immunoreactive (ir) fibers are distributed widely in the hypothalamus, neither LPXRFa-ir fibers nor LPXRFa-receptor are closely associated or co-expressed with GnRH1 or GnRH3 (52). Likewise, these peptides are not associated with kisspeptin (Kiss2) neurons, but they do appear to act on pituitary gonadotropes (see below). In contrast, LPXRFa dose-dependently suppresses tongue sole Kiss2-elicited cAMP responsive element-dependent luciferase activity in COS-7 cells (53). In general, these studies suggest that the role of GnIH as an inhibitory neuropeptide is maintained in fish species, although further studies of LPXRFa in other species are necessary to fully understand the role of this neuropeptide across fish.
GnIH/RFRP-3 action in the anterior pituitary
Equivocal findings have been obtained regarding whether RFRP-3 has direct actions on pituitary gonadotropes. For example, in hamsters, sheep, macaque, and humans, RFRP-3 projections have been observed in the outer layer of the median eminence (26, 27, 44, 45), a result not observed in rats (54). In other cases, fiber labeling in the median eminence is sparse or absent (17, 54-57). Consistent with the absence of RFRP-3 fibers in the outer layer of the median eminence in rats, for example, systemic injections of Fluorogold, a technique used to label hypophysiotropic cells via retrograde transport to cells contacting the portal blood supply, does not label RFRP cells in this species (54). In support of possible actions of RFRP-3 at the level of the hypophysis, RFRP-3 receptor is expressed in hamster (27) and human (45) pituitary and RFRP-3 inhibits gonadotropin synthesis and/or release in sheep (32), cattle (29), and rat (58) cultured pituitaries. Additionally, in ewes, RFRP-3 is detected in hypophyseal portal blood, with pulse amplitude and frequency being higher during the non-breeding season, suggesting direct actions on the pituitary in the seasonal control of reproduction ((59); and see below). However, one recent study found that intravenous infusion of RFRP-3 does not suppress plasma luteinizing hormone (LH) pulsatility in ewes or the estrogen-induced LH surge, drawing into question whether or not RFRP-3 acts on pituitary gonadotropes to suppress LH secretion in ewes, despite being detectable in portal blood (60). The GnIH homolog in teleost fish, LPXRF, acts on the anterior pituitary gland across species. In pituitary explants of zebrafish, LPXRFa-3 downregulates LHβ subunit and gonadotropin common α subunit expression (50). In tilapia, actions of GnIH on the anterior pituitary are likely more widespread, with LPXRFa-receptor expressed in LH, adrenocorticotropin-releasing hormone (ACTH), and α-melanocyte-stimulating hormone (α-MSH) cells (52), and LPXRFa in this species appears to be a positive regulator of reproduction (61).
GnIH/RFRP-3 control of the ovulatory cycle and female reproduction
In spontaneously ovulating mammals, estrogen secretion from maturing ovarian follicles maintains LH at low concentrations through estrogen negative feedback during the follicular phase of the ovulatory cycle. During the ovulatory phase, estrogen negative feedback fails and estrogen acts through positive feedback to stimulate the LH surge that initiates ovulation. Our group hypothesized that RFRP-3 cells might represent a locus at which estrogen acts to drive estrogen negative feedback and that the transient inhibition of this negative feedback is necessary for the preovulatory LH surge (27, 30). For these studies, we used Syrian hamsters due to the precision in the timing of their LH surge. We first found that RFRP-3 cells express estrogen receptor-α (ERα) and appear to mediate estradiol negative feedback (30), with similar expression of ERα later shown in mice (62, 63) and rats (64). Estradiol negative feedback was examined by removing the ovaries of hamsters to release the reproductive axis from sex steroid negative feedback and RFRP-3 peptide was administered in the early part of the light cycle, a time when negative feedback is maximal (30). These injections led to a rapid, dose-dependent suppression of LH, pointing to a likely role in estradiol negative feedback regulation. Additionally, our studies found that the removal of estradiol negative feedback at the time of the LH surge is mediated, at least in part, through circadian-controlled inhibition of the RFRP-3 system (27), with analogous findings recently reported in mice (65). These studies showing coordinated suppression of RFRP-3 cellular activity with proestrus were conducted in an estrogen-clamped LH surge model. However, RFRP-3 cellular activity is suppressed during proestrus in naturally cycling hamsters as well (66).
We next explored whether or not that the same cell phenotype(s) by which the master circadian clock in the suprachiasmatic nucleus (SCN) stimulates the LH surge (i.e., vasopressin (AVP) and vasoactive-intestinal polypeptide (VIP)) simultaneously coordinate the removal of RFRP-3 inhibition. We found that both AVP- and VIP-ergic SCN fibers project to RFRP-3 cells in the DMH (67). Additionally, central VIP injections suppress RFRP-3 cellular activity at the time of the LH surge, but not prior to the LH surge (67). RFRP-3 cells express the core clock protein, Period 1, in a rhythmic fashion, suggesting that autonomous RFRP-3 cell timekeeping underlies this time-dependent sensitivity to VIP signaling (67). RFRP-3 suppresses VIP-induced GnRH secretion from GT1-7 cells, further pointing to the necessity of inhibiting RFRP-3 at the LH surge (68). Together, these findings suggest that the SCN serves a dual role, both coordinating gonadotropic axis stimulation with negative feedback disinhibition to initiate the LH surge and ovulation and point to a novel, circadian-controlled neural circuit that participates in the transition from estradiol negative to positive feedback.
It is striking that the RFRP-3 system projects broadly to limbic structures driving motivated behavior, in addition to directly projecting to the GnRH system (30). RFRP-3 infusions on the day of proestrus grossly reduce sexual motivation in Syrian hamsters (69), suggesting that suppression of RFRP-3 system activity on the day of proestrus is not only necessary for the LH surge, but for sexual motivation as well. RFRP-3 reduces sexual motivation even when animals are primed with estradiol-17 β/progesterone (69), pointing to actions on motivational circuitry rather than through inhibition of the HPG axis. RFRP-3 administration also alters cellular activity in key neural loci implicated in female reproductive behavior, including the medial preoptic area, medial amygdala and bed nucleus of the stria terminalis, independent of changes in circulating gonadal steroids (69). These results are consistent with additional reports of GnIH/RFRP-3 induced inhibition of sexual behavior in avian and rodent species (28, 47, 70, 71). Together, these data point to a neuromodulatory role for RFRP-3 in female sexual motivation, independent of downstream alterations in sex steroid production.
The suppression of RFRP-3 at the time of the surge appears to be common across mammalian species. In mice, RFRP-3 inhibits LH secretion when administered at the time of the preovulatory LH surge in intact mice, or during an estradiol-induced LH surge in ovariectomized mice (38). In rats, Rfrp mRNA is lower during proestrus than during diestrus (72) and treatment with RFRP-3 during proestrus suppresses GnRH cellular activity (73). In ewes, RFRP-3 expression is reduced during the preovulatory period and an 8 h infusion of RFRP-3 was shown to block estrogen-induced LH surges (74). In contrast, one recent study showed that 24 h infusion of RFRP-3 had no effect on the estradiol-induced LH surge in in ewes (60). Whether or not this disparity between studies is due to dose and time of administration of RFRP-3 requires further investigation. Unlike findings in hamsters, however, RFRP-3 infusion did not suppress female sexual behavior in this species. These findings indicate the need for suppression of RFRP-3 activity at the time of the LH surge in spontaneously-ovulating species in which RFRP-3 is inhibitory.
GnIH in the seasonal control of reproduction
Seasonal control of reproduction in birds
Given the pronounced effects of GnIH/RFRP-3 on the reproductive axis across species, researchers interested in the neural mechanisms driving seasonal changes in reproduction began to explore the possibility that the GnIH/RFRP-3 system represents a key locus at which transduced day length information is decoded to appropriately drive the GnRH system. Initial studies of GnIH in avian seasonal cycles of reproduction examined the pattern of GnIH across the seasons to determine whether any changes observed were consistent with a role for GnIH in the seasonal involution and regrowth of the reproductive axis. Early studies in house sparrows and song sparrows found that GnIH-ir neurons are larger in birds at the end of the breeding season than at other times of year, consistent with an inhibitory role for this neuropeptide in seasonal breeding (15). Similarly, GnRH mRNA levels and GnRH-ir cell numbers are higher during the non-breeding period and in short days relative to the breeding season and long days in Eurasian tree sparrows (75). In contrast, GnIH-ir cell number and size and Gnih mRNA do not differ between the breeding and non-breeding seasons in wild Australian zebra finch populations (76). This disparity is likely due to the fact that sparrows have distinct breeding seasons whereas zebra finches adopt an opportunistic breeding strategy based on water availability.
