Abstract
To catalog the diversity and abundance of halogenated organic compounds (HOCs) accumulating in high trophic marine species from the southwestern Atlantic Ocean, tissue from bottlenose dolphins (Tursiops truncatus) stranded or incidentally captured along the coast of Rio de Janeiro, Brazil, were analyzed by a non-targeted approach based on GC×GC/TOF-MS. A total of 158 individual HOCs from 32 different structural classes were detected in the blubber of 4 adult male T. truncatus. Nearly 90 percent of the detected compounds are not routinely monitored in the environment. DDT-related and mirex/dechlorane-related compounds were the most abundant classes of anthropogenic origin. Methoxy-brominated diphenyl ethers and chlorinated methyl- and dimethyl bipyrroles (MBPs and DMBPs) were the most abundant natural products. Reported for the first time in southwestern Atlantic cetaceans and in contrast to North American marine mammals, chlorinated MBPs and DMBPs were more diverse and abundant than their brominated and/or mixed halogenated counterparts. HOC profiles in coastal T. tursiops from Brazil and California revealed a distinct difference, with a higher abundance of mirex/dechloranes and chlorinated bipyrroles in the Brazilian dolphins. Thirty-six percent of the detected HOCs had an unknown structure. These results suggest broad geographical differences in the patterns of bioaccumulative chemicals found in the marine environment, and indicate the need to develop more complete catalogs of HOCs from various marine environments.
Graphic Abstract
1. INTRODUCTION
Many environmental monitoring programs that measure halogenated organic compounds (HOCs) are focused on coastal marine ecosystems because the ocean is the final destination of urban and agricultural runoff by fluvial and estuarine systems. Typical monitoring includes anthropogenic contaminants accumulating in sediments and biological tissue; for example, invertebrates, fish and top predators. Due to their persistence and potential for biomagnification in aquatic food webs, legacy organohalogen contaminants, or persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs), organochlorine biocides (DDTs, chlordanes) and polybrominated diphenyl ethers (PBDEs) are routinely measured in these marine surveys.
The Stockholm Convention initially identified 12 organochlorine chemicals as POPs of environmental concern in 2001. New chemicals have since been added, and nine new POPs were listed in 2009 (α-, β- and γ-HCH, chlordecone, hexabromobenzene, pentachlorobenzene, PFAS, octa- and penta-BDE)1. As a result, the global production of organochlorines and organobromines identified by the Convention has either ceased or has been restricted2.
Whereas regulations such as the Stockholm Convention identify a short list of POPs to monitor, thousands of organic chemicals are produced and a fraction may exhibit environmental persistence and/or toxicity. Among such “neglected” chemicals are chlorinated flame retardants (dechloranes), polychlorinated styrenes (PCS) and polychlorinated terphenyls (PCTs), as well as degradation products of legacy POPs including transformation products of DDT, hexachlorobenzene (HCB) and PCBs (hydroxyl- and methylsulfonyl-PCB). Over time, new chemicals are developed or the production volumes of “old” chemicals are increased to replace those that have been banned or deemed obsolete. A subset of these emerging chemicals can be found in human and marine mammal tissue and have been suspected of possessing, for example, endocrine activity that may lead to higher order effects3,4.
In contrast to anthropogenic contaminants, a host of marine natural products also contain chlorine and bromine. Many of these complex organohalogen compounds are synthesized by bacteria, phytoplankton, algae and/or soft-bodied invertebrates and associated microorganisms5. Such compounds can exhibit antibiotic, antibacterial, antifungal, antitumor, antimicrobial, anti-inflammatory, anti-fouling, neurotoxic, and ichthyotoxic activities6–10. Some have been shown to bioaccumulate in marine/aquatic food webs11–13, but their ecological roles and effects remain largely unknown. Natural organohalogens are rarely, if ever, included in routine monitoring of POPs.
Non-targeted analysis (NTA) attempts to detect all contaminants with properties amenable to analysis by mass spectrometry. This is in contrast to targeted analysis, which is designed to exclusively measure specific compounds. Thus, NTA is useful for developing geographical and/or species-specific contaminant profiles as well as discovering new contaminants. The common bottlenose dolphin (Tursiops truncatus) is a cosmopolitan marine mammal species, distributed worldwide with site fidelity in coastal regions14. Due to its top predator status, life history, and presence near densely populated areas, cetaceans have been studied via targeted analyses15, and more recently using NTA. Hoh and coworkers identified a total of 270 halogenated organic compounds (HOCs) in a blubber sample of a common dolphin (Delphinus delphis) stranded in the NW Atlantic16; a second study using the same methodology reported 327 HOCs in the blubber of bottlenose dolphins (T. truncates) frequenting the southern California coast in the NE Pacific17. Comparison of the HOCs catalogued in these geographically disparate studies revealed differences in the relative distribution of anthropogenic contaminants, halogenated natural products, and unknown HOCs.
Guanabara Bay, located in southeastern Brazil near Rio de Janeiro (population 12 million), has received discharges of industrial, domestic and agricultural waste effluents since the 1950’s. This estuarine complex is home to more than 14,000 industrial establishments and is one of the most contaminated areas in Latin America18. Elevated concentrations of persistent bioaccumulative substances19,20, as well as emerging contaminants21,22 have been documented in cetaceans frequenting this coastal region. However, these studies have relied on targeted analysis, and thus no comprehensive cataloguing of bioaccumulative contaminants has been conducted to date on any marine species, including the marine sentinel T. truncatus23.
The aim of this study was to identify and catalog bioaccumulative HOCs occurring in blubber from T. truncatus stranded or incidentally captured along the Rio de Janeiro coast using a NTA approach. The study provides new information on anthropogenic, natural, and unknown HOCs missed by previous targeted monitoring surveys, and generates a comprehensive bioaccumulative HOC profile for the Brazilian dolphins that allows for comparison to profiles recently generated by NTA representing cetaceans in other parts of the world.
2. METHODS
A list of defined acronyms is given in the Supporting Information (SI).
2.1. Chemicals and reagents.
Dichloromethane, hexane, ethyl acetate, cyclohexane and toluene were exclusively residue-analysis grade. Standard solutions of extraction surrogates 3,3’,4,4’tetrabromodiphenyl ether (BDE-077S); 2,3,4,4’,5,6-hexabromodiphenyl ether (BDE-166S); 2,2’,4,4’,5,5’-hexachlorobiphenyl (C-153S); 4-fluoro-2,3’,4,6-tetrabromodiphenyl ether (FBDE-4001S); and 6-fluoro-2,2’,4,4’-tetrabromodiphenyl ether (FBDE-4003S) were purchased from AccuStandard (New Haven, CT, USA).
2.2. Samples.
Blubber samples were collected from four male T. truncatus fatally stranded or incidentally captured in fishing operations along the Rio de Janeiro coast, Brazil (SW Atlantic), between 2004 and 2011 (See Table 1 and Figure S1). The dolphins were collected with appropriate permission from Brazilian Environmental Agencies (Brazilian Institute of the Environment/Ministry of the Environment, permission number 11495). Male dolphins were analyzed to eliminate bias resulting from the transfer of female contaminant loads to their progeny21. Rio de Janeiro State University, Aquatic Mammals and Bioindicator Laboratory, staff performed the necropsies and collected tissues using a sterilized stainless steel scalpel. Samples were stored in aluminum foil and kept frozen at −20 ºC. To allow for a semi-quantitative comparison of HOC abundance among samples, the extracted blubber mass was kept uniform at 3g wet mass (Table 1).
Table 1.
