Abstract
Purpose of the Review:
Bisphosphonates have well-established effects on suppressing bone resorption and slowing bone loss yet the effects on bone mechanical properties are less clear. We review recent data from pre-clinical and clinical experiments that assessed mechanical properties of bisphosphonate-treated specimens.
Recent Findings:
Pre-clinical work has utilized new techniques to show reduced fatigue life and transfer of stress from the mineral-to-collagen. Several notable studies having examined mechanical properties of tissue from patients treated with bisphosphonates with mixed results. Pre-clinical data suggest effects on mechanics may be independent of remodeling suppression.
Summary:
The direct effect of bisphosphonates on bone mechanics remains unclear but recent work has set a solid foundation for the coming years.
Keywords: atypical fracture, zoledronate, remodeling suppression, anti-remodeling, biomechanics
Today’s clinicians have plenty of options for reducing the risk of fracture in their patients. Bisphosphonates, selective estrogen receptor modulators (SERM), parathyroid hormone, hormone replacement therapy, Rank-L inhibitors, and parathyroid hormone related protein analogs are all FDA-approved and generally effective. Each drug (or drug class) works through a slightly different mechanism but in general affect bone cell activity to either make more bone or slow the loss. Moving from altered bone mass to altered mechanics is more challenging. A population-based positive correlation between bone mass and fracture risk has led to the assumption that changes in bone mass reflect changes in actual mechanical properties. Yet as the London playwright Oscar Wilde wrote, “The truth is rarely pure and never simple”.
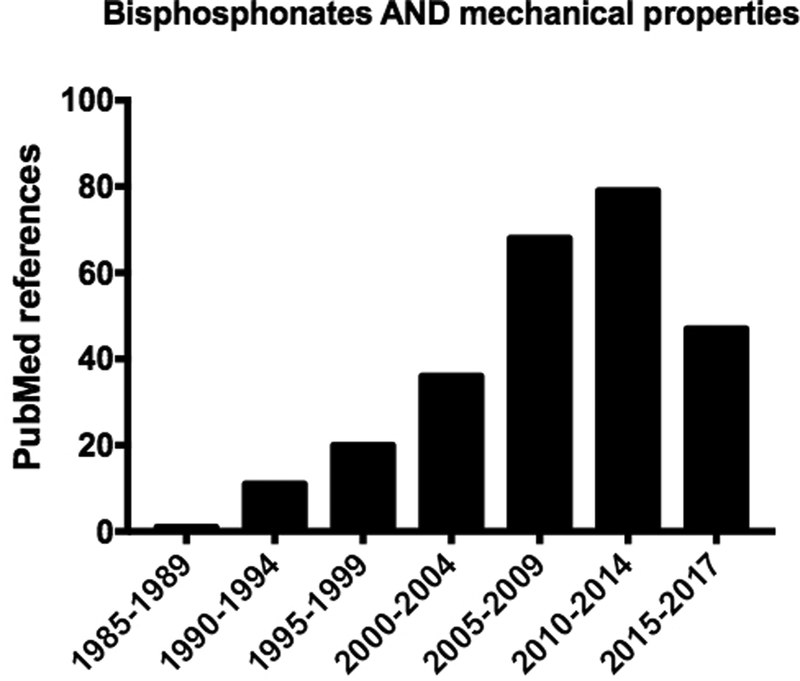
The bisphosphonate pamidronate was FDA approved in 1991 for treatment of malignancy-induced hypercalcemia, Paget’s disease, and osteolytic bone lesions. Four years later alendronate received FDA approval for treatment/prevention of osteoporosis. Prior to 1995, less than 20 papers described the effects of bisphosphonates on bone mechanical properties, only two of which used alendronate (others focused on pamidronate, etidronate, and tiludronate). Thus, the vast majority of work describing ‘bisphosphonates and mechanical properties’ (>250 PubMed citations) has been performed in the 20+ years following their entry into the osteoporosis clinic (Figure 1). Even now as data continues to emerge the answer to the question ‘How does bisphosphonate treatment affect mechanical properties’ does not appear simple. The goal of this brief review is to highlight work related to this question of the past few years (2014-now).
Figure 1.