Photoperiod is transduced into a melatonin signal inversely proportional to day length (i.e., secreted at night) and inhibits the reproductive axis in birds (e.g., (77, 78). Removal of the major sites of melatonin production, the pineal gland and eyes (79), decreases the expression of Gnih precursor mRNA and the GnIH peptide content in the hypothalamus in quail (80). In contrast, melatonin administration to quail leads increases Gnih mRNA expression and peptide. Melatonin likely acts directly on GnIH cells as Mel1c, a melatonin receptor subtype, is expressed on GnIH-ir neurons in this species (80). Additional support for direct actions of melatonin comes from the observation that melatonin administration dose-dependently increases GnIH release from quail hypothalamic explants in vitro, with GnIH release exhibiting a nightly peak when melatonin is produced (81). Moreover, LH release is inversely correlated with the pattern of GnIH, providing further evidence for a stimulatory role of melatonin on the GnIH network. Finally, GnIH release is increased in explants from short-day (SD) animals relative to explants from long-day (LD) quail. Together, these findings point to the GnIH system as a significant mediator of seasonal reproduction in birds, with melatonin acting directly on GnIH cells to inhibit the reproductive axis during the non-breeding season.
In addition to acting on cells upstream of the GnRH system, including GnIH, to regulate avian seasonal cycles of reproduction, melatonin may also act directly on the gonads to regulate local GnIH activity. In European starling, for example, the testes express mRNA for GnIH, GnIH receptor and the melatonin receptors, Mel1b and Mel1c (82). Consistent with a role in regulating seasonal breeding, GnIH and GnIH receptor expression levels in the testes are higher during the non-breeding season. Additionally, in vitro, melatonin administration dose-dependently increases Gnih mRNA and decreases testosterone release from gonadotropin-stimulated testes (82). Together, these results suggest that sex steroid secretion is regulated seasonally in starling testes by direct actions of melatonin on gonadal GnIH production ((82); reviewed in (83, 84)).
Seasonal control of reproduction in mammals
As in birds, given the conserved nature of RFRP-3 across species, this peptide emerged as an attractive candidate system guiding seasonal reproduction in mammals. Because increased negative feedback by gonadal steroids is required for seasonal reproductive inhibition (85-87), prior to the discovery of RFRP-3, studies of photoperiodic rodents searched for target sites at which melatonin signaling might alter sex steroid receptors to modify negative feedback. In Syrian hamsters, the dorsomedial hypothalamus (DMH), the site where RFRP-3 cells are localized in rodents, was found to express both sex steroid and melatonin receptors and be essential for short-day (SD) or melatonin-induced reproductive regression (88-91). These findings pointed to the possibility that melatonin signaling is decoded in the DMH, possibly through RFRP-3 neurons, to alter negative feedback across the seasons. However, despite being necessary for reproductive regression in Syrian hamsters, the DMH is not essential for enhanced gonadal steroid negative feedback following SD exposure in this species (92). Although these findings do not rule out a role for the DMH as a locus participating in seasonal changes in Syrian hamster reproductive function, they do suggest that melatonin acts upstream of the DMH to drive changes in RFRP-3, a possibility supported by findings described further below (93, 94).
As was the strategy in birds, initial studies of seasonally-breeding rodents explored the effects of photoperiod on RFRP-3 expression in Syrian and Siberian hamsters to determine whether or not patterns observed are consistent with a role for this neuropeptide in seasonal breeding. Contrary to expectation, extended exposure to inhibitory day lengths leads to reductions in RFRP-3 peptide and mRNA (95, 96) as well as decreased fiber density and projections to GnRH cells in Syrian and Siberian hamsters (33, 95). Analogous seasonal patterns of RFRP-3 expression are seen in European (97) and Turkish (98) hamsters, as well as the semi-desert rodent, jerboa (99). Recently, a study in Syrian hamsters revealed that expression RFRP-3 receptor, GPR147, also varies seasonally (23). In females, gpr147 expression is decreased under SD in multiple hypothalamic loci, mirroring SD suppression of the RFRP-3 peptide. However, hypothalamic gpr147 expression in males was comparatively stable across photoperiods.
In contrast to LD-breeding rodents, exposure to SD stimulates the HPG axis in sheep. RFRP-3 expression, RFRP-3 projections to GnRH neurons, and release of RFRP-3 into the hypophyseal blood portal system are all reduced in SD animals in this species (36, 59, 100). As in hamsters, photoperiodic changes in RFRP-3 are not a result of altered sex steroids in sheep (36). This pattern of RFRP-3, in sheep, is consistent with a role in reproductive disinhibition during SD, whereas the pattern of expression in rodents is at odds with such a clear-cut role for this neuropeptide. As discussed previously, however, RFRP-3 can bind to both stimulatory and inhibitory G-proteins. Although G-protein binding to RFRP-3 receptors has not been examined in a seasonal context, suggestive evidence points to differential G-protein coupling in hamsters based on sex and season. For example, RFRP-3 inhibits release of LH in female Syrian hamsters, but stimulates LH in males (30, 37, 101). The stimulatory effect of RFRP-3 on LH secretion in males was recently also observed in mice (38). In male Siberian hamsters, RFRP-3 is inhibitory in long-day (LD) animals but stimulatory in SD hamsters (33). Consistent with these stimulatory effects, chronic treatment of SD male Syrian hamsters with RFRP-3 reactivates the HPG axis and accelerates gonadal growth (37, 101). Thus, there are species-, sex-, and photoperiod-specific aspects to RFRP-3 and control of the HPG axis. These contextual changes in the effects of RFRP-3 may be essential to its role in mediating photoperiodic control of reproduction and potentially explain why both sheep and hamsters experience SD-induced suppression of RFRP-3 despite the opposing effects of SD on their breeding condition (93). A complete understanding of the role of RFRP-3 in photoperiodism requires continuing investigation into the effects of species, sex, and photoperiod on RFRP-3 in the control of the HPG axis.
As more mammalian species were investigated, it became apparent that SD does not universally inhibit the RFRP-3 system. For example, expression of RFRP-3 does not differ between the breeding and non-breeding seasons in mares or either sex of red deer (102, 103). Likewise, the brushtail possum, a SD-breeding marsupial, displays increased expression of RFRP-ir under SD photoperiod (55). Furthermore, although Siberian hamsters reduce Rfrp under SD photoperiods, Rfrp expression is increased by intermediate day lengths, relative to LD (104). Thus, although RFRP-3 is regulated by photoperiod in the majority of species studied, continued investigation is necessary to elucidate the role of this peptide in seasonal reproduction. Studies in which RFRP-3 cells are genetically manipulated to examine the impact of RFRP-3 communication to cell-specific targets are needed to help clarify the role of this peptide seasonal breeding, as pharmacological manipulations may have off-target effects that make interpretation incomplete.
In sheep and rodents, SD suppression of RFRP-3 is driven by melatonin and not by downstream changes in circulating sex steroids; treatment with melatonin to mimic SD release reduces RFRP-3 expression (33, 96). Conversely, removing the source of endogenous melatonin via pinealectomy before transfer into SD prevents changes in RFRP-3 expression (33, 96). The effects of melatonin on RFRP-3 are consistent with the pattern of reproductive changes under natural conditions, with RFRP-3 expression unchanged by 3 weeks of SD exposure (95) and daily melatonin treatment leading to maximal RFRP-3 suppression after 60 days (96). Although RFRP-3 cells express estrogen receptor-α (30), neither hormone replacement nor gonadectomy influences RFRP-3 expression in SD or LD animals, indicating that changes in Rfrp/RFRP-3 are not the result of changing sex steroid feedback (33, 36, 95, 96, 101). Finally, in photorefractory hamsters (i.e., animals undergoing reproductive recrudescence that are insensitive to melatonin), Rfrp expression is suppressed, reflecting photoperiod rather than reproductive state (96). Thus, despite the observation that treatment with RFRP-3 can hasten hamster reproductive recrudescence (37, 101), this finding argues against a stimulatory role for RFRP-3 in spontaneous regrowth of the reproductive axis in spring, or at least in the initial neuroendocrine cascades initiating recrudescence.
Because the pars tuberalis (PT) of the anterior pituitary has the highest density of melatonin receptors across species (105-109) and has been implicated in seasonal breeding (110-113), this site has received significant attention as a locus at which melatonin acts to drive seasonal cycles of reproduction. In hamsters, melatonin acts on the PT to inhibit thyroid-stimulating hormone (TSH) synthesis which, in turn, acts in the mediobasal hypothalamus to alter the expression of type 2 deiodinase (Dio2), an enzyme that catalyzes the conversion of thyroxine (T4) prohormone into the more biologically active triiodothyronine (T3) hormone, and type 3 deiodinase (Dio3), which converts T3 into the bio-inactive 3,5-diio-L-thyronine (T2) (111). Exposure to SD photoperiod decreases the ratio of Dio2:Dio3 and decreases local concentrations of T3, whereas transfer to LD increases Dio2:Dio3 (114-119). As spring approaches and melatonin duration is decreased, increased T3 acts on downstream circuitry, presumably including RFRP-3 cells, to modify GnRH secretion and guide the transition from the winter to summer breeding phenotype (120). The precise means by which thyroid hormones signal the RFRP-3 system remains to be determined. Chronic central administration of TSH or T3 to SD hamsters for several weeks restores the LD pattern of RFRP-3, indicating that RFRP-3 cells are either direct or indirect targets of this thyroid hormone pathway (94, 121).