Year of sampling, wet and lipid mass of blubber analyzed from adult male bottlenose dolphins (T. truncatus) stranded along Rio de Janeiro coast, Brazil.
| Sample | Year | Wet Mass (g) | Lipid Mass (g) | Lipid (%) | BL (cm)* | Location (Lat., Long.)** |
|---|---|---|---|---|---|---|
| 1 | 2013 | 3.0041 | 0.5 | 16.64 | 240 | Barra da Tijuca, RJ (23.0130º S, 43.3201º W) |
| 2 | 2013 | 3.0148 | 0.31 | 10.28 | 315 | Camboinhas, Niterói (22.9597º S, 43.0659º W) |
| 3 | 2013 | 3.0074 | 0.52 | 17.29 | 260 | Ponta Negra, Maricá (22.9606º S, 42.6928º W) |
| 4 | 2014 | 3.0569 | 0.31 | 10.14 | 300 | Recreio dos Banderantes, RJ (23.0213º S, 43.4411º W) |
BL – Body length (cm)
Lat., Long. – Latitude and Longitude
2.3. Extraction and cleanup.
Blubber samples were homogenized with kilned Na2SO4 and Soxhlet extracted with dichloromethane/hexane (1:1 v/v) for 8h. The extracts were spiked with 13C-PCB-153, BDE-77, BDE-166 and 6-FBDE-47 as internal standards prior to automated gel permeation chromatography (24 g BioBeads S-X3 eluted with 1:1 ethyl acetate/cyclohexane at a flow rate of 5 mL/min), as described previously16,17. The eluent fraction between 8.5 and 20.5 min was collected and evaporated to 1 mL under N2 gas, and the GPC purification step was repeated to remove residual lipids. The GPC extract was solvent-exchanged to hexane and reduced to a final volume of 100 μL. Fifty μL of the recovery standard 4-FBDE-69 was added to each sample extract prior to instrumental analysis.
2.4. Instrumental analysis.
Blubber sample extracts were analyzed utilizing a Pegasus 4D GC×GC/TOF-MS (LECO, St. Joseph, MI, USA). The first-dimension (1D) column was a Restek (Bellefonte, PA) Rtx-5MS (30m × 0.25mm i.d. × 0.25μm film thickness) with a 5 m guard column, and the second dimension (2D) column was a Restek Rxi-17 (1m × 0.10mm i.d.× 0.10μm film thickness). Two μL of extract was introduced into the splitless mode auto injector at 300 °C. Research grade helium (Airgas, Radnor, PA) was used as the carrier gas at 1 mL/min. The primary oven temperature started at 60 °C (1 min hold), ramped at 10 °C/min to 300 °C (3 min hold), and ramped at 20 °C/min to 320 °C (20 min hold). The secondary oven temperature was maintained 20 °C higher than the primary oven temperature. For GC×GC, the modulation period was 3.5 s with a 0.9 s hot pulse duration, and the modulator temperature offset was 35 °C relative to the primary oven temperature. The MS transfer line and ion source temperatures were 285 °C and 250 °C, respectively. The MS was operated in the electron ionization (EI) mode with a detector voltage of 1600 V, electron energy of −70 eV and data acquisition rate of 150 spectra/s.
2.5. Data Analysis.
Automatic peak finding and mass spectral deconvolution routines in the LECO ChromaTOF software (version 4.50.8.0) were used to isolate chromatographic peaks and associated mass spectra. PCB congeners were excluded from data analysis due their well-documented occurrence.
A total of 7,763 chromatographic features present at a signal-to-noise ratio (S/N) of 50 or above in the first sample analyzed. A threshold of S/N ≥ 50 was necessary to select peaks with reasonable mass spectral quality. A total of 150 peaks were identified as potential HOCs based on the identification of characteristic ion clusters and using two rules. 1) The mass spectrum must contain at least two halogenated isotopic clusters, with the observed isotopic profiles matching theoretical profiles (http://orgmassspec.github.io/). 2) The fragmentation pattern must indicate a halogenated loss (either bromine and/or chlorine). A reference data processing method was created from the 150 peaks and used to search the remaining three samples17. The allowed retention time deviation, set based on the modulation time, was ± 3.5 s in the 1st dimension and ± 0.05 s in the 2nd dimension. The peak S/N was required to be ≥ 50. The result was a list of peaks for the remaining samples that either matched those in the reference processing method, or were classified as new identifications. New identifications were manually checked for occurrence in the other three samples. A total of 158 unique HOCs were identified among all four dolphin samples. These compounds were not present in the procedural blank.
The unique HOC mass spectra were searched against the NIST 2011 EI Mass Spectral Library, the Southern California Dolphin Blubber Contaminant Library (SpecLibDolphin2014), and the Massachusetts Dolphin Blubber Contaminant Library (SpecLibDolphin2011), the last two of which are open access (http://orgmassspec.github.io/libraries.html ).
The compound identification categories in the mass spectra library were defined previously in Hoh et al.16 as “(1) The experimental mass spectrum and retention times were matched to those of a reference standard analyzed under the same conditions [authentic MS RT]. (2) The experimental spectrum, but not the retention times, was matched to a reference standard, indicating the experimental spectrum is that of an isomer [authentic MS]. (3) The experimental spectrum was matched to one within the NIST Electron Ionization Mass Spectral Library [reference database MS]. (4) The experimental spectrum was matched to one found in the literature [literature MS]. (5) The experimental mass spectrum was identified as potentially belonging to a class of congeners on the basis of comparison to that of a reference standard within the same class of congeners [manual-congener group]. (6) A presumptive identification was made by manual interpretation of the experimental spectrum [manual]. (7) The experimental spectrum was identified as belonging to a halogenated compound, but the chemical structure could not be further identified [unknown].” Isomers were numbered based on the elution order.
Mass spectra of interest and auxiliary information (e.g., retention times, categorization, and fragment ion identifications) were stored in a custom library. Development of the software used to generate the library was described previously16. The library is available as a PDF report in the SI, and at http://OrgMassSpec.github.io/ as a text file in the NIST MSP format for import into other software, and as the R package SpecLibDolphin2016. The library contains all mass spectra for the set of unique compounds, including unknowns, and information for the complete set of identifications across all samples.
The relative abundance of each identified compound was estimated by selection of an abundant fragment ion with minimal interference as the quantification ion. Next, the quantification ion peak area was divided by that of the internal standard (BDE-77). This value was then divided by the mass of extracted lipid for each sample to give the normalized relative abundance. Due to the unavailability of synthetic standards for most of compounds, the normalized abundances are considered semi-quantitative17. Variation between the normalized abundance profiles of the Californian and Brazilian dolphins was investigated by principal component analysis (PCA) using R24 function prcomp and visualized using function biplot. Prior to PCA, the abundance of non-detected compounds was set to zero, and all results were transformed as log10(x + 1), where x is the abundance. Separation of dolphin contaminant profiles by region was separately confirmed by k-means analysis using function kmeans with two centers (groups) specified.
3. RESULTS AND DISCUSSION
3.1. Origin and abundance of detected HOCs.
One hundred and fifty-eight unique HOCs (125 ± 32; n=4) in 32 structural classes were detected (Table 2). More than half of the HOCs were chlorinated (95 or 60%, in 22 classes), with a smaller percentage of brominated (43 or 27%, in 12 classes) and mixed halogenated compounds (16 or 10%, in 5 classes). Thirty-six percent (57 compounds in 6 classes) were unknown. PCB congeners were excluded from our cataloguing effort because they were investigated previously in dolphins from the sample location25; but other legacy POPs such as PBDEs, DDTs and chlordane-related HOCs were included. HOCs reported herein were classified as anthropogenic, naturally-occurring, or of unknown origin. Sixty-seven compounds (42%) were classified as anthropogenic; 26 (16%) were of natural origin; and 61 (39%) were of unknown origin. Four compounds (2.5%) were deemed to have both anthropogenic and natural sources (Table 2).
Table 2.
