Abstract
Many neurological disorders stem from defects in or the loss of specific neurons. Neuron transplantation has tremendous clinical potential for central nervous system therapy as it may allow for the targeted replacement of those cells that are lost in diseases. Normally, most neurons are added during restricted periods of embryonic and fetal development. The permissive milieu of the developing brain promotes neuronal migration, neuronal differentiation, and synaptogenesis. Once this active period of neurogenesis ends, the chemical and physical environment of the brain changes dramatically. The brain parenchyma becomes highly packed with neuronal and glial processes, extracellular matrix, myelin, and synapses. The migration of grafted cells to allow them to home into target regions and become functionally integrated is a key challenge to neuronal transplantation. Interestingly, transplanted young telencephalic inhibitory interneurons are able to migrate, differentiate, and integrate widely throughout the postnatal brain. These grafted interneurons can also functionally modify local circuit activity. These features have facilitated the use of interneuron transplantation to study fundamental neurodevelopmental processes including cell migration, cell specification, and programmed neuronal cell death. Additionally, these cells provide a unique opportunity to develop interneuron-based strategies for the treatment of diseases linked to interneuron dysfunction and neurological disorders associated to circuit hyperexcitability.
Keywords: Interneuron, Ganglionic eminence, Transplantation, Plasticity, Epilepsy
1. INTRODUCTION
Normal brain function requires balanced levels of excitation and inhibition. In the mammalian cerebral cortex, these functions are respectively attributed to excitatory glutamatergic pyramidal cells and inhibitory interneurons expressing GABA (γ-aminobutyric acid) that together represent about 20% of all cortical cells (Lodato and Arlotta, 2015). While pyramidal neurons make long-range connections within and outside the cortex, interneurons synchronize the activity of local projection neuron ensembles and gate excitatory and inhibitory inputs that they receive (Klausberger and Somogyi, 2008; Klausberger et al., 2003; Lewis et al., 2012; Vogels and Abbott, 2009). Interneurons are thus considered to be the main cellular components for the control of brain excitability. Accordingly, a wide range of neurological and psychiatric disorders stem from cortical interneuron dysfunction (Marín, 2012). Notably, these conditions include epilepsy, schizophrenia, and autism, and have been referred to as interneuropathies (Kato and Dobyns, 2005).
Neuronal transplantation has been extensively studied as a potential therapeutic strategy for the treatment of various neurological conditions. For such an approach to be successful, the candidate cell should be able to disperse following transplantation and functionally integrate within the diseased host circuitry in a manner that recapitulates the properties of the endogenous cells targeted for replacement. However, most cell types display very little dispersal upon transplantation in the postnatal central nervous system (CNS) (Dunnett and Björklund, 2012; Gage, 2012; Lindvall et al., 1990), which is a prerequisite for the functional integration of the transplants. The discovery of the origin of telencephalic interneurons in mice (Anderson et al., 1997; Tamamaki et al., 1997), as well as the capacity for their precursors to functionally integrate as inhibitory interneurons upon transplantation in the postnatal mouse brain (Alvarez-Dolado et al., 2006; Wichterle et al., 1999), provided an opportunity to test the potential of interneuron transplantation as a therapy for interneuropathies and other conditions associated to circuit hyperexcitability. Here, we summarize advances in the emerging field of interneuron biology and transplantation and also review some work on the potential clinical relevance of interneuron transplantation. First, we briefly summarize telencephalic interneuron development and discuss their behavior upon transplantation in the postnatal mouse CNS. We then touch upon how transplantation has been used for the study of CNS development and eventually examine the disease-modifying properties of interneuron transplants from studies based on mouse models of epilepsy, Parkinson’s disease (PD), Alzheimer’s disease (AD), and psychiatric disorders.
2. DEVELOPMENT OF TELENCEPHALIC GABAergic INTERNEURONS
2.1. TANGENTIAL MIGRATION
The ability of interneurons to migrate after heterochronic transplantation into the post-natal mouse brain likely stems from the extensive migration they undergo during development. Excitatory and inhibitory cortical neurons emerge from two distinct compartments in the developing brain. Excitatory neurons are produced locally in the ventricular zone of the pallium and invade the cortex radially using the radial-glial scaffold as a migratory substrate (Götz and Huttner, 2005; Molyneaux et al., 2007).
In contrast, cortical inhibitory neurons are generated outside of the cortex in the ventral telencephalon and must migrate tangentially over long distances to reach their final position in the cortex (Anderson et al., 1997; de Carlos et al., 1996; DeDiego et al., 1994; Tamamaki et al., 1997; Wichterle et al., 2001). It is precisely this capacity to migrate across the radial-glial scaffold that may allow young interneurons to disperse through the postnatal brain parenchyma, making them a strong candidate for transplantation and cell-based therapy in the CNS. While the subcortical origin of telencephalic interneurons and their migratory route to reach the cortex was originally described in mouse, nonradial migration from the ventral forebrain also applies to interneurons in the developing primate cortex (Hansen et al., 2013; Ma et al., 2013), which challenges earlier reports suggesting a cortical origin for these cells (Jones, 2009; Letinic et al., 2002; Yu and Zecevic, 2011).
2.2. ORIGINS AND DIVERSITY
In the mammalian cortex, inhibitory interneurons are less numerous than pyramidal cells by a ratio of ~1:5. However, this small population of local circuit nerve cells displays high diversity in shape and function. Deciphering the meaning and origin of this interneuron diversity is key for our understanding of how information is processed in the brain and how defects in specific interneuron circuits may give rise to diseases. Consequently, many groups have worked toward characterizing this cellular diversity as well as its functional relevance for cortical circuit physiology. Accordingly, interneurons can be classified in more than 20 subtypes based upon various criteria (partially overlapping for some of them) including morphology, physiology, patterns of local connectivity, and molecular identity (DeFelipe et al., 2013; Gonchar and Burkhalter, 1997; Markram et al., 2004; Petilla Interneuron Nomenclature Group et al., 2008). While such an approach is necessary to fully appreciate the complexity of this heterogeneous cell population (DeFelipe et al., 2013; Petilla Interneuron Nomenclature Group et al., 2008), it is noteworthy that the expression of the calcium-binding protein parvalbumin (PV), the neuropeptide somatostatin (SST), and the ionotropic serotonin receptor 5HT3a (5HT3aR) defines three nonoverlapping groups of cells that account for nearly 100% of interneurons in the mouse primary somatosensory cortex (Rudy et al., 2011).
Subtype identity is dictated by the spatiotemporal origin of cortical interneurons during development (Butt et al., 2005; Flames et al., 2007; Fogarty et al., 2007; Gelman et al., 2009; Ghanem et al., 2007; Miyoshi et al., 2010; Xu, 2004). The majority of mouse cortical interneurons are generated between E10.5 and E16.5 by progenitors located in the ventricular and subventricular zones of the subpallium, within the ganglionic eminences. This highly proliferative compartment of the embryonic brain can be anatomically and molecularly divided into three regions, namely, the lateral-, the medial-, and the caudal ganglionic eminences (LGE, MGE, and CGE, respectively). While it is commonly accepted that the LGE does not contribute to the mouse cortical interneuron population (Wichterle et al., 2001; Wonders and Anderson, 2006), the MGE and CGE are the two major sources of cortical interneurons and give rise to anatomically and functionally distinct subsets of cells (Anderson et al., 2001; Butt et al., 2005; Fogarty et al., 2007; Lavdas et al., 1999; Miyoshi et al., 2010; Nery et al., 2002, 2003; Rubin et al., 2010; Wichterle et al., 2001). MGE and CGE also produce interneurons that migrate to other brain regions, including striatum, septum, hippocampus, and amygdala, thus illustrating the heterogeneity and importance of these germinal zones.
MGE-derived neurons represent ≈60–70% of all cortical interneurons in rodents. These cells express either PV or SST and are born for the most part during early neurogenesis and preferentially locate to deep layers of the neocortex (Anderson et al., 2001; Marín, 2013; Wichterle et al., 2001; Xu et al., 2008). From a molecular standpoint, MGE-derived interneurons are specified by transcription factors including the Dlx genes, Lhx6, Sox6, and Nkx2.1 (Chédotal and Rijli, 2009; Flandin et al., 2011; Kessaris et al., 2014; McKinsey et al., 2013; Sussel et al., 1999; Vogt et al., 2014). In contrast to the early production of MGE-derived interneurons, interneuron generation in the mouse CGE has been shown to peak at around E16.5 (Miyoshi et al., 2010). Progenitors in the CGE express the orphan nuclear receptors COUP-TF I/II (Kanatani et al., 2008) and generate ≈30% of mouse cortical interneurons (Miyoshi et al., 2010; Nery et al., 2002; Rudy et al., 2011). CGE-derived neurons represent a very heterogeneous pool of cells expressing vasoactive intestinal poly-peptide (VIP) and calretinin (CR) as well as a group of cells that do not express VIP and include neurogliaform reelin (RLN)-expressing cells (Rudy et al., 2011). Virtually all CGE-derived interneurons express 5HT3aR in the neocortex (Lee et al., 2010; Vucurovic et al., 2010). CGE-derived neurons mostly target the superficial layers of the neocortex independently of their time of birth (Lee et al., 2010; Miyoshi et al., 2010). Interestingly, more than half of human cortical interneurons are thought to originate from CGE progenitors (Hansen et al., 2013), which could reflect the evolutionary expansion of the upper layers of the cortex that are highly enriched in late-born CGE-derived neurons (Hansen et al., 2013; Miyoshi et al., 2010). In addition to the major contributions from both MGE and CGE, the preoptic area (POA) accounts for ≈10% of all cortical interneurons (Gelman et al., 2009). This group includes some neuropeptide Y (NPY)-expressing multipolar cells, as well as some PV- and SST-positive cells. Two distinct progenitor domains have been identified so far in the POA, one expressing Nkx5.1 and another Dbx1 (Gelman et al., 2009, 2011).