Although not specifically investigated in the context of seasonal breeding in mammals, it is likely that, as in birds, local gonadal RFRP-3 contributes to seasonal changes in gonadal functioning. For example, RFRP-3 and GPR147 are expressed in the gonads of Syrian hamsters and mice (122-124). RFRP-3 is expressed in spermatocytes and in round to early elongated spermatids in hamster testis, whereas GPR147 protein is seen in myoid cells, pachytene spermatocytes, maturation division spermatocytes, and in round and late elongated spermatids (122). In mice, testicular RFRP-3 synthesis is increased during reproductive senescence and may contribute to the decline in testicular GnRH at this time (123). Finally, in mice, RFRP-3 and GPR147 are present in ovarian granulosa cells of healthy and antral follicles during proestrus and estrus and in luteal cells during diestrus (124), suggesting participation in follicular development and atresia.
Interactions between the stress axis and GnIH/RFRP-3 Systems
Unfavorable environmental conditions and psychosocial stress suppress reproductive function by acting at all levels of the HPG axis (125, 126). Given that transient adverse environmental conditions can temporarily inhibit breeding during spring and summer in birds, Calisi et al. examined whether or not GnIH participates in stress-induced reproductive suppression in house sparrows (127). Indeed, GnIH-ir neuron numbers are increased in response to capture-handling stress in spring and cellular activation is increased by this stressor in both spring and fall. These findings pointed to GnIH as a mechanism contributing to the transient suppression of reproductive function during unpredictable, ‘stressful’ conditions (127). Further evidence for a role of GnIH in the avian stress response comes from studies showing that, in 14-day old chicks, exposure to high temperatures leads to increased, diencephalic Gnih precursor mRNA at 24 and 48 h (128). In mice, Rfrp gene expression is reduced by cold temerpatures, suggesting that temperature might alter Rfrp mRNA independent of effects on the stress axis (129). In zebra finches, restraint stress reduces GnIH-ir cell numbers concomitant with lower Fshβ mRNA, together suggesting that stress enhances GnIH release in this species (130). Glucocorticoids likely mediate this temporary reproductive inhibition through direct actions on GnIH neurons; glucocorticoid receptors (GR) mRNA is expressed in GnIH neurons in quail and treatment with corticosterone (CORT) increases Gnih mRNA expression this species (131). Finally, stress might also influence reproductive function through direct actions on the gonads; in European starlings, CORT up-regulates GnIH expression in the testis and metabolic stress up-regulate GnIH expression in the ovaries (132). Likewise, restraint stress increases Gnih mRNA expression in the testes of zebra finches (130).
As in birds, stress has pronounced inhibitory actions on the HPG axis of mammals. Analogous to findings in sparrows and quail, acute immobilization stress increases Rfrp/RFRP-3 mRNA and protein expression rapidly following stress in rats, returning to baseline 24 hours later (133). Stress-induced glucocorticoid secretion appears to act directly on GR-expressing RFRP-3 cells, as observed in birds (133). Similar findings are seen following mild foot shock stress in rats, with this stressor increasing FOS protein expression in RFRP-3-ir cells (134). Conversely, administration of RFRP-3 to control animals increased anxiety-like behavior, as measured by an open field test, in this same study (134). The impact of stress in rodents is likely conserved as restraint stress also increases RFRP-3 cell activation and expression in female mice (135). In addition to CORT increasing RFRP-3 cell activity, RFRP-3 appears to, in turn, further potentiate the stress response through increasing CORT release in mice, potentially via increases in corticotropin-releasing hormone (136). Consistent with this finding, RFRP-3 infusions induce anxiety like behavior that can be blocked by co-infusion with the recently discovered RFRP-3 receptor antagonist, GJ14 (136). Immune activation stress can also stimulate the RFRP-3 system; administration of lipopolysaccharide (LPS) to female rats elevates Rfrp and Gpr147 mRNA expression (137). Finally, in one study, stress did not affect RFRP-3/Rfrp peptide or mRNA expression in sheep (138). However, a subsequent study by the same group showed that pseudostress through daily intramuscular injections of adrenocorticotropin increased RFRP-3 cells numbers, gene expression/cell, and close contacts of RFRP-3 fibers onto GnRH cells (139). Together, these findings point to an evolutionarily conserved mechanism of control across species (140).
Recent findings using an RFRP-3-expressing neuronal cell line, rHypoE-23 cells derived from rat hypothalamus further confirm that glucocorticoids act directly on RFRP-3 cells to mediate the effects of stress (131, 141). rHypoE-23 cells express GR mRNA and increase Rfrp and Gpr147 mRNA expression in response to CORT treatment. DNA deletion analysis revealed a CORT-responsive region upstream of the Rfrp precursor coding region that includes two GC response elements (GREs) at -1665 and -1530 bp. Mutation of the -1530 bp GRE abolished CORT responsiveness. These results provide a molecular basis by which glucocorticoids alter transcriptional activation of RFRP-3 cells during times of stress (131).
It is well established that chronic stress negatively affects reproductive functioning in females (142). However, whether female reproduction continues to be negatively affected following cessation of the stress is virtually unexplored. Geraghty et al. (143) recently found that even under conditions where stress is terminated 4 days prior to mating in female rats, there is persistent and marked reproductive dysfunction, with fewer successful copulation events, fewer pregnancies in those that successfully mated, and increased embryo resorption (143). Remarkably, genetic silencing via shRNA of Rfrp during stress completely rescues stress-induced infertility in female rats, resulting in mating and pregnancy success rates indistinguishable from non-stress controls (143). Importantly, genetic silencing of Rfrp occurred only during the stress procedure, establishing that stress-induced increases in RFRP-3 prior to pregnancy mediate the stress-induced dysfunction. Thus, these findings reveal that chronic stress has long-term effects on pregnancy success, even post-stressor, that are mediated by RFRP-3.
VI. Conclusions and Considerations
The present overview points to GnIH/RFRP-3 as an important modulator of the reproductive axis across species (Figure 1). In birds, the data consistently establish GnIH as an inhibitor of the reproductive axis through actions on the GnRH system and anterior pituitary gonadotropin regulation. In other species, the actions of GnIH/RFRP-3 are more complex, varying in function based on species, sex, season, and reproductive condition. These differences reveal an exciting opportunity to explore the marked diversity across mammalian species in the role(s) of RFRP-3. Likewise, within mammals, the disparity across studies in the actions of RFRP-3 at the level of the pituitary, including those within the same species, require further investigation to clarify the putative hypophysiotropic role of RFRP-3 in some cases. Researchers working together and sharing resources to standardize investigation within a species would help to clarify some of these discrepancies.
Figure 1.
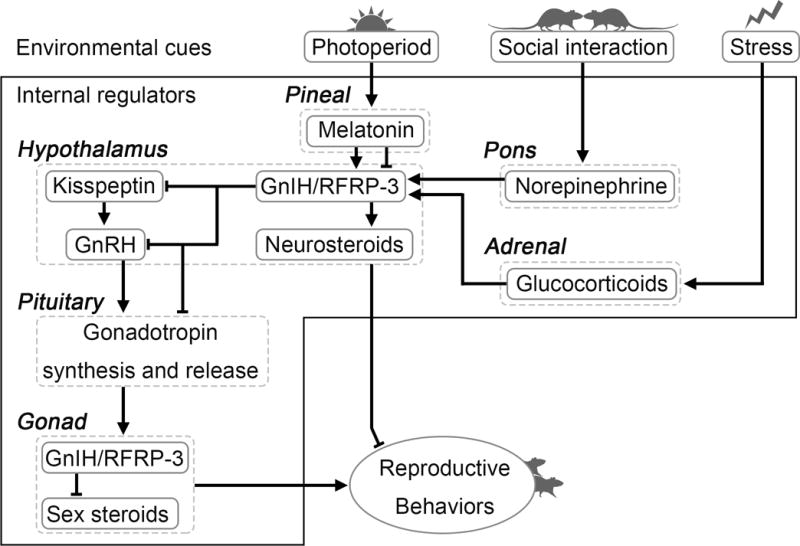
Model of GnIH/RFRP-3 actions as described herein in birds and mammals. The GnIH/RFRP-3 system acts as a central locus at which internal and external factors relevant for reproduction converge. In turn, GnIH/RFRP-3 acts on the reproductive axis to regulate physiology and behavior via actions at the GnRH and kisspeptin systems, the anterior pituitary, and through alterations in neurosteroid synthesis.
Investigating the influence of RFRP-3 in behavior also represents an important area for further investigation. In addition to the suppressive effects on sexual behavior described above, GnIH/RFRP has also been found to modulate displays of aggression in white crown sparrows and Japanese quail (71, 144, 145), and is associated with aggression in mice (146). Additionally, RFRP/GnIH stimulates feeding or food hoarding in a variety of avian and mammalian species (28, 74, 147-150). This has led some to the proposition that RFRP may act to balance reproductive and metabolic needs (74, 151, 152). However, additional exploration of the effects of GnIH/RFRP-3 at specific neural loci remains necessary, as micro-infusion experiments suggest that effects of RFRP-3 on feeding may depend on the targeted region (153).