Classes of compounds identified in the blubber of adult male bottlenose dolphins (T. truncatus) from the Rio de Janeiro coast, Brazil. Shown are the number of congeners or isomers within each class, the number of bromines and chlorines, source, and the number of compounds that are not typically monitored in environmental studies.
| Class* | No. compounds | No. bromines | No. chlorines | Source | Authentic Standard | No. not typically monitored |
|---|---|---|---|---|---|---|
| 2MeO-Biphenyl-N | 1 | 4 | 0 | natural | 1 | 1 |
| 2MeO-Biphenyl-U | 1 | 3 | 1 | unknown | NA | 1 |
| B/CDE | 1 | 3 | 1 | unknown | NA | 1 |
| Brominated anisole | 1 | 3 | 0 | mixed | 1 | 1 |
| Brominated indole | 2 | 1,2 | 0 | natural | 2 | 2 |
| Bromophenol-M | 2 | 1,3 | 0 | mixed | NA | 2 |
| Bromophenol-U | 1 | 2 | 0 | unknown | NA | 1 |
| Chlordane-related | 2 | 0 | 8,9 | anthropogenic | 1 | NA |
| Chlorinated benzene | 3 | 0 | 3,4,6 | anthropogenic | 2 | 2 |
| Chlorinated styrene | 2 | 0 | 6,7 | anthropogenic | NA | 2 |
| Chlorophenol-M | 1 | 0 | 1 | mixed | NA | 1 |
| Chlorophenol-U | 1 | 0 | 2 | unknown | NA | 1 |
| DDT-related | 11 | 0,NA | 3,4,7,8,NA | anthropogenic | 3 | 8 |
| DMBP | 9 | 0,1,2,3,4,6 | 0,2,3,4,5,6 | natural | 6 | 9 |
| HCH-related | 1 | 0 | 6 | anthropogenic | 1 | NA |
| Heptachlor-related | 1 | 0 | 7 | anthropogenic | 1 | NA |
| MBP | 6 | 0 | 6,7 | natural | 1 | 6 |
| MeO-B/CDE | 2 | 3 | 1 | natural | NA | 2 |
| MeO-BDE | 6 | 3,4,5 | 0 | natural | 3 | 6 |
| Methylsulfonyl-PCB | 4 | 0 | 5 | anthropogenic | 1 | 4 |
| Mirex-related | 7 | 0,NA | 10,11,12,NA | anthropogenic | 3 | 6 |
| PBDE | 12 | 3,4,5,6 | 0 | anthropogenic | 8 | 5 |
| PCT | 20 | 0 | 5,6,7 | anthropogenic | NA | 20 |
| TCPM | 2 | 0 | 3 | anthropogenic | 1 | 2 |
| TCPMOH | 1 | 0 | 3 | anthropogenic | 1 | 1 |
| Toxaphene | 1 | 0 | 8 | anthropogenic | 1 | NA |
| Unknown | 32 | 0,NA | 7,NA | unknown | NA | 32 |
| Unknown-1 | 5 | 3,4 | 1 | unknown | NA | 5 |
| Unknown-2 | 14 | 0 | 6,7,8,9 | unknown | NA | 14 |
| Unknown-3 | 1 | NA | NA | unknown | NA | 1 |
| Unknown-4 | 2 | NA | NA | unknown | NA | 2 |
| Unknown-5 | 3 | NA | NA | unknown | NA | 3 |
The class Unknowns contains the unidentified compounds. NA = not applicable, -N = natural source, -U = unknown source, -M = mixed source.
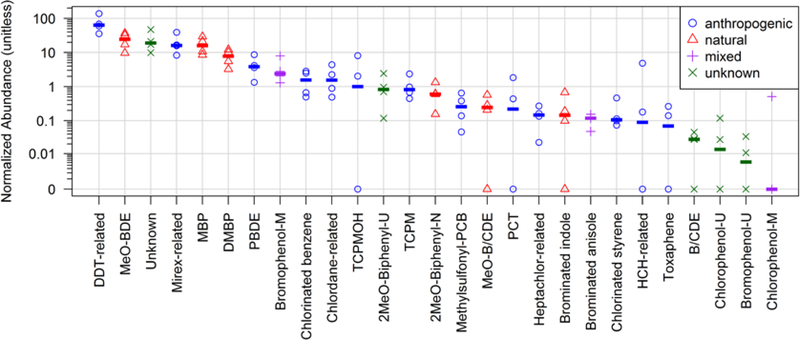
The normalized relative abundance of HOCs summed by class was as follows: 1) DDT-related > 2) MeO-BDE > 3) Unknown > 4) Mirex-related > 5) MBP > 6) DMBP > 7) PBDE > 8) Bromophenol-M (mixed) > 9) Chlorinated benzene > 10) Chlordane-related (Figure 1). Of these, the top 5 classes fell within an order of magnitude between 10 and 100 on the relative abundance scale, whereas the next 5 most abundant classes fell within between 1 and 10. The remaining classes ranged in abundance from 1 to 0.001 of the relative response. Five of the top 10 HOC classes were anthropogenic (DDT- and mirex-related, PBDE, chlorinated benzene and chlordane-related), 3 were of natural origin (MeO-BDE, MBP and DMBP), one was of unknown origin and the remaining class (bromophenol-M) has both natural and anthropogenic sources10,26,27.
Figure 1.
Relative abundance of HOCs by chemical class detected in the blubber of adult male bottlenose dolphins (T. truncatus) from the Rio de Janeiro coast, Brazil. Symbols represent individual samples (n=4) and the dash represents the median. –M, –U and –N refer to mixed, unknown and natural sources, respectively. The acronyms are listed in the SI.
3.2. Novelty and monitoring relevance of individual HOCs.
The large majority of detected HOCs (141 or 89% of the total) are not widely and/or routinely monitored in the environment (Table 2). This high percentage is similar to previous non-targeted studies, where 62% and 86% of the HOCs detected in blubber of dolphins stranded in the NW Atlantic16 and NE Pacific Oceans17, respectively, were not typically monitored. These results also suggest that most anthropogenic compounds present are not typically monitored (51 of 67, or 76%). For individual HOCs, p-p’-DDE (p. 7 in the mass spectral library PDF report in the SI, or “Library”) was the most abundant compound, followed by 6-MeO-BDE-47, MBP-Cl7, mirex, 2’-MeOBDE-68, p,p’-DDD, unknown-15, and DMBP-Cl6 (Figure 2, Figure 3, and p. 76, 93, 24, 75, 9, 137, and 79 in the Library).
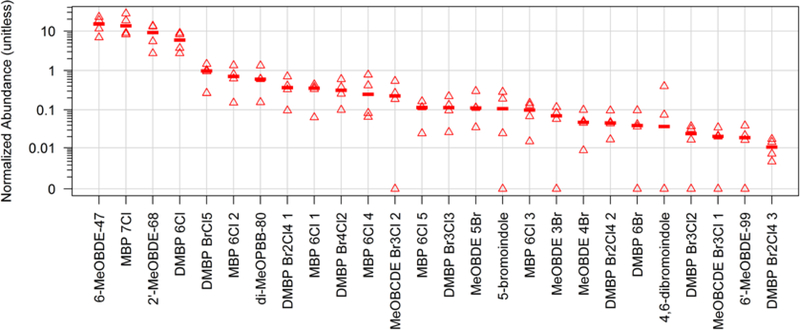
Figure 2.
Relative abundance of naturally occurring HOCs detected in the blubber of adult male bottlenose dolphins (T. truncatus) from the Rio de Janeiro coast, Brazil. The acronyms are listed in the SI.
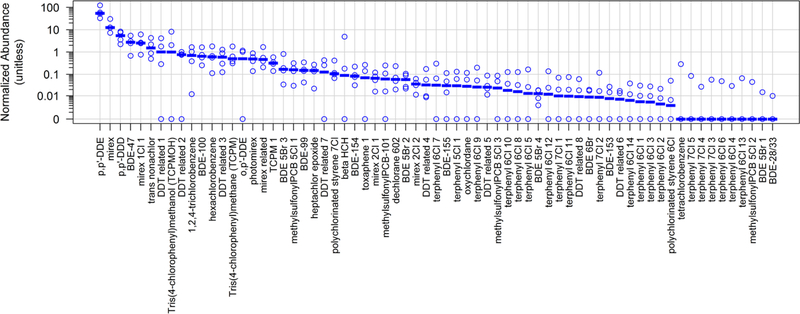
Figure 3.
