Abstract
Background:
Gait and balance impairment is common in secondary progressive multiple sclerosis (SPMS). Lipoic acid (LA), an over-the-counter antioxidant, is effective in MS animal models and may improve walking speed, but effects on mobility are unreported.
Objective:
Examine the effects of 1200 mg daily oral dose of LA versus placebo (PLA) on gait and balance in a 2-year, randomized, double-blind pilot study.
Methods:
134 participants were screened for eligibility before assignment to LA (n = 28) or PLA (n = 26). Included here were, 21 participants with SPMS who took LA (N = 11) or PLA (N = 10) capsules for 2 years (enrolled May 2, 2011 – August 14, 2015) and completed all tasks without the use of an assistive device. Participants completed the Timed Up and Go (TUG) and quiet standing tasks every 6 months while wearing inertial sensors (APDM Opals) to quantify mobility.
Results:
LA had a medium effect on time to complete TUG at 2 years (g = 0.51; 95% CI = - 0.35, 1.38). In a subset of 18 participants with less disability (EDSS < 6, no use of ambulatory device), turning time was significantly shorter with LA (p = 0.048, ∆= 0.48 s). No differences in balance metrics were found between groups.
Conclusions:
LA had an effect on walking performance in people with SPMS, particularly in those with lower baseline disability.
Keywords: antioxidant, inertial sensors, posture, rehabilitation, sway, timed up and go
Among physical functions impaired by multiple sclerosis (MS),walking ability s frequently considered most important.1 Walking impairment is associated with greater use of healthcare resources2 and increased fall risk. People with MS have larger postural sway and slower walking speed than age-matched control subjects.3 these impairments are often worse in those with secondary progressive MS (SPMS)4 than other forms of MS due to cerebellar and vestibular lesions.5
Few therapies have been shown to improve or maintain gait and balance in MS. Efficacious treatments might be expected to increase somatosensory conduction speed,6 or improve the ability of the central nervous system to integrate sensory information.3 Previous interventions to improve walking in MS have focused on physical training and have rarely been supported by an understanding of the neural mechanisms.7 Dalfampridine, a potassium channel antagonist, has been shown to improve walking speed in MS, but in clinical trials was deemed effective in only a third of participants.8 The efficacy of complementary and alternative medicine interventions for gait and balance has also been explored, but there is currently insufficient evidence to support practice recommendations for any modality.9 Few of the interventions for gait and balance have focused on SPMS, further limiting the applicability and generalizability of these prior findings.10
There is a need for therapies that slow the deterioration of gait and balance in SPMS. Lipoic acid (LA) is a readily available dietary supplement that serves as an antioxidant, can stimulate glucose uptake, and supports mitochondrial function.11 In the animal model of MS, experimental autoimmune encephalomyelitis, LA results in a dose-dependent reduction in disability accompanied by a MANUSCRIPT reduction in inflammation, demyelination, and central nervous system (CNS)-entering T cells.12,13 LA is well tolerated in people with MS and increases serum LA with daily oral doses of 1200 mg.14 pilot randomized controlled trial suggested LA improves the T25FW at 2 years.15 The purpose of this study was to examine the specific gait and balance metrics that contributed to suggested improvement in walking speed in the ambulatory SPMS participants enrolled in the 2-year pilot trial.
Methods
Design
The 2-year study15 from which participants for this analysis were drawn used a randomized, placebo-controlled, double -blind design. The Veterans Affairs Portland Health Care System (VAPORHCS) and Oregon Health & Science University (OHSU) Institutional Review Boards approved all study methods (FDA IND #110132, NCT0118881).
Participants
Participants were recruited from the VAPORHCS MS Center of Excellence clinic and the general public. Included participants in this analysis were 40–70 years old, diagnosed with SPMS as defined per the primary paper as “prior RRMS (2005 McDonald criteria), and current SPMS defined by MS disability progression in the absence of clinical relapse during the prior 5 years as determined by the principal investigator (PI) based on history and chart review”,15,16 Expanded Disability Status Scale (EDSS) ≤ 6.0, and able to walk at least 25 feet without an aid. Participants were allowed to choose to take glatiramer acetate, B-interferon, or no disease-modifying therapy during the study. Additional inclusion and exclusion criteria less relevant to the present study analyses are described elsewhere.15
Outcome Measures
The expanded Timed Up and Go (TUG) is a task used to assess function and rehabilitation progress in MS and is highly reliable.17 Participants are seated in a chair and then told to stand up, walk forward 7 meters to a tape mark on the floor, make a 180° turn, walk back to the chair, and sit down. Participants completed three different conditions of the TUG. First a TUG was completed at “normal walking speed” (TUG-normal) followed by a TUG done “walking as quickly as possible” (TUG-fast) and then with a cognitive dual-task. The dual-task TUG involved having participants subtract 3 from a starting number (e.g., 139) and continue subtracting 3 from the answer, while completing the TUG “as quickly as possible” (TUG-dual task). Participants were instructed to do their best on both tasks (i.e., walking and subtracting) rather than prioritizing one task and were permitted 30 seconds rest between each trial.