Whereas rapid progress has been made uncovering the functional significance of GnIH/RFRP-3 since its discovery almost two decades ago, numerous opportunity exists to further explore the roles played by this novel peptide across species. While most studies to date have examined the impact of GnIH/RFRP-3 on neural functioning, physiology and behavior, or patterns of GnIH/RFRP-3 expression associated with changes in physiology and behavior, there are fewer findings directly confirming the role of this peptide in normal functioning. This challenge represents further opportunity for researchers working across levels of analysis to combine their efforts to uncover the specific roles played by GnIH/RFRP-3 in typical physiological and behavioral conditions. Finally, the recent finding that RFRP-3 inhibits LH secretion in women (34) points to potential translational significance in the treatment of infertility in humans and the importance of continued investigation of this novel peptide.
Table 1.
Primary structures of avian and mammalian GnIHs [LPXRFamide (X = L or Q) peptides].
| Animal | Name | Sequence | Reference | |
|---|---|---|---|---|
| Birds | Quail | GnIH | SIKPSAYLPLRFa | Tsutsui et al. (2000) |
| GnIH-RP-1* | SLNFEEMKDWGSKNFMKVNTPTVNKVPNSVANLPLRFa | Satake et al. (2001) | ||
| GnIH-RP-2 | SSIQSLLNLPQRFa | Satake et al. (2001) | ||
| Chicken | GnIH | SIRPSAYLPLRFa | McConn et al., 2014 | |
| GnIH-RP-1* | SLNFEEMKDWGSKNFLKVNTPTVNKVPNSVANLPLRFa | Ikemoto et al. (2005) | ||
| GnIH-RP-2* | SSIQSLLNLPQRFa | Ikemoto et al. (2005) | ||
| Sparrow | GnIH* | SIKPFSNLPLRFa | Osugi et al. (2004) | |
| GnIH-RP-1* | SLNFEEMEDWGSKDIIKMNPFTASKMPNSVANLPLRFa | Osugi et al. (2004) | ||
| GnIH-RP-2* | SPLVKGSSQSLLNLPQRFa | Osugi et al. (2004) | ||
| Starling | GnIH | SIKPFANLPLRFa | Ubuka et al. (2008) | |
| GnIH-RP-1* | SLNFDEMEDWGSKDIIKMNPFTVSKMPNSVANLPLRFa | Ubuka et al. (2008) | ||
| GnIH-RP-2* | GSSQSLLNLPQRFa | Ubuka et al. (2008) | ||
| Zebra finch | GnIH | SIKPFSNLPLRFa | Tobari et al. (2010) | |
| GnIH-RP-1* | SLNFEEMEDWRSKDIIKMNPFAASKMPNSVANLPLRFa | Tobari et al. (2010) | ||
| GnIH-RP-2* | SPLVKGSSQSLLNLPQRFa | Tobari et al. (2010) | ||
| Mammals | Human | RFRP-1 | MPHSFANLPLRFa | Ubuka et al. (2009) |
| RFRP-3 | VPNLPQRFa | Ubuka et al. (2009) | ||
| Macaque | RFRP-1* | MPHSVTNLPLRFa | Ubuka et al. (2009) | |
| RFRP-3 | SGRNMEVSLVRQVLNLPQRFa | Ubuka et al. (2009) | ||
| Bovine | RFRP-1 | SLTFEEVKDWAPKIKMNKPVVNKMPPSAANLPLRFa | Fukusumi et al. (2001) | |
| RFRP-3 | AMAHLPLRLGKNREDSLSRWVPNLPQRFa | Yoshida et al. (2003) | ||
| Ovine | RFRP-1* | SLTFEEVKDWGPKIKMNTPAVNKMPPSAANLPLRFa | Clarke et al. (2008) | |
| RFRP-3* | VPNLPQRFa | Clarke et al. (2008) | ||
| Rat | RFRP-1* | SVTFQELKDWGAKKDIKMSPAPANKVPHSAANLPLRFa | Ukena et al. (2002) | |
| RFRP-3 | ANMEAGTMSHFPSLPQRFa | Ukena et al. (2002) | ||
| Hamster | RFRP-1 | SPAPANKVPHSAANLPLRFa | Ubuka et al. (2012) | |
| (Siberian) | RFRP-3 | TLSRVPSLPQRFa | Ubuka et al. (2012) | |
| Hamster | RFRP-1* | SPAPANKVPHSAANLPLRFa | Kriegsfeld et al., (2006) | |
| (Syrian) | RFRP-3* | TLSRVPSLPQRFa | Kriegsfeld et al., (2006) |
Putative peptides. The C-terminal LPXRFamide (X = L or Q) motifs are shown in bold.
Acknowledgments
Findings presented herein and preparation of this review supported by NIH R01 HD-050470 and NSF IOS-1257638 (to L.J.K.) and Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (22132004 and 22227002 to K.T.).
Footnotes
DR L J KRIEGSFELD (Orcid ID : 0000-0002-6361-1496)
References
- 1.Matsuo H, Arimura A, Nair RM, Schally AV. Synthesis of the porcine LH- and FSH-releasing hormone by the solid-phase method. Biochem Biophys Res Commun. 1971;45(3):822–7. doi: 10.1016/0006-291x(71)90491-8. [DOI] [PubMed] [Google Scholar]
- 2.Baba Y, Matsuo H, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. II. Confirmation of the proposed structure by conventional sequential analyses. Biochem Biophys Res Commun. 1971;44(2):459–63. doi: 10.1016/0006-291x(71)90623-1. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43(6):1334–9. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- 4.Schally AV, Arimura A, Baba Y, Nair RM, Matsuo H, Redding TW, Debeljuk L. Isolation and properties of the FSH and LH-releasing hormone. Biochem Biophys Res Commun. 1971;43(2):393–9. doi: 10.1016/0006-291x(71)90766-2. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275(2):661–7. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 6.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 7.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satake H, Hisada M, Kawada T, Minakata H, Ukena K, Tsutsui K. Characterization of a cDNA encoding a novel avian hypothalamic neuropeptide exerting an inhibitory effect on gonadotropin release. The Biochemical journal. 2001;354(Pt 2):379–85. doi: 10.1042/0264-6021:3540379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsutsui K. A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): Biosynthesis, mode of action and functional significance. Progress in neurobiology. 2009;88(1):76–88. doi: 10.1016/j.pneurobio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Frontiers in neuroendocrinology. 2010;31(3):284–95. doi: 10.1016/j.yfrne.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Tsutsui K, Bentley GE, Kriegsfeld LJ, Osugi T, Seong JY, Vaudry H. Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. Journal of neuroendocrinology. 2010;22(7):716–27. doi: 10.1111/j.1365-2826.2010.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsutsui K, Ubuka T, Bentley GE, Kriegsfeld LJ. Review: regulatory mechanisms of gonadotropin-inhibitory hormone (GnIH) synthesis and release in photoperiodic animals. Frontiers in neuroscience. 2013;7:60. doi: 10.3389/fnins.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ubuka T, Kim S, Huang YC, Reid J, Jiang J, Osugi T, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology. 2008;149(1):268–78. doi: 10.1210/en.2007-0983. [DOI] [PubMed] [Google Scholar]
- 14.Tobari Y, Iijima N, Tsunekawa K, Osugi T, Okanoya K, Tsutsui K, Ozawa H. Identification of gonadotropin-inhibitory hormone in the zebra finch (Taeniopygia guttata): Peptide isolation, cDNA cloning and brain distribution. Peptides. 2010;31(5):816–26. doi: 10.1016/j.peptides.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. Journal of neuroendocrinology. 2003;15(8):794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- 16.Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nature cell biology. 2000;2(10):703–8. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 17.Ukena K, Tsutsui K. Distribution of novel RFamide-related peptide-like immunoreactivity in the mouse central nervous system. Neurosci Lett. 2001;300(3):153–6. doi: 10.1016/s0304-3940(01)01583-x. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsui K, Ubuka T. Gonadotropin-inhibitory Hormone. In: Kastin AJ, Vaudry H, editors. Handbook of biologically active peptides Section on brain peptides. London: Academic Press; 2012. pp. 802–11. [Google Scholar]
- 19.Tsutsui K, Ubuka T, Bentley GE, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect. General and comparative endocrinology. 2012;177(3):305–14. doi: 10.1016/j.ygcen.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ubuka T, Son YL, Tsutsui K. Molecular, cellular, morphological, physiological and behavioral aspects of gonadotropin-inhibitory hormone. General and comparative endocrinology. 2016:22727–50. doi: 10.1016/j.ygcen.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Dave A, Krishna A, Tsutsui K. Direct effects of RFRP-1, a mammalian GnIH ortholog, on ovarian activities of the cyclic mouse. General and comparative endocrinology. 2017:252193–9. doi: 10.1016/j.ygcen.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs A, Laszlo K, Zagoracz O, Ollmann T, Peczely L, Galosi R, Lenard L. Effects of RFamide-related peptide-1 (RFRP-1) microinjections into the central nucleus of amygdala on passive avoidance learning in rats. Neuropeptides. 2017:6281–6. doi: 10.1016/j.npep.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Henningsen JB, Poirel VJ, Mikkelsen JD, Tsutsui K, Simonneaux V, Gauer F. Sex differences in the photoperiodic regulation of RF-Amide related peptide (RFRP) and its receptor GPR147 in the syrian hamster. The Journal of comparative neurology. 2016;524(9):1825–38. doi: 10.1002/cne.23924. [DOI] [PubMed] [Google Scholar]