Relative abundance of the anthropogenic HOCs detected in adult male bottlenose dolphins (T. truncatus) from Rio de Janeiro, Brazil. Circles represent individual samples (n=4) and the dash represents the median.
Fifty-nine HOCs were identified for the first time in marine mammal tissue (the following page numbers refer to the Library). Among these, 14 were from anthropogenic sources, including one DDT related compound (DDT related 5, p. 12), a MeSO2-PCB (5Cl 1, p. 59), two mirex/dechlorane related products (p. 20 and 21), as well as 3 penta-BDE isomers (p. 32, 33 and 34) and 7 polychlorinated terphenyl congeners (6 hexa-CT on p. 40, 42, 47, 50, 51, 52 and one hepta-CT on p. 57). For natural products, 4 novel halogenated bipyrroles were identified for the first time in marine mammal tissues, including 2 isomers each of MBP-Cl6 (p. 90 and 92) and DMBP Br2Cl4 (p. 83 and 84).
Anthropogenic HOCs.
DDT use was restricted in Brazil beginning in 1971, with a total ban in 2009, but its abundance and persistence still results in widespread contamination of marine biota18,28,29. The relative abundance of p,p’-DDE, one of the most persistent components of technical DDT, was up to 120 times higher than other detectable DDTs (Figure 3). The next most abundant DDT isomer was p,p’-DDD, followed by DDT related 1, 2, 3 (p. 6, 8 and 10). The elemental formula for DDT related 1, which was also observed previously in dolphins16,17 and rays30, is C14H11Cl3, suggesting loss of chlorine from one or more of the Cl4 and Cl5 isomers found in technical DDT. Conversely, several DDT-related compounds contained a DDE backbone with 7 and 8 chlorines (p. 11–15). These HOCs also occurred in the NW Atlantic and NE Pacific dolphin samples referred to previously16,17.
Of the 7 mirex-related compounds detected (Figure S2 and p. 20–26), 4 contained 1 or 2 fewer chlorines than the parent mirex. Mirex 2Cl 1 and Mirex 2Cl 2 were assigned the elemental formula C10H2Cl10, are considered to be novel, and represent possible transformation products as dihydro C10H2Cl10 derivatives31,32. Mirex 1Cl 1 (p. 23) with a relative abundance similar to that of BDE-47 (Figure 3) is another possible dechlorination product of mirex. The other compound containing 11 chlorines (C10HCl11) was identified as photomirex and confirmed by authentic standard (p. 22). Photomirex was previously reported as a mirex metabolite31–34. Dechlorane 602 (Dec 602) was also identified and confirmed by authentic standard (p. 25).
Mirex and Dec 602 were previously detected in Franciscana (Pontoporia blainvillei) dolphin liver from Brazilian waters, along with two related flame-retarding compounds we did not detect, Dechlorane Plus and Declorane 60335. Mirex related compounds had a higher relative abundance compared to other compound classes in dolphins from Brazil (this study), in contrast to those collected from NE Pacific dolphins17. Mirex was used primarily as a pesticide in the Southeastern region of the US, and to a lesser degree as a flame retardant additive under the trade name Dechlorane, until it was banned in the 1970s36–38. Dechlorane was replaced by the chlorinated compounds Dechlorane Plus, Dec 602, Dec 603, and Dec 604. It follows that the occurrence of mirex and related compounds in the Brazilian samples are also related to past regional use, perhaps in higher abundance and/or closer proximity than in the case of the NE Pacific dolphins.
Other anthropogenic HOCs detected in our samples were: polychlorinated terphenyls (PCTs) (n=20, p. 39–58), PBDEs (n=12, p. 27–38), methylsulfonyl-PCBs (n=4, p. 59–62), polychlorinated styrenes (p. 67 and 68), HCB and likely HCB metabolites (p. 63–65), tri-(4chlorophenyl)methane and its isomer (p. 16 and 17) (Table 2). PBDEs were the most abundant of these classes (p. 27–38), with BDE-47, −100 and −99 among the most abundant congeners (Figure 3). The PCB derivatives are likely transformation products of parent PCBs. These HOC classes were also found in the NW Atlantic and NE Pacific dolphin data reported previously16,17, indicating their ubiquity across three different oceanic regions.
Natural products.
The naturally produced methoxy-BDEs (MeO-BDE, p. 73–78) and chlorinated bipyrroles (MBP-Cl7 and DMBP-Cl6, p. 93 and 79) were comparable in abundance to DDT- and mirex-related compounds (Figure 1). These two natural product classes were also detected in marine mammals from other parts of the world17,23. We detected 6 isomers of MeO-BDE, 2 brominated indoles (5-bromo and 4,6-dibromo, p. 94 and 95), two mixed halogenated methoxy-diphenyl ethers (MeO-B/CDE 1 and 2, p. 71 and 72), and a single isomer of dimethoxy-polybrominated biphenyl (di−MeOPBB−80, p. 80). On an individual compound basis, 6-MeO-BDE-47, MBP-Cl7, 2’-MeO-BDE-68 and DMBP-Cl6 were among the most abundant natural products, followed by DMBP BrCl5, MBP-Cl6 2, di-MeO-PBB-80, DMBP Br2Cl4 1, MBP-Cl6 1 and DMBP Br4Cl2 (Figure 2, p. 81, 89, 70, 82, 88, and 86). The production of MeO-BDE is associated with marine cyanobacteria39, (mostly red) algae39–42 and invertebrates including corals5, sponges5,43 and their associated bacterial assemblages43.
Bromoindoles are reported in Brazilian cetacean tissues for the first time. These compounds are also synthesized by algae44 and invertebrates, including Tubastrea spp43. which, perhaps not coincidentally, are invasive species along the coast of Rio de Janeiro45. Bromoindoles have been shown to be toxic to various organisms, suggesting a role in protecting the host (such as corals, sponges, algae and other invertebrates) from predation and/or disease5,10,43. The 2 mixed halogenated MeO-BDEs (MeO-BCDE Br3Cl 1 and 2) identified herein were previously found in NW Atlantic dolphin16 and in NE Pacific bottlenose dolphins17.
Our detection of halogenated MBPs and DMBPs is, to our knowledge, the first in cetaceans from Brazil and more broadly the SW Atlantic. In total, we catalogued 5 isomers of MBP-Cl6 (p. 88–92), MBP-Cl7 (p. 93), 9 isomers of DMBPs (DMBP-Cl6, DMBP-Br6, DMBPBrCl5, DMBP-Br3Cl2, DMBP-Br3Cl3, DMBP-Br4Cl2 and 3 isomers of DMBP-Br2Cl4, p. 79–87). In contrast to the predominance of DMBPs relative to MBPs in dolphins from the northern hemisphere46–48, our samples exhibited the opposite trend, with MBPs in greater abundance than DMBPs. This trend is consistent with results reported for marine mammals from Australia48–50.
Hoh et al.16 originally found 2 isomers of MBP-Cl6 in a NW Atlantic common dolphin, a discovery that was followed by the detection of 3 MBP-Cl6 isomers by Shaul et al.17 in T. truncatus from the NE Pacific. Herein we describe two additional MBP-Cl6 congeners (Figure S3), for a total of 5 isomers that are possible degradation products of MBP-Cl7 homologs. The MBP-Cl6 congeners were among the most abundant HOCs in our samples (Figure 2). Interestingly, the samples in the present study were dominated by chlorinated MBPs, contrary to those from the NW Atlantic16,51 and NE Pacific17 that were dominated by brominated MBPs. Pangallo et al.51 suggested that MBPs may have an ocean-basin specific distribution, as evidenced by the dominance of brominated MBPs in the North Atlantic and highly-chlorinated derivatives in the South Pacific. The addition of our results, however, suggests a difference between the northern and southern hemisphere52, where SE Pacific and SW Atlantic odontocetes exhibit a dominance of chlorinated MBPs48,49 and NE Pacific and NW Atlantic odontocetes exhibit a dominance of brominated MBPs12,17,51,53.