A quiet standing task measured balance as postural sway. Participants stood as still and as quietly as possible with arms crossed across the chest and feet separated by a template.18 Quiet standing was done for 30 seconds under three conditions: eyes open (EO), eyes closed (EC), and eyes closed with a dual-task, i.e. subtracting (EC-dual task) by 3 from a starting number (e.g., 165). Trials were required to last ≥ 5 seconds to be considered valid and were not conducted if the participant or administrator believed they could not complete them safely and without the use of an assistive device. During all tasks participants wore wireless inertial sensors (APDM Opals) on the ankles, wrists, sternum and lumbar back to quantify movement, as described previously.18,19 To reduce risk of Type 1 error, the study only tested for group differences in gait variables previously found different in MS (mixed types) from control participants as measured by inertial sensors.18
Procedure
Informed written consent was obtained at the screening visit. The study principal investigator confirmed study eligibility and completed a neurological exam to determine disability level using the EDSS. During this screening visit, participants then completed 3 practice trials of each TUG and sway condition, results of which were not used in the longitudinal data analysis. After the screening visit, participant study treatment was block-randomized according to baseline EDSS (≤ 4.5 vs. > 4.5) by a research pharmacist not otherwise involved in the study. Study drug was from Pure Encapsulations®, who provided capsules containing 600 mg of racemic LA or placebo (PLA; Avicel™, microcellulose crystal and 4.3 mg quercetin) in gelatin capsules. Participants were instructed to take 2 capsules with food daily. Participants and study staff (including primary care physicians and neurologists) were blinded to treatment assignments.
The baseline visit was scheduled within 30 days of the screening visit. Subsequent study visits occurred every 3 months ± 2 weeks until study completion at 2 years for a total of 5 visits that included outcome measures. At each visit, participants completed 3 trials of each TUG and sway task. All tasks were completed in 3 cycles (rather than completing each trial consecutively) to minimize practice effects and fatigue.
Statistical Analysis
Data were analyzed in SPSS version 22 (IBM Corp., Armonk, NY) before the blind was broken. Reliability of each 3 trial TUG and quiet standing condition block was assessed at each time point for each treatment (LA, PLA) using intraclass correlation coefficients with a two-way mixed model for consistency. Reliability was acceptable (α ≥ .85) at each time point and the median of each trial block was used in subsequent analyses. Data were checked for outliers (> 3 SDs from the mean) and normality using residual and Q-Q plots, and Shapiro-Wilk tests. Outlying values were transformed to the next most extreme score plus one. When data did not meet normality assumptions after the transformation of outlying values for several variables (e.g., jerk, RMS sway), a square root transformation was applied to yield a normal distribution. Mixed models were used to test and estimate treatment X time interactions for each variable, with EDSS score entered as a covariate. Mixed models allow for consideration of the correlation between measures in longitudinal data and can be used with missing data . 20 A subgroup analysis was also conducted that included only participants with EDSS< 6 who were on treatment for the entire 2-year study (n = 18), because baseline disability and adherence could influence the treatment response. series of one-way ANCOVAs, controlling for group differences in EDSS, tested if change scores (2 years – baseline) were different according to treatment group (LA, PLA). Hedges’ g and corresponding 95% confidence intervals were calculated using complete data to estimate effect size,21 which are considered small and medium at values of .20 and .50, respectively.22 To calculate g, the change in the PLA group (baseline – post) was subtracted from the change in the LA group, and divided by the pooled standard deviation from baseline.
Results
Demographic characteristics of participants that completed the full study are provided in Table 1. Treatment compliance (measured by pill counts) was high (> 80%) and not different between LA and PLA. 25 participants were assigned to treatment (and included in the mixed models), with 21 completing the full 2-year study.
Table 1.
Demographic Characteristics of Study Participants
| Variable | Lipoic Acid (N = 11) | Placebo (N = 10) |
|---|---|---|
| Age (years) | 55.8 ± 5.7 | 55.7 ± 4.1 |
| Male/Female | 5/6 | 5/5 |
| Height (cm) | 170.9 ± 9.6 | 168.9 ± 8.8 |
| Weight (kg) | 73.9 ± 18.4 | 75.0 ± 13.7 |
| EDSS (median, range) | 3.5 (3–6) | 4 (3–6) |
| MS Duration (years) | 28.9 ± 8.6 | 22.7 ± 8.5 |
| # Taking Glatiramer acetate | 3 | 3 |
| # Taking B-Interferon | 3 | 3 |
| # Taking No DMT | 5 | 4 |
Timed Up and Go
There were no statistically significant treatment X time interactions (all p ≥ 0.88) for time to complete the TUG in any of the 3 conditions. However, LA had a small effect on time to complete the TUG-fast at 6 months (g = 0.23; 95% CI = −0.62, 1.09) that increased by 2 years (g = 0.51; 95% CI = −0.35, 1.38). The effect at 2 years equates to over a 2 second difference in TUG completion time, and is due to the PLA group getting slower (12.87%, 95% CI = −7.88%, 33.62%) over time and the LA group only slowing by 0.60% (95% CI = - 3.96%, 5.16%) as shown (Figure 1). Individual data (Figure 2) for the TUG-fast shows that LA resultedin 4/11(36%)participants walking faster after 2 years, whereas only 1/10 (10%) of those taking PLA walked faster.
Figure 1.
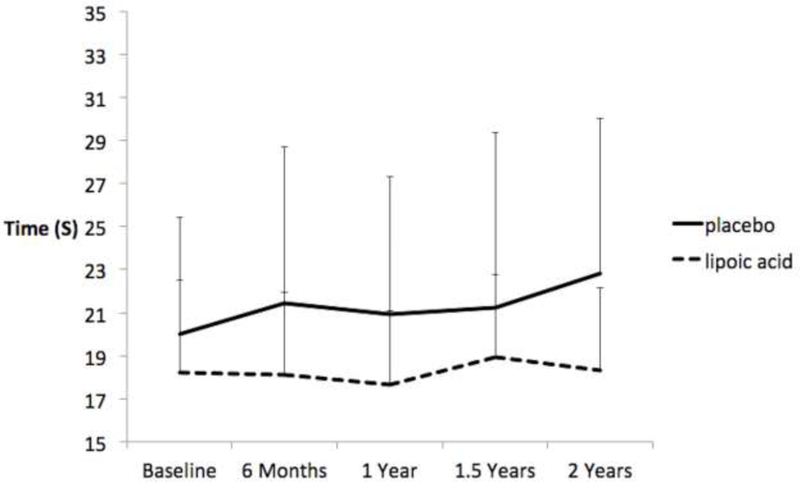
Effects of lipoic acid and placebo on time to complete the 7-meter timed up and go in the fast speed walking condition. Bars are standard deviations.
Figure 2.
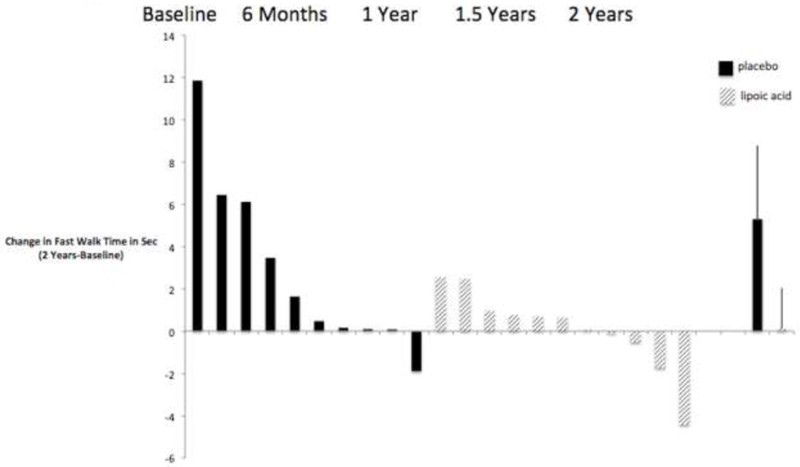
Change from baseline in time to complete the 7-meter timed up and go in the fast walking condition for each participant taking lipoic acid and place bo.A positive change indicates slowing from baseline and a negative change indicates improvement from baseline. The last two columns on the far right show the mean change (bars are standard deviations). Only participants with data at all visits are shown.
A similar effect was seen for the TUG-dual task condition at 6 months (g = 0.25, 95% CI = −0.60, 1.11) that also increased at 2 years (g = 0.44; 95% CI = −0.42, 1.31; Figure 3). The group difference at 2 years amounted to over 2 seconds due to a slowing of the PLA group. Individually, 8/11 (73%) of participants taking LA walked faster by 2 years while 5/10 (50%) of those taking PLA walked faster (data not shown). However, there was no effect of LA on time to complete the TUG-normal at 6 months (g = −0.14; 95% CI = −1.00, 0.71) or 2 years (g = 0.05; 95% CI = −0.79, 0.91).
Figure 3.
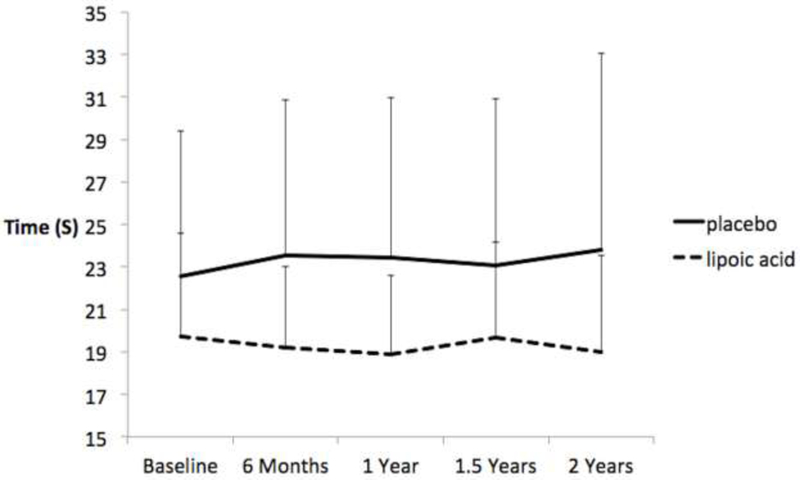
Effects of lipoic acid and placebo on time to complete the 7-meter timed up and go in the walking while subtracting condition (dual task). Bars are standard deviations.
The subgroup analysis, including only those participants with EDSS < 6 who completed the full study, found a significant difference in TUG-fast turning time, F(1,15) = 4.65, p = 0.048. At the end of 2 years, participants in the LA condition turned an adjusted average of 0.30 (SE = .14) seconds faster by study end, whereas those taking PLA turned 0.18 (SE = .16) seconds slower (effect size g=1.07;95%CI=0.08,2.06).individual data demonstrate that 8/10 (80%) participants taking LA turned faster by study end versus 2/8 (25%) participants taking PLA turned faster, as shown in Figure 4. There were no differences between the LA and PLA for any other TUG outcomes in the sub-analysis (all p ≥ 0.115).
Figure 4.

Change from baseline in turning time during the 7-meter timed up and go in the fast walking condition for each participant taking lipoic acid and placebo. positive change indicates slowing from baseline and a negative change indicates improvement from baseline. Only participants who completed the 2 year study with EDSS < 6 are shown.
Descriptive characteristics (M, SD) are presented in Table 2, and effect sizes (g) in Table 3. There were no significant treatment X time interactions for any gait measures captured by inertial sensors (all p ≥ 0.15).
Table 2.
Means and Standard Deviations for Selected Gait and Balance Measures
| Task | Lipoic Acid | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|
| Measurement Time | Baseline | 6 Months | 1 Year | 2 Years | Baseline | 6 Months | 1 Year | 2 Years |
| Fast Walking | ||||||||
| Trunk Horizontal RoM (º) | 5.25 ± 1.59 | 5.85 ± 2.32 | 5.37± 1.41 | 5.93 ± 1.65 | 6.06 ± 2.26 | 6.51 ± 2.53 | 6.52 ± 2.13 | 6.80 ±1.94 |
| Trunk Saggital RoM (º) | 4.97 ± 1.13 | 5.12 ± 0.99 | 5.30± 1.31 | 5.38 ± 1.53 | 6.36 ± 1.35 | 5.91 ± 1.22 | 5.39 ± 0.97 | 5.99 ±1.20 |
| Trunk Frontal RoM (º) | 10.24 ± 2.38 | 10.24 ± 2.91 | 10.48 ± 2.26 | 10.38 ± 3.19 | 11.17 ± 2.43 | 11.37 ± 2.52 | 10.77 ± 2.05 | 11.6±3.03 |
| Turn Duration (s) | 2.59 ± 1.00 | 2.37 ± 0.64 | 2.50± 0.85 | 2.30 ± 0.78 | 2.89 ± 0.85 | 2.90 ± 1.08 | 3.22 ± 1.04 | 3.16 ±1.35 |
| Walking + Subtracting (dual task) | ||||||||
| Trunk Horizontal RoM (º) | 5.46 ± 1.74 | 6.26 ± 2.29 | 6.06±1.73 | 6.08 ± 1.56 | 6.97 ± 2.75 | 7.12 ± 2.30 | 6.75 ± 2.67 | 7.33 ±2.67 |
| Trunk Saggital RoM (º) | 5.09 ± 1.04 | 5.31 ± 1.18 | 5.40± 1.18 | 5.33 ± 1.74 | 6.46 ± 1.13 | 6.06 ± 1.15 | 5.57 ± 1.18 | 6.19 ±1.33 |
| Trunk Frontal RoM (º) | 11.06 ± 2.65 | 11.20 ± 2.60 | 10.50 ± 2.44 | 11.04 ± 2.26 | 11.14 ± 2.55 | 10.69 ± 2.47 | 10.20 ± 2.25 | 11.00±3.00 |
| Turn Duration (s) | 2.60 ± 0.96 | 2.26 ± 0.54 | 2.67± 1.05 | 2.58 ± 0.72 | 2.86 ± 1.11 | 3.02 ± 1.13 | 3.34 ± 1.16 | 3.10 ±1.33 |
|
Quiet Stance, Eyes-Closed Sway Acceleration (m/s2) |
0.15 ± 0.06 | 0.19 ± 0.11 | 0.14± 0.05 | 0.18 ± 0.11 | 0.14 ± 0.06 | 0.14 ± 0.04 | 0.14 ± 0.11 | 0.12 ±0.06 |
| Normalized Jerk ML | 1.96 ± 0.36 | 2.25 ± 0.56 | 2.02± 0.35 | 1.74 ± 0.28 | 2.15 ± 0.43 | 2.02 ± 0.39 | 2.24 ± 0.63 | 2.19 ±0.46 |
| Quiet Stance, Eyes-Closed, Subtraction | ||||||||
| Sway Acceleration (m/s2) | 0.13 ± 0.04 | 0.19 ± 0.12 | 0.14± 0.06 | 0.16 ± 0.07 | 0.15 ± 0.09 | 0.17 ± 0.12 | 0.14 ± 0.06 | 0.15±0.09 |
| Normalized Jerk ML | 2.24 ± 0.27 | 2.43 ± 0.67 | 2.11± 0.51 | 1.99 ± 0.25 | 2.40 ± 0.46 | 2.05 ± 0.46 | 2.35 ± 0.44 | 2.37 ±0.61 |
Table 3.
Effect Size (Hedges g) of Lipoic Acid Calculated from Baseline
| Task | 6 Months | 1 Year | 1.5 Years | 2 Years |
|---|---|---|---|---|
| Fast Walking | ||||
| Trunk Horizontal RoM | −0.07 (−0.95, 0.80) | 0.17 (−0.70, 1.06) | −0.15 (−1.03, 0.73) | 0.02 (−0.84, 0.91) |
| Trunk Saggital RoM | −0.50 (−1.39, 0.39) | −1.06 (−2.00, −0.12) | −0.77 (−1.69, 0.13) | −0.63 (−1.54, 0.26) |
| Trunk Frontal RoM | 0.08 (−0.79, 0.96) | −0.27 (−1.15, 0.61) | −0.20 (−1.09, 0.67) | 0.14 (−0.74, 1.02) |
| Turn Duration | 0.23 (−0.64, 1.11) | 0.44 (−0.44, 1.33) | 0.33 (−0.55, 1.21) | 0.59 (−0.30, 1.49) |
| Walking + Subtracting (dual task) | ||||
| Trunk Horizontal RoM | −0.28 (−1.17, 0.59) | −0.36(−1.25, 0.52) | −0.30 (−1.19, 0.58) | −0.11 (−0.99, 0.77) |
| Trunk Saggital RoM | −0.57 (−1.47, 0.32) | −1.11(−2.06, −0.16) | −0.33 (−1.21, 0.55) | −0.47 (−1.36, 0.41) |
| Trunk Frontal RoM | −0.23 (−1.11, 0.65) | −0.14(−1.02, 0.73) | −3.88 (−5.37, −2.39) | 0.09 (−0.78, 0.97) |
| Turn Duration | 0.48 (−0.41, 1.37) | 0.39 (−0.49, 1.28) | 0.20 (−0.67, 1.09) | 0.24 (−0.63, 1.12) |
| Quiet Stance, Eyes-Closed | ||||
| Sway Acceleration Amplitude | −0.72(−1.77, 0.32) | 0.26 (−0.75, 1.28) | −0.75(−1.80, 0.29) | −0.65 (−1.69, 0.38) |
| Normalized Jerk ML | −1.08(−2.16, 0.003) | 0.05 (−0.95, 1.07) | −0.37(−1.39, 0.65) | 0.62 (−0.40, 1.66) |
| Quiet Stance, Eyes-Closed, dual task | ||||
| Sway Acceleration Amplitude | −0.47(−1.50, 0.55) | −0.28(−1.30, 0.73) | −1.36(−2.49, −0.23) | −0.46 (−1.48, 0.56) |
| Normalized Jerk ML | −1.48(−2.63, −0.34) | 0.20 (−0.80, 1.22) | −1.50(−2.64, −0.35) | 0.58 (−0.44, 1.62) |
Postural Sway
There were no statistically significant treatment X time interactions (all p ≥ 0.09) for any of the sway measures in any quiet standing condition (EO, EC, EC-dual task). Descriptive characteristics (M, SD) are presented in Table 2, and effect size (g) is shown in Table 3 specifically for sway measures expected from previous research to be sensitive to change.18 The subgroup analysis also did not find any significant group differences on measures hypothesized to change (all p ≥ 0.10).
Discussion
This secondary analysis of a 2 year, double-blind, placebo-controlled study suggests that daily oral intake of 1200mg LA may preserve walking ability in people with SPMS, in that the time to complete the TUG-fast and the TUG- dual task was maintained. In a subgroup of participants with EDSS < 6 who completed the entire study, turning time in the TUG-fast was significantly better for LA compared to PLA. This suggests that a faster turning time among those taking LA may have helped to maintain TUG completion time. These preliminary findings are important given the need for therapies that maintain gait and balance in people with SPMS. Results here provide important information necessary for designing large clinical trials that are optimally designed to maximize treatment effects.
Inertial sensors in this study detected a significant difference in turning duration when there was not a statistically significant difference in overall TUG completion time. Body-worn sensors have been shown to be more sensitive than walking time alone in detecting gait differences in MS, suggesting that the responsible parameter or group of parameters can be identified by this method.18 Previous research has found that persons with MS turn slower, even when they have straight walking speed that is not discernable from control participants.18 Inertial sensors have also found that turn duration may slow over time in people with Parkinson’s disease23 and turning is different in people with mild traumatic brain injury when compared to control participants.24 Turning duration is also an ecologically valid outcome because nearly half of daily steps are not straight25, with people in the general population turning about 70 times per hour.26 Turning time, captured by inertial sensors, may be a particularly sensitive outcome in future large clinical trials of LA for persons with SPMS.
There are several possible explanations why LA could have a small effect on time to complete walking tasks and on turn duration. First, LA could improve walking performance due to enhanced skeletal muscle function rather than altered CNS function. LA supports mitochondrial function as an essential co-factor for pyruvate dehydrogenase during aerobic respiration, and is involved in synthesis of mitochondrial nucleic acids.27,28 Completion time differences could result from preserved cardiopulmonary function and faster walking during the course of the study, in addition to CNS neuroprotection. Indeed, people with MS have slowed phosphocreatine resynthesis, which is influenced by mitochondrial function and represents a measure of skeletal muscle oxidative capacity.29 It is also possible that time to complete walking tasks is influenced by task motivation, but that balance during walking and standing is not. Further investigation evaluating the LA mechanisms of action is necessary so that future clinical trials can be designed to induce the greatest effects.
This study has several limitations. The greatest limitation is the small sample size with large confidence intervals around study point estimates. Second, time to complete the walking tasks was highly variable in this study sample with the coefficient of variation often > 20%. Prior studies have noted the high variability of performances on physical measures in MS studies and suggested that a change of at least 20% is needed to assure clinical meaningfulness.30,31 This study did not account for factors such as sleep, depression, and concurrent level of fatigue that could have impacted results. Future studies should attempt to reduce this variability by controlling these confounds to physical performance as much as possible. Based on our pilot study results, future trials testing the effect of LA on walking in SPMS may detect the largest effects by recruiting participants with EDSS < 6 and using turning time in fast walking tasks as the primary outcome variable.
In summary, our results suggest that LA may preserve walking performance in people with SPMS. The maintenance of the time to complete the TUG may be driven by an increase in turning speed as seen in those with EDSS < 6. The findings warrant further investigation of use of LA as a treatment for walking impairment given the burden of walking speed and turning limitations in people with SPMS,32 and the limited number of treatments that preserve walking speed.8
Highlights.
Two-year randomized, double-blind trial of lipoic acid
Secondary-progressive multiple sclerosis (n = 21) with no assistive device use
Timed up and go and standing balance measured by wireless inertial sensors
Moderate effect of lipoic acid on preserving walking speed.
Subgroup analysis found significantly faster turning time with lipoic acid.
Acknowledgements
Work supported by NIH-NCCIH T32 AT002688 (BDL) and the National Multiple Sclerosis Society MB0011 (FBH). The research reported here was supported by grant B7493-W (R. Spain) from the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service. Additional support came from NIH grant UL1TR000128. Pure Encapsulations (Sudbury, MA) provided the LA and placebo. The authors also thank the study participants, Dr. Elizabeth Heriza for study management, and Charles Murchison for feedback on statistical analyses. This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI) grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Role of Funding Source
Work supported by NIH-NCCIH T32 AT002688 (BDL) and the National Multiple Sclerosis Society MB0011 (FBH). The research reported here was supported by grant B7493-W (R. Spain) from the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service. Additional support came from NIH grant UL1TR000128. Pure Encapsulations (Sudbury, MA) provided the LA and placebo. This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI) grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: Lipoic Acid for Secondary Progressive Multiple Sclerosis https://clinicaltrials.gov/ct2/show/NCT01188811?term=spain+lipoic+acid&rank=1 NCT01188811
Conflict of Interest
B. Loy and B. Fling declare no conflicts of interest. OHSU and Dr. Horak have a significant financial interest in ADPM, a company that may have a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OHSU. D. Bourdette received travel funding from National Multiple Sclerosis Society, Consortium of MS Centers, and Paralyzed Veterans of America; serves on the editoral board for Neurology; holds patents for treatment of multiple sclerosis with cyclic peptide derivatives of cyclosporin and thyromimetic drugs for stimulating remyelination in multiple sclerosis; consulted for Magellan Health, Best Doctors, Inc.; and received research support from National MS Society. R. Spain received research support from Department of Veterans Affairs, Oregon Clinical and Translational Research Institute, VA ort land Health Care System, Oregon Health & Science University ,National MS Society ,Conrad Hilton Foundation ,Medical Research Foundation of Oregon, and Raceto Erase MS.
References:
- 1.Heesen C, Bohm J, Reich C, Kasper J, Goebel M and Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Multiple sclerosis 2008; 14: 988–91. [DOI] [PubMed] [Google Scholar]
- 2.Pike J, Jones E, Rajagopalan K, Piercy J and Anderson P. ocial and economic burden of walking and mobility problems in multiple sclerosis. BMC Neurology 2012; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron MH and Lord S. Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep 2010; 10: 407–12. [DOI] [PubMed] [Google Scholar]
- 4.Confavreux C, Vukusic S, Moreau T and Adeleine P. Relapses and progression of disability in multiple sclerosis. The New England Journal of Medicine 2000; 343: 1430–8. [DOI] [PubMed] [Google Scholar]
- 5.Soyuer F, Mirza M and Erkorkmaz U. Balance performance in three forms of multiple sclerosis. Neurological Research 2006; 28: 555–62. [DOI] [PubMed] [Google Scholar]
- 6.Cameron MH, Horak FB, Herndon RR and Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosensory & motor research 2008; 25: 113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunn H,Markevics S,Haas B,Marsden J and Freeman J Systematic Review: The Effectiveness of Interventions to Reduce Falls and Improve Balance in Adults With Multiple Sclerosis. Arch Phys Med Rehabil 2015; 96: 1898–912. [DOI] [PubMed] [Google Scholar]
- 8.Dunn J and Blight . Dalfampridine: a brief review of its mechanism of action and efficacy as a treatment to improve walking in patients with multiple sclerosis. Curr Med Res Opin 2011; 27: 1415–23. [DOI] [PubMed] [Google Scholar]
- 9.Yadav V, Bever C, Bowen J, et al. Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis. Neurology 2014; 82: 1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinstein A, Freeman J and Lo AC. Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. The Lancet Neurology 2015; 14: 194–207. [DOI] [PubMed] [Google Scholar]
- 11.Salinthone S, Yadav V, Bourdette D and Carr DW. Lipoic acid: a novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the cns. Endocrine, Metabolic & Immune Disorders-Drug Targets 2008; 8: 132–42. [DOI] [PubMed] [Google Scholar]
- 12.Marracci GH, Jones RE, McKeon GP and Bourdette D. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. Journal of Neuroimmunology 2002; 131: 104–14. [DOI] [PubMed] [Google Scholar]
- 13.Morini M, Roccatagliata L, Dell’Eva R, et al. Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J Neuroimmunol 2004; 148: 146–53. [DOI] [PubMed] [Google Scholar]
- 14.Yadav V, Marracci G, Lovera J, et al. Lipoic acid in multiple sclerosis: a pilot study. Multiple sclerosis 2005; 11: 159–65. [DOI] [PubMed] [Google Scholar]
- 15.Spain RI, Powers K, Murchison C, et al. Lipoic acid in secondary progressive multiple sclerosis: A randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm 2017; 4: e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Annals of Neurology 2005; 58: 840–6. [DOI] [PubMed] [Google Scholar]
- 17.Nilsagard Y, Lundholm C, Gunnarsson L- G and Denison E. Clinical relevance using timed walk tests and ‘timed up and go’ testing in persons with Multiple Sclerosis. Physiotherapy Research International 2007; 12: 105–14. [DOI] [PubMed] [Google Scholar]
- 18.Spain RI, St George RJ, Salarian A, et al. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait & posture 2012; 35: 573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancini M, King L, Salarian A, Holmstrom L, McNames J and Horak FB. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. Journal of bioengineering & biomedical science 2011; Suppl 1: 007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molenberghs G and Kenward MG. Missing Data in Clinical tudies. The Atrium, Southern Gate, Chichester, West Sussex: PO19 8SQ, England: John Wiley & Sons, Ltd., 2007. [Google Scholar]
- 21.Hedges LV and Olkin I. Statistical methods for meta-analysis New York: Academic Press, 1985. [Google Scholar]
- 22.Cohen J A power primer. Psychological bulletin 1992; 112: 155–9. [DOI] [PubMed] [Google Scholar]
- 23.Mancini M and Horak FB. Potential of PDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert review of medical devices 2016; 13: 455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fino PC, Parrington L, Walls M, et al. Abnormal Turning and Its Association with Self-Reported Symptoms in Chronic Mild Traumatic Brain Injury. Journal of neurotrauma 2018 [DOI] [PMC free article] [PubMed]
- 25.Glaister BC, Bernatz GC, Klute GK and Orendurff MS. Video task analysis of turning during activities of daily living. Gait & posture 2007; 25: 289–94. [DOI] [PubMed] [Google Scholar]
- 26.Mancini M, El-Gohary M, Pearson S, et al. Continuous monitoring of turning in Parkinson’s disease: Rehabilitation potential. NeuroRehabilitation 2015; 37: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Packer L, Tritschler HJ and Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radical Biology & Medicine 1997; 22: 359–78. [DOI] [PubMed] [Google Scholar]
- 28.Biewenga G , Dorstijn MA, Verhagen JV, Haenen GR and Bast A. Reduction of lipoic acid by lipoamide dehydrogenase. Biochemical Pharmacology 1996; 51: 233–8. [DOI] [PubMed] [Google Scholar]
- 29.Kent-Braun JA, Sharma KR, Miller RG and Weiner MW. Postexercise phosphocreatine resynthesis is slowed in multiple sclerosis. Muscle & Nerve 1994; 17: 835–41. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman M, Moyer J. The significant change for the timed 25-foot walk in the multiple sclerosis functional composite. Multiple sclerosis 2000; 6: 286–90. [DOI] [PubMed] [Google Scholar]
- 31.Goldman MD, Motl RW, Scagnelli J, Pula JH, Sosnoff JJ and Cadavid D. Clinical meaningful performance benchmarks in MS: Timed 25-foot walk and the real world. Neurology 2013; 81: 1856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yildiz M The impact of slower walking speed on activities of daily living in patients with multiple sclerosis. International journal of clinical practice 2012; 66: 1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