- 24.Fukusumi S, Habata Y, Yoshida H, Iijima N, Kawamata Y, Hosoya M, Fujii R, Hinuma S, Kitada C, Shintani Y, Suenaga M, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. Characteristics and distribution of endogenous RFamide-related peptide-1. Biochim Biophys Acta. 2001;1540(3):221–32. doi: 10.1016/s0167-4889(01)00135-5. [DOI] [PubMed] [Google Scholar]
- 25.Jorgensen SR, Andersen MD, Overgaard A, Mikkelsen JD. Changes in RFamide-related peptide-1 (RFRP-1)-immunoreactivity during postnatal development and the estrous cycle. Endocrinology. 2014;155(11):4402–10. doi: 10.1210/en.2014-1274. [DOI] [PubMed] [Google Scholar]
- 26.Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149(11):5811–21. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- 27.Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149(10):4958–69. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51(1):171–80. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadokawa H, Shibata M, Tanaka Y, Kojima T, Matsumoto K, Oshima K, Yamamoto N. Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domestic animal endocrinology. 2009;36(4):219–24. doi: 10.1016/j.domaniend.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103(7):2410–5. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. The Journal of endocrinology. 2008;199(1):105–12. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- 32.Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology. 2009;150(12):5549–56. doi: 10.1210/en.2009-0775. [DOI] [PubMed] [Google Scholar]
- 33.Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, Tsutsui K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology. 2012;153(1):373–85. doi: 10.1210/en.2011-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George JT, Hendrikse M, Veldhuis JD, Clarke IJ, Anderson RA, Millar RP. Effect of gonadotropin-inhibitory hormone on luteinizing hormone secretion in humans. Clin Endocrinol (Oxf) 2017;86(5):731–8. doi: 10.1111/cen.13308. [DOI] [PubMed] [Google Scholar]
- 35.Clarke IJ, Qi Y, Puspita Sari I, Smith JT. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Frontiers in neuroendocrinology. 2009;30(3):371–8. doi: 10.1016/j.yfrne.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–82. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ancel C, Bentsen AH, Sebert ME, Tena-Sempere M, Mikkelsen JD, Simonneaux V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule. Endocrinology. 2012;153(3):1352–63. doi: 10.1210/en.2011-1622. [DOI] [PubMed] [Google Scholar]
- 38.Ancel C, Inglis MA, Anderson GM. Central RFRP-3 Stimulates LH Secretion in Male Mice and Has Cycle Stage-Dependent Inhibitory Effects in Females. Endocrinology. 2017;158(9):2873–83. doi: 10.1210/en.2016-1902. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, Zhong M, Xue HL, Ding JS, Wang S, Xu JH, Chen L, Xu LX. Effect of RFRP-3 on reproduction is sex- and developmental status-dependent in the striped hamster (Cricetulus barabensis) Gene. 2014;547(2):273–9. doi: 10.1016/j.gene.2014.06.054. [DOI] [PubMed] [Google Scholar]
- 40.Gouarderes C, Mazarguil H, Mollereau C, Chartrel N, Leprince J, Vaudry H, Zajac JM. Functional differences between NPFF1 and NPFF2 receptor coupling: high intrinsic activities of RFamide-related peptides on stimulation of [35S]GTPgammaS binding. Neuropharmacology. 2007;52(2):376–86. doi: 10.1016/j.neuropharm.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 41.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 42.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587(Pt 7):1401–11. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizwan MZ, Poling MC, Corr M, Cornes PA, Augustine RA, Quennell JH, Kauffman AS, Anderson GM. RFamide-related peptide-3 receptor gene expression in GnRH and kisspeptin neurons and GnRH-dependent mechanism of action. Endocrinology. 2012;153(8):3770–9. doi: 10.1210/en.2012-1133. [DOI] [PubMed] [Google Scholar]
- 44.Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. The Journal of comparative neurology. 2009;517(6):841–55. doi: 10.1002/cne.22191. [DOI] [PubMed] [Google Scholar]
- 45.Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PloS one. 2009;4(12):e8400. doi: 10.1371/journal.pone.0008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poling MC, Quennell JH, Anderson GM, Kauffman AS. Kisspeptin neurones do not directly signal to RFRP-3 neurones but RFRP-3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. Journal of neuroendocrinology. 2013;25(10):876–86. doi: 10.1111/jne.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peragine DE, Pokarowski M, Mendoza-Viveros L, Swift-Gallant A, Cheng HM, Bentley GE, Holmes MM. RFamide-related peptide-3 (RFRP-3) suppresses sexual maturation in a eusocial mammal. Proc Natl Acad Sci U S A. 2017;114(5):1207–12. doi: 10.1073/pnas.1616913114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinelli C, Jadhao AG, Biswas SP, Tsutsui K, D’Aniello B. Neuroanatomical organization of the brain gonadotropin-inhibitory hormone and gonadotropin-releasing hormone systems in the frog Pelophylax esculentus. Brain Behav Evol. 2015;85(1):15–28. doi: 10.1159/000368594. [DOI] [PubMed] [Google Scholar]
- 49.Choi YJ, Habibi HR, Kil GS, Jung MM, Choi CY. Effect of cortisol on gonadotropin inhibitory hormone (GnIH) in the cinnamon clownfish, Amphiprion melanopus. Biochem Biophys Res Commun. 2017;485(2):342–8. doi: 10.1016/j.bbrc.2017.02.078. [DOI] [PubMed] [Google Scholar]
- 50.Spicer OS, Zmora N, Wong TT, Golan M, Levavi-Sivan B, Gothilf Y, Zohar Y. The gonadotropin-inhibitory hormone (Lpxrfa) system’s regulation of reproduction in the brain-pituitary axis of the zebrafish (Danio rerio) Biol Reprod. 2017;96(5):1031–42. doi: 10.1093/biolre/iox032. [DOI] [PubMed] [Google Scholar]
- 51.Ogawa S, Parhar IS. Anatomy of the kisspeptin systems in teleosts. General and comparative endocrinology. 2013:181169–74. doi: 10.1016/j.ygcen.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa S, Sivalingam M, Biran J, Golan M, Anthonysamy RS, Levavi-Sivan B, Parhar IS. Distribution of LPXRFa, a gonadotropin-inhibitory hormone ortholog peptide, and LPXRFa receptor in the brain and pituitary of the tilapia. The Journal of comparative neurology. 2016;524(14):2753–75. doi: 10.1002/cne.23990. [DOI] [PubMed] [Google Scholar]
- 53.Wang B, Yang G, Liu Q, Qin J, Xu Y, Li W, Liu X, Shi B. Inhibitory action of tongue sole LPXRFa, the piscine ortholog of gonadotropin-inhibitory hormone, on the signaling pathway induced by tongue sole kisspeptin in COS-7 cells transfected with their cognate receptors. Peptides. 2017:9562–7. doi: 10.1016/j.peptides.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 54.Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150(3):1413–20. doi: 10.1210/en.2008-1287. [DOI] [PubMed] [Google Scholar]
- 55.Harbid AA, McLeod BJ, Caraty A, Anderson GM. Seasonal changes in RFamide-related peptide-3 neurons in the hypothalamus of a seasonally breeding marsupial species, the brushtail possum (Trichosurus vulpecula) The Journal of comparative neurology. 2013;521(13):3030–41. doi: 10.1002/cne.23328. [DOI] [PubMed] [Google Scholar]
- 56.Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod. 2010;83(4):568–77. doi: 10.1095/biolreprod.110.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yano T, Iijima N, Kakihara K, Hinuma S, Tanaka M, Ibata Y. Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res. 2003;982(2):156–67. doi: 10.1016/s0006-8993(03)02877-4. [DOI] [PubMed] [Google Scholar]
- 58.Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, Dijcks FA, Blomenrohr M, Pinilla L, van Noort PI, Tena-Sempere M. Characterization of the inhibitory roles of RFRP3, the mammalian ortholog of GnIH, in the control of gonadotropin secretion in the rat: in vivo and in vitro studies. Am J Physiol Endocrinol Metab. 2010;299(1):E39–46. doi: 10.1152/ajpendo.00108.2010. [DOI] [PubMed] [Google Scholar]
- 59.Smith JT, Young IR, Veldhuis JD, Clarke IJ. Gonadotropin-inhibitory hormone (GnIH) secretion into the ovine hypophyseal portal system. Endocrinology. 2012;153(7):3368–75. doi: 10.1210/en.2012-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Decourt C, Anger K, Robert V, Lomet D, Bartzen-Sprauer J, Caraty A, Dufourny L, Anderson G, Beltramo M. No Evidence That RFamide-Related Peptide 3 Directly Modulates LH Secretion in the Ewe. Endocrinology. 2016;157(4):1566–75. doi: 10.1210/en.2015-1854. [DOI] [PubMed] [Google Scholar]