Nine DMBPs were identified in the Brazilian T. truncatus. In contrast to MBPs, both brominated and chlorinated DMBPs were detected, although a slight predominance of chlorinated versus brominated homologs were observed (Table 2). DMBP Br2Cl4 2 and DMBP Br2Cl4 3 (p. 83 and 84) are reported for the first time, along with DMBP Br2Cl4 1 (p. 82), which had the highest abundance in this homolog group (Figure 2). Like MBPs, dolphins from the northern hemisphere contained higher proportions as well as a more diverse suite of brominated DMBPs compared to chlorinated DMBPs13,16,17,47. In the Brazilian case, chlorinated DMBP congeners were more prevalent than brominated DMBPs.
HOCs of mixed origin.
The present study detected 5 halogenated phenolic compounds in marine mammals: bromo-, dibromo-, and tribromophenol, dichlorophenol, and methoxy chlorophenol (p. 97–101). Bromophenols (C6H5BrO) are present as impurities in synthetic chemicals54 and are produced by marine algae26 and bacteria55. They have been detected in a variety of marine organisms, including polychaetes, sponges, bryozoans, and fishes26,56, and are of interest due to their antioxidant and anticancer properties57. Tribromophenol and 2,4,6tribromo anisole (p. 98 and 96), a closely related structural homolog, were also detected in our samples (Table 2). These compounds, detected previously in cetacean blood58,59, are found in synthetic flame retardant formulations27 and are also marine natural products26,60.
Unknown origin.
Unknown HOCs were classified in the mass spectral library based on structural similarity (p.103–159). Unknowns in the present study that were identified in dolphin blubber from the NW Atlantic Ocean or the NE Pacific Ocean were indicated as such in the library and were discussed in detail previously16,17. The class referred to as Unknown-1 is comprised of compounds with elemental formulas C9H6OBr3Cl and C9H5OBr4Cl (p.103–107), including two novel HOCs referred to as Unknown-1–2 and Unknown-1–4 (p.104 and 106, respectively). Determination of the elemental formulas and potential chemical structures were proposed previously61. Based on mass spectral similarity, some compounds from this class may have been previously detected in sponges from Croatia62, in water of the Great Barrier Reef63, in NW Atlantic dolphin tissue16 and fish oil61. Class Unknown-2 compounds may be HO-PCB or PCDE homologs based on similar fragmentation patterns. Five new Cl7 – Cl9 homologs (unknowns 2–4, 2–6, 2–8, 2–12, and 2–14 in p.111, 113, 115, and 121, respectively) were also assigned to class Unknown 2. The compound di−MeOBB−Br3Cl (p. 69) has an unknown source; however, it is structurally similar to the natural product di-MeOPBB-80 (p.70).
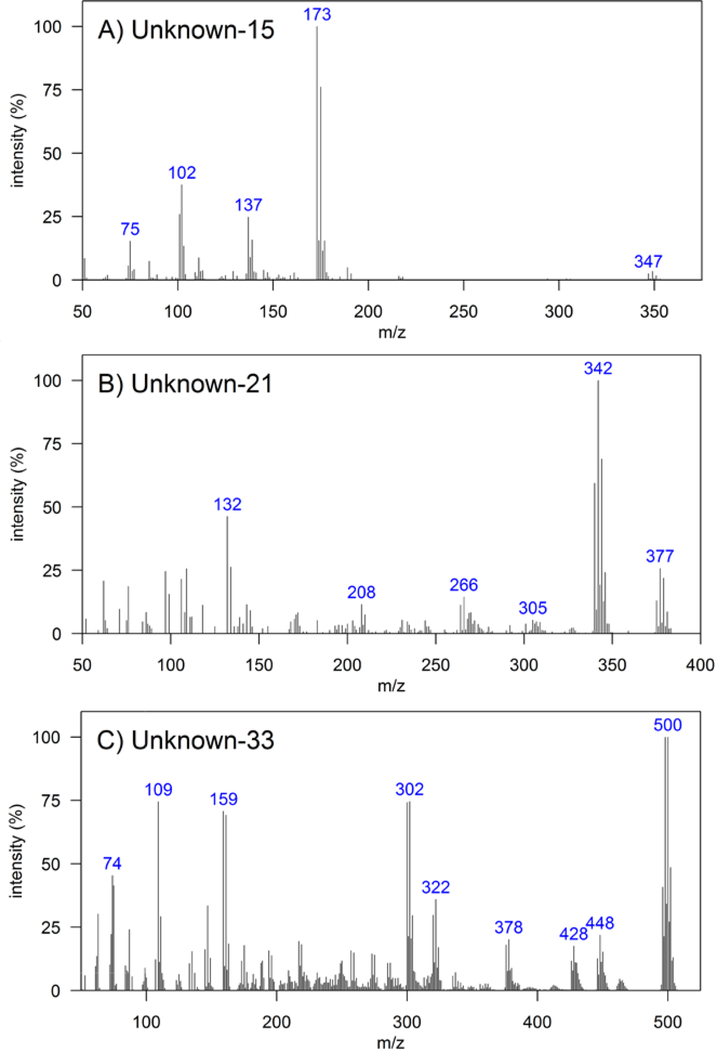
Unknowns 15, 21, and 33 (Figure 4 and p.137, 143, and 155, respectively) were the most abundant unknown HOCs (Figure S4), with relative abundances in the range of the eight most abundant HOCs of anthropogenic origin (Figure 3). Unknown-15 was previously found in dolphin blubber of the NW Atlantic Ocean16, whereas Unknown-21 was previously found in dolphin blubber of the NE Pacific Ocean17 and in dietary supplemental fish oil61. No previous reports of compounds that resembled Unknown 33 were found.
Figure 4.
Electron ionization mass spectra of a) Unknown-15, b) Unknown-21, and c) Unknown-33
3.3. Comparison of non-targeted HOC profiles.
Noting the sample type and analytical procedures used in the present study were essentially the same as those used by Shaul et al.17, we present a comparison of the non-targeted HOC distributions observed among the two studies. There were 8 specimens of T. truncatus from the NE Pacific (California Bight) and 4 T. truncatus samples from the SW Atlantic (this study, Rio de Janeiro Coast).
The number of individual HOCs identified in the SW Atlantic bottlenose dolphins (158; n=4) was less than that in NE Pacific (327; n=8)17. The diversity of HOCs, measured by the number of structural classes (32 in the SW Atlantic), was similar to NE Pacific (34). Far fewer legacy organohalogens were detectable in the present study compared to the NE Pacific study17. For example, 23 chlordane-related and 19 toxaphene-related HOCs were identified in NE Pacific dolphins, compared to only 2 chlordane-related compounds and 1 toxaphene in this study. Twenty-nine DDT-related compounds and 12 TCPMs were observed in the NE Pacific study, compared to only 11 and 2, respectively, in this study. Thirteen PBBs and one chlorophosphate were found in NE Pacific dolphins; in contrast, none of these flame retardant compounds were detected in dolphins from the present study. In contrast, mirex/dechlorane related HOCs were more numerous (7 vs. 5) and had a greater relative abundance in this study compared to the NE Pacific study.
The diversity of natural products between the two regions was mixed, depending on the HOC class. Eight MBP and 20 DMBP isomers were reported in the NE Pacific study, compared to 6 MBPs and 9 DMBPs identified in the SW Atlantic. Polybrominated hexahydroxanthene derivatives (PBHD; n=4) were detected in the NE Pacific samples but were not detected in the present study. On the other hand, the relative abundances of MBP-Cl7 and DMBP-Cl6 were up to 10 and 5 times higher, respectively, compared to the NE Pacific dolphins. Moreover, the two predominant MeO-BDEs (2’-MeO-BDE-68 and 6-MeO-BDE-47) were more than 120-fold higher in abundance than was found in the NE Pacific study.
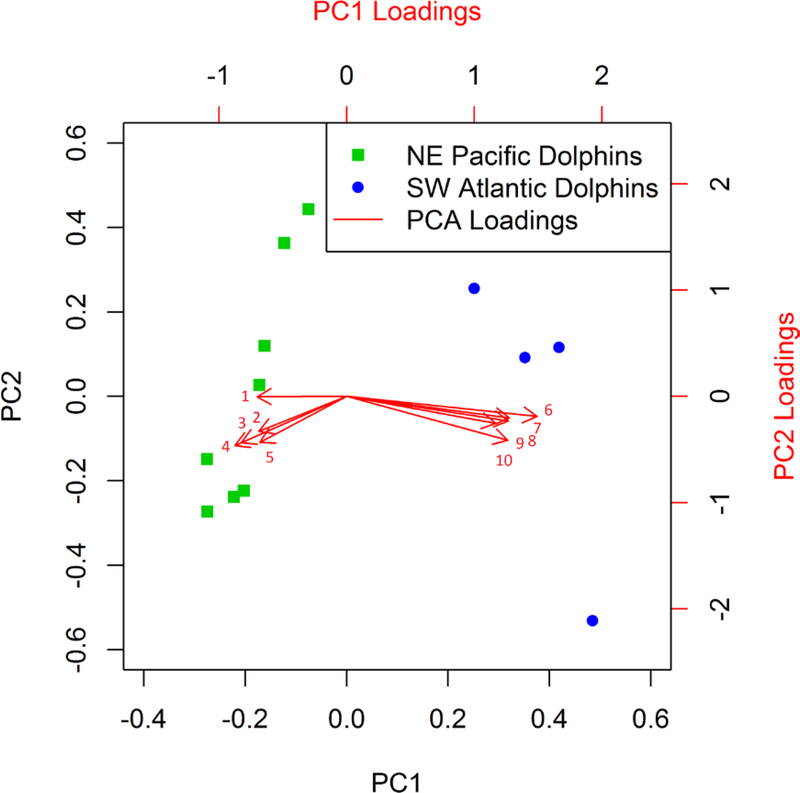
Principal components analysis comparing the relative abundances of detected HOCs in the SW Atlantic bottlenose dolphin samples show a distinct difference from the profile associated with the NE Pacific bottlenose dolphins (Figure 5). In particular, the five compounds with the greatest relative abundance in the Brazilian dolphins, as identified by the PCA loadings, were mirex (p. 24), unknown-15 (p. 137), and the natural products 6-MeO-BDE-47 (p. 76), 2’MeO-BDE-68 (p. 75), and MBP-Cl7 (p. 93). The five contaminants with the greatest relative abundance in the NE Pacific samples were all breakdown products or impurities from technical DDT: o,p’-DDD, DDMU-3, 4,4’-dichlorobenzophenone (these first three were not detected in the Brazilian samples), TCPM (p. 17)., and TCPM-1 (p. 16).
Figure 5.
Biplot of the NE Pacific (California Bight) and SW Atlantic (Rio de Janeiro Coast) contaminant profiles. The cumulative proportion of the variance represented by principal component 1 (PC1) and PC2 was 60% and 74%, respectively. Dolphin groups were separated primarily along PC1. The arrows show the relative loadings (influence) of contaminants on the first and second principal components. The five largest negative loadings and five largest positive loading on PC1 are shown and discussed in the text. 1 = o,p’-DDD, 2 = 4,4’dichlorobenzophenone, 3 = TCPM, 4 = DDMU-3, 5 = TCPM-1, 6 = 6-MeO-BDE-47, 7 = unknown-15, 8 = 2’-MeO-BDE-68, 9 = MBP-Cl7, 10 = mirex. Negative loadings indicate relatively high contaminant abundance in the NE Pacific samples, and positive loadings indicate relatively high contaminant abundance in the SW Atlantic samples. The assignment of dolphin samples to each PCA cluster was confirmed by k-means analysis.
Seventeen of the 57 unknowns detected in the SW Atlantic dolphins were also found in the NE Pacific dolphins. Future structural elucidation of these compounds will help complete the HOC exposure profile for these marine mammal sentinels, and will enhance the capability to compare contaminant exposure profiles among other oceanic and/or geographic regions.
Supplementary Material
Acknowledgements
We thank Dr. Wenjian (Wayne) Lao (SCCWRP), Dr. Susan Mackintosh (SDSU), and Kayo Watanabe (SDSU) for assisting with the analysis.
Funding Sources
This research was funded in part by Brazilian Research Council CNPq (fellowship to M.B. Alonso Post Doctorate Abroad), Rio de Janeiro State Government Research Agency FAPERJ (Jovem Cientista do Nosso Estado #101.449/2010), Mount Sinai School of Medicine (NY/USA), Fogarty International Center NIH/USA (1D43TW0640), the National Science Foundation (OCE-1313747) and National Institute of Environmental Health Sciences (P01-ES021921) through the Oceans and Human Health Program, the NOAA Prescott Grant Program (NA14NMF4390177), and the member agencies of SCCWRP.
Footnotes
Notes
The authors declare no competing financial interest.
Supporting Information
Glossary of acronyms, supplemental figures, and a mass spectral library are available free of charge via internet at http://pubs.acs.org/.
REFERENCES
- (1).UNEP. The 9 new POPs; 2010; Vol. 2008. [Google Scholar]
- 2).European Court of Justice. Commission Decision 010/571/EU. Off. J. Eur. Union 2010, 56, 30–36. [Google Scholar]
- 3).Alonso MB; Feo ML; Corcellas C; Vidal LG; Bertozzi CP; Marigo J; Secchi ER; Bassoi M; Azevedo AF; Dorneles PR; et al. Pyrethroids: a new threat to marine mammals? Environ. Int. 2012, 47, 99–106. [DOI] [PubMed] [Google Scholar]
- 4).Gago-Ferrero P; Alonso MB; Bertozzi CP; Marigo J; Barbosa L; Cremer M; Secchi ER; Domit C; Azevedo A; Lailson-Brito J; et al. First determination of UV filters in marine mammals. Octocrylene levels in Franciscana dolphins. Environ. Sci. Technol. 2013, 47, 5619–5625. [DOI] [PubMed] [Google Scholar]
- 5).Blunt JW; Copp BR; Hu WP; Munro MH; Northcote PT; Prinsep MR Marine natural products. Nat Prod Rep 2009, 26, 170–244. [DOI] [PubMed] [Google Scholar]
- 6).Keyzers R; Davies-Coleman MT Anti-inflammatory metabolites from marine sponges. Chem. Soc. Rev. 2005, 34, 355–365. [DOI] [PubMed] [Google Scholar]
- 7).Simmons TL; Andrianasolo E; McPhail K; Flatt P; Gerwick WH Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [PubMed] [Google Scholar]
- 8).Rodrigues D; Alves C; Horta A; Pinteus S; Silva J; Culioli G; Thomas O; Pedrosa R Antitumor and antimicrobial potential of bromoditerpenes isolated from the red alga, Sphaerococcus coronopifolius. Mar. Drugs 2015, 13, 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Qian P-Y; Li Z; Xu Y; Li Y; Fusetani N Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [DOI] [PubMed] [Google Scholar]
- 10).Gribble GW Naturally Occurring Organohalogen Compounds - A Comprehensive Update, Progress in the Chemistry of Organic Natural Products; 1st ed.; Springer: Vienna, 2010; Vol. 91. [DOI] [PubMed] [Google Scholar]
- 11).Alonso MB; Eljarrat E; Gorga M; Secchi ER; Bassoi M; Barbosa L; Bertozzi CP; Marigo J; Cremer M; Domit C; et al. Natural and anthropogenically-produced brominated compounds in endemic dolphins from Western South Atlantic: another risk to a vulnerable species. Environ. Pollut. 2012, 170, 152–160. [DOI] [PubMed] [Google Scholar]
- 12).Pangallo KC; Reddy CM Distribution patterns suggest biomagnification of halogenated 1 ‘-Methyl-1,2 ‘-Bipyrroles (MBPs). Environ. Sci. Technol. 2009, 43, 122–127. [DOI] [PubMed] [Google Scholar]