PubMed results for a search on ‘bisphosphonates AND mechanical properties’ show a steady rise in publications since the late 1980’s. A near doubling of publications occurred around 2005, coinciding with the publication of the first paper describing atypical femoral fractures [1]. Note, the final bar denotes only 3 years while all other bars are 5 year increments.
Three atypical fractures re-ignited the flame of interest around bisphosphonates and bone mechanics
In 2005, Odvina and colleagues described a series of patients that had ‘severely suppressed bone turnover’ associated with bisphosphonate treatment [1]. A sub-set of these patients (n=3) experienced what the authors called ‘atypical fractures’ of the femur shaft. In the years to follow there emerged a growing number of reports described similar atypical femoral fractures that were associated with bisphosphonate treatment. Collectively, these report spurred several consensus statements, most of which highlighted that there was a gap in our understanding of how bisphosphonates affect bone mechanical properties – most notably after prolonged treatment [2–6]. The number of papers related to bisphosphonates and mechanical properties nearly doubled in the years following the work of Odvina compared to a similar period prior (Figure 1). This has moved the field forward by spurring novel approaches to studying the question in preclinical work and, most importantly, pushing for mechanical data in clinical samples.
Pre-clinical mechanical data – Finally moving beyond monotonic tests
The majority of work underpinning the skeletal mechanisms of bisphosphonates was carried out in rodent models. Because of this, the fundamental effects these drugs impart on bone mechanical properties came from rodents. Mechanical data in rodents, while informative, has limitations [7]. Most notably, the absence of intracortical remodeling in normal rodents does not provide a realistic model reflecting how remodeling suppression in cortical bone occurs in humans. Assuming that the effects bisphosphonates have on mechanical properties are driven by remodeling suppression (see final section of this review), this limitation is significant.
Large animal models (those with intracortical remodeling such as dog, sheep, non-human primates) provide a cloudy picture of bisphosphonate-induced effects on mechanical properties [8]. Results differ depending on species, skeletal site, duration of treatment, mechanical parameter being assessed, mode of testing, and on and on and on. Etidronate, an early generation bisphosphonate, resulted in negative effects on bone structural-level mechanical properties in large animal models [9]. Subsequently-developed bisphosphonates, including those approved for reducing fracture risk, have mostly documented increased structural mechanical properties in large animal models based on whole bone tests (see summary of papers here [10]). Material properties, estimated through back-calculation of structure tests or through testing machined specimens, have shown positive, neutral, and negative effects from bisphosphonate treatment in various studies [10].
Two recent papers have investigated the mechanical effects of bisphosphonates using novel testing techniques on canine bone. The first advances our understanding of mechanical properties beyond monotonic properties, the mode of testing in the majority of previous experiments. In this work, cyclic loading tests were performed on bone beams machined from the cortical bone of dog ribs [11]. This skeletal site has high bone turnover (~20%/year) which has been shown to be significantly suppressed following 3-years of alendronate treatment at clinically relevant doses [12]. Fatigue life in these cortical bone beams was 3-fold lower in animals that had been treated with high doses of alendronate compared to control. There was no significant effect at the lower dose (representing an estimation of the dose used clinically on a mg/kg basis). The reduction in the high dose animals was associated with a number of differences in structure, such as the size of osteons and lacunar density, suggesting that changes manifesting from the suppression of remodeling might play a role in the compromised fatigue life. The major advance of this work is that it attempts to model failure of bone in a more life-like situation. While it can be argued that fatigue studies are not realistic, they certainly test how bone fails when exposed to sub-damaging loads over time, most notably in conditions where remodeling activity is significantly suppressed. Outside of impact testing (which would be really valuable to assess in bisphosphonate-treated tissue), fatigue tests produce some of the most useful data with respect to how drugs affect functional bone properties.