3. TRANSPLANTATION AND THE STUDY OF BRAIN DEVELOPMENT
The initial studies that unraveled the subpallial origin of cortical interneurons were mostly based on dye labeling of discrete groups of cells in cultured mouse brain slices (Anderson et al., 1997; Tamamaki et al., 1997). Before the advent of genetic fate mapping techniques, transplantation allowed for the in vivo confirmation of migratory routes and also provided valuable information on the fate and functions of cortical interneurons. Additionally, transplantation studies demonstrated the remarkable ability for embryonic MGE and CGE cells to functionally integrate into both neonatal and adult host circuits (Fig. 1), and also provided key information on many aspects of interneuron development.
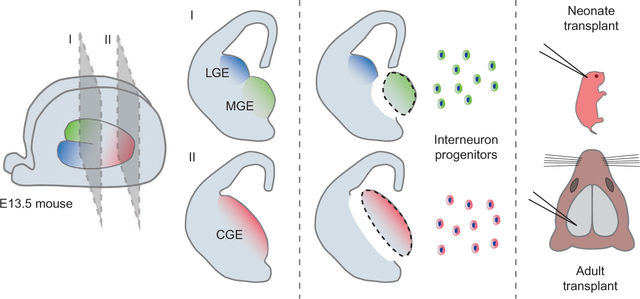
FIG. 1:
Heterochronic transplantation of interneuron progenitors. The MGE or CGE is dissected from the embryonic mouse brain. The MGE is anatomically separated from the LGE by a large sulcus; the CGE is a caudal extension of both LGE and MGE. Dissociated cells from these ganglionic eminences can be transplanted using beveled glass needles into both neonatal and adult nervous system (see text). MGE and CGE interneuron progenitors have the ability to migrate and differentiate into multiple interneuron subtypes that become integrated into functional circuits; dispersal is more robust in the permissive neonatal brain.
3.1. INTERNEURON INTRINSIC DEVELOPMENTAL PROGRAM
The extraordinary migratory potential of MGE cells was first demonstrated in vitro (Wichterle et al., 1999). Using embryonic mouse brain explants grown in matrigel, MGE-derived neuroblasts were found to migrate extensively, as opposed to cells derived from neocortical explants. Upon homotopic and isochronic transplantation in utero using ultrasound guided injection, MGE cells were shown to migrate dorsally perpendicular to the radial-glial scaffold via both the neocortical subventricular and marginal zones. These homotopic and isochronic MGE transplant-derived cells primarily populated the neocortex but also contributed significantly to the globus pallidus, the striatum, the amygdala, and the CA1 region of the hippocampus (Wichterle et al., 2001). Transplanted MGE cells persisted into adulthood and mostly differentiated into aspiny local interneurons immunoreactive for GABA, PV, and SST, illustrating that the fate of interneurons was determined prior to their exit of the ganglionic eminence (Flames et al., 2007; Fogarty et al., 2007; Wonders et al., 2008). In contrast, LGE transplant-derived cells were found to migrate ventrally and anteriorly to give rise to medium spiny neurons in the striatum, nucleus accumbens, and olfactory tubercle, as well as granule and periglomerular cells in the olfactory bulb (Wichterle et al., 2001). Interestingly, upon isochronic transplantation in the MGE, LGE cells did not modify their migratory behavior and remained in the ventral forebrain, with very few cells populating the neocortex, thus suggesting that at least some aspects of the development of ventral forebrain neuronal progenitors are intrinsically determined.
Heterochronic transplantation studies further confirmed that transplanted immature interneurons from the ganglionic eminences preserve their internal developmental program (Fig. 1). First, when injected in the postnatal brain, MGE cells display an initial highly migratory phase reminiscent of their distant origins (Fig. 2). Accordingly, transplant-derived cells have been shown to migrate distances up to 2.5 mm in the adult rodent brain (Davis et al., 2015; De la Cruz et al., 2011; Hunt et al., 2013; Martínez-Cerdeño et al., 2010) and 5 mm in the neonate (Alvarez-Dolado et al., 2006; Southwell et al., 2010). Second, in line with the postnatal maturation of inter-neurons in vivo (Okaty et al., 2009), following their migratory phase, transplanted MGE interneurons develop over the course of several weeks in the heterochronic environment before acquiring mature morphology, marker expression, and electro-physiological properties (Alvarez-Dolado et al., 2006; Howard and Baraban, 2016; Southwell et al., 2010) (Fig. 2). Finally, while transplanted MGE cells can develop in regions they normally migrate to, they similarly differentiate into mature interneurons in regions of the CNS they are not fated to populate. For instance, upon hetero-chronic transplantation in the spinal cord, MGE precursor cells migrate away from the injection site, survive, and eventually display molecular marker expression, morphology, and electrophysiological properties similar to those of cortical interneurons (Bráz et al., 2012, 2014, 2015).
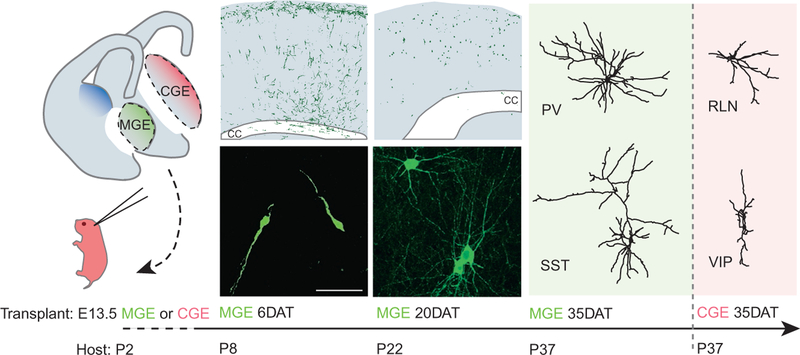
FIG. 2:
Transplant-derived interneuron development in the heterochronic environment. MGE and CGE progenitors were transplanted into the cortex of a P2 host. At 6 days posttransplantation (6DAT), many MGE and CGE transplant-derived cells are found within the superficial layers of the cortex and are tangentially oriented, a behavior reminiscent of endogenous interneuron migration within the marginal zone of the developing neocortex (top). A large number of cells have also started to invade the cortex at 6DAT and display a radial orientation (top). At this stage, virtually all transplant-derived cells display a typical migratory morphology, with a long leading process and a short trailing process (bottom). At 20DAT, many transplanted interneurons have undergone programmed cell death. The transplanted MGE cells that survive usually stay clear of cortical layer I (as opposed to transplanted CGE cells), distribute across all cortical layers (top), and display a more mature morphology (bottom). At 35DAT, the vast majority of MGE transplant-derived cells differentiate into GABAergic interneurons expressing either PV or SST. CGE transplants give rise to many neurogliaform neurons that express RLN and to VIP-expressing interneurons. Neurogliaform interneurons mostly localize to layer I. CC, corpus callosum. Scale bar: 50 μm.
Recent results have shown that the development of CGE cells is also intrinsically determined. Indeed, despite the known ontogeny of cortical interneurons since the late 1990s, it is only recently that studies addressed whether CGE cells, like their MGE counterparts, might be amenable to transplantation in the postnatal brain (Fig. 1). Not surprisingly, CGE transplant-derived cells were found to migrate extensively following heterochronic transplantation in the neonatal brain, with a dispersal similar to that of MGE cells (Hunt and Baraban, 2015; Larimer et al., 2016), thus suggesting that tangential migration of ventral forebrain inter-neuron precursors is a determinant factor for their dispersal upon transplantation. However, conflicting results were found following transplantation in the adult brain, with one report describing the failure of CGE cells to disperse in the mature cortex (Davis et al., 2015) and another study showing accentuated dispersal of CGE cells compared to that of MGE cells (Isstas et al., 2016). Such inconsistencies may be due to the lack of a clear anatomical distinction between MGE/LGE and CGE (Fig. 1). As the CGE is a caudal extension of both LGE and MGE, using the most rostral aspect of the CGE for transplantation may give rise to grafts enriched in LGE-derived cells that exhibit poor dispersal (Wichterle et al., 1999). However, in line with genetic fate mapping studies (Miyoshi et al., 2010; Rudy et al., 2011), CGE transplant-derived interneurons were more likely to localize to cortical layer I (Larimer et al., 2016) and express VIP, CR, and RLN (Hunt and Baraban, 2015; Isstas et al., 2016; Larimer et al., 2016) (Fig. 2). Altogether, this work shows that the fates of both MGE and CGE cells are instructed by developmental programs established in the embryo and that these grafts can be used to complement existing circuits with specific subsets of interneurons.
Starting at 35 days after transplantation, transplanted MGE progenitors display electrophysiological properties and intrinsic firing patterns similar to those of endogenous MGE-derived interneurons (Howard and Baraban, 2016; Larimer et al., 2016). Their synaptic integration was first illustrated using electron microscopy (Baraban et al., 2009; Southwell et al., 2010; Wichterle et al., 1999) and was confirmed by intracellular recordings on acute brain slices showing spontaneous and evoked post-synaptic currents (Alvarez-Dolado et al., 2006; Howard and Baraban, 2016; Martínez-Cerdeño et al., 2010; Southwell et al., 2010), thus demonstrating that grafted cells receive functional inputs from host neurons. Paired recordings further showed inhibitory synapses made by transplanted MGE or CGE cells onto host pyramidal neurons (Howard and Baraban, 2016; Larimer et al., 2016; Southwell et al., 2012), as well as reciprocal inhibitory connections between CGE transplant-derived cells and host interneurons (Larimer et al., 2016). In agreement with their endogenous inhibitory function in the CNS and their functional integration upon transplantation, grafted MGE-derived interneurons can modify synaptic inhibition in the host brain (Alvarez-Dolado et al., 2006; Baraban et al., 2009; Bráz et al., 2012; Howard et al., 2014; Southwell et al., 2010) and are thus of a great clinical interest for the manipulation of inhibition in disorders that display circuit hyperexcitability (Chohan and Moore, 2016; Southwell et al., 2014; Tyson and Anderson, 2014). The therapeutic potential of CGE transplants has not been as extensively evaluated as that of MGE transplants so far. However, the expanded repertoire of transplantable interneurons allowed by these grafts will undoubtedly offer further insight into interneuron development.
3.2. INTERNEURON FATE AND SURVIVAL
Cortical interneuron precursor transplantation has been used to study the origin of interneuron subtypes. While MGE and CGE transplantation showed the variety of cortical interneurons generated by ventral forebrain progenitors, grafts of microdomains of the MGE provided insight into precursor cell heterogeneity. First, marker expression analysis revealed that each subregion of the subpallium can be further divided into distinct progenitor domains (Flames et al., 2007). In order to address whether these domains indeed corresponded to functionally distinct progenitor pools, homotopic and isochronic in utero transplants of the most dorsal and the most ventral domains of the MGE were generated. The neurochemical composition of the transplants was analyzed at postnatal day 14 (P14) and showed a strong bias for the generation of SST interneurons from the most dorsal region, as opposed to a bias for the production of PV interneurons by the ventral region (Flames et al., 2007). These results were corroborated using neonatal transplants (Inan et al., 2012; Wonders et al., 2008). Neonatal transplantation of microdomains has also been very useful to study the ontogeny of specific interneuron subtypes. Chandelier cells have attracted much attention due to their ability to both depolarize and hyperpolarize pyramidal neurons (Glickfeld et al., 2009; Szabadics et al., 2006; Woodruff et al., 2009, 2011) and their potential implication in schizophrenia (Howard et al., 2005). While genetic fate mapping techniques show that virtually all chandelier cells are generated in the MGE (Xu et al., 2008), transplantation experiments demonstrate that there is a strong bias for the production of these cells by ventral MGE progenitors at late stages of neurogenesis (Inan et al., 2012). These findings were further confirmed using a tamoxifen-dependent lineage tracing strategy showing that chandelier cell production by Nkx2.1-positive progenitors peaks at E16.5 (Taniguchi et al., 2013). Taken together, these findings suggest that cortical interneuron diversity may stem from the heterogeneity in progenitor populations found in the embryo.
The mechanisms of interneuron lamination in the neocortex have also been studied using transplantation techniques (Pla et al., 2006). While the secretion of RLN by Cajal Retzius cells (D’Arcangelo et al., 1995; Ogawa et al., 1995; Soriano et al., 2005) is required for the proper lamination of the neocortex (Caviness, 1982), homo-topic and isochronic transplants of MGE cells lacking the intracellular adaptor Dab1 required for RLN signaling revealed normal lamination of the mutant MGE cells, suggesting that cortical interneuron lamination does not depend on cell-autonomous RLN signaling. By contrast, WT MGE cells that are transplanted in the MGE of Dab1-deficient embryos fail to adopt a normal lamination and display a distribution that highly correlates that of misplaced pyramidal cells. Considering that interneurons invade the cortical plate after pyramidal cells have reached their final position and that synchronically born interneurons and pyramidal cells tend to locate in the same cortical layers, this work provides evidence that interneurons laminate in the cortex using cues presented by synchronically born pyramidal cells (Pla et al., 2006). This notion has been reinforced by (i) the aberrant lamination of both PV and SST interneurons in Fezf2-null mice that are lacking layer V corticofugal projection neurons (Lodato et al., 2011) and (ii) the recruitment of additional inhibitory synapses from PV interneurons by layer II–III callosal neurons converted into corticofugal projection neurons following Fezf2 overexpression (Ye et al., 2015). Nevertheless, the molecular nature of factors that govern interneuron positioning remains unknown.
Finally, interneuron transplantation has been employed to address the rules governing waves of programmed cell death that occur in the developing CNS and help sculpt neural circuits (Southwell et al., 2012). Throughout the development of the nervous system, great numbers of neurons are eliminated at a time that coincides with synaptogenesis (Dekkers et al., 2013). In the case of mouse cortical interneurons, this occurs at around P7. While the role played by supernumerary cells during CNS development is still unknown, it was widely accepted that such a selection is driven by limited trophic support for which developing neurons would have to compete: the so-called neurotrophin hypothesis (Hamburger and Levi-Montalcini, 1949; Levi-Montalcini, 1949). If we were to extend this notion to MGE transplants, the host brain should thus be able to only accommodate a finite number of transplanted interneurons. In contrast, transplantation studies strongly suggest that interneuron survival is independent from signals arising from the host (Southwell et al., 2012). Upon transplantation in the neonatal brain, MGE cells display developmental apoptosis coinciding with their own age, and not that of the host, and therefore asynchronously from endogenous interneurons (Fig. 2). Interestingly, the proportion of interneurons undergoing cell death remained constant across grafts of various sizes and was similar to the extent of cell death found among endogenous interneurons during normal development. Additionally, transplanted interneuron cell death was found to be independent of the neurotrophin receptor TrkB and was temporally recapitulated by cultured MGE cells in vitro. Altogether this work indicates that interneuron developmental programmed cell death is intrinsically determined and that interneuron survival does not depend on extrinsic cues from the host cells. However, it is possible that transplanted interneuron survival could depend on competition for survival signals emanating from interneurons themselves. Self-regulated cell death, at the individual cell level or at the population level, offers unique advantages for interneuron transplantation as the number of surviving transplanted cells is not adjusted with respect to the population of host interneurons, but mostly by the number of transplanted cells themselves. This work suggests that grafted cells execute their own endogenous program to determine the timing of cell death and the final number of surviving interneurons.
4. TRANSPLANTATION AND CORTICAL PLASTICITY
In recent years, interneuron transplantation has been used to induce cortical plasticity in mature animals that normally exhibit minimal plasticity (Fig. 3) (Davis et al., 2015; Isstas et al., 2016; Larimer et al., 2016; Southwell et al., 2010; Tang et al., 2014). This line of work has reinforced the importance of the intrinsic programs that govern interneuron development and their integration upon transplantation. The model chosen for this work is the mouse visual system that displays a developmental critical period of plasticity for ocular dominance during which thalamic afferents compete for space and synaptic strength in the binocular zone of the primary visual cortex (Espinosa and Stryker, 2012; Wiesel and Hubel, 1963). During the critical period, but neither before nor after, imbalancing visual inputs by suturing one eye induces a shift in cortical responsiveness in favor of the open eye (Fagiolini et al., 1994; Prusky and Douglas, 2003). The induced rewiring of intracortical connectivity eventually leads to a loss of visual acuity for the closed eye, which mimics amblyopia, a condition found in humans and that affects ~4% of the population (Hensch, 2005). The opening of the critical period is governed by the maturation of cortical GABAergic circuits (Di Cristo et al., 2007; Fagiolini and Hensch, 2000; Hanover et al., 1999; Hensch et al., 1998; Iwai et al., 2003; Kanold et al., 2009; Katagiri et al., 2007; Sugiyama et al., 2008). Accordingly, plasticity can be triggered ahead of time by promoting interneuron development (Di Cristo et al., 2007; Hanover et al., 1999; Sugiyama et al., 2008) or by pharmacologically enhancing inhibitory transmission in the visual cortex (Fagiolini and Hensch, 2000; Fagiolini et al., 2004). Interestingly, heterochronic transplantation of immature interneurons in the neonatal (Southwell et al., 2010) or adult (Davis et al., 2015) brain induces a second period of plasticity in the recipient visual cortex (Fig. 3). In both studies monocular deprivation was found to induce ocular dominance plasticity in MGE transplant recipients only if performed ~5 weeks after the transplantation of E13.5 MGE cells, well after the endogenous critical period has ended (Fig. 3). Interestingly, the age of the transplanted cells at the time of transplant-induced plasticity corresponds to that of host interneurons when endogenous ocular dominance plasticity reaches its maximum, suggesting that interneuron intrinsic developmental programs regulate critical period timing.
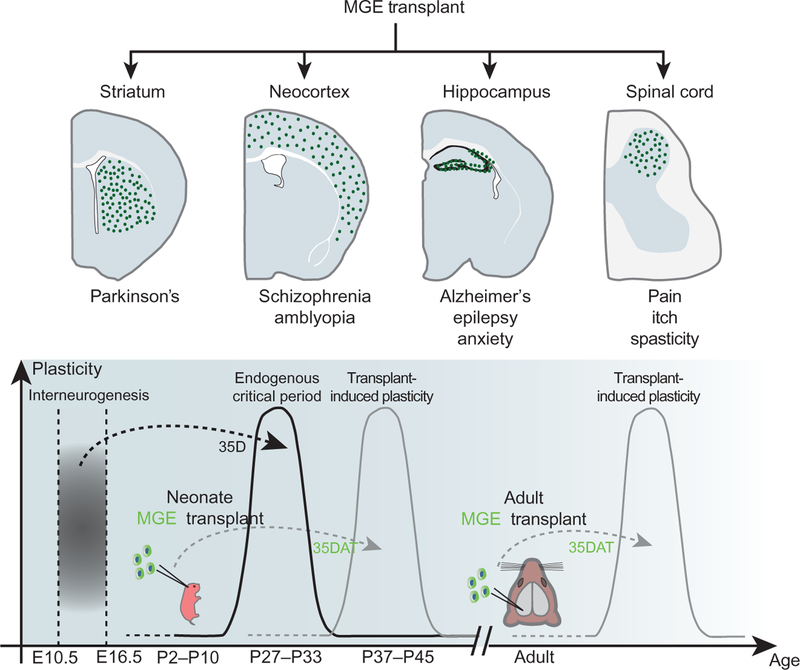
FIG. 3:
Immature interneuron transplantation and therapeutic applications. (Top) Immature interneurons can be obtained directly from the embryonic MGE or in vitro from embryonic stem (ES) or induced pluripotent stem (IPS) cells directed to differentiate into MGE-like progenitors. Interneurons have been transplanted into multiple regions of the CNS, including the striatum, neocortex, hippocampus, and spinal cord. Transplanted interneurons display disease-modifying activity in animal models of Parkinson’s disease, Alzheimer’s disease, epilepsy, schizophrenia, anxiety, spasticity, chronic pain, and neuropathic itch. (Bottom) Interneuron transplantation has also been used to study and manipulate cortical plasticity. The timing of native critical period of plasticity in the mouse visual cortex is dictated by the maturation of endogenous interneurons. Ocular dominance plasticity peaks at around P30 when inhibitory neurons are approximately 35 days of age (35D). Upon transplantation into both neonatal and adult visual cortex, interneurons induce ocular dominance plasticity when they reach a similar cellular age at approximately 35 days after transplantation (35DAT). Transplant-induced plasticity allows functional recovery of visual acuity in mouse models of developmentally acquired amblyopia. These findings suggest that interneuron development is governed by molecular programs established in the embryo and that these programs are retained and executed by embryonic interneurons upon heterochronic transplantation.
As MGE grafts primarily generate PV and SST interneurons, Tang et al. (2014) sought to determine the respective contribution of these two interneuron populations in MGE transplant-induced plasticity. Using the R26-GDTA allele that allows ablation of specific cell types through Cre-dependent diphtheria toxin alpha subunit expression, PV and/or SST cells were specifically eliminated from MGE transplants. Surprisingly, PV- and SST-depleted transplants each induced robust plasticity, thus indicating that both PV and SST interneurons can induce ODP. However, elimination of both PV and SST cells prevented transplant-induced plasticity, suggesting that a sufficient number of either cell type has to be present for MGE transplants to induce plasticity. It has been suggested that PV interneurons are a central hub for cortical plasticity gating (Bernard et al., 2016; Beurdeley et al., 2012; Chattopadhyaya et al., 2004; Fagiolini et al., 2004; Huang et al., 1999; Kuhlman et al., 2013; Maffei et al., 2006; Miyata et al., 2012; Pizzorusso et al., 2002; Spatazza et al., 2013; Sugiyama et al., 2008; Takesian and Hensch, 2013; Yazaki-Sugiyama et al., 2009). However, the role of SST interneurons in this system had been overlooked so far. The work by Tang et al. (2014) does not preclude the possibility that the induction of plasticity by transplanted SST interneurons may be mediated in part by their action on host PV cells. The demonstration that SST interneurons can also induce plasticity highlights the power of transplantation experiments to decipher the cellular mechanisms of plasticity.
The ability of SST neurons to induce cortical plasticity raised the question as to whether interneurons are in general capable of reopening sensory critical periods. To test this hypothesis, recent studies tested whether CGE-derived interneurons might be competent (Davis et al., 2015; Isstas et al., 2016; Larimer et al., 2016). The CGE gives rise to a pool of interneurons different from those originating in the MGE (Rudy et al., 2011). Just like MGE transplants, grafted CGE cells disperse and differentiate in the heterochronic environment. However, in contrast to the MGE, CGE-derived interneurons do not reactivate plasticity (Davis et al., 2015; Isstas et al., 2016; Larimer et al., 2016) despite being functionally integrated in the host brain (Larimer et al., 2016). One study found that PV and SST interneurons account for ~20% of CGE transplant-derived cells (Larimer et al., 2016), suggesting that some young interneurons generated in the MGE migrate through the CGE (Butt et al., 2005). Such mixed transplants were found to reopen plasticity, although plasticity induction is solely attributable to PV and SST interneurons as it is fully abolished following genetic ablation of these cells from CGE transplants (Larimer et al., 2016).
Taken together, these results suggest that transplant-induced plasticity is restricted to MGE-derived PV and SST interneurons. It is unlikely that transplant-induced plasticity results from increased inhibition as pharmacological enhancement of inhibition does not trigger plasticity after the critical period (Fagiolini et al., 2004). The inability for CGE-derived neurons to induce plasticity also suggests that the mechanisms required to home the transplanted cells into the host cortical network are not enough to elicit functional reorganization. Endogenous critical period closure has been associated with the expression of molecular brakes that stabilize mature cortical networks (Bavelier et al., 2010; Morishita et al., 2010; Sajo et al., 2016). Transplanted PV and SST cells may alter the expression of such brakes and thus allow host cells to rewire upon sensory deprivation. Undoubtedly, the study of both MGE and CGE transplants provides a powerful new tool to investigate the molecular and synaptic mechanisms enabling transplant-induced plasticity. The induction of plasticity by heterochronically transplanted interneurons also offers an opportunity to modify neural circuits for clinical gains.
5. DISEASE-MODIFYING PROPERTIES OF MGE TRANSPLANTS
Removing plasticity brakes in adulthood holds great promises for lifelong learning and the treatment of neurodevelopmental disorders (Bavelier et al., 2010; Takesian and Hensch, 2013). Studies of transplant-induced cortical plasticity suggest that young interneuron transplantation could be employed to promote the functional reorganization of cortical networks following brain injury or trauma. While this hypothesis remains to be tested, it is supported by recent results showing that MGE transplantation in the adult visual cortex rescues amblyopia acquired upon visual deprivation in juvenile mice (Davis et al., 2015). Whether the plasticity-inducing effect of MGE transplants can be similarly applied to brain regions other than the visual cortex remains unknown. For example, the basolateral amygdala displays a critical period of plasticity during which fear memories can be erased by extinction training (Gogolla et al., 2009). Importantly, plasticity in the amygdala shares many features with that in the visual cortex, suggesting that common molecular and cellular determinants may be gating plasticity in both systems. Most notably, amygdala plasticity is constrained by the expression of perineuronal nets (Gogolla et al., 2009) and can be reactivated by antidepressant drugs (Karpova et al., 2011). It will be interesting to investigate whether interneuron transplantation can modify fear memory resiliency, which bears strong therapeutic implications for patients suffering from posttraumatic stress disorders. Interestingly, MGE cell transplants have been shown to reduce anxiety levels in WT animals (Valente et al., 2013).
Interneuropathies constitute a wide range of neurological disorders that directly result from interneuron dysfunctions (Kato and Dobyns, 2005). Whether they are caused by a reduction in interneuron number or more specific deficits in the firing properties of individual neurons, these syndromes share impaired GABAergic transmission (Kato and Dobyns, 2005; Marín, 2012). Other conditions such as PD, Huntington’s disease, or neuropathic pain originate from imbalance between excitation and inhibition levels secondary to defects in other neuronal populations (Kato and Dobyns, 2005; Marín, 2012; Southwell et al., 2014). These diseases are all associated with network hyperexcitability, which led to the proposal that interneuron transplantation could be used to restore inhibition and thus alleviate the symptoms observed in animal models of these conditions (Fig. 3).
5.1. SCHIZOPHRENIA
Schizophrenia has been associated with impaired GABA signaling (Inan et al., 2013; Marín, 2012). Injection of the NMDA receptor antagonist phencyclidine (PCP) triggers schizophreniform cognitive deficits in both healthy humans (Javitt and Zukin, 1991) and rodents (Mouri et al., 2007). PCP is thought to primarily act on NMDA receptors localized to cortical interneurons (Korotkova et al., 2010), which would result in altered activity of projection neurons in the prefrontal cortex (PFC). In order to address whether cortical interneurons could potentially help in the treatment of schizophrenia-related symptoms, MGE transplants were performed in the neonatal mouse brain 6 weeks before PCP acute administration (Tanaka et al., 2011). Interestingly, PFC transplants can prevent PCP-induced cognitive deficits, as opposed to visual cortex transplants that are ineffective in this system. Immediate early gene expression in PFC projection neurons was increased in MGE recipients, suggesting that MGE transplant benefits on PCP-induced deficits are not simply linked to modulation of PFC neuron activity.
Disinhibition in the hippocampus is also thought to be determining schizophrenia-related positive symptoms (e.g., delusions and hallucinations) and psychosis (Heckers and Konradi, 2010; Schobel et al., 2013). To study this aspect of the disease, Gilani et al. (2014) used the cyclin D2 (CCND2) genetic mouse model (Glickstein et al., 2007) that displays a reduction in hippocampal interneurons and increased hippocampal output in vivo. Interestingly, MGE transplants can restore normal hippocampal activity and rescue the hippocampus-related cognitive deficits exhibited by this model. These findings point to the importance of interneuron development and survival in the pathogenesis of psychotic disorders and demonstrate the procognitive effects of interneuron-based strategies, which could benefit treatment-resistant patients that demonstrate hippocampal hyperactivation at rest.
5.2. EPILEPSY
Epilepsy is a heterogeneous neurological disorder affecting more than 50 million people and characterized by repeated episodes of seizure activity (de Boer et al., 2008). While known genetic mutations are responsible for a small proportion of cases (Pandolfo, 2013), epilepsy can also develop as a result of traumatic brain injury, stroke, tumor, or surgery (Chang and Lowenstein, 2003). Hyperexcitability is a key feature of epilepsies and often reflects impaired inhibition, in both animal models (Cossart et al., 2001; Sloviter, 1987) and human patients (de Lanerolle et al., 1989; Mathern et al., 1995). Seizure reduction following interneuron transplantation was first demonstrated in a genetic mouse model of epilepsy displaying severe spontaneous seizures by the second to third postnatal week of age (Baraban et al., 2009). MGE progenitors were transplanted in the neonatal cortex and seizure events were recorded by video EEG starting 30 days after transplantation. A 90% reduction in seizure events was observed in grafted mutant animals over the course of a month-long monitoring period. In another model of acquired epilepsy (Hammad et al., 2015), neonatal transplantation of MGE cells also yielded a significant decrease in the frequency and duration of epilepsy episodes, as early as 3 weeks posttransplantation. Concomitantly, transplantation seemed to promote survival of the mutant animals, as 80% of the MGE graft recipients survived up to 4 months compared to an average survival of 29 days for control animals. Taken together, these findings demonstrate that neonatal MGE grafts can have a prophylactic effect in two distinct congenital seizure disorders.
MGE precursor cells also demonstrated efficacy in mouse models of induced epilepsy. Calcagnotto et al. (2010) injected MGE cells in the mouse neonatal cortex and employed maximum electroconvulsive shock (MES) in adult mice to address whether MGE grafts could protect against the induction of tonic seizures. MES acutely induces a single seizure and is often used as a platform to screen antiepileptic drugs. Upon MES induction at 2 months posttransplant, the incidence of tonic seizure was significantly lower in the MGE grafts group compared to controls. Accordingly, animal survival rate was also increased among transplant recipients compared to controls. Interneuron transplantation in the adult cortex also reduces seizure propagation as indicated by local field potential measurement upon focal administration of 4-aminopyridine (4-AP), a potent convulsant and potassium channel blocker (De la Cruz et al., 2011). Here, the beneficial impact of MGE grafts on epileptiform activity was detected as early as 2.5 weeks posttransplant and was also found to be independent of the extent of transplanted cell survival, which suggests that a critical amount of grafted cells is required for optimal tuning of neuronal inhibition (Southwell et al., 2010; Tang et al., 2014).
Upon saporin-induced elimination of hippocampal interneurons, MGE transplantation restored inhibitory postsynaptic currents onto CA1 pyramidal cells and reduced pharmacologically induced seizure susceptibility of the grafted animals (Zipancic et al., 2010). The impact of interneuron transplantation on spontaneous recurrent seizures was also tested in the pilocarpine mouse model of temporal lobe epilepsy (Henderson et al., 2014; Hunt et al., 2013). In both studies, hippocampal MGE transplants were performed approximately 2 weeks after the animals reached status epilepticus. In the hippocampus of epileptic mice, grafted MGE cells differentiate into GABAergic interneurons, acquire mature electrophysiological properties, and form functional synapses onto endogenous granule cells. MGE transplant-induced seizure suppression was found at ~60 days after transplantation. Interestingly, prolonged video EEG monitoring of grafted animals showed that this effect did not persist in time despite the presence of functional transplanted cells (Henderson et al., 2014), which raises the question of the long-lasting effect of MGE grafts on the control of seizure phenotype.
Taken together, these findings indicate that local interneuron precursor transplants have a potent impact on seizures. However, further work is required to determine the molecular and cellular mechanisms of transplant-induced seizure suppression in order to guide the development of transplant-based strategies for the treatment of refractory seizures. Inconsistencies in the timing of MGE graft efficacy suggest that those mechanisms may not be conserved across seizure models. For instance, while the kinetics of seizure suppression observed in the pilocarpine model strongly suggest a role for synaptic mechanisms (Henderson et al., 2014; Hunt et al., 2013), the early effects observed in the 4-AP model (De la Cruz et al., 2011) would indicate a possible role for nonsynaptic mechanisms. MGE grafts enhance both synaptic and extrasynaptic inhibition (Baraban et al., 2009). Interestingly, the requirement of extrasynaptic GABA-A receptors for the transplant-mediated dampening of seizure propagation in the 4-AP model was recently shown (Jaiswal et al., 2015). Given the heterogeneity of MGE transplant-derived interneurons, it will be important to identify whether specific subtypes may prove therapeutic for specific forms of seizures.
5.3. PARKINSON’S DISEASE
Interneuron transplantation as a cell-based therapeutic strategy has also been tested in a mouse model of PD (Martínez-Cerdeño et al., 2010). PD affects a large population worldwide and is characterized by motor impairments as well as cognitive and autonomic dysfunctions. The motor symptoms result from the degeneration of substantia nigra pars compacta dopaminergic neurons that normally extend axonal projections to the striatum. Reduced dopamine release in the striatum induces a cascade of neurotransmitter release imbalance that inhibits the output of the basal ganglia and leads to motor dysfunctions (DeLong and Wichmann, 2007). Striatal GABAergic interneurons have been shown to gate basal ganglia output (Tepper and Bolam, 2004) and, consequently, worsen striatal imbalance in the dopamine-depleted striatum (Mallet et al., 2006), thus contributing to the pathophysiology of PD. These findings indicate that striatal inhibition can be used as a nondopamine-based lever to alleviate some of the symptoms characteristic of PD. The 6-hydroxydopamine (6-OHDA) rat model recapitulates the deterioration of the nigrostriatal pathway observed in PD patients. MGE progenitors transplanted into the striatum of 6-OHDA-treated adult rats are able to disperse, differentiate into GABAergic interneurons, synaptically integrate locally, and survive for up to a year (Martínez-Cerdeño et al., 2010). Importantly, MGE grafts were able to dampen the motor deficits displayed in this animal model of PD. Of note, overall locomotor activity of naïve control animals was increased following striatal MGE transplantation, thus suggesting that added interneurons can also exert a strong influence on striatal-dependent behaviors in an intact environment. The mechanisms of MGE cell effects in this system remain unknown and further work will be required for their identification. Previous studies in rodents have reported amelioration of PD-associated symptoms upon modulation of basal ganglia activity by enhancement of GABA stimulation (Lee et al., 2005; Luo, 2002; Winkler et al., 1999). Accordingly, motor deficits were likely improved as a consequence of transplant-induced increase in striatal inhibitory transmission. Alternatively, it is plausible that interneuron transplantation induces secondary changes mediating behavioral improvements or provides exogenous trophic support to remaining dopaminergic processes in the striatum. Finally, 25% of MGE transplant-derived cells differentiate into oligodendrocytes in the striatum, which raises the possibility of additional nonneuronal trophic support.
5.4. ALZHEIMER’S DISEASE
Approximately 40 million people are affected by AD, a number that is predicted to triple by 2050 (Wimo et al., 2013). Memory deficits in patients suffering from AD are associated to an excitation–inhibition imbalance in the dentate gyrus that leads to hippocampal hyperactivity (Huang and Mucke, 2012). While amyloid-β (Aβ) overproduction or accumulation leads to interneuron dysfunction (Palop et al., 2007; Verret et al., 2012), the expression of apolipoprotein (apo) E4, a strong genetic risk factor for AD, causes hippocampal hyperactivity in humans (Filippini et al., 2009) and a progressive decrease in hilar interneuron number in mice (Andrews-Zwilling et al., 2010; Leung et al., 2012; Li et al., 2009). As a result of aberrant neural network activity, AD patients have also been found to display increased incidence of epileptic events (Amatniek et al., 2006). Given the GABAergic dysfunctions observed both in patients and in mouse models of AD, it was tested whether interneuron replacement therapy leads to improvement of both cognitive and behavioral deficits in two widely used AD mouse models (Tong et al., 2014). Bilateral MGE transplantation was performed in the hilus of aged apoE4 knock-in mice. Transplanted cells were found to disperse throughout the hilus, extend dendrites into the molecular layer of the dentate gyrus, predominantly differentiate in GABAergic interneurons expressing SST, and survive for at least 90 days. Grafted cells functionally integrated and increased inhibitory transmission onto excitatory granule cells, thus compensating for the reduction of hilar interneurons in these mice. MGE cell transplantation rescued learning and memory deficits in apoE4 knock-in mice, both with and without Aβ plaques, to levels similar to those of wild-type mice. These findings suggest that interneuron replacement could ameliorate AD-related symptoms. The lack of significant modification of Aβ levels and plaques suggests that cognitive improvements are mediated by restored synaptic inhibition, although trophic support emanating from the transplanted cells onto remaining hilar interneurons cannot be ruled out. In this context it would be interesting to test whether early wild-type interneuron transplantation could have a prophylactic effect on interneuron decrease in apoE4 knock-in mice. Cell-based strategies for the treatment of AD appear as a viable approach given that wild-type MGE transplant-derived cells can survive in the apoE4-Aβ toxic environment.
5.5. NEUROPATHIC PAIN
Neuropathic pain is provoked by nerve injury and is characterized by both allodynia (where nonnoxious stimuli are painful) and hyperalgesia (where pain behaviors caused by normally painful stimuli are increased). While the complex molecular, biochemical, and cellular changes that occur upon nerve injury are central for both mechanical and thermal hypersensitivity, how they contribute to the pain described by patients is not fully resolved. It is accepted that peripheral nerve injury results in decreased GABAergic neurotransmission within the spinal cord dorsal horn. A loss of inhibitory interneurons has been reported in the lesioned spinal cord (Moore et al., 2002; Scholz et al., 2005), as well as the reduced expression of postsynaptic GABAA receptors (Fukuoka et al., 1998), decreased GABA release (Lever et al., 2003), and diminished glutamic acid decarboxylase (GAD) expression (Eaton et al., 1998; Lever et al., 2003). In agreement with the long-standing idea that disinhibition in the spinal dorsal horn could underlie neuropathic pain (Loeser and Ward, 1967), GABA agonists have been found to improve allodynia and hyperalgesia (Munro et al., 2009).
A recent set of studies explored whether transplantation of MGE cells in the spinal cord could mitigate the behavioral features of two mouse models of neuropathic pain (see also chapter “Interneuron transplantation in spinal cord for treatment of pain” by Basbaum). Transplanted MGE cells can survive in the adult spinal cord for at least 6 months, differentiate into GABAergic interneurons that display a “cortical” signature, and functionally integrate within the host spinal circuitry. Importantly, mechanical responsiveness is returned to baseline levels following MGE transplantation in mouse models of both sciatic nerve lesion where animals develop a severe hypersensitivity (Bráz et al., 2012) and chemotherapy-induced neuropathic pain (Bráz et al., 2015). In the latter, MGE cells deficient for the vesicular GABA transporter (VGAT) failed to rescue the pain behavior, which suggests that synaptic GABA release is important for MGE transplant-induced behavioral improvements in the paclitaxel model. As observed in other studies (De la Cruz et al., 2011; Southwell et al., 2010; Tang et al., 2014), there was no correlation between transplanted interneuron number and functional effect (Bráz et al., 2012). Taken together, this work highlights the efficacy of MGE cell transplants for the management of neuropathic pain. Considering the known side effects of the traditional pharmacological approaches used in patients, interneuron-based therapy may thus represent a promising avenue for future therapeutic strategies.
6. CONCLUSION
The ontogeny of ventral telencephalon interneurons undoubtedly endows this cell population with the remarkable ability to disperse upon transplantation in the neonatal, juvenile, or even adult brain. Following migration, grafted interneurons have the ability to differentiate and synaptically integrate into functional circuits. Grafted interneurons can modify the activity of host target cells, which has strengthened the potential of interneuron-based therapies for the treatment of various conditions characterized by hyperexcitability. While the numerous preclinical studies discussed here offer promises for such approaches to be taken out of the laboratory and into the clinic, specific limitations still remain to be addressed. Notably, the mechanisms by which MGE transplants control target cell activity need further investigation. Moreover, a better understanding of each disease’s etiology will be required so that the composition of the transplants may be adapted to achieve optimal efficacy. The generation of safe human interneurons will also be crucial for clinical transition. Recent reports demonstrating the in vitro production of transplantable MGE-like interneurons from human pluripotent stem cells (Maroof et al., 2013; Nicholas et al., 2013) have generated great excitement, which was further reinforced by studies showing therapeutic efficacy for such cells in mouse models of disease (Cunningham et al., 2014; Fandel et al., 2016; Liu et al., 2013).
Fundamental knowledge has also been gained from the remarkable ability of young interneurons to disperse and functionally integrate upon heterochronic transplantation. Here too, we anticipate that transplantation of interneurons will continue revealing basic insights about how these neurons find their way through complex and heterogeneous environments, how they choose to make connections with other neurons, and how they determine whether they survive or die. Additionally, the ability of transplanted interneurons to induce juvenile-like plasticity offers a powerful tool to study basic mechanisms of critical periods of plasticity. Future work may also reveal what cell-intrinsic information within young interneurons endows them with their unique ability to migrate through the parenchyma of the postnatal brain.
ACKNOWLEDGMENTS
We would like to thank Marianna Di Lullo and Shawn F. Sorrells for their careful reading of this manuscript and useful comments. We apologize to the authors of many additional relevant papers we could not cite here due to space constraints. Work in the Alvarez-Buylla’s laboratory is supported by the NIH (EY025174, NS028478, HD032116) and the John G. Bowes Research Fund. A.A.B. is the Heather and Melanie Muss Endowed Chair of Neurological Surgery at UCSF. A.A.B. is on the scientific advisory board and is co-founder of Neurona Therapeutics.
REFERENCES
- Alvarez-Dolado M, Calcagnotto ME, Karkar KM, Southwell DG, Jones-Davis DM, Estrada RC, Rubenstein JLR, Alvarez-Buylla A, Baraban SC, 2006. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J. Neurosci 26, 7380–7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y, 2006. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia 47, 867–872. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL, 1997. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474–476. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL, 2001. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development 128, 353–363. [DOI] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y, 2010. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci 30, 13707–13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, García-Verdugo JM, Rubenstein JLR, Alvarez-Buylla A, 2009. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc. Natl. Acad. Sci. U.S.A 106, 15472–15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK, 2010. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J. Neurosci 30, 14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Vincent C, Testa D, Bertini E, Ribot J, Di Nardo AA, Volovitch M, Prochiantz A, 2016. A mouse model for conditional secretion of specific single-chain antibodies provides genetic evidence for regulation of cortical plasticity by a non-cell autonomous homeoprotein transcription factor. PLoS Genet 12, e1006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurdeley M, Spatazza J, Lee HHC, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK, Prochiantz A, 2012. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J. Neurosci 32, 9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bráz JM, Reza S-N, Daniel V, Arnold K, Arturo A-B, Rubenstein JL, Basbaum AI, 2012. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron 74, 663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bráz JM, Dina J-S, Ross SE, Basbaum AI, 2014. Transplant restoration of spinal cord inhibitory controls ameliorates neuropathic itch. J. Clin. Invest 124, 3612–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bráz JM, Wang X, Guan Z, Rubenstein JL, Basbaum AI, 2015. Transplant-mediated enhancement of spinal cord GABAergic inhibition reverses paclitaxel-induced mechanical and heat hypersensitivity. Pain 156, 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJB, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G, 2005. The temporal and spatial origins of cortical interneurons predict their physiological sub-type. Neuron 48, 591–604. [DOI] [PubMed] [Google Scholar]
- Calcagnotto ME, Ruiz LP, Blanco MM, Santos-Junior JG, Valente MF, Patti C, Frussa-Filho R, Santiago MF, Zipancic I, Alvarez-Dolado M, Mello LE, Longo BM, 2010. Effect of neuronal precursor cells derived from medial ganglionic eminence in an acute epileptic seizure model. Epilepsia 51 (Suppl. 3), 71–75. [DOI] [PubMed] [Google Scholar]
- Caviness VS, 1982. Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Dev. Brain Res 4, 293–302. [DOI] [PubMed] [Google Scholar]
- Chang BS, Lowenstein DH, 2003. Epilepsy. N. Engl. J. Med 349, 1257–1266. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ, 2004. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J. Neurosci 24, 9598–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chédotal A, Rijli FM, 2009. Transcriptional regulation of tangential neuronal migration in the developing forebrain. Curr. Opin. Neurobiol 19, 139–145. [DOI] [PubMed] [Google Scholar]
- Chohan MO, Moore H, 2016. Interneuron progenitor transplantation to treat CNS dysfunction. Front. Neural Circuits 10, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C, 2001. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat. Neurosci 4, 52–62. [DOI] [PubMed] [Google Scholar]
- Cunningham M, Cho J-H, Leung A, Savvidis G, Ahn S, Moon M, Lee PKJ, Han JJ, Azimi N, Kim K-S, Bolshakov VY, Chung S, 2014. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell 15, 559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T, 1995. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374, 719–723. [DOI] [PubMed] [Google Scholar]
- Davis MF, Figueroa Velez DX, Guevarra RP, Yang MC, Habeeb M, Carathedathu MC, Gandhi SP, 2015. Inhibitory neuron transplantation into adult visual cortex creates a new critical period that rescues impaired vision. Neuron 86, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer HM, Marco M, Sander JW, 2008. The global burden and stigma of epilepsy. Epilepsy Behav 12, 540–546. [DOI] [PubMed] [Google Scholar]
- de Carlos JA, López-Mascaraque L, Valverde F, 1996. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J. Neurosci 16, 6146–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDiego I, Smith-Fernández A, Fairén A, 1994. Cortical cells that migrate beyond area boundaries: characterization of an early neuronal population in the lower intermediate zone of prenatal rats. Eur. J. Neurosci 6, 983–997. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, López-Cruz PL, Benavides-Piccione R, Bielza C, Larrañaga P, Anderson S, Burkhalter A, Cauli B, Fair én A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, González-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvárday Z, Kubota Y, Lewis DA, Marín O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JLR, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamás G, Thomson A, Wang Y, Yuste R, Ascoli GA, 2013. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci 14, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers MPJ, Nikoletopoulou V, Barde Y-A, 2013. Cell biology in neuroscience: death of developing neurons: new insights and implications for connectivity. J. Cell Biol 203, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Cruz E, Zhao M, Guo L, Ma H, Anderson SA, Schwartz TH, 2011. Interneuron progenitors attenuate the power of acute focal ictal discharges. Neurotherapeutics 8, 763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD, 1989. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res 495, 387–395. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T, 2007. Circuits and circuit disorders of the basal ganglia. Arch. Neurol 64, 20–24. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Chattopadhyaya B, Kuhlman SJ, Fu Y, B elanger M-C, Wu CZ, Rutishauser U, Maffei L, Huang ZJ, 2007. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci 10, 1569–1577. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Björklund A, 2012. Introduction (part II). Prog. Brain Res 201, 3–5. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K, 1998. Changes in GAD- and GABA- immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J. Chem. Neuroanat 16, 57–72. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP, 2012. Development and plasticity of the primary visual cortex. Neuron 75, 230–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK, 2000. Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404, 183–186. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L, 1994. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res 34, 709–720. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy J-M, Löw K, Möhler H, Rudolph U, Hensch TK, 2004. Specific GABAA circuits for visual cortical plasticity. Science 303, 1681–1683. [DOI] [PubMed] [Google Scholar]
- Fandel TM, Trivedi A, Nicholas CR, Zhang H, Chen J, Martinez AF, Noble-Haeusslein LJ, Kriegstein AR, 2016. Transplanted human stem cell-derived interneuron precursors mitigate mouse bladder dysfunction and central neuropathic pain after spinal cord injury. Cell Stem Cell 19 (4), 544–557. http://dx.doi.org/10.1016/j.stem.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE, 2009. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U.S.A 106, 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JLR, Puelles L, Marín O, 2007. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci 27, 9682–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin P, Zhao Y, Vogt D, Jeong J, Long J, Potter G, Westphal H, Rubenstein JLR, 2011. Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron 70, 939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marín O, Pachnis V, Kessaris N, 2007. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci 27, 10935–10946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K, 1998. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain 78, 13–26. [DOI] [PubMed] [Google Scholar]
- Gage FH, 2012. Transplantation in the future. Prog. Brain Res 201, 7–13. [DOI] [PubMed] [Google Scholar]
- Gelman DM, Martini FJ, Nobrega-Pereira S, Pierani A, Kessaris N, Marin O, 2009. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J. Neurosci 29, 9380–9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, Pla R, Pierani A, Marín O, 2011. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J. Neurosci 31, 16570–16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem N, Yu M, Long J, Hatch G, Rubenstein JLR, Ekker M, 2007. Distinct cis-regulatory elements from the Dlx1/Dlx2 locus mark different progenitor cell populations in the ganglionic eminences and different subtypes of adult cortical interneurons. J. Neurosci 27, 5012–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani AI, Chohan MO, Inan M, Schobel SA, Chaudhury NH, Paskewitz S, Chuhma N, Glickstein S, Merker RJ, Xu Q, Small SA, Anderson SA, Ross ME, Moore H, 2014. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc. Natl. Acad. Sci. U.S.A 111, 7450–7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P, Scanziani M, 2009. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat. Neurosci 12, 21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein SB, Alexander S, Ross ME, 2007. Differences in cyclin D2 and D1 protein expression distinguish forebrain progenitor subsets. Cereb. Cortex 17, 632–642. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Lüthi A, Herry C, 2009. Perineuronal nets protect fear memories from erasure. Science 325, 1258–1261. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A, 1997. Three distinct families of GABAergic neurons in rat visual cortex. Cereb. Cortex 7, 347–358. [DOI] [PubMed] [Google Scholar]
- Götz M, Huttner WB, 2005. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol 6, 777–788. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Levi-Montalcini R, 1949. Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. J. Exp. Zool 111, 457–501. [DOI] [PubMed] [Google Scholar]
- Hammad M, Schmidt SL, Zhang X, Bray R, Frohlich F, Ghashghaei HT, 2015. Transplantation of GABAergic interneurons into the neonatal primary visual cortex reduces absence seizures in stargazer mice. Cereb. Cortex 25, 2970–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP, 1999. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J. Neurosci 19, RC40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Pierre F, Kazuaki Y, Rubenstein JL, Arturo A-B, Kriegstein AR, 2013. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat. Neurosci 16, 1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Konradi C, 2010. Hippocampal pathology in schizophrenia. Curr. Top. Behav. Neurosci 4, 529–553. [DOI] [PubMed] [Google Scholar]
- Henderson KW, Gupta J, Tagliatela S, Litvina E, Zheng X, Van Zandt MA, Woods N, Grund E, Lin D, Royston S, Yanagawa Y, Aaron GB, Naegele JR, 2014. Long-term seizure suppression and optogenetic analyses of synaptic connectivity in epileptic mice with hippocampal grafts of GABAergic interneurons. J. Neurosci 34, 13492–13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, 2005. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci 6, 877–888. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Michela F, Nobuko M, Steinunn B, Kash SF, 1998. Competitive plasticity via intrinsic GABAergic circuits in the developing visual cortex. Neurosci. Res 31, S325. [Google Scholar]
- Howard MA, Baraban SC, 2016. Synaptic integration of transplanted interneuron progenitor cells into native cortical networks. J. Neurophysiol 116, 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I, 2005. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci 28, 310–316. [DOI] [PubMed] [Google Scholar]
- Howard MA, Rubenstein JLR, Baraban SC, 2014. Bidirectional homeostatic plasticity induced by interneuron cell death and transplantation in vivo. Proc. Natl. Acad. Sci. U.S.A 111, 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L, 2012. Alzheimer mechanisms and therapeutic strategies. Cell 148, 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S, 1999. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755. [DOI] [PubMed] [Google Scholar]
- Hunt RF, Baraban SC, 2015. Interneuron transplantation as a treatment for epilepsy. Cold Spring Harb. Perspect. Med 5, 1–13. http://dx.doi.org/10.1101/cshperspect.a022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Girskis KM, Rubenstein JL, Arturo A-B, Baraban SC, 2013. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat. Neurosci 16, 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan M, Welagen J, Anderson SA, 2012. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier sub-type in the medial ganglionic eminence. Cereb. Cortex 22, 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan M, Melis I, Petros TJ, Anderson SA, 2013. Losing your inhibition: linking cortical GABAergic interneurons to schizophrenia. Neurobiol. Dis 53, 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isstas M, Teichert M, Bolz J, Lehmann K, 2016. Embryonic interneurons from the medial, but not the caudal ganglionic eminence trigger ocular dominance plasticity in adult mice. Brain Struct. Funct http://dx.doi.org/10.1007/s00429-016-1232-y. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK, 2003. Rapid critical period induction by tonic inhibition in visual cortex. J. Neurosci 23, 6695–6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal MK, Keros S, Zhao M, Inan M, Schwartz TH, Anderson SA, Homanics GE, Goldstein PA, 2015. Reduction in focal ictal activity following transplantation of MGE interneurons requires expression of the GABAA receptor α4 subunit. Front. Cell. Neurosci 9, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR, 1991. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 148, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Jones EG, 2009. The origins of cortical interneurons: mouse versus monkey and human. Cereb. Cortex 19, 1953–1956. [DOI] [PubMed] [Google Scholar]
- Kanatani S, Yozu M, Tabata H, Nakajima K, 2008. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J. Neurosci 28, 13582–13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Kim YA, GrandPre T, Shatz CJ, 2009. Co-regulation of ocular dominance plasticity and NMDA receptor subunit expression in glutamic acid decarboxylase-65 knock-out mice. J. Physiol 587, 2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agústsdóttir A, Antila H, Popova D, Akamine Y, Bahi A, Sullivan R, Hen R, Drew LJ, Castrén E, 2011. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 334, 1731–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Fagiolini M, Hensch TK, 2007. Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron 53, 805–812. [DOI] [PubMed] [Google Scholar]
- Kato M, Dobyns WB, 2005. X-linked lissencephaly with abnormal genitalia as a tangential migration disorder causing intractable epilepsy: proposal for a new term, “interneuronopathy” J. Child Neurol 20, 392–397. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Magno L, Rubin AN, Oliveira MG, 2014. Genetic programs controlling cortical interneuron fate. Curr. Opin. Neurobiol 26, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P, 2008. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Márton LF, Roberts JDB, Cobden PM, Buzsáki G, Somogyi P, 2003. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421, 844–848. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H, 2010. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68, 557–569. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT, 2013. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501, 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer P, Spatazza J, Espinosa JS, Tang Y, Kaneko M, Hasenstaub AR, Stryker MP, Alvarez-Buylla A, 2016. Caudal ganglionic eminence precursor transplants disperse and integrate as lineage-specific interneurons but do not induce cortical plasticity. Cell Rep 16, 1391–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG, 1999. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J. Neurosci 19, 7881–7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Lee H, Nam YR, Oh JH, Cho YH, Chang JW, 2005. Enhanced expression of glutamate decarboxylase 65 improves symptoms of rat parkinsonian models. Gene Ther 12, 1215–1222. [DOI] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B, 2010. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci 30, 16796–16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P, 2002. Origin of GABAergic neurons in the human neocortex. Nature 417, 645–649. [DOI] [PubMed] [Google Scholar]
- Leung L, Andrews-Zwilling Y, Yoon SY, Jain S, Ring K, Dai J, Wang MM, Tong L, Walker D, Huang Y, 2012. Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice. PLoS One 7, e53569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever I, Cunningham J, Grist J, Yip PK, Malcangio M, 2003. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur. J. Neurosci 18, 1169–1174. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, 1949. The development to the acoustico-vestibular centers in the chick embryo in the absence of the afferent root fibers and of descending fiber tracts. J. Comp. Neurol 91, 209–241. illust, incl. 3 pl. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW, 2012. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y, 2009. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell 5, 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Marsden CD, Björklund A, 1990. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science 247, 574–577. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yan L, Weick JP, Huisheng L, Robert K, Xiaoqing Z, Lixiang M, Guo-min Z, Melvin A, Su-Chun Z, 2013. Medial ganglionic eminence–like cells derived from human embryonic stem cells correct learning and memory deficits. Nat. Biotechnol 31, 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Arlotta P, 2015. Generating neuronal diversity in the mammalian cerebral cortex. Annu. Rev. Cell Dev. Biol 31, 699–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Rouaux C, Quast KB, Jantrachotechatchawan C, Studer M, Hensch TK, Arlotta P, 2011. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron 69, 763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser JD, Ward AA Jr., 1967. Some effects of deafferentation on neurons of the cat spinal cord. Arch. Neurol 17, 629–636. [DOI] [PubMed] [Google Scholar]
- Luo J, 2002. Subthalamic GAD gene therapy in a Parkinson’s disease rat model. Science 298, 425–429. [DOI] [PubMed] [Google Scholar]
- Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y, Liu F, You Y, Chen C, Campbell K, Song H, Ma L, Rubenstein JL, Yang Z, 2013. Subcortical origins of human and monkey neocortical interneurons. Nat. Neurosci 16, 1588–1597. [DOI] [PubMed] [Google Scholar]
- Maffei A, Arianna M, Kiran N, Nelson SB, Turrigiano GG, 2006. Potentiation of cortical inhibition by visual deprivation. Nature 443, 81–84. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F, 2006. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J. Neurosci 26, 3875–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O, 2012. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci 13 (2), 107–120. http://dx.doi.org/10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- Marín O, 2013. Cellular and molecular mechanisms controlling the migration of neocortical interneurons. Eur. J. Neurosci 38, 2019–2029. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C, 2004. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci 5, 793–807. [DOI] [PubMed] [Google Scholar]
- Maroof AM, Keros S, Tyson JA, Ying S-W, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, Widmer HR, Eggan K, Goldstein PA, Anderson SA, Studer L, 2013. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 12, 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cerdeño V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S, Daadi MM, Bankiewicz K, Alvarez-Buylla A, Kriegstein AR, 2010. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell 6, 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Pretorius JK, Melendez M, Lévesque MF, 1995. The path-ophysiologic relationships between lesion pathology, intracranial ictal EEG onsets, and hippocampal neuron losses in temporal lobe epilepsy. Epilepsy Res 21, 133–147. [DOI] [PubMed] [Google Scholar]
- McKinsey GL, Lindtner S, Trzcinski B, Visel A, Pennacchio LA, Huylebroeck D, Higashi Y, Rubenstein JLR, 2013. Dlx1&2-dependent expression of Zfhx1b (Sip1, Zeb2) regulates the fate switch between cortical and striatal interneurons. Neuron 77, 83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H, 2012. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat. Neurosci 15 (414–22), S1–S2. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJB, Battiste J, Johnson JE, Machold RP, Fishell G, 2010. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J. Neurosci 30, 1582–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD, 2007. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci 8, 427–437. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ, 2002. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci 22, 6724–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, Hensch TK, 2010. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 330, 1238–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T, 2007. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem. Int 51, 173–184. [DOI] [PubMed] [Google Scholar]
- Munro G, Ahring PK, Mirza NR, 2009. Developing analgesics by enhancing spinal inhibition after injury: GABAA receptor subtypes as novel targets. Trends Pharmacol. Sci 30, 453–459. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG, 2002. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat. Neurosci 5, 1279–1287. [DOI] [PubMed] [Google Scholar]
- Nery S, Corbin JG, Fishell G, 2003. Dlx2 progenitor migration in wild type and Nkx2.1 mutant telencephalon. Cereb. Cortex 13, 895–903. [DOI] [PubMed] [Google Scholar]
- Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen Y-JJ, Stanley EG, Elefanty AG, Sasai Y, Alvarez-Buylla A, Rubenstein JLR, Kriegstein AR, 2013. Functional maturation of hPSC-derived fore-brain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 12, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Masaharu O, Takaki M, Kazunori N, Kenichi Y, Masahiro S, Kazuhiro I, Hiroshi Y, Katsuhiko M, 1995. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 14, 899–912. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB, 2009. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J. Neurosci 29, 7040–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu G-Q, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L, 2007. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfo M, 2013. Pediatric epilepsy genetics. Curr. Opin. Neurol 26, 137–145. [DOI] [PubMed] [Google Scholar]
- Petilla Interneuron Nomenclature Group, Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, Fairén A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvárday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Muñoz A, Packer A, Petersen CCH, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R, 2008. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci 9, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L, 2002. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251. [DOI] [PubMed] [Google Scholar]
- Pla R, Borrell V, Flames N, Marín O, 2006. Layer acquisition by cortical GABAergic interneurons is independent of reelin signaling. J. Neurosci 26, 6924–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM, 2003. Developmental plasticity of mouse visual acuity. Eur. J. Neurosci 17, 167–173. [DOI] [PubMed] [Google Scholar]
- Rubin AN, Alfonsi F, Humphreys MP, Choi CKP, Rocha SF, Kessaris N, 2010. The germinal zones of the basal ganglia but not the septum generate GABAergic interneurons for the cortex. J. Neurosci 30, 12050–12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J, 2011. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol 71, 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajo M, Ellis-Davies G, Morishita H, 2016. Lynx1 limits dendritic spine turnover in the adult visual cortex. J. Neurosci 36, 9472–9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Beatriz P, Styner MA, Iris A, Inbar BP, Corcoran CM, Lieberman JA, Holly M, Small SA, 2013. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn D-H, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ, 2005. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J. Neurosci 25, 7317–7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, 1987. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science 235, 73–76. [DOI] [PubMed] [Google Scholar]
- Soriano E, Eduardo S, del Río JA, 2005. The cells of Cajal-Retzius: still a mystery one century after. Neuron 46, 389–394. [DOI] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP, 2010. Cortical plasticity induced by inhibitory neuron transplantation. Science 327, 1145–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, Alfaro-Cervello C, Tang Y, Garcia-Verdugo JM, Rubenstein JL, Baraban SC, Alvarez-Buylla A, 2012. Intrinsically determined cell death of developing cortical interneurons. Nature 491, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Nicholas CR, Basbaum AI, Stryker MP, Kriegstein AR, Rubenstein JL, Alvarez-Buylla A, 2014. Interneurons from embryonic development to cell-based therapy. Science 344, 1240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatazza J, Lee HHC, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK, Prochiantz A, 2013. Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep 3, 1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK, 2008. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134, 508–520. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL, 1999. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126, 3359–3370. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnár G, Oláh S, Barzó P, Tamás G, 2006. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311, 233–235. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Hensch TK, 2013. Balancing plasticity/stability across brain development. Prog. Brain Res 207, 3–34. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori KE, Takauji R, 1997. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J. Neurosci 17, 8313–8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka DH, Toriumi K, Kubo K-I, Nabeshima T, Nakajima K, 2011. GABAergic precursor transplantation into the prefrontal cortex prevents phencyclidine-induced cognitive deficits. J. Neurosci 31, 14116–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Stryker MP, Alvarez-Buylla A, Espinosa JS, 2014. Cortical plasticity induced by transplantation of embryonic somatostatin or parvalbumin interneurons. Proc. Natl. Acad. Sci. U.S.A 111, 18339–18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Lu J, Huang ZJ, 2013. The spatial and temporal origin of chandelier cells in mouse neocortex. Science 339, 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP, 2004. Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol 14, 685–692. [DOI] [PubMed] [Google Scholar]
- Tong LM, Djukic B, Arnold C, Gillespie AK, Yoon SY, Wang MM, Zhang O, Knoferle J, Rubenstein JLR, Alvarez-Buylla A, Huang Y, 2014. Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Aβ accumulation. J. Neurosci 34, 9506–9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson JA, Anderson SA, 2014. GABAergic interneuron transplants to study development and treat disease. Trends Neurosci 37, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente MF, Romariz S, Calcagnotto ME, Ruiz L, Mello LE, Frussa-Filho R, Longo BM, 2013. Postnatal transplantation of interneuronal precursor cells decreases anxiety-like behavior in adult mice. Cell Transplant 22, 1237–1247. [DOI] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ, 2012. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Abbott LF, 2009. Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat. Neurosci 12, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt D, Hunt RF, Mandal S, Sandberg M, Silberberg SN, Nagasawa T, Yang Z, Baraban SC, Rubenstein JLR, 2014. Lhx6 directly regulates Arx and CXCR7 to determine cortical interneuron fate and laminar position. Neuron 82, 350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, Geoffroy H, Monyer H, Rossier J, Vitalis T, 2010. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb. Cortex 20, 2333–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A, 1999. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat. Neurosci 2, 461–466. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A, 2001. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development 128, 3759–3771. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH, 1963. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol 26, 1003–1017. [DOI] [PubMed] [Google Scholar]
- Wimo A, Jönsson L, Bond J, Prince M, Winblad B, Disease International, Alzheimer, 2013. The worldwide economic impact of dementia 2010. Alzheimers Dement 9, 1–11.e3. [DOI] [PubMed] [Google Scholar]
- Winkler C, Bentlage C, Nikkhah G, Samii M, Björklund A, 1999. Intranigral transplants of GABA-rich striatal tissue induce behavioral recovery in the rat Parkinson model and promote the effects obtained by intrastriatal dopaminergic transplants. Exp. Neurol 155, 165–186. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA, 2006. The origin and specification of cortical interneurons. Nat. Rev. Neurosci 7, 687–696. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA, 2008. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev. Biol 314, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R, 2009. Depolarizing effect of neocortical chandelier neurons. Front. Neural Circuits 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, McGarry LM, Vogels TP, Inan M, Anderson SA, Yuste R, 2011. State-dependent function of neocortical chandelier cells. J. Neurosci 31, 17872–17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, 2004. Origins of cortical interneuron subtypes. J. Neurosci 24, 2612–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA, 2008. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J. Comp. Neurol 506, 16–29. [DOI] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Yoko Y-S, Siu K, Hideyuki C, Tomoki F, Hensch TK, 2009. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature 462, 218–221. [DOI] [PubMed] [Google Scholar]
- Ye Z, Mostajo-Radji MA, Brown JR, Rouaux C, Tomassy GS, Hensch TK, Arlotta P, 2015. Instructing perisomatic inhibition by direct lineage reprogramming of neocortical projection neurons. Neuron 88, 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zecevic N, 2011. Dorsal radial glial cells have the potential to generate cortical interneurons in human but not in mouse brain. J. Neurosci 31, 2413–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipancic I, Calcagnotto ME, Piquer-Gil M, Mello LE, Alvarez-Dolado M, 2010. Transplant of GABAergic precursors restores hippocampal inhibitory function in a mouse model of seizure susceptibility. Cell Transplant 19, 549–564. [DOI] [PubMed] [Google Scholar]