- 13).Teuten EL; Reddy CM Halogenated organic compounds in archived whale oil: A pre-industrial record. Environ. Pollut. 2007, 145, 668–671. [DOI] [PubMed] [Google Scholar]
- 14).Fruet PF; Secchi ER; Daura-Jorge F; Vermeulen E; Flores PAC; SimõesLopes PC; Genoves RC; Laporta P; Di Tullio JC; Freitas TRO; et al. Remarkably low genetic diversity and strong population structure in common bottlenose dolphins (Tursiops truncatus) from coastal waters of the Southwestern Atlantic Ocean. Conserv. Genet 2014, 879–895. [Google Scholar]
- 15).Kucklick J; Schwacke L; Wells R; Hohn A; Guichard A; Yordy J; Hansen L; Zolman E; Wilson R; Litz J; et al. Bottlenose dolphins as indicators of persistent organic pollutants in the western North Atlantic Ocean and northern Gulf of Mexico. Environ. Sci. Technol. 2011, 45, 4270–4277. [DOI] [PubMed] [Google Scholar]
- 16).Hoh E; Dodder NG; Lehotay SJ; Pangallo KC; Reddy CM; Maruya KA Nontargeted comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry method and software for inventorying persistent and bioaccumulative contaminants in marine environments. Environ. Sci. Technol. 2012, 46, 8001–8008. [DOI] [PubMed] [Google Scholar]
- 17).Shaul NJ; Dodder NG; Aluwihare LI; Mackintosh SA; Maruya KA; Chivers SJ; Danil K; Weller DW; Hoh E Nontargeted biomonitoring of halogenated organic compounds in two ecotypes of bottlenose dolphins (Tursiops truncatus) from the southern California bight. Environ. Sci. Technol. 2015, 49, 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Lailson-Brito J; Dorneles PR; Azevedo-Silva CE; Azevedo AF; Vidal LG; Zanelatto RC; Lozinski CPC; Azeredo A; Fragoso ABL; Cunha HA; et al. High organochlorine accumulation in blubber of Guiana dolphin, Sotalia guianensis, from Brazilian coast and its use to establish geographical differences among populations. Environ. Pollut. 2010, 158, 1800–1808. [DOI] [PubMed] [Google Scholar]
- 19).Dorneles PR; Lailson-Brito J; Fernandez MAS; Vidal LG; Barbosa LA; Azevedo AF; Fragoso ABL; Torres JPM; Malm O Evaluation of cetacean exposure to organotin compounds in Brazilian waters through hepatic total tin concentrations. Environ. Pollut. 2008, 156, 1268–1276. [DOI] [PubMed] [Google Scholar]
- 20).Dorneles PR; Sanz P; Eppe G; Azevedo AF; Bertozzi CP; Martínez MA; Secchi ER; Barbosa LA; Cremer M; Alonso MB; et al. High accumulation of PCDD, PCDF, and PCB congeners in marine mammals from Brazil: A serious PCB problem. Sci. Total Environt. 2013, 463–464, 309–318. [DOI] [PubMed] [Google Scholar]
- 21).Alonso MB; Feo ML; Corcellas C; Gago-Ferrero P; Bertozzi CP; Marigo J; Flach L; Meirelles ACO; Carvalho VL; Azevedo AF; et al. Toxic heritage: Maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil. Environ. Pollut. 2015, 207, 391–402. [DOI] [PubMed] [Google Scholar]
- 22).Dorneles PR; Lailson-Brito J; Azevedo AF; Meyer J; Vidal LG; Fragoso AB; Torres JP; Malm O; Blust R; Das K High accumulation of perfluorooctane sulfonate (PFOS) in marine tucuxi dolphins (Sotalia guianensis) from the Brazilian coast. Environ. Sci. Technol. 2008, 42, 5368–5373. [DOI] [PubMed] [Google Scholar]
- 23).Alonso MB; Azevedo A; Torres JPM; Dorneles PR; Eljarrat E; Barceló D; Lailson-Brito J; Malm O Anthropogenic (PBDE) and naturally-produced (MeO-PBDE) brominated compounds in cetaceans - A review. Sci. Total Environ. 2014, 481C, 619–634. [DOI] [PubMed] [Google Scholar]
- 24).R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria, 2016, URL https://www.R-project.org/ (accessed December 1, 2016) [Google Scholar]
- 25).Lailson-Brito J; Dorneles PR; Azevedo-Silva CE; Bisi TL; Vidal LG; Legat LN; Azevedo AF; Torres JPM; Malm O Organochlorine compound accumulation in delphinids from Rio de Janeiro State, southeastern Brazilian coast. Sci. Total Environ. 2012, 433C, 123–131. [DOI] [PubMed] [Google Scholar]
- 26).Gribble GW The natural production of organobromine compounds. Environ. Sci. Pollut. Res. Int. 2000, 7, 37–47. [DOI] [PubMed] [Google Scholar]
- 27).Wang H-S; Chen Z-J; Ho K-L; Ge L-C; Du J; Lam MH-W; Giesy JP; Wong M-H; Wong CK-C Hydroxylated and methoxylated polybrominated diphenyl ethers in blood plasma of humans in Hong Kong. Environ. Int. 2012, 47, 66–72. [DOI] [PubMed] [Google Scholar]
- 28).D’Amato C; Torres JPM; Malm O DDT (Dicloro difenil tricloroetano): Toxicidade e contaminação ambiental - Uma revisão. Quim. Nova 2002, 25, 995–1002. [Google Scholar]
- 29).Brazilian Court of Justice. Federal Law No. 11936/2009 DOU 15.5.2009. Off. J. Brazilian Repub. 2009, 1, (In Portuguese). [Google Scholar]
- 30).Rosenfelder N; Lehnert K; Kaffarnik S; Torres JPM; Vianna M; Vetter W Thorough analysis of polyhalogenated compounds in ray liver samples off the coast of Rio de Janeiro, Brazil. Environ. Sci. Pollut. Res 2012, 19, 379–389. [DOI] [PubMed] [Google Scholar]
- 31).Andrade P; Wheeler WB; Carlson DA Identification of a mirex metabolite. 1975, 14. [DOI] [PubMed]
- 32).Stein VB; Pittman KA; Kennedy MW Characterization of a mirex metabolite from monkeys. Bull. Environ. Contam. Toxicol. 1976, 15. [DOI] [PubMed] [Google Scholar]
- 33).Stein VB; Pittman KA Identification of a mirex metabolite from monkeys. 1977, 18, 425–427. [DOI] [PubMed] [Google Scholar]
- 34).Norstrom RJ; Hallett DJ; Onuska F; Comba ME Mirex and its degradation products in Great Lakes herring gulls. 1980.