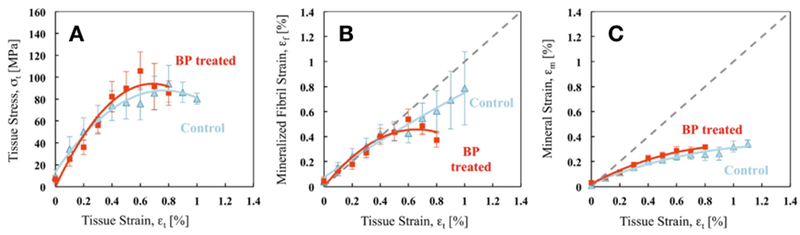
The second recent study that provides novel data assessed cortical bone beams from canine humeri (3 years of treatment with alendronate at a clinically relevant dose) [13]. The authors performed a wide range of mechanical tests on the cortical beams including monotonic bending, fracture toughness testing (using notched specimens) and small-angle x-ray scattering/wide-angle x-ray diffraction (SAXS/WAXS) during tensile testing. The monotonic and tensile tests showed lower post-yield properties in alendronate-treated animals, although only some parameters reached statistical significance. Fracture toughness tests revealed no difference between control and bisphosphonate-treated groups for crack initiation or crack growth toughness among groups. SAXS/WAXS tests, in tension, showed the two groups had identical responses of tissue stress/strain at small strains while at higher strains there was a significant degradation in post-yield deformation in bone from alendronate-treated animals (Figure 2). Partitioning this out into how the two components of bone handle the strain, the authors concluded that the strain carried by the collagen, but not the mineral, was compromised in treated animals. This was hypothesized to be related to the increase in non-enzymatic collagen cross-linking, associated with bisphosphonate treatment which would limit the ability of the collagen fibers to slide and dissipate energy prior to breaking. Increased non-enzymatic collagen cross-linking was noted in the samples tested in this work, similar to what has been documented in other pre-clinical bisphosphonate studies [14–16]. Certainly, it remains plausible that other alterations in collagen or the collagen/mineral interface, besides cross-linking, are responsible for these effects. The major advance of this work is that it attempts to dive a bit deeper into the mechanical effects, partitioning out collagen and mineral. These sorts of studies have the potential to truly determine the mechanical mechanisms at play with bisphosphonate treatment.
Figure 2.
Pre-clinical data showing the effects of bisphosphonates on mechanical properties, partitioned out into effects on the mineral and collagen using small-angle x-ray scattering/wide-angle x-ray diffraction (SAXS/WAXS). Cortical bone beams from canine treated with alendronate (BP-treated) or vehicle (control) were assessed using uniaxial tensile testing. (A) Stress/Strain curves showing the strains measured in the bone tissue and compared with strains measured in (B) the mineralized collagen fibril (stagger in the gap zone) using SAXS and (C) the HA mineral crystalline lattice using WAXD experiments. Reproduced with permission from Bone. [13].
Mechanical data from bisphosphonate-treated patients – More anticipated than a Star Wars movie
The most exciting work emerging in the field over the past few years comes from the handful of studies describing effects of bisphosphonates on mechanical properties using human tissue. Obtaining and working with human tissue is challenging. Sample sizes tend to be low, control tissue often involves use of cadaver material, and duration of bisphosphonate use is often variable. Despite these limitations (and others) such data provide the closest look we have to what is happening in the patients we are ultimately trying to benefit with treatment.
A series of two papers, by the same laboratory, have described properties of femoral head trabecular bone tissue from patients that experienced a femoral neck fracture and were undergoing surgery [17,18]. Groups included those that had taken bisphosphonates and those that had not, along with cadaveric non-fracture controls. Treated patients had taken weekly oral alendronate for 1–9 years (average 3), based on patient records. Cylindrical cores (~10 × 7 mm) were taken from the region directly superior to the trabecular chiasma in the primary compressive trabecular arcade of the femoral heads. Samples were assessed using synchrotron imaging (to examine microdamage) and compression mechanical testing. Bone samples from bisphosphonate-treated individuals had significantly lower strength and modulus, both at the whole core level and at the tissue level (correcting for the amount of bone volume) compared to both the fracture and non-fracture controls. Bisphosphonate-treated patients had higher microcrack density and volume compared to both non-bisphosphonate fracture and non-fracture control groups. The authors concluded that the femoral head bone of bisphosphonate-treated patients was weaker and this could be due to the associated higher amount of microdamage in the tissue, presumably from reduced remodeling. The most notable weakness of this paper is that the tissues were from those patients that fractured. Given that the bisphosphonate group didn’t have higher trabecular bone volume compared to controls (at least in one of the reports [17]), combined with the fact that they fractured, raises the question of whether these results are generalizable to the larger population of treated patients or if these represent non-responders to the treatment.