- 61.Biran J, Golan M, Mizrahi N, Ogawa S, Parhar IS, Levavi-Sivan B. LPXRFa, the piscine ortholog of GnIH, and LPXRF receptor positively regulate gonadotropin secretion in Tilapia (Oreochromis niloticus) Endocrinology. 2014;155(11):4391–401. doi: 10.1210/en.2013-2047. [DOI] [PubMed] [Google Scholar]
- 62.Poling MC, Kim J, Dhamija S, Kauffman AS. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology. 2012;153(4):1827–40. doi: 10.1210/en.2011-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molnar CS, Kallo I, Liposits Z, Hrabovszky E. Estradiol down-regulates RF-amide-related peptide (RFRP) expression in the mouse hypothalamus. Endocrinology. 2011;152(4):1684–90. doi: 10.1210/en.2010-1418. [DOI] [PubMed] [Google Scholar]
- 64.Iwasa T, Matsuzaki T, Murakami M, Kinouchi R, Osugi T, Gereltsetseg G, Yoshida S, Irahara M, Tsutsui K. Developmental changes in the mammalian gonadotropin-inhibitory hormone (GnIH) ortholog RFamide-related peptide (RFRP) and its cognate receptor GPR147 in the rat hypothalamus. Int J Dev Neurosci. 2012;30(1):31–7. doi: 10.1016/j.ijdevneu.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Poling MC, Luo EY, Kauffman AS. Sex Differences in Steroid Receptor Coexpression and Circadian-Timed Activation of Kisspeptin and RFRP-3 Neurons May Contribute to the Sexually Dimorphic Basis of the LH Surge. Endocrinology. 2017;158(10):3565–78. doi: 10.1210/en.2017-00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henningsen JB, Ancel C, Mikkelsen JD, Gauer F, Simonneaux V. Roles of RFRP-3 in the Daily and Seasonal Regulation of Reproductive Activity in Female Syrian Hamsters. Endocrinology. 2017;158(3):652–63. doi: 10.1210/en.2016-1689. [DOI] [PubMed] [Google Scholar]
- 67.Russo KA, La JL, Stephens SB, Poling MC, Padgaonkar NA, Jennings KJ, Piekarski DJ, Kauffman AS, Kriegsfeld LJ. Circadian Control of the Female Reproductive Axis Through Gated Responsiveness of the RFRP-3 System to VIP Signaling. Endocrinology. 2015;156(7):2608–18. doi: 10.1210/en.2014-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Son YL, Ubuka T, Soga T, Yamamoto K, Bentley GE, Tsutsui K. Inhibitory action of gonadotropin-inhibitory hormone on the signaling pathways induced by kisspeptin and vasoactive intestinal polypeptide in GnRH neuronal cell line, GT1-7. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30(6):2198–210. doi: 10.1096/fj.201500055. [DOI] [PubMed] [Google Scholar]
- 69.Piekarski DJ, Zhao S, Jennings KJ, Iwasa T, Legan SJ, Mikkelsen JD, Tsutsui K, Kriegsfeld LJ. Gonadotropin-inhibitory hormone reduces sexual motivation but not lordosis behavior in female Syrian hamsters (Mesocricetus auratus) Horm Behav. 2013;64(3):501–10. doi: 10.1016/j.yhbeh.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006;49(4):550–5. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Ubuka T, Mizuno T, Fukuda Y, Bentley GE, Wingfield JC, Tsutsui K. RNA interference of gonadotropin-inhibitory hormone gene induces aggressive and sexual behaviors in birds. General and comparative endocrinology. 2013:181179–86. doi: 10.1016/j.ygcen.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 72.Salehi MS, Jafarzadeh Shirazi MR, Zamiri MJ, Pazhoohi F, Namavar MR, Niazi A, Ramezani A, Tanideh N, Tamadon A, Zarei A. Hypothalamic Expression of KiSS1 and RFamide-related Peptide-3 mRNAs during The Estrous Cycle of Rats. Int J Fertil Steril. 2013;6(4):304–9. [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology. 2009;150(4):1834–40. doi: 10.1210/en.2008-1359. [DOI] [PubMed] [Google Scholar]
- 74.Clarke IJ, Smith JT, Henry BA, Oldfield BJ, Stefanidis A, Millar RP, Sari IP, Chng K, Fabre-Nys C, Caraty A, Ang BT, Chan L, Fraley GS. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology. 2012;95(4):305–16. doi: 10.1159/000332822. [DOI] [PubMed] [Google Scholar]
- 75.Dixit AS, Singh NS, Byrsat S. Role of GnIH in photoperiodic regulation of seasonal reproduction in the Eurasian tree sparrow. J Exp Biol. 2017;220(Pt 20):3742–50. doi: 10.1242/jeb.164541. [DOI] [PubMed] [Google Scholar]
- 76.Perfito N, Zann R, Ubuka T, Bentley G, Hau M. Potential roles for GNIH and GNRH-II in reproductive axis regulation of an opportunistically breeding songbird. General and comparative endocrinology. 2011;173(1):20–6. doi: 10.1016/j.ygcen.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 77.Ohta M, Kadota C, Konishi H. A role of melatonin in the initial stage of photoperiodism in the Japanese quail. Biol Reprod. 1989;40(5):935–41. doi: 10.1095/biolreprod40.5.935. [DOI] [PubMed] [Google Scholar]
- 78.Guyomarc’h C, Lumineau S, Vivien-Roels B, Richard J, Deregnaucourt S. Effect of melatonin supplementation on the sexual development in European quail (Coturnix coturnix) Behavioural processes. 2001;53(1–2):121–30. doi: 10.1016/s0376-6357(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 79.Underwood H, Binkley S, Siopes T, Mosher K. Melatonin rhythms in the eyes, pineal bodies, and blood of Japanese quail (Coturnix coturnix japonica) General and comparative endocrinology. 1984;56(1):70–81. doi: 10.1016/0016-6480(84)90063-7. [DOI] [PubMed] [Google Scholar]
- 80.Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc Natl Acad Sci U S A. 2005;102(8):3052–7. doi: 10.1073/pnas.0403840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chowdhury VS, Yamamoto K, Ubuka T, Bentley GE, Hattori A, Tsutsui K. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology. 2010;151(1):271–80. doi: 10.1210/en.2009-0908. [DOI] [PubMed] [Google Scholar]
- 82.McGuire NL, Kangas K, Bentley GE. Effects of melatonin on peripheral reproductive function: regulation of testicular GnIH and testosterone. Endocrinology. 2011;152(9):3461–70. doi: 10.1210/en.2011-1053. [DOI] [PubMed] [Google Scholar]
- 83.Bentley GE, Wilsterman K, Ernst DK, Lynn SE, Dickens MJ, Calisi RM, Kriegsfeld LJ, Kaufer D, Geraghty AC, viviD D, McGuire NL, Lopes PC, Tsutsui K. Neural Versus Gonadal GnIH: Are they Independent Systems? A Mini-Review. Integr Comp Biol. 2017 doi: 10.1093/icb/icx085. [DOI] [PubMed] [Google Scholar]
- 84.Ubuka T, Son YL, Tobari Y, Narihiro M, Bentley GE, Kriegsfeld LJ, Tsutsui K. Central and direct regulation of testicular activity by gonadotropin-inhibitory hormone and its receptor. Front Endocrinol (Lausanne) 2014;5:8. doi: 10.3389/fendo.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Legan SJ, Karsch FJ, Foster DL. The endocrin control of seasonal reproductive function in the ewe: a marked change in response to the negative feedback action of estradiol on luteinizing hormone secretion. Endocrinology. 1977;101(3):818–24. doi: 10.1210/endo-101-3-818. [DOI] [PubMed] [Google Scholar]
- 86.Tamarkin L, Hutchison JS, Goldman BD. Regulation of serum gonadotropins by photoperiod and testicular hormone in the Syrian hamster. Endocrinology. 1976;99(6):1528–33. doi: 10.1210/endo-99-6-1528. [DOI] [PubMed] [Google Scholar]
- 87.Turek FW. The interaction of the photoperiod and testosterone in regulating serum gonadotropin levels in castrated male hamsters. Endocrinology. 1977;101(4):1210–5. doi: 10.1210/endo-101-4-1210. [DOI] [PubMed] [Google Scholar]
- 88.Bae HH, Mangels RA, Cho BS, Dark J, Yellon SM, Zucker I. Ventromedial hypothalamic mediation of photoperiodic gonadal responses in male Syrian hamsters. Journal of biological rhythms. 1999;14(5):391–401. doi: 10.1177/074873099129000795. [DOI] [PubMed] [Google Scholar]
- 89.Lewis D, Freeman DA, Dark J, Wynne-Edwards KE, Zucker I. Photoperiodic control of oestrous cycles in Syrian hamsters: mediation by the mediobasal hypothalamus. Journal of neuroendocrinology. 2002;14(4):294–9. doi: 10.1046/j.1365-2826.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- 90.Maywood ES, Bittman EL, Hastings MH. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol Reprod. 1996;54(2):470–7. doi: 10.1095/biolreprod54.2.470. [DOI] [PubMed] [Google Scholar]
- 91.Maywood ES, Hastings MH. Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology. 1995;136(1):144–53. doi: 10.1210/endo.136.1.7828525. [DOI] [PubMed] [Google Scholar]
- 92.Jarjisian SG, Piekarski DJ, Place NJ, Driscoll JR, Paxton EG, Kriegsfeld LJ, Zucker I. Dorsomedial hypothalamic lesions block Syrian hamster testicular regression in short day lengths without diminishing increased testosterone negative-feedback sensitivity. Biol Reprod. 2013;89(2):23. doi: 10.1095/biolreprod.113.109587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henningsen JB, Gauer F, Simonneaux V. RFRP Neurons - The Doorway to Understanding Seasonal Reproduction in Mammals. Front Endocrinol (Lausanne) 2016;7:36. doi: 10.3389/fendo.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klosen P, Sebert ME, Rasri K, Laran-Chich MP, Simonneaux V. TSH restores a summer phenotype in photoinhibited mammals via the RF-amides RFRP3 and kisspeptin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(7):2677–86. doi: 10.1096/fj.13-229559. [DOI] [PubMed] [Google Scholar]
- 95.Mason AO, Duffy S, Zhao S, Ubuka T, Bentley GE, Tsutsui K, Silver R, Kriegsfeld LJ. Photoperiod and reproductive condition are associated with changes in RFamide-related peptide (RFRP) expression in Syrian hamsters (Mesocricetus auratus) Journal of biological rhythms. 2010;25(3):176–85. doi: 10.1177/0748730410368821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Revel FG, Saboureau M, Pevet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149(3):902–12. doi: 10.1210/en.2007-0848. [DOI] [PubMed] [Google Scholar]
- 97.Simonneaux V, Ancel C, Poirel VJ, Gauer F. Kisspeptins and RFRP-3 Act in Concert to Synchronize Rodent Reproduction with Seasons. Frontiers in neuroscience. 2013;7:22. doi: 10.3389/fnins.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piekarski DJ, Jarjisian SG, Perez L, Ahmad H, Dhawan N, Zucker I, Kriegsfeld LJ. Effects of Pinealectomy and Short Day Lengths on Reproduction and Neuronal RFRP-3, Kisspeptin, and GnRH in Female Turkish Hamsters. Journal of biological rhythms. 2014;29(3):181–91. doi: 10.1177/0748730414532423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janati A, Talbi R, Klosen P, Mikkelsen JD, Magoul R, Simonneaux V, El Ouezzani S. Distribution and seasonal variation in hypothalamic RF-amide peptides in a semi-desert rodent, the jerboa. Journal of neuroendocrinology. 2013;25(4):402–11. doi: 10.1111/jne.12015. [DOI] [PubMed] [Google Scholar]
- 100.Dardente H, Birnie M, Lincoln GA, Hazlerigg DG. RFamide-related peptide and its cognate receptor in the sheep: cDNA cloning, mRNA distribution in the hypothalamus and the effect of photoperiod. Journal of neuroendocrinology. 2008;20(11):1252–9. doi: 10.1111/j.1365-2826.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- 101.Henningsen JB, Ancel C, Mikkelsen JD, Gauer F, Simonneaux V. Roles of RFRP-3 in the daily and seasonal regulation of reproductive activity in female Syrian hamsters. Endocrinology. doi: 10.1210/en.2016-1689. 2016en20161689. [DOI] [PubMed] [Google Scholar]
- 102.Thorson JF, Prezotto LD, Cardoso RC, Sharpton SM, Edwards JF, Welsh TH, Jr, Riggs PK, Caraty A, Amstalden M, Williams GL. Hypothalamic distribution, adenohypophyseal receptor expression, and ligand functionality of RFamide-related peptide 3 in the mare during the breeding and nonbreeding seasons. Biol Reprod. 2014;90(2):28. doi: 10.1095/biolreprod.113.112185. [DOI] [PubMed] [Google Scholar]
- 103.Barrell GK, Ridgway MJ, Wellby M, Pereira A, Henry BA, Clarke IJ. Expression of regulatory neuropeptides in the hypothalamus of red deer (Cervus elaphus) reveals anomalous relationships in the seasonal control of appetite and reproduction. General and comparative endocrinology. 2016:2291–7. doi: 10.1016/j.ygcen.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 104.Paul MJ, Pyter LM, Freeman DA, Galang J, Prendergast BJ. Photic and nonphotic seasonal cues differentially engage hypothalamic kisspeptin and RFamide-related peptide mRNA expression in Siberian hamsters. Journal of neuroendocrinology. 2009;21(12):1007–14. doi: 10.1111/j.1365-2826.2009.01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bittman EL, Weaver DR. The distribution of melatonin binding sites in neuroendocrine tissues of the ewe. Biol Reprod. 1990;43(6):986–93. doi: 10.1095/biolreprod43.6.986. [DOI] [PubMed] [Google Scholar]
- 106.Weaver DR, Provencio I, Carlson LL, Reppert SM. Melatonin receptors and signal transduction in photorefractory Siberian hamsters (Phodopus sungorus) Endocrinology. 1991;128(2):1086–92. doi: 10.1210/endo-128-2-1086. [DOI] [PubMed] [Google Scholar]
- 107.Williams LM, Morgan PJ, Hastings MH, Lawson W, Davidson G, Howell HE. Melatonin Receptor Sites in the Syrian Hamster Brain and Pituitary. Localization and Characterization Using [|]lodomelatonin*. Journal of neuroendocrinology. 1989;1(5):315–20. doi: 10.1111/j.1365-2826.1989.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 108.Skene DJ, Masson-Pevet M, Pevet P. Characterization of melatonin binding sites in the pars tuberalis of the European hamster. Journal of neuroendocrinology. 1992;4(2):189–92. doi: 10.1111/j.1365-2826.1992.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 109.Masson-Pevet M, Gauer F. Seasonality and melatonin receptors in the pars tuberalis in some long day breeders. Biological signals. 1994;3(2):63–70. doi: 10.1159/000109527. [DOI] [PubMed] [Google Scholar]
- 110.Dardente H, Wyse CA, Birnie MJ, Dupre SM, Loudon AS, Lincoln GA, Hazlerigg DG. A molecular switch for photoperiod responsiveness in mammals. Curr Biol. 2010;20(24):2193–8. doi: 10.1016/j.cub.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 111.Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18(15):1147–52. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- 112.Nakao N, Ono H, Yoshimura T. Thyroid hormones and seasonal reproductive neuroendocrine interactions. Reproduction. 2008;136(1):1–8. doi: 10.1530/REP-08-0041. [DOI] [PubMed] [Google Scholar]
- 113.Nakane Y, Yoshimura T. Universality and diversity in the signal transduction pathway that regulates seasonal reproduction in vertebrates. Frontiers in neuroscience. 2014;8:115. doi: 10.3389/fnins.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yasuo S, Yoshimura T, Ebihara S, Korf HW. Photoperiodic control of TSH-beta expression in the mammalian pars tuberalis has different impacts on the induction and suppression of the hypothalamo-hypopysial gonadal axis. Journal of neuroendocrinology. 2010;22(1):43–50. doi: 10.1111/j.1365-2826.2009.01936.x. [DOI] [PubMed] [Google Scholar]
- 115.Revel FG, Saboureau M, Pevet P, Mikkelsen JD, Simonneaux V. Melatonin regulates type 2 deiodinase gene expression in the Syrian hamster. Endocrinology. 2006;147(10):4680–7. doi: 10.1210/en.2006-0606. [DOI] [PubMed] [Google Scholar]
- 116.Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, Jethwa P, Boelen A, Visser TJ, Ozanne DM, Archer ZA, Mercer JG, Morgan PJ. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology. 2007;148(8):3608–17. doi: 10.1210/en.2007-0316. [DOI] [PubMed] [Google Scholar]
- 117.Watanabe M, Yasuo S, Watanabe T, Yamamura T, Nakao N, Ebihara S, Yoshimura T. Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology. 2004;145(4):1546–9. doi: 10.1210/en.2003-1593. [DOI] [PubMed] [Google Scholar]
- 118.Saenz de Miera C, Hanon EA, Dardente H, Birnie M, Simonneaux V, Lincoln GA, Hazlerigg DG. Circannual variation in thyroid hormone deiodinases in a short-day breeder. Journal of neuroendocrinology. 2013;25(4):412–21. doi: 10.1111/jne.12013. [DOI] [PubMed] [Google Scholar]
- 119.Yasuo S, Yoshimura T, Ebihara S, Korf HW. Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology. 2007;148(9):4385–92. doi: 10.1210/en.2007-0497. [DOI] [PubMed] [Google Scholar]
- 120.Saenz de Miera C, Monecke S, Bartzen-Sprauer J, Laran-Chich MP, Pevet P, Hazlerigg DG, Simonneaux V. A circannual clock drives expression of genes central for seasonal reproduction. Curr Biol. 2014;24(13):1500–6. doi: 10.1016/j.cub.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 121.Henson JR, Carter SN, Freeman DA. Exogenous T(3) elicits long day-like alterations in testis size and the RFamides Kisspeptin and gonadotropin-inhibitory hormone in short-day Siberian hamsters. Journal of biological rhythms. 2013;28(3):193–200. doi: 10.1177/0748730413487974. [DOI] [PubMed] [Google Scholar]
- 122.Zhao S, Zhu E, Yang C, Bentley GE, Tsutsui K, Kriegsfeld LJ. RFamide-related peptide and messenger ribonucleic acid expression in mammalian testis: association with the spermatogenic cycle. Endocrinology. 2010;151(2):617–27. doi: 10.1210/en.2009-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anjum S, Krishna A, Sridaran R, Tsutsui K. Localization of gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), kisspeptin and GnRH receptor and their possible roles in testicular activities from birth to senescence in mice. Journal of experimental zoology Part A, Ecological genetics and physiology. 2012;317(10):630–44. doi: 10.1002/jez.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singh P, Krishna A, Sridaran R, Tsutsui K. Immunohistochemical localization of GnRH and RFamide-related peptide-3 in the ovaries of mice during the estrous cycle. Journal of molecular histology. 2011;42(5):371–81. doi: 10.1007/s10735-011-9340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. Ecological bases of hormone–behavior interactions: the “Emergency Life History Stage”. Am Zool. 1998:38191–206. [Google Scholar]
- 126.Nargund VH. Effects of psychological stress on male fertility. Nat Rev Urol. 2015;12(7):373–82. doi: 10.1038/nrurol.2015.112. [DOI] [PubMed] [Google Scholar]
- 127.Calisi RM, Rizzo NO, Bentley GE. Seasonal differences in hypothalamic EGR-1 and GnIH expression following capture-handling stress in house sparrows (Passer domesticus) General and comparative endocrinology. 2008;157(3):283–7. doi: 10.1016/j.ygcen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 128.Chowdhury VS, Tomonaga S, Nishimura S, Tabata S, Cockrem JF, Tsutsui K, Furuse M. Hypothalamic gonadotropin-inhibitory hormone precursor mRNA is increased during depressed food intake in heat-exposed chicks. Comp Biochem Physiol A Mol Integr Physiol. 2012;162(3):227–33. doi: 10.1016/j.cbpa.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 129.Jaroslawska J, Chabowska-Kita A, Kaczmarek MM, Kozak LP. Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status. PLoS Genet. 2015;11(6):e1005287. doi: 10.1371/journal.pgen.1005287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ernst DK, Lynn SE, Bentley GE. Differential response of GnIH in the brain and gonads following acute stress in a songbird. General and comparative endocrinology. 2016:22751–7. doi: 10.1016/j.ygcen.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 131.Son YL, Ubuka T, Narihiro M, Fukuda Y, Hasunuma I, Yamamoto K, Belsham DD, Tsutsui K. Molecular basis for the activation of gonadotropin-inhibitory hormone gene transcription by corticosterone. Endocrinology. 2014;155(5):1817–26. doi: 10.1210/en.2013-2076. [DOI] [PubMed] [Google Scholar]
- 132.McGuire NL, Koh A, Bentley GE. The direct response of the gonads to cues of stress in a temperate songbird species is season-dependent. PeerJ. 2013:1e139. doi: 10.7717/peerj.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A. 2009;106(27):11324–9. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaewwongse M, Takayanagi Y, Onaka T. Effects of RFamide-related peptide (RFRP)-1 and RFRP-3 on oxytocin release and anxiety-related behaviour in rats. Journal of neuroendocrinology. 2011;23(1):20–7. doi: 10.1111/j.1365-2826.2010.02077.x. [DOI] [PubMed] [Google Scholar]
- 135.Yang JA, Song CI, Hughes J, Kreisman MJ, Parra RA, Haisenleder DJ, Kauffman AS, Breen KM. Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice. Endocrinology. 2017 doi: 10.1210/en.2017-00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim JS, Brownjohn PW, Dyer BS, Beltramo M, Walker CS, Hay DL, Painter GF, Tyndall JD, Anderson GM. Anxiogenic and Stressor Effects of the Hypothalamic Neuropeptide RFRP-3 Are Overcome by the NPFFR Antagonist GJ14. Endocrinology. 2015;156(11):4152–62. doi: 10.1210/en.2015-1532. [DOI] [PubMed] [Google Scholar]
- 137.Iwasa T, Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Kawami T, Kato T, Kuwahara A, Yasui T, Irahara M. Effects of ovariectomy on the inflammatory responses of female rats to the central injection of lipopolysaccharide. J Neuroimmunol. 2014;277(1–2):50–6. doi: 10.1016/j.jneuroim.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 138.Papargiris MM, Rivalland ET, Clarke IJ, Smith JT, Pereira A, Tilbrook AJ. Evidence that RF-amide related peptide-3 is not a mediator of the inhibitory effects of psychosocial stress on gonadotrophin secretion in ovariectomised ewes. Journal of neuroendocrinology. 2011;23(3):208–15. doi: 10.1111/j.1365-2826.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 139.Clarke IJ, Bartolini D, Conductier G, Henry BA. Stress Increases Gonadotropin Inhibitory Hormone Cell Activity and Input to GnRH Cells in Ewes. Endocrinology. 2016;157(11):4339–50. doi: 10.1210/en.2016-1513. [DOI] [PubMed] [Google Scholar]
- 140.Iwasa T, Matsuzaki T, Yano K, Irahara M. Gonadotropin-Inhibitory Hormone Plays Roles in Stress-Induced Reproductive Dysfunction. Front Endocrinol (Lausanne) 2017;8:62. doi: 10.3389/fendo.2017.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gojska NM, Belsham DD. Glucocorticoid receptor-mediated regulation of Rfrp (GnIH) and Gpr147 (GnIH-R) synthesis in immortalized hypothalamic neurons. Mol Cell Endocrinol. 2014;384(1–2):23–31. doi: 10.1016/j.mce.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 142.Louis GM, Lum KJ, Sundaram R, Chen Z, Kim S, Lynch CD, Schisterman EF, Pyper C. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil Steril. 2011;95(7):2184–9. doi: 10.1016/j.fertnstert.2010.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Geraghty AC, Muroy SE, Zhao S, Bentley GE, Kriegsfeld LJ, Kaufer D. Knockdown of hypothalamic RFRP3 prevents chronic stress-induced infertility and embryo resorption. Elife. 2015;4 doi: 10.7554/eLife.04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ubuka T, Mukai M, Wolfe J, Beverly R, Clegg S, Wang A, Hsia S, Li M, Krause JS, Mizuno T, Fukuda Y, Tsutsui K, Bentley GE, Wingfield JC. RNA interference of gonadotropin-inhibitory hormone gene induces arousal in songbirds. PloS one. 2012;7(1):e30202. doi: 10.1371/journal.pone.0030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ubuka T, Haraguchi S, Tobari Y, Narihiro M, Ishikawa K, Hayashi T, Harada N, Tsutsui K. Hypothalamic inhibition of socio-sexual behaviour by increasing neuroestrogen synthesis. Nat Commun. 2014;5:3061. doi: 10.1038/ncomms4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jennings KJ, Chang J, Cho H, Piekarski DJ, Russo KA, Kriegsfeld LJ. Aggressive interactions are associated with reductions in RFamide-related peptide, but not kisspeptin, neuronal activation in mice. Horm Behav. 2016:78127–34. doi: 10.1016/j.yhbeh.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 147.Benton NA, Russo KA, Brozek JM, Andrews RJ, Kim VJ, Kriegsfeld LJ, Schneider JE. Food restriction-induced changes in motivation differ with stages of the estrous cycle and are closely linked to RFamide-related peptide-3 but not kisspeptin in Syrian hamsters. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fraley GS, Coombs E, Gerometta E, Colton S, Sharp PJ, Li Q, Clarke IJ. Distribution and sequence of gonadotropin-inhibitory hormone and its potential role as a molecular link between feeding and reproductive systems in the Pekin duck (Anas platyrhynchos domestica) General and comparative endocrinology. 2013:184103–10. doi: 10.1016/j.ygcen.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 149.McConn BR, Yi J, Gilbert ER, Siegel PB, Chowdhury VS, Furuse M, Cline MA. Stimulation of food intake after central administration of gonadotropin-inhibitory hormone is similar in genetically selected low and high body weight lines of chickens. General and comparative endocrinology. 2016:23296–100. doi: 10.1016/j.ygcen.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 150.Tachibana T, Sato M, Takahashi H, Ukena K, Tsutsui K, Furuse M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res. 2005;1050(1–2):94–100. doi: 10.1016/j.brainres.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 151.Schneider JE, Benton NA, Russo KA, Klingerman CM, Williams WP, Simberlund J, Abdulhay A, Brozek JM, Kriegsfeld LJ. RFamide-related Peptide-3 and the Trade-off between Reproductive and Ingestive Behavior. Integrative and Comparative Biology. 2017 doi: 10.1093/icb/icx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Klingerman CM, Williams WP, 3rd, Simberlund J, Brahme N, Prasad A, Schneider JE, Kriegsfeld LJ. Food Restriction-Induced Changes in Gonadotropin-Inhibiting Hormone Cells are Associated with Changes in Sexual Motivation and Food Hoarding, but not Sexual Performance and Food Intake. Front Endocrinol (Lausanne) 2011;2:101. doi: 10.3389/fendo.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kovacs A, Laszlo K, Galosi R, Ollmann T, Peczely L, Zagoracz O, Bencze N, Lenard L. Intraamygdaloid microinjection of RFamide-related peptide-3 decreases food intake in rats. Brain Res Bull. 2014;107:61–8. doi: 10.1016/j.brainresbull.2014.07.002. [DOI] [PubMed] [Google Scholar]