- 35).De La Torre A; Alonso MB; Martínez MA; Sanz P; Shen L; Reiner EJ; Lailson-Brito J; Torres JPM; Bertozzi C; Marigo J; et al. Dechlorane-related compounds in franciscana dolphin (Pontoporia blainvillei) from southeastern and southern coast of Brazil. Environ. Sci. Technol. 2012, 46, 12364–12372. [DOI] [PubMed] [Google Scholar]
- 36).ATSDR - Agency for toxic substances and diseases registry. Mirex and chlordecone Toxicol. profile mirex chlordecone. Atlanta, GA: U.S. Dep. Heal. Hum. Serv. Public Heal. Serv. 1995, 0–2. [Google Scholar]
- 37).Hoh E; Zhu L; Hites RA Dechlorane plus, a chlorinated flame retardant, in the Great Lakes. Environ. Sci. Technol. 2006, 40, 1184–1189. [DOI] [PubMed] [Google Scholar]
- 38).Shen L; Reiner EJ; MacPherson KA; Kolic TM; Helm PA; Richman LA; Marvin CH; Burniston DA; Hill B; Brindle ID; et al. Dechloranes 602, 603, 604, dechlorane plus, and chlordene plus, a newly detected analogue, in tributary sediments of the Laurentian great lakes. Environ. Sci. Technol. 2011, 45, 693–699. [DOI] [PubMed] [Google Scholar]
- 39).Malmvärn A; Zebühr Y; Kautsky L; Bergman Å; Asplund L. Hydroxylated and methoxylated polybrominated diphenyl ethers and polybrominated dibenzo-p-dioxins in red alga and cyanobacteria living in the Baltic Sea. Chemosphere 2008, 72, 910–916. [DOI] [PubMed] [Google Scholar]
- 40).Haraguchi K; Kotaki Y; Relox JR; Romero MLJ; Terada R Monitoring of naturally produced brominated phenoxyphenols and phenoxyanisoles in aquatic plants from the Philippines. J. Agric. Food Chem. 2010, 12385–12391. [DOI] [PubMed] [Google Scholar]
- 41).Kuniyoshi M; Yamada K; Higa T A biologically active diphenyl ether from the green alga Cladophora fascicularis. Experientia 1985, 41, 523–524. [Google Scholar]
- 42).Malmvärn A; Marsh G; Kautsky L; Athanasiadou M; Bergman A; Asplund L Hydroxylated and methoxylated brominated diphenyl ethers in the red algae Ceramium tenuicorne and blue mussels from the Baltic Sea. Environ. Sci. Technol. 2005, 39, 2990–2997. [DOI] [PubMed] [Google Scholar]
- 43).Puglisi MP; Sneed JM; Sharp K; Ritson-Williams R; Paul VJ Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2014, 31, 1510–1553. [DOI] [PubMed] [Google Scholar]
- 44).Suárez-Castillo OR; Beiza-Granados L; Meléndez-Rodríguez M; ÁlvarezHernández A; Morales-Ríos MS; Joseph-Nathan P Synthesis of bromoindole alkaloids from Laurencia brongniartii. J. Nat. Prod. 2006, 69, 1596–1600. [DOI] [PubMed] [Google Scholar]
- 45).Sampaio CLS; Miranda RJ; Maia-Nogueira R; Nunes J de A CC New occurrences of the nonindigenous orange cup corals tubastraea coccinea and T. tagusensis (Scleractinia: Dendrophylliidae) in southwestern Atlantic. Check List 2012, 8, 528–530. [Google Scholar]
- 46).Teuten EL; Pedler BE; Hangsterfer AN; Reddy CM Identification of highly brominated analogues of Q1 in marine mammals. Environ. Pollut. 2006, 144, 336–344. [DOI] [PubMed] [Google Scholar]
- 47).Tittlemier S; Borrell A; Duffe J; Duignan PJ; Fair P; Hall A; Hoekstra P; Kovacs KM; Krahn MM; Lebeuf M; et al. Global distribution of halogenated dimethyl bipyrroles in marine mammal blubber. Arch. Environ. Contam. Toxicol. 2002, 43, 244–255. [DOI] [PubMed] [Google Scholar]
- 48).Hauler C; Martin R; Knolker HJ; Gaus C; Mueller JF; Vetter W Discovery and widespread occurrence of polyhalogenated 1,1’-dimethyl-2,2’-bipyrroles (PDBPs) in marine biota. Environ. Pollut. 2013, 178, 329–335. [DOI] [PubMed] [Google Scholar]
- 49).Vetter W; Scholz E; Gaus C; Müller JF; Haynes D Anthropogenic and natural organohalogen compounds in blubber of dolphins and dugongs (dugong dugon) from northeastern Australia. Arch. Environ. Contam. Toxicol. 2001, 41, 221–231. [DOI] [PubMed] [Google Scholar]
- 50).Vetter W; Alder L; Kallenborn R; Schlabach M Determination of Q1, an unknown organochlorine contaminant, in human milk, Antarctic air, and further environmental samples. Environ. Pollut. 2000, 110, 401–409. [DOI] [PubMed] [Google Scholar]
- 51).Pangallo K; Nelson RK; Teuten EL; Pedler BE; Reddy CM Expanding the range of halogenated 1’-methyl-1,2’-bipyrroles (MBPs) using GC/ECNI-MS and GC x GC/TOF-MS. Chemosphere 2008, 71, 1557–1565. [DOI] [PubMed] [Google Scholar]
- 52).Vetter W; Alder L; Palavinskas R Mass Spectrometric Characterization of Q1, a C9H3Cl7N2 contaminant in environmental samples. Rapid Commun. Mass Spectrom. 1999, 13, 2118–2124. [DOI] [PubMed] [Google Scholar]
- 53).Pangallo KC; Reddy CM Marine natural products, the halogenated 1’-methyl-1,2’bipyrroles, biomagnify in a northwestern Atlantic food web. Environ. Sci. Technol. 2010, 44, 5741–5747. [DOI] [PubMed] [Google Scholar]
- 54).Adams JB; Lock SJ; Toward MR; Williams BM Bromophenol formation as a potential cause of “disinfecant” taint in foods. Food Chem. 1999, 64, 377–381. [Google Scholar]
- 55).Agarwal V; El Gamal AA; Yamanaka K; Poth D; Kersten RD; Schorn M; Allen EE; Moore BS Biosynthesis of polybrominated aromatic organic compounds by marine bacteria. Nat. Chem. Biol. 2014, 10, 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Oliveira AS; Silva VM; Veloso MCC; Santos GV Andrade JB de. Bromophenol concentrations in fish from Salvador, BA, Brazil. An. Acad. Bras. Cienc. 2009, 81, 165–172. [DOI] [PubMed] [Google Scholar]
- 57).de Oliveira ALL; de Felício R; Debonsi HM Marine natural products: Chemical and biological potential of seaweeds and their endophytic fungi. Brazilian J. Pharmacogn. 2012, 22, 906–920. [Google Scholar]
- 58).Nomiyama K; Eguchi A; Mizukawa H; Ochiai M; Murata S; Someya M; Isobe T; Yamada TK; Tanabe S Anthropogenic and naturally occurring polybrominated phenolic compounds in the blood of cetaceans stranded along Japanese coastal waters. Environ. Pollut. 2011, 159, 3364–3373. [DOI] [PubMed] [Google Scholar]
- 59).Nomiyama K; Kanbara C; Ochiai M; Eguchi A; Mizukawa H; Isobe T; Matsuishi T; Yamada TK; Tanabe S Halogenated phenolic contaminants in the blood of marine mammals from Japanese coastal waters. Mar. Environ. Res. 2014, 93, 15–22. [DOI] [PubMed] [Google Scholar]
- 60).Vetter W; Hahn ME; Tomy G; Ruppe S; Vatter S; Chahbane N; Lenoir D; Schramm K-W; Scherer G Biological activity and physicochemical parameters of marine halogenated natural products 2,3,3’,4,4’,5,5’-heptachloro-1’-methyl-1,2’-bipyrrole and 2,4,6-tribromoanisole. Arch. Environ. Contam. Toxicol. 2004, 48, 1–9. [DOI] [PubMed] [Google Scholar]
- 61).Hoh E; Lehotay SJ; Mastovska K; Ngo HL; Vetter W; Pangallo KC; Reddy CM Capabilities of direct sample introduction-comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry to analyze organic chemicals of interest in fish oils. Environ. Sci. Technol. 2009, 43, 3240–3247. [DOI] [PubMed] [Google Scholar]
- 62).Melcher J; Janussen D; Garson MJ; Hiebl J; Vetter W Polybrominated hexahydroxanthene derivatives (PBHDs) and other halogenated natural products from the Mediterranean sponge Scalarispongia scalaris in marine biota. Arch. Environ. Contam. Toxicol 2007, 52, 512–518. [DOI] [PubMed] [Google Scholar]
- 63).Rosenfelder N; Van Zee NJ; Mueller JF; Gaus C; Vetter W Gas chromatography/electron ionization-mass spectrometry-selected ion monitoring screening method for a thorough investigation of polyhalogenated compounds in passive sampler extracts with quadrupole systems. Anal. Chem. 2010, 82, 9835–9842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.