Abstract
Neutrophils, basophils, and monocytes are continuously produced in bone marrow via myelopoiesis, circulate in blood, and are eventually removed from circulation to maintain homeostasis. To quantitate the kinetics of myeloid cell movement during homeostasis, we applied 5-bromo-2′-deoxyuridine (BrdU) pulse labeling in healthy rhesus macaques (Macaca mulatta) followed by hematology and flow cytometry analyses. Results were applied to a mathematical model and the blood circulating half-life and daily production, respectively, of each cell type from macaques aged 5–10 years old were calculated for; neutrophils (1.63 ± 0.16 days, 1.42 × 109 cells/L/day), basophils (1.78 ± 0.30 days, 5.89 × 106 cells/L/day), and CD14+CD16− classical monocytes (1.01 ± 0.15 days, 3.09 × 108 cells/L/day). Classical monocytes were released into the blood circulation as early as one day after dividing, while neutrophils remained in bone marrow 4-5 days before being released. Among granulocytes, neutrophils and basophils exhibited distinct kinetics in bone marrow maturation time and blood circulation. With increasing chronological age, there was a significant decrease in daily production of neutrophils and basophils but the half-life of these granulocytes remained unchanged between 3-19 years of age. In contrast, daily production of classical monocytes remained stable through 19 years of age but exhibited a significant decline in half-life. These results demonstrated relatively short half-lives and continuous replenishment of neutrophils, basophils, and classical monocytes during homeostasis in adult rhesus macaques with compensations observed during increasing chronological age.
Introduction
Granulocytes (including neutrophils, eosinophils, basophils) and monocytes are myeloid-lineage cells that differentiate from myeloblasts in bone marrow and are well known for participation in innate immune responses for pathogen clearance and inflammation. Their production in bone marrow, circulation kinetics, and subsequent removal are tightly regulated to maintain homeostasis (1, 2). Qualitative descriptions of myeloid cell development and regulatory mechanisms have been reported, but quantitative measures such as half-life and production rate in vivo are less clearly understood (3, 4).
During homeostasis, myeloid cells are continuously produced via bone marrow hematopoiesis. Precursor cells are generated from hematopoietic stem cells and retained in bone marrow while undergoing differentiation. Neutrophils are the predominant white blood cell (WBC) population ranging from 20% to 50% in rhesus macaques. Mature neutrophils leave bone marrow, enter and circulate in blood, and then leave circulation after a specified period of time (3). In contrast, basophils comprise less than 1% of WBC during homeostasis, although their numbers may increase during parasite infections and allergic inflammation (5, 6). Little is known about basophil development and kinetics during homeostasis, mainly due to their scarcity at steady state. Monocytes comprise between 2% and 10% of WBC. At least three subsets have been identified in humans and rhesus macaques consisting of CD14+/CD16− classical monocytes, CD14+/CD16+ intermediate monocytes, and CD14−/CD16+ non-classical monocytes, and these subsets exhibit distinctive kinetics (7, 8).
Limitations exist for defining the half-life of myeloid cells in humans due to potential toxicity of in vivo labels and difficulty in consecutive bone marrow and blood sampling needed to validate cell kinetics. Translating results from rodents is confounded by the more predominant lymphocytes in mouse blood compared to the higher percent of neutrophils in human blood (9, 10). Thus, nonhuman primates are relevant because they are genetically and physiologically similar to humans, exhibit a similar WBC composition as humans, and can be applied to more effective in vivo cell proliferation labeling and specimen sampling. The purpose of this study therefore, was to; 1) develop a mathematical model to investigate and compare the kinetics of neutrophils, basophils and classical monocytes in adult rhesus macaques using in vivo pulse BrdU labeling, 2) confirm the biological relevance of the model by sampling bone marrow after the administration of the label, and 3) apply the model to a cohort of older animals to investigate the chronological aging effects on the kinetics of myeloid cells.
Materials and methods
Rhesus macaques
Forty-five Indian origin rhesus macaques specific pathogen-free for SIV, Type D Simian Retrovirus, and Simian T-cell Leukemia Virus type 1 from the Tulane National Primate Research Center (TNPRC) were studied (Table II). One group comprised 11 adult males between 5 and 10 years of age to study myeloid cell kinetics. A second group of 33 males and females ranging from 3 to 20 years of age were used to consider the effects of chronological aging on myeloid cell kinetics. All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approval by the Tulane University Institutional Animal Care and Use Committee (IACUC) (11).
Table II.
Animals used in the study
| Group 1 | Group 2 | ||||
|---|---|---|---|---|---|
| ID | Sex1 | Age (years) | ID | Sex1 | Age (years) |
| DG09 | M | 10.3 | GP56 | M | 3.0 |
| DR67 | M | 9.2 | GM77 | M | 3.1 |
| EM89 | M | 8.2 | GL96 | M | 3.2 |
| ER17 | M | 8.3 | GH64 | M | 3.3 |
| FI38 | M | 7.8 | FG54 | M | 5.2 |
| GK40 | M | 5.5 | FF01 | F | 5.3 |
| GN17 | M | 5.8 | EM66 | F | 6.2 |
| GN24 | M | 5.2 | EL53 | M | 6.2 |
| HA52 | M | 5.0 | EF03 | F | 6.3 |
| IR99 | M | 6.2 | EM25 | F | 6.9 |
| IT27 | M | 5.7 | EE66 | F | 7.5 |
| DJ87 | F | 8.3 | |||
| DG85 | F | 8.3 | |||
| BR31 | F | 10.5 | |||
| BJ22 | F | 11.0 | |||
| BG19 | F | 11.4 | |||
| AM92 | F | 12.3 | |||
| AI70 | F | 12.4 | |||
| T053 | M | 14.4 | |||
| P306 | M | 16.3 | |||
| P153 | M | 16.3 | |||
| CN73 | M | 16.4 | |||
| HF74 | F | 17.8 | |||
| IR93 | F | 18.5 | |||
| IR86 | F | 18.9 | |||
| IR91 | F | 18.9 | |||
| HF73 | F | 19.0 | |||
| IR89 | F | 19.0 | |||
| IR87 | F | 19.4 | |||
| IR94 | F | 19.4 | |||
| IR85 | F | 19.4 | |||
| IR92 | F | 19.5 | |||
| IR88 | F | 19.5 | |||
| IR90 | F | 19.5 | |||
M indicates male; F indicates female.