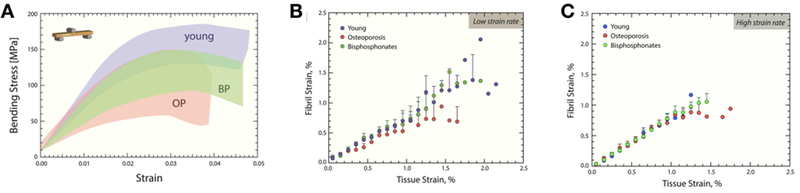
In a similar study design, albeit using mid-femoral diaphysis tissue from cadavers, properties were examined from three distinct cohorts: young healthy individuals (no disease, mean age of 35), individuals with osteoporosis based on medical records but without a history of bisphosphonates, and individuals with osteoporosis and a medical record of taking bisphosphonates (alendronate) for 6 years [19]. The mean age of the latter two groups was 80. A number of analyses were conducted including high resolution CT, 3-pt bending, SAXS and fractography. Unlike the above studies, this work focused on cortical bone, taken from the lateral cortex of the femur and machined into beam specimens. Various morphological differences existed between the specimens from young individuals and those from the two aged cohorts (such as porosity), but there were no mechanical property differences between those with osteoporosis and those with osteoporosis treated with bisphosphonates either at low or high strain (Figure 3). This includes no difference in fibril strain, which was the property shown above (in canine studies) to be most affected by bisphosphonates.
Figure 3.
Clinical specimen data showing the effects of bisphosphonates on mechanical properties, partitioned out into effects on the mineral and collagen using small-angle x-ray scattering/wide-angle x-ray diffraction (SAXS/WAXS). Human cortical bone samples from the mid- diaphysis of the femur were used for assessment. (A) Stress-strain curves are shown for young, osteoporosis (OP) and bisphosphonate-treated (BP) cases. The shaded area contains all of the stress-strain curves for each group. (B) Fibril strain at low strain rate (B) and high strain rate (C), was assessed using synchrotron SAXS to determine the elastic stretching of the fibrils during the initial linear portion of the curve. At low strain rates (B) osteoporosis cases have a more pronounced plateau in the fibril strain (p < 0.001), indicating lower fibril deformation than the young cases. In contrast, the bisphosphonate-treated cases exhibit more fibrillar deformation, which corresponds to the behavior of the young cases, and may explain the improvements in strength. At the high strain rates (C) the fibril versus tissue strain is very linear for all three conditions. Reproduced from [19] under the creative commons license.
Single trabeculae from the L5 of cadavers were assessed for mechanical properties in three groups: control (osteoporosis but no treatment), short term bisphosphonate treatment (1–5 years) and long-term bisphosphonate treatment (6+ years) [20]. Details of bisphosphonate treatment were obtained from the family and medical records and consisted of weekly alendronate in all cases. Microdamage, assessed histologically, was non-significantly lower in the two treated groups compared to controls. Young’s modulus, ultimate stress, work to failure and toughness of single trabeculae, tested in bending, did not different among the three groups. Based on these data the authors concluded that alendronate treatment, even beyond 6 years, has no adverse impact on vertebral trabecular bone mechanical properties. Two main considerations in this work are the efficacy of the treatment and the tissue assessment. Trabecular BV/TV was lower in those on bisphosphonate compared to control, questioning the effectiveness of treatment, although erosion surface was significantly lower and tissue mineral density was significantly higher compared to controls. The latter effects would be consistent with drug efficacy. The second consideration is the method of mechanical testing. Although there is great value in determining the mechanics of single trabeculae, the isolation of a single trabeculae may not reflect the properties of the larger network.
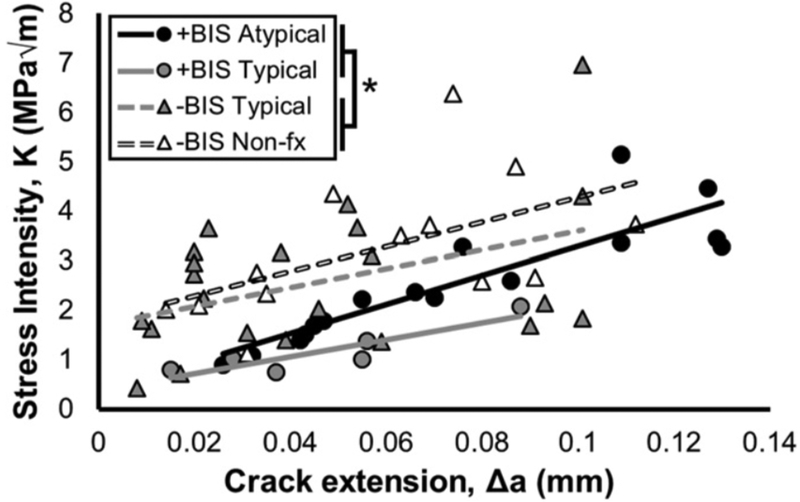
The most direct work aimed at understanding bone mechanical properties in the context of atypical femoral fracture bone mechanics utilizes tissue from individuals who have experienced these fractures [21]. Cortical bone tissue from the lateral aspect of the proximal femur was collected from individuals treated with bisphosphonates that had experienced an atypical fracture, those treated with bisphosphonates that experienced a typical fracture (intertrochanteric), those treated with bisphosphonates without a fracture (undergoing total hip arthroplasty as treatment for osteoarthritis), those without a history of bisphosphonate treatment that experienced a typical fracture (intertrochanteric), or those without a history of bisphosphonate treatment who had not fractured. Obtaining such a collection of tissues is amazing, as is the fact that the authors could perform mechanical tests on such tissue. Bisphosphonate-treated patients had been using alendronate, ibandronate, or risedronate for an average duration of ~ 7 years. All tissue was embedded in plastic and then subjected to various assessments, including fracture toughness and nanoindentation. Mechanics on embedded tissue has notable limitations which should not be ignored (plastic carries some load, the in-situ collagen/mineral organization may be disrupted, tissue is dehydrated prior to embedding affecting water, etc), yet given that all tissues were treated the same, there remains utility in the relative effects across treatments. That said, it cannot be discounted that embedding has differential effects across treatment groups which could affect results and interpretation of this work. Based on nanoindentation, cortical bone from patients with atypical fractures had hardness values similar to that of other bisphosphonate-treated patients. Hardness of patients treated with bisphosphonates was collectively higher than non-fracture non-bisphosphonate patient tissue but not non-bisphosphonate fracture patients. Fracture toughness tests on notched specimens revealed that cortical bone from patients on bisphosphonates had reduced fracture resistance (Figure 4) compared to treatment naïve patients; no difference existed between those with and without fracture within the bisphosphonate cohort. The authors also described different patterns of crack growth, with cracks in bisphosphonate-treated bone being less tortuous and following a more distinct crack path. Similar to the works above, there are few pieces of data in the work that document ‘efficacy’ of bisphosphonates, that is properties of mineralization are mostly unchanged compared to non-treated individuals. It’s possible that the lack of effects on parameters such as bone volume, or mineral/matrix ratio (mean and/or distribution heterogeneity) are not showing bisphosphonate effects due to low sample size, yet the commonality of this finding among all these human studies makes one stop and ponder – are the effects observed in animals reflective of what is happening in humans? Additional work on human tissue will be needed to reveal the answer.
Figure 4.
Fracture resistance R-curves of stress intensity (K) as a function of crack extension, Δa, based on in situ fracture toughness tests. Lines represent a fit of the data for each group. Tissue from patients treated with bisphosphonates (+BIS groups) was less tough than that from bisphosphonate-naïve patients (−BIS groups); *P = 0.01. In addition, tissue from patients without fractures (−BIS Nonfx) was tougher than that from patients with typical or atypical fractures (P = 0.03 by linear fixed effects model). Reproduced with permission (Proceedings of the National Academy of Sciences of the United States of America). [21]
A fundamentally different type of human tissue assessment, using finite element analyses, asked the question of how estimated bone mechanical properties might look like with long term bisphosphonate treatment [22]. Iliac crest bone biopsies were obtained from treatment naïve patients or patients treated with oral bisphosphonate (alendronate, risedronate, ibandronate) for up to 16 years (range 1–16 years). Biopsies were imaged with high resolution computed tomography and the central portion of the scan (mid-4mm trabecular section) was computationally meshed, assigned homogeneous material properties, and subjected to computational loading. The assumption of homogenous material properties’, which is commonly used in similar models, has limitations in the context of bisphosphonates as these would be expected to be altered (although its unknown exactly how – the point of this review). The computational models showed that mechanical properties such as apparent stiffness and failure stress had second-order polynomial relationship, with increasing properties up to about 7 years and decreasing properties thereafter. These results are most conservatively interpreted as suggesting that despite bisphosphonate-treatment there are changes in the structure over time that result in a loss of the benefits conferred during early treatment.
in vivo assessment of bisphosphonate-induced tissue properties
The section above highlights studies based on biopsy or other tissue sample that were removed from the patient during surgery (or post-mortem). A limitation of this work is that the study design is cross-sectional and thus the variability can be large. Over the past several years, indentation-based techniques have emerged that have the potential to measure tissue properties in vivo [23]. These techniques have recently been used to explore the effects of long-term bisphosphonate treatment [24]. Patients that had taken bisphosphonates for more than four years were separated into two groups, those that fractured on treatment and those that did not. The majority of fractures were vertebral fractures. In vivo impact microindentation was performed on the mid-tibia cortical bone and the lone parameter, bone material strength index (BMSi) was calculated. Individuals who had taken bisphosphonates and fractured had significantly lower BMSi compared to treated patients that had not fractured. This is consistent with previous data in which the indentation properties of long-term bisphosphonate-treated patients were assessed using a different type of impact microindentation [25]. In that work the indentation properties of those treated with bisphosphonates were not different from controls, and it was the presence of fracture that dictated whether indentation properties were different or not [25]. While these data suggest that in vivo indentation measures may be able to detect differential properties in fractured versus non-fractured bone there is no evidence to date that bisphosphonate treatment is detectible in humans using this method.
Bisphosphonates and mechanics – Where do we go from here?
The generation of human data in the realm of bisphosphonates and mechanics represents a significant step forward for the field. While it hasn’t provided a definitive answer to if/how these agents affect mechanics, it has laid a solid foundation on which we can build future questions and experiments. The next step will be to continue exploring mechanics, using a variety of techniques and tissues while at the same time looking deeper into other tissue properties to begin to answer the question of why bisphosphonates may be affecting mechanics (either universally or in select situations). Some studies have looked at properties of remodeling alterations, some at physical differences (lacunar density and microdamage) and others at properties of mineral or collagen. The incorporation of these tissue assessments is essential in an attempt to find some common ground of effects and the more of these properties that can be measured in each study the better. Obviously, the most efficient way to achieve such a goal is to foster collaborations across groups.
The basic premise of this whole concept is that bisphosphonates suppress remodeling and that, with longer term exposure, there are consequences of reduced remodeling that leave the tissue more potentially brittle. A recent experiment by our laboratory explored the effects of bisphosphonate treatment on two different commonly used mouse strains (A/J and B6) [26]. While we found that the response of mechanical properties was highly divergent (B6 animals had significant lower post-yield properties while A/J were unchanged) an equally interesting outcome is the fact that mice have reduced mechanics at all. As noted at the start of this review, mice don’t experience intracortical remodeling, certainly not at the age used in this work. So how exactly could bisphosphonates alter mechanical properties in such an experiment (and numerous other rodent papers in the literature)?
It is well accepted that bisphosphonates bind to the skeleton (it’s how they impart their anti-remodeling effect) and that this retention is dose dependent [27]. They bind to exposed bone surfaces and also penetrate deep into the matrix through the canalicular system [28]. Based on this we asked the question of whether incorporation of bisphosphonate in the bone matrix could affect mechanical properties. In a series of unpublished experiments, one where we dosed animals with bisphosphonate and then examined acute effects on mechanics (within 48 hrs of dosing) and another where we exposed bones to bisphosphonate in situ, we found significantly lower mechanical properties, specifically those in the post-yield region (Figure 5). Furthermore, we have quantified bisphosphonate accumulation differences in the cortex of A/J and B6 mice which follow patterns consistent with strain-specific effects on bone mechanics noted above [26]. Although more work is necessary, these results set up the provocative hypothesis that physical effects of the bisphosphonate accumulation in the matrix could play a role in altering mechanical properties.
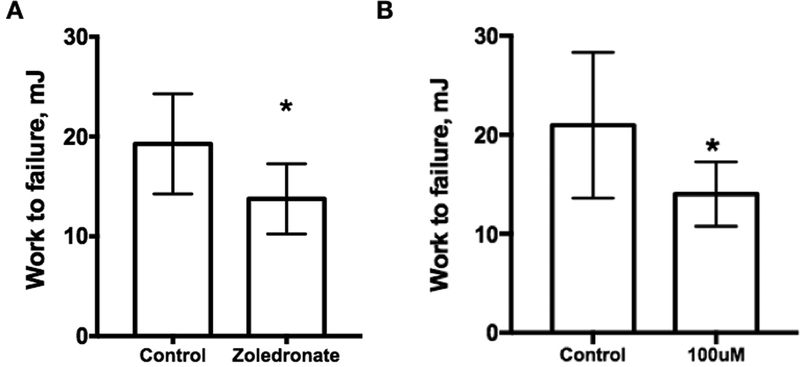
Figure 5.
Acute effect of zoledronate on mechanical properties. (A) Zoledronate was administered to 16-week-old male C57/B6 male mice on two consecutive days after which femora were extracted and tested in 4-point bending. Work to failure was significantly lower in animals treated with 48 hours of zoledronate compared to control (n=10/gp). (B) Femoral bones from 16-week-old male C57/B6 male mice were soaked in situ in control (phosphate buffered saline) or zoledronic acid (100 μm concentration) in a 37-degree incubator for 48 hours (n=12 bones/condition). Bones were then subjected to mechanical testing. Work to failure was significantly lower in bones soaked in zoledronate compared to control (n=10/gp). Together, these data suggest bisphosphonates, through binding within the bone matrix, could be having a physical effect on bone mechanics. Both experiments were approved by the Indiana University Institutional Animal Care and Use Committee prior to activity.
“The truth is rarely pure and never simple”. This certainly seems to concisely frame the question of how bisphosphonates affect bone mechanical properties. Yet it’s a question that still deserves investigation given that these drugs are highly effective at reducing fracture risk. Hopefully there continues to be investment in answering the question and the slope of publications shown in Figure 1 continues on a steep upward trajectory.
Acknowledgements
This work was supported by a United States (U.S.) Department of Veterans Affairs Merit Award (BX003025) and a NIH grant AR62002 to MRA.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CYC. Severely Suppressed Bone Turnover: A Potential Complication of Alendronate Therapy. Journal of Clinical Endocrinology & Metabolism. 2005;90:1294–301. [DOI] [PubMed] [Google Scholar]
- 2.Giusti A, Hamdy NAT, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy. Bone. Elsevier Inc; 2010;47:169–80. [DOI] [PubMed] [Google Scholar]
- 3.Dell RM, Adams AL, Greene DF, Funahashi TT, Silverman SL, Eisemon EO, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544–50. [DOI] [PubMed] [Google Scholar]
- 4.Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures: lessons from materials research. Bone. 2013.**A high-level review of atypical femoral fractures with proposed mechanisms, written by thought-leaders in the field.
- 5.Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23. [DOI] [PubMed] [Google Scholar]
- 6.Brown JP, Morin S, Leslie W, Papaioannou A, Cheung AM, Davison KS, et al. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician. 2014;60:324–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Allen MR. Preclinical Models for Skeletal Research: How Commonly Used Species Mimic (or Don’t) Aspects of Human Bone. Toxicol Pathol. 2017;4:019262331773392–4. [DOI] [PubMed] [Google Scholar]
- 8.Allen MR, Burr DB. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: What we think we know and what we know that we don’t know. Bone. 2011;49:56–65. [DOI] [PubMed] [Google Scholar]
- 9.Mashiba T, Turner CH, Hirano T, Forwood M, Jacob D, Johnston C, et al. Effects of high-dose etidronate treatment on microdamage accumulation and biomechanical properties in beagle bone before occurrence of spontaneous fractures. Bone. 2001;29:271–8. [DOI] [PubMed] [Google Scholar]
- 10.Kostenuik P On the Evolution and Contemporary Roles of Bone Remodeling. Osteoporosis. Elsevier; 2013. pp. 873–914.**A review of preclinical studies focused on bisphosphonates (actually on anti-osteoporosis agents in general) and mechanical properties.
- 11.Bajaj D, Geissler JR, Allen MR, Burr DB, Fritton JC. The resistance of cortical bone tissue to failure under cyclic loading is reduced with alendronate. Bone. 2014;64:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen MR, Reinwald S, Burr DB. Alendronate Reduces Bone Toughness of Ribs without Significantly Increasing Microdamage Accumulation in Dogs Following 3 Years of Daily Treatment. Calcif Tissue Int. 2008;82:354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acevedo C, Bale H, Gludovatz B, Wat A, Tang SY, Wang M, et al. Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone. Bone. 2015;81:352–63. [DOI] [PubMed] [Google Scholar]
- 14.Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2008;20:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2007;19:329–37. [DOI] [PubMed] [Google Scholar]
- 16.Saito M, Mori S, Mashiba T, Komatsubara S, Marumo K. Collagen maturity, glycation induced-pentosidine, and mineralization are increased following 3-year treatment with incadronate in dogs. Osteoporos Int. 2008;19:1343–54. [DOI] [PubMed] [Google Scholar]
- 17.Jin A, Cobb J, Hansen U, Bhattacharya R, Reinhard C, Vo N, et al. The effect of long-term bisphosphonate therapy on trabecular bone strength and microcrack density. Bone Joint Res. 2017;6:602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma S, Goh EL, Jin A, Bhattacharya R, Boughton OR, Patel B, et al. Long-term effects of bisphosphonate therapy: perforations, microcracks and mechanical properties. Sci. Rep. Nature Publishing Group; 2017;:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmermann EA, Schaible E, Gludovatz B, Schmidt FN, Riedel C, Krause M, et al. Intrinsic mechanical behavior of femoral cortical bone in young, osteoporotic and bisphosphonate- treated individuals in low- and high energy fracture conditions. Sci. Rep. Nature Publishing Group; 2016;:1–12.**Detailed mechanical assessment of clinical tissue from bisphosphonate (and control) treated individuals.
- 20.Krause M, Soltau M, Zimmermann EA, Hahn M, Kornet J, Hapfelmeier A, et al. Effects of long-term alendronate treatment on bone mineralisation, resorption parameters and biomechanics of single human vertebral trabeculae. Eur Cell Mater. 2014;28:152–63–discussion163–5. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd AA, Gludovatz B, Riedel C, Luengo EA, Saiyed R, Marty E, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proceedings of the National Academy of Sciences of the United States of America. 2017;49:201704460–6.**Detailed mechanical assessment of clinical tissue from bisphosphonate (and control) treated individuals. A sub-set of these samples were from patients who experienced atypical femoral fractures - the only data that currently exists from such patients.
- 22.Ward J, Wood C, Rouch K, Pienkowski D, Malluche HH. Stiffness and strength of bone in osteoporotic patients treated with varying durations of oral bisphosphonates. Osteoporos Int. Osteoporosis International; 2016:1–8. [DOI] [PubMed] [Google Scholar]
- 23.Allen MR, McNerny EM, Organ JM, Wallace JM. True Gold or Pyrite: A Review of Reference Point Indentation for Assessing Bone Mechanical Properties In Vivo. J Bone Miner Res. 2015;30:1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nogues X, Prieto-Alhambra D, Güerri-Fernández R, Garcia Giralt N, Rodriguez-Morera J, Cos L, et al. Fracture during oral bisphosphonate therapy is associated with deteriorated bone material strength index. Bone. 2017;103:64–9. [DOI] [PubMed] [Google Scholar]
- 25.Güerri-Fernández RC, Nogués X, Quesada Gómez JM, Torres del Pliego E, Puig L, García-Giralt N, et al. Microindentation for in vivo measurement of bone tissue material properties in atypical femoral fracture patients and controls. J Bone Miner Res. 2012;28:162–8. [DOI] [PubMed] [Google Scholar]
- 26.Aref MW, McNerny EMB, Brown D, Jepsen KJ, Allen MR. Zoledronate treatment has different effects in mouse strains with contrasting baseline bone mechanical phenotypes. Osteoporos Int. 2016;27:3637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin J Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18:75. [DOI] [PubMed] [Google Scholar]
- 28.Turek J, Ebetino FH, Lundy MW, Sun S, Kashemirov BA, Mckenna CE, et al. Bisphosphonate Binding Affinity Affects Drug Distribution in Both Intracortical and Trabecular Bone of Rabbits. Calcif Tissue Int. 2012;90:202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]