BrdU administration and collection of blood and bone marrow specimens
BrdU (Sigma, B5002-100G), a thymidine analogue, was prepared at 30 mg/ml in endotoxin-free PBS (EMD Millipore Corporation, TMS-012-A), filter sterilized through 0.2 μm polyethersulfone membranes (Steriflip, Fisher Scientific, SCGP00525 or Fisher Scientific, 09-740-2A), and administered intravenously at a dose of 60 mg/kg body weight. Blood and bone marrow aspirates were obtained between 8 AM and 11 AM on collection days after BrdU injections to minimize effects of daily oscillations on the kinetics studies.
Flow cytometry and hematology analyses
Cellular immuno-phenotyping and staining for BrdU incorporation were performed as described.(8, 12) Briefly, 200 μl EDTA (ethylenediaminetetraacetic acid)–anticoagulated whole blood was washed and stained with surface monoclonal antibody (mAb) cocktails (Table I). Red blood cells (RBC) were lysed with FACS lysing solution (BD Bioscience, San Jose, CA, USA) and remaining cells were permeabilized with a three-step Cytofix/Cytoperm protocol per manufacturer instructions (BD Biosciences). Bone marrow aspirates were washed, filtered through a 100 μm cell strainer (Corning, Corning, NY, USA), and treated with 10 ml ammonium-chloride-potassium lysing buffer (ACK Lysing Buffer, Lonza) to remove RBC. Approximately one million bone marrow cells were then stained as for whole blood. Samples were acquired with a LSR II or LSRFortessa flow cytometer (BD Bioscience, San Jose, CA, USA) and data were analyzed with FlowJo software (version 10 FlowJo, LLC, Ashland, OR, USA). Hematology analyses were performed on a Sysmex XT-2000iV automated hematology analyzer (Sysmex America, Inc., Lincolnshire, IL). Absolute cell counts for neutrophils and basophils were directly measured from the automated hematology analyzer. The absolute cell counts for classical monocytes were calculated from the percentages of classical monocytes in the overall monocyte population (including classical monocytes, intermediate monocytes, and nonclassical monocytes) by flow cytometry analysis and multiplied by the absolute cell counts for all monocytes.
Table I.
Antibodies used in the study
| Antibody | Clone | Source |
|---|---|---|
| CD1c | AD5-8E7 | Miltenyi Biotec |
| CD3 | SP34-2 | BD Biosciences |
| CD8 | SK1 | BD Biosciences |
| CD11b | ICRF44 | BD Biosciences |
| CD11c | 3.9 | eBioscience |
| CD14 | M5E2 | BD Biosciences |
| CD16 | 3G8 | BD Biosciences |
| CD20 | B9E9 | Beckman Coulter |
| CD45 | MB4-6D6 | Miltenyi Biotec |
| CD123 | 7G3 | BD Biosciences |
| CD163 | Mac2-158 | Trillium |
| CD169 | 7-239 | Biolegend |
| HLA-DR | L243 | BD Biosciences |
| Myeloperoxidase (MPO) | 5B8 | BD Biosciences |
| BrdU | 3D4 | BD Biosciences |
Mathematical modeling
We developed a mathematical model applying the BrdU incorporation kinetics data to estimate the turnover rates and half-lives of each myeloid cell population. This was based on the following consensus assumptions of myeloid cell development. 1) Myeloid cells divide in bone marrow, enter blood circulation as G0 phase (without further proliferation), and subsequently leave the blood circulation to enter tissues or undergo clearance. 2) BrdU is incorporated into dividing myeloid progenitor cells in bone marrow almost immediately after injection and unincorporated BrdU decays relatively soon after injection (13, 14). 3) After division, BrdU-labeled precursor cells remain in the bone marrow for T days (defined as bone marrow transit time). 4) Only mature cells egress from bone marrow and enter the blood circulation in a one-phase decay rate. 5) During homeostasis, the numbers of each cell population in blood and precursor cell population in bone marrow remain stable. For example, it is assumed that neutrophils leave the blood circulation at the same rate that bone marrow-mature neutrophils enter blood circulation to retain stable numbers during homeostasis.
We designated p as the proliferation production rate for each type of cells in bone marrow, Nm as the number of each type of mature myeloid cell (i.e. neutrophil, basophil, and monocyte) in bone marrow, k1Nm as the rate at which mature cells from bone marrow enter blood circulation, Nb as the number of each type of labeled myeloid cell in blood, k2Nb as the rate of each type of myeloid cell leaving blood circulation, and T as bone marrow transit time. Therefore, we calculated the kinetics of mature cells in bone marrow as,
| (1) |
in the blood, since mature neutrophils enter after transit time T,
| (2) |
During homeostasis, each type of cell numbers in blood remain stable (Assumption 5) which means , so .
We assumed labeled cells have the same kinetics as above. Because we used single bolus injection of BrdU and the unincorporated BrdU was cleared quickly after injection (Assumption 2), dividing cells were labeled over a short time. Thus, we can ignore the proliferation input over time (p=0). We then let Lm be the number of mature labeled cells in bone marrow, and Lb be the labeled cells in blood. Similarly, we calculated the kinetics of labeled cells as,
| (3) |
| (4) |
Dividing (3) and (4) by Nm and Nb respectively, and using ,
| (5) |
| (6) |
Let M = be the ratio of labeled/total precursor cells in bone marrow, B = be the ratio of labeled/total cells in blood, k=k2, and R = , assuming Nm and Nb are constant, then (5) and (6) transform to
| (7) |
| (8) |
Solution of (7) is
| (9) |
where M0 is M at t=0. Assuming B=0 at t=T, solution of (8) for t > T is,
| (10) |
Applying Tayler series approximation around t ~ T to the term,
| (11) |
Thus, the simplified expression is,
| (12) |
which is
| (13) |
We fitted equation (13) to the blood BrdU kinetics data using the scipy.optimize.curve_fit function from the Scipy library for non-linear least squares (15). Calculations were performed using iPython notebook (Python version 2.7) and best-fit curves are shown in Supplemental Figure S2C. Calculated parameters in Supplemental Table S1 are blood half-life = , blood average lifespan = 1/k, and daily production = absolute number of cells in blood/average lifespan of cells in blood.
Statistical analyses
Spearman’s correlation test was used to compare half-lives or daily productions in relation to ages of the monkeys. Kruskal-Wallis analyses were used to compare results between different age groups of monkeys. Graphs were prepared using Graphpad Prism (Version 7, San Diego, CA), and P < 0.05 was considered significant.
Results
Phenotype analysis of neutrophils, basophils, and monocytes in blood of rhesus macaques
Healthy rhesus macaques were administered a single intravenous bolus of BrdU (60 mg/kg body weight) to label dividing progenitor cells in bone marrow. Flow cytometry phenotyping was used to identify neutrophil, basophil, and monocyte populations in blood of rhesus macaques (Fig. 1A). Granulocytes and monocytes were first gated from intermediate to high forward scatter (FSC) and side scatter (SSC) fractions and then separated based on the expression of HLA-DR. Granulocytes were CD3−/CD8−/CD20−/HLA-DR− and monocytes/dendritic cells were CD3−/CD8−/CD20−/HLA-DR+. Within granulocytes, basophils stained brightly for CD123 expression whereas neutrophils were negative for CD123. Interestingly, basophils in rhesus macaques appeared higher in the SSC region (Supplemental Fig. 1) whereas human basophils usually were in the lower SSC area with lymphocyte lineage populations under homeostatic conditions (16). This gating strategy for neutrophils was confirmed by expression of myeloperoxidase (MPO). As expected, MPO was detected at high levels in neutrophils and classical monocytes but not in lymphocytes and basophils (Fig. 1B).
FIGURE 1. Phenotype analysis of neutrophils and basophils in peripheral blood of rhesus macaques.
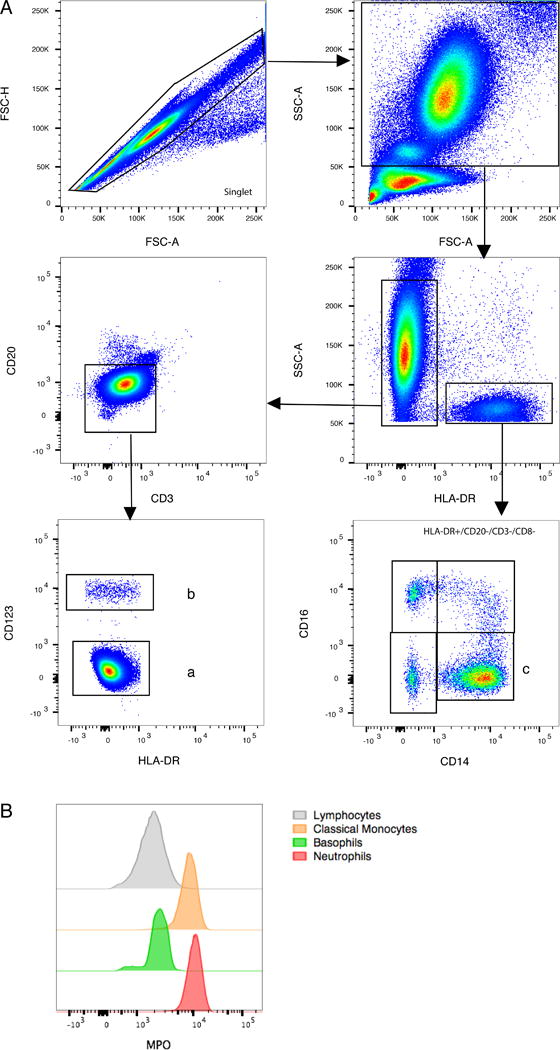
EDTA-treated blood samples were processed, stained with antibodies (Table I), and analyzed by flow cytometry as described in the Materials and Methods. (A) The gating strategies of myeloid-lineage cells were indicated for; (a) neutrophils = single cells, FSC/SSChigh/dim, HLA-DR−, CD3−, CD20−, CD123− ; (b) basophils = single cells, FSC/SSChigh/dim, HLA-DR−, CD123+; and (c) classical monocytes = single cells, FSC/SSCdim, HLA-DR+, CD14+CD16−. (B) Myeloperoxidase (MPO) expression was shown for neutrophils, basophils, classical monocytes, and lymphocytes gated from the above strategy.
Monocyte populations were gated as intermediate in forward and side scatter and for staining CD3−/CD8−/CD20−/HLA-DR+. From these non-lymphocytic HLA-DR+ cells, monocyte subsets were separated as CD14+/CD16− classical monocytes, CD14+/CD16+ intermediate monocytes, and CD14−/CD16+ non-classical monocytes (Fig. 1A). We previously described the continuous differentiation from classical monocytes to intermediate and non-classical monocytes (8) and here focused on classical monocytes which directly traffic from bone marrow to blood. Additional markers for immune-phenotyping neutrophils, basophils, and classical monocytes in rhesus macaques are shown in Supplemental Fig. 1.
Neutrophils, basophils, and classical monocytes exhibit distinct kinetics in blood during homeostasis
At the time of BrdU administrations, dividing hematopoietic stem cells and granulocyte-monocyte progenitor cells in bone marrow will incorporate the BrdU thymidine analogue. Based on phenotyping and gating strategies shown in Fig. 1A, we measured the fraction of BrdU-labeled cells within each population and followed their movement into blood 1, 2, 4, 7, 10, and 14 days after BrdU administration. The length of time each cell type remains in the bone marrow differed. BrdU-labeled neutrophils began to be observed in blood 4 days post-BrdU, increased on day 5, reached the highest observed percentage on about day 7, and were cleared from blood by about 14 days post-BrdU (Fig. 2A and Supplemental Fig. 2A-B). Labeled basophils were first observed in blood 2 days post-BrdU, reached the highest observed percentage on day 4, and were cleared over the course of about 14 days (Fig. 2B and Supplemental Figure 2A). BrdU-labeled classical monocytes appeared in blood as soon as one day later, reached the highest observed percentage 2 days post-BrdU, and were cleared by 8-10 days post-BrdU, as also reported previously by Sugimoto et al (8) and shown here for comparison (Fig. 2C and Supplemental Fig. 2A). This suggested that basophil- and neutrophil-differentiating cells require at least 2 and 4 days in bone marrow after division, respectively, until trafficking to blood while monocyte-differentiating cells enter the blood as early as one day after division. These results were consistent among the adult rhesus macaques examined and suggested that; (1) BrdU-labeled cells could be readily detected and measured in blood following a single bolus injection of BrdU, and (2) during homeostasis, neutrophils, basophils, and monocytes each exhibited consistently distinct kinetics of cell differentiation in bone marrow and trafficking to blood.
FIGURE 2. Neutrophils, basophils, and monocytes exhibit distinct kinetics in blood during homeostasis.
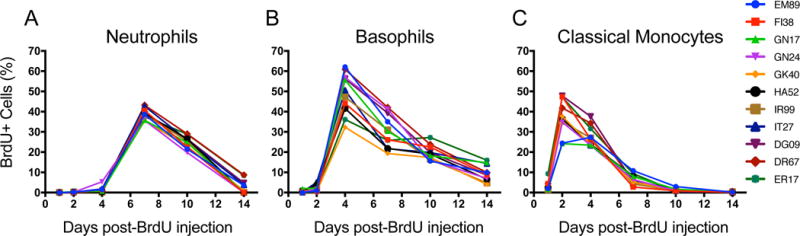
Single-bolus BrdU (60 mg/kg body weight) was administrated intravenously to eleven rhesus macaques aged 5 – 10 years old and EDTA-treated blood samples were collected 1, 2, 4, 7, and 14 days later for staining and flow cytometry analysis. Neutrophils (A), basophils (B), and classical monocytes (C) were gated based on the strategy described in Figure 1A and the percentages of BrdU-labeled cells were measured in each subset. Results from monocytes in panel C were previously published by Sugimoto et al. (8) and presented here for comparison with permission of the publisher.
Mathematical kinetics modeling of blood half-life and daily production of myeloid cells
To estimate half-life and daily production of each myeloid cell population in the blood, we developed and applied a mathematical model to fit kinetics data from BrdU incorporation by neutrophils, basophils, and classical monocytes. Unincorporated BrdU is cleared in vivo within a short time (13, 14) and only progenitor cells proliferating within the first 1-2 hours after administration incorporate detectable levels of BrdU. We fitted the data to equation (13), allowing M0, k, T to be free parameters. In the second group of 3 through 19 year-old animals, we allowed M0, k to be free parameters and fixed T to the results from the first group of animals for improved calculations. Turnover rates (k values as described in the Material and Methods section) were calculated from the best-fit curves (Supplemental Fig. 2C) for the BrdU incorporation kinetics of 11 animals in group 1 (Fig. 3A). The results estimated the half-life of neutrophils as 1.63 ± 0.16 days, and the daily production as 1.42 ± 0.44 × 109 cells/L/day. Half-life and daily production of basophils were estimated as 1.78 ± 0.30 days and 5.89 × 106 cells/L/day, respectively. The half-life of classical monocytes was estimated at 1.01 ± 0.15 days with daily production as 3.09 ± 1.3 × 108 cells/L/day (Fig. 3B-C; Supplemental Table 1). The half-lives for neutrophils and basophils were similar at approximately 1.6 and 1.7 days, respectively that were slightly longer than the 1-day half-life of classical monocytes.
FIGURE 3. Rapid turnover of neutrophils, basophils and classical monocytes, and massive production of neutrophils in rhesus macaques during homeostasis.
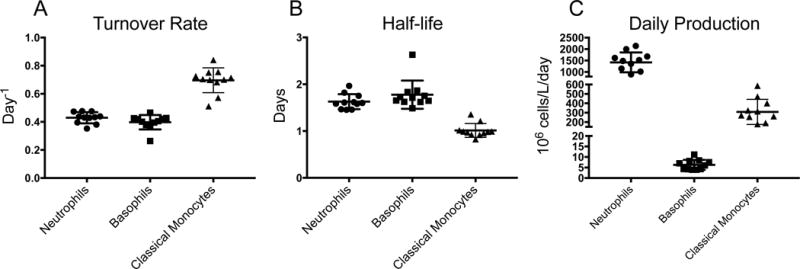
(A) Turnover rates for neutrophils, basophils, and classical monocytes (k values as described in the Material and Methods section) were calculated from the best-fit curves to fit the mathematical model to the BrdU incorporation kinetics. (B) Half-life values for neutrophils, basophils, and classical monocytes were calculated as ln(2)/k. (C) Daily cell production numbers for neutrophils, basophils, and classical monocytes were calculated as absolute cell counts divided by the average circulating lifespans. Each data point represents results from one animal (n=11). All results were expressed as the mean ± SD for each cell population.
BrdU kinetics of neutrophil- and basophil-differentiating progenitor cells in bone marrow
To further assess the kinetics and validate biological relevance of our mathematical model, we examined bone marrow aspirates collected 24 hours, and 4, 7, and 10 days post BrdU administration from 8 of the 11 adult animals. Based on information from humans in which bone marrow neutrophil-differentiating cells started to express CD11b at the myelocyte stage and monocytes/macrophages express HLA-DR (17), neutrophil-differentiating cells in the rhesus macaque bone marrow were gated by singlet/CD45+/FSC-SSChigh/CD3−/CD20−/CD11b+/HLA-DR− (Fig. 4A). This population appeared to mainly contain the post-mitotic pool of neutrophils and was not the initial progenitor cells that incorporated BrdU since only a low percentage of these cells were labeled 24 hours post-BrdU. In the bone marrow, the neutrophil-differentiating progenitor cells that incorporated BrdU reached the highest observed percentage levels at Day 4 and then gradually decreased at 7-10 days post-BrdU (Fig. 4B).
FIGURE 4. Gating strategy and BrdU kinetics of neutrophil- and basophil-committed progenitor cells in bone marrow.
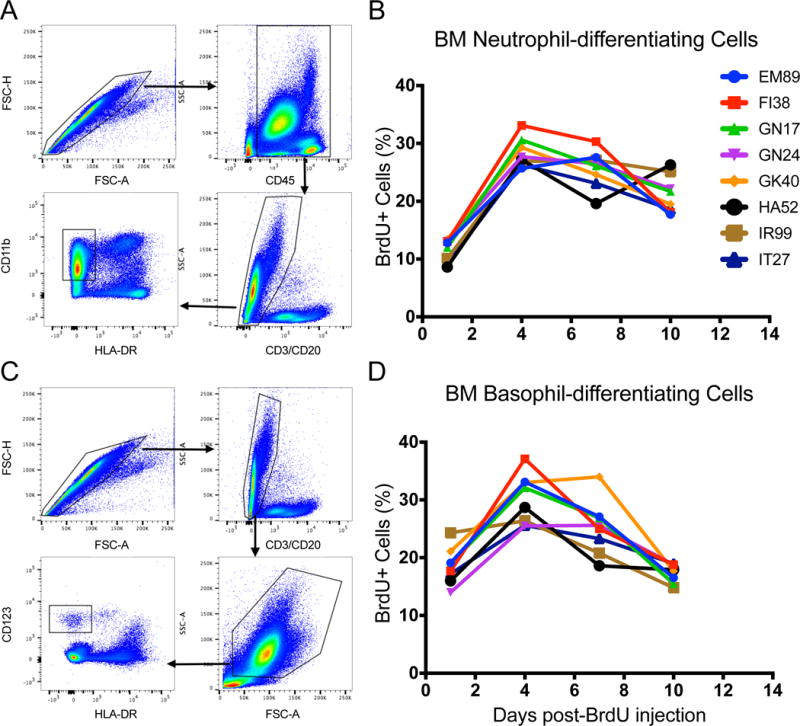
Bone marrow aspirates were collected, stained and analyzed 1, 4, 7, and 10 days after intravenous BrdU administration from 8 of the 11 animals assessed in Fig. 2. (A) Neutrophil-differentiating cells were gated as Singlet/CD45+/FSC-SSChigh/CD3−/CD20−/CD11b+/HLA-DR−. (C) Basophil-differentiating cells were gated as singlet/FSC-SSChigh/CD3−/CD20−/CD123bright/HLA-DR−. The percent of BrdU-labeled (B) neutrophil- and (D) basophil-committed cells in bone marrow was low at day 1, increased at day 4, and then was declining by days 7 - 10.
Basophil-differentiating cells in bone marrow were gated by singlet/FSC-SSChigh/CD3−/CD20−/CD123bright/HLA-DR− expression (Fig. 4C). Although CD123 is expressed on other types of precursor cells in normal human bone marrow such as B cell precursors and common myeloid/granulocyte-macrophage progenitor cells (18). CD123 expression on basophils and basophil-differentiating cells is higher and at higher FSC/SSC (19, 20). The results also demonstrated that a higher percent of basophil-committed progenitor cells in bone marrow were BrdU-labeled 1 day post-BrdU compared to the neutrophil-differentiating cells (Fig. 4B and D).
Chronological aging correlated with lower production of neutrophils and basophils, and shorter half-life of classical monocytes
We showed that myeloid cells required constant replenishment from bone marrow hematopoiesis during homeostasis. In humans, the bone marrow cellularity declines with age (21) and the hematopoietic stem cells (HSCs) from the elderly have shown skewing towards myelopoiesis potential versus lymphopoiesis potential ex vivo (22). However, the effect of aging on the myeloid cell kinetics in vivo is still not clear. So we examined this in a group of clinically healthy rhesus macaques of both sexes between 3 through 19 years of age. EDTA-blood was collected at 1, 4, 7, 10, and 14 days after single-bolus intravenous BrdU administration for immunostaining and flow cytometry as described in Fig. 1. Interestingly, granulocytes (neutrophils, basophils) and classical monocytes exhibited distinct shifting kinetics with increasing age. The half-life of neutrophils and basophils remained similar at about 1.75 and 1.81 days, respectively, across all ages (Fig. 5A-B, D-E) while there was a significant negative correlation between the numbers of neutrophils and basophils in blood and ages (Supplemental Fig. 3 A-B). The similar half-life but lower number of neutrophils and basophils in blood calculated to a significant negative correlation between daily productions with age (Fig. 5H-G). On the other hand, there was a significant negative correlation between half-lives of classical monocytes and age (Fig. 5 C, F) as well as a significant negative correlation between absolute counts of classical monocytes in blood and age (Supplemental Fig. 3 C). As a result, daily production of classical monocytes remained similar across all ages (Fig. 5I). Similar results were observed after comparing differences among animals divided into four age groups of 3-5 years old (n = 4), 5-10 years old (n = 8), 10-18 years old (n = 10), and above 18 years old (n = 11) by Kruskal-Wallis test (Supplemental Fig. 3 D-I), except that the change of basophil’s daily productions among groups didn’t reach statistical significance (p=0.076).
FIGURE 5. Chronological aging correlated with lower production of neutrophils and basophils, and a shorter half-life of classical monocytes.
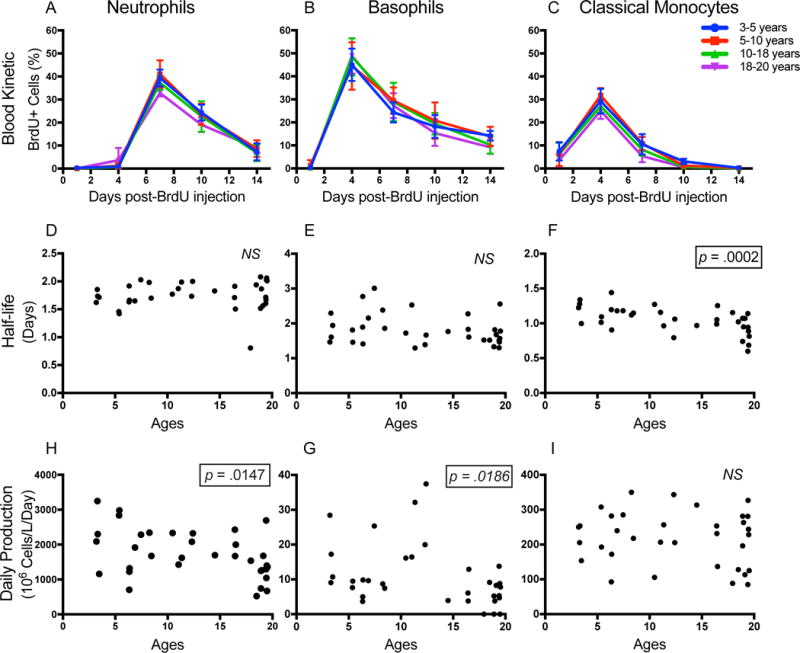
EDTA-treated blood samples from rhesus macaques were collected and stained (group 2; Table II) 1, 4, 7, 10, and 14 days after iv BrdU administration. The BrdU+ cell fraction, half-life, and daily production were measured and calculated respectively for neutrophils (A, D, H), basophils (B, E, G), and classical monocytes (C, F, I). Blood kinetics were expressed as the mean ± SD of the BrdU+ cell fraction for each age group. Nonparametric Spearman correlation tests were used to compare half-life and daily production of cell types against age of the animals. P < 0.05 was considered statistically significant.
Discussion
Conflicting results have been reported about the half-life of neutrophils (23–26) and monocytes (27) in humans while such information is essentially absent about basophils (28, 29). Here, we applied in vivo labeling with the thymidine analogue, BrdU, to investigate myeloid lineage cell development and kinetics in rhesus macaques that are more closely related genetically and physiologically to humans than are rodents, and thereby provides a good model to better understand human myeloid cell kinetics. Furthermore, studies in humans are more limited by the use and administration route of labeling agents, as well as accessibility to repeated bone marrow, blood, and tissue sampling, than are rhesus macaques. We demonstrated relatively short half-lives of circulating neutrophils, basophils, and classical monocytes, and massive production of neutrophils and classical monocytes during homeostasis via BrdU in vivo labeling. Furthermore, the distinct kinetics patterns of neutrophils, basophils, and classical monocytes were internally consistent among the monkeys evaluated.
Neutrophils are the predominant myeloid and WBC component in blood of humans and nonhuman primates. The half-life of blood neutrophils in rhesus macaques was calculated at approximately 1.63 days compared to previous estimated circulating half-lives of neutrophils ranging between 7 hours and 3.8 days in humans (23–26). The earlier studies on human neutrophil kinetics used adoptive transfer techniques and/or toxic radioactive labeling methods which likely altered the active state of neutrophils resulting in an under-estimated half-life of neutrophils (23). Two recent in vivo studies that utilized nontoxic labeling methods estimated the half-lives of circulating neutrophils in humans at 3.8 days and 19 hours, respectively (24, 25). This discrepancy was considered by Lahoz-Beneytez et al. (25) to be due to their mathematical models rather than the labeling methods being used. Lahoz-Beneytez’s model utilized an empirical parameter R representing the ratio of blood neutrophils to bone marrow mitotic neutrophil precursors; the estimation of R was based on several other studies that examined the maturation stage of neutrophil precursors in bone marrow by morphology rather than by direct labeling to assess cell division (25, 26, 30). The longer and slower uptake phase of the 2H2O and deuterium-labeled glucose uptake via drinking water in the Pillay et al. (24) and Lahoz-Beneytez et al. (25) studies, respectively may also have influenced the neutrophil half-life estimations. Our calculated neutrophil half-life of 1.6 days in rhesus macaques was intermediate to the two values reported by the above two groups and was based on a single bolus intravenous labeling strategy which simplified the label uptake phase calculations.
We also examined the differentiation phase of neutrophils after proliferation in bone marrow. BrdU incorporation occurs prior to expression of the CD11b+HLA-DR− phenotype in neutrophil precursors in bone marrow and this CD11b+/HLA-DR− subset in bone marrow exhibited low levels of BrdU incorporation one day after BrdU injection with increasing levels observed at day 4, followed by gradual declines at days 7 and 10. The estimated daily production of neutrophils at 1.42 ± 0.44 × 109 cells/L/day in rhesus macaques was lower than the previously reported 0.87 × 109 cells/kg body weight/day in humans (or 11.98 × 109 cells cells/L/day based on 5.3 L blood in a 73 kg-weight man) that was likely based on the longer estimation of neutrophil half-life at 1.63 days in our study compared to 7 hours estimated by Dancey et al. (26). Calculation for neutrophil bone marrow transit of 4-5 days in rhesus macaques was similar to the 5-6 days reported for humans (24–26).
Our results also suggested that neutrophils subsequently traffic to tissues after circulating in blood. For example, BrdU-labeled neutrophils were detected in lung biopsies 7 days after BrdU injection of rhesus macaques (data not shown). In addition, the fraction of BrdU-labeled neutrophils in the bone marrow remained at about 20% which is higher than we originally expected, and this could have resulted from homing of the “aged” neutrophils back to bone marrow for clearance (31, 32).
Like neutrophils, monocytes contribute to the innate immune system and in rhesus macaques, differentiate from CD14+/CD16− classical monocytes to CD14+/CD16+ intermediate monocytes and to CD14−/CD16+ non-classical monocytes in blood (8). Thus, earlier estimates of monocyte half-life (27) probably represented the average monocyte half-life rather than the kinetics of specific monocyte sub-populations. We calculated the half-life of classical monocytes in blood at just over one day in adult macaques that were produced at a rate of approximately 3 × 108 cells/L/day during homeostasis. The transit time of monocytes in the bone marrow after proliferation is less than 1 day which is much shorter than for neutrophils and basophils. Classical monocytes were predominant over intermediate and non-classical monocytes and appeared to leave blood circulation to transition into tissue macrophages, consistent with two recent reports on humans (33, 34). In our earlier studies (35), BrdU-labeled monocytes that trafficked into interstitial lung tissues of rhesus macaques were relatively shorter-lived. Conversely, alveolar lung macrophages that had not incorporated BrdU within the 7–10 day time course were considered longer-lived. Thus, in vivo BrdU labeling studies can be applied for examining monocyte and tissue macrophage subpopulation kinetics that influence immune responses.
Less is known about the kinetics of basophils, mainly due to their paucity in blood. Ohnmacht et al. (36) used BrdU labeling in mice and estimated the lifespan of basophils from blood to tissues at 60 hours based on the assumption that the labeled basophils enter tissues at a constant rate and independent of the concentration of cells. To our knowledge, the present study is the first to demonstrate that basophils exhibit distinct kinetics and shorter bone marrow transit time compared to neutrophils in nonhuman primates. Surprisingly, basophils also have a relatively short half-life in blood at about 1.7 days (slightly longer than neutrophils) and are replenished from bone marrow at a rate of 5.89 × 106 cells/L/day during homeostasis. BrdU incorporation into the CD123+HLA-DR− basophil- differentiating cell subset in bone marrow was higher one day post BrdU than for neutrophil-differentiating cells and the decay four days after BrdU injection also was faster to coincide with basophil kinetics in peripheral blood. Although we were limited from collecting bone marrow samples at daily intervals between 1 and 4 days after BrdU injection, the results suggested that peak BrdU labeling of basophils in bone marrow occurred within this timeframe.
We calculated the daily production of the different myeloid cells described above described above based on their average circulating lifespan and absolute count in blood circulation as well as on the assumption that each gated cell population is homogenous in the blood. That is to say, it is still possible that small fractions of cells exist in the above cell populations that have a much slower kinetics or recirculate back from tissue to blood. Such subsets, if they exist, are considered to comprise small fractions of the whole given that we didn’t observe any consistent BrdU labeling in the above populations long-term after label injection (data not shown).
The initial myeloid cell BrdU kinetics were evaluated in rhesus macaques between 5-10 years old that are considered young adults equivalent to humans of approximately 18-40 years of age (or 3.5-4x older than rhesus macaques). A set of rhesus macaques aged 3 through 19 years of age (approximately 10-75 years of age in humans) of both sexes were then evaluated to examine effects of chronological aging on myeloid cell kinetics. Elderly people and animals are more susceptible to infections (37) and hematopoietic stem cells (HSCs) in bone marrow from the elderly exhibit a trend for less regenerative potential compared to HSC from younger adults (22). This might partially be reflected by the slight decrease of innate immune cell numbers observed in elderly humans (37–39), as well as in rhesus macaques (40). Absolute cell numbers, however, do not reflect the movement or the production of the cells. Throughout the age range of 3-19 years of age in the rhesus macaques, neutrophil production declined in daily output while neutrophil half-life remained unchanged. Interestingly, daily production of classical monocytes remained unchanged while the half-life of monocytes declined with increasing age. These may reflect different mechanisms for neutrophils and monocytes to compensate for limited bone marrow hematopoiesis or output as animals age. We didn’t observe significant differences in kinetics, half-life, or daily production in all three cell types between age-matched male and female animals between 3 and 18 years of age in our study (data not shown). However, the combined effect of age and sex is still not clear because the animals above 18 years old were all females in our study based on availability. Thus, it is important to investigate the potential sex effects especially in the context of aging in future studies.
Overall, these results demonstrated the massive production of neutrophils and monocytes in bone marrow and rapid turnover of neutrophils, basophils, and classical monocytes in blood of rhesus macaques during homeostasis. Also, the rapid “turnover” rates of neutrophils, basophils, and classical monocytes were maintained in the older animals albeit with a decrease of neutrophil production with aging. Consistency observed between animals suggested a tight regulation of the half-life and massive production that may be required to maintain homeostasis.
Supplementary Material
Acknowledgments
We are grateful for the assistance of Toni P. Penny, Edith M. Walker, Erin M. Haupt, Nadia Slisarenko, Jeanne M. Perkins, Kelly A. Goff, Julie A. Bruhn, and Calvin R. Lanclos in the Division of Immunology, Robert Blair in the Division of Comparative Pathology, and Jason P. Dufour in the Division of Veterinary Medicine at Tulane National Primate Research Center (TNPRC).
Grant support: this study was supported by research funding from the National Institutes of Health (AI097059 and AI110163 to M.J.K., MH108458 and MH107333 to W.-K.K., AG052349 to E.S.D., HL139278 to E.S.D. and M.J.K., and OD011104 to the TNPRC). This study was also supported by Virginia’s Commonwealth Health Research Board Grant #11-09 to W.-K.K.
Footnotes
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 2.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–74. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–24. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol. 2013;34:398–409. doi: 10.1016/j.it.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. J Allergy Clin Immunol. 2013;132:789–801. doi: 10.1016/j.jaci.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voehringer D. The role of basophils in helminth infection. Trends Parasitol. 2009;25:551–6. doi: 10.1016/j.pt.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJM, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto C, Hasegawa A, Saito Y, Fukuyo Y, Chiu KB, Cai Y, Breed MW, Mori K, Roy CJ, Lackner AA, Kim WK, Didier ES, Kuroda MJ. Differentiation Kinetics of Blood Monocytes and Dendritic Cells in Macaques: Insights to Understanding Human Myeloid Cell Development. J Immunol. 2015;195:1774–81. doi: 10.4049/jimmunol.1500522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doeing DC, Borowicz JL, Crockett ET. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol. 2003;3:3. doi: 10.1186/1472-6890-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 11.Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, D.C: 2011. [Google Scholar]
- 12.Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, Vinet-Oliphant H, Kim WK, Williams KC, Ribeiro RM, Lackner AA, Veazey RS, Kuroda MJ. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–25. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.KRISS JP, MARUYAMA Y, TUNG LA, BOND SB, REVESZ L. The fate of 5-bromodeoxyuridine, 5-bromodeoxycytidine, and 5-iododeoxycytidine in man. Cancer Res. 1963;23:260–8. [PubMed] [Google Scholar]
- 14.Matiašová A, Sevc J, Mikeš J, Jendželovský R, Daxnerová Z, Fedoročko P. Flow cytometric determination of 5-bromo-2′-deoxyuridine pharmacokinetics in blood serum after intraperitoneal administration to rats and mice. Histochem Cell Biol. 2014;142:703–12. doi: 10.1007/s00418-014-1253-7. [DOI] [PubMed] [Google Scholar]
- 15.Oliphant TE. Python for Scientific Computing. Comput Sci Eng. 2007;9:10–20. [Google Scholar]
- 16.Sharma M, Hegde P, Aimanianda V, Beau R, Maddur MS, Sénéchal H, Poncet P, Latgé JP, Kaveri SV, Bayry J. Circulating human basophils lack the features of professional antigen presenting cells. Sci Rep. 2013;3:1188. doi: 10.1038/srep01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terstappen L, Safford M, Loken MR. Flow cytometric analysis of human bone marrow. III. Neutrophil maturation. Leukemia. 1990;4:657–63. [PubMed] [Google Scholar]
- 18.Hassanein NM, Alcancia F, Perkinson KR, Buckley PJ, Lagoo AS. Distinct Expression Patterns of CD123 and CD34 on Normal Bone Marrow B-Cell Precursors (“Hematogones”) and B Lymphoblastic Leukemia Blasts. Am J Clin Pathol. 2009;132:573–580. doi: 10.1309/AJCPO4DS0GTLSOEI. [DOI] [PubMed] [Google Scholar]
- 19.Toba K, Koike T, Shibata A, Hashimoto S, Takahashi M, Masuko M, Azegami T, Takahashi H, Aizawa Y. Novel technique for the direct flow cytofluorometric analysis of human basophils in unseparated blood and bone marrow, and the characterization of phenotype and peroxidase of human basophils. Cytometry. 1999;35:249–59. [PubMed] [Google Scholar]
- 20.Han X, Jorgensen JL, Brahmandam A, Schlette E, Huh YO, Shi Y, Awagu S, Chen W. Immunophenotypic study of basophils by multiparameter flow cytometry. Arch Pathol Lab Med. 2008;132:813–9. doi: 10.5858/2008-132-813-ISOBBM. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev. 2000;117:57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 22.Pang WW, Price Ea, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tak T, Tesselaar K, Pillay J, Borghans JaM, Koenderman L. Whatˈs your age again? Determination of human neutrophil half-lives revisited. J Leukoc Biol. 2013;94:595–601. doi: 10.1189/jlb.1112571. [DOI] [PubMed] [Google Scholar]
- 24.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JAM, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 25.Lahoz-Beneytez J, Elemans M, Zhang Y, Ahmed R, Salam A, Block M, Niederalt C, Asquith B, Macallan D. Human neutrophil kinetics: Modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood. 2016;127:3431–3438. doi: 10.1182/blood-2016-03-700336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58:705–15. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitelaw DM. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet. 1972;5:311–7. doi: 10.1111/j.1365-2184.1972.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 28.Siracusa MC, Perrigoue JG, Comeau MR, Artis D. New paradigms in basophil development, regulation and function. Immunol Cell Biol. 2010;88:275–284. doi: 10.1038/icb.2010.1. [DOI] [PubMed] [Google Scholar]
- 29.Min B, Brown MA, Legros G. Understanding the roles of basophils: breaking dawn. Immunology. 2012;135:192–7. doi: 10.1111/j.1365-2567.2011.03530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison WJ. The total cellularity of the bone marrow in man. J Clin Pathol. 1962;15:254–259. doi: 10.1136/jcp.15.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008;22:3111–3119. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, A-González N, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A, A-gonza N, Nagasawa T, Frenette PS, Castrillo A, Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, A-González N, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–35. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D, Yona S. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. 2017;214:1913–1923. doi: 10.1084/jem.20170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tak T, Drylewicz J, Conemans L, de Boer RJ, Koenderman L, Borghans JAM, Tesselaar K. Circulatory and maturation kinetics of human monocyte subsets in vivo. Blood. 2017;130:1474–1477. doi: 10.1182/blood-2017-03-771261. [DOI] [PubMed] [Google Scholar]
- 35.Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. In Vivo Characterization of Alveolar and Interstitial Lung Macrophages in Rhesus Macaques: Implications for Understanding Lung Disease in Humans. J Immunol. 2014;192:2821–2829. doi: 10.4049/jimmunol.1302269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–2825. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 37.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–87. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Martinis M, Modesti M, Ginaldi L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol Cell Biol. 2004;82:415–20. doi: 10.1111/j.0818-9641.2004.01242.x. [DOI] [PubMed] [Google Scholar]
- 39.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:30. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Didier ES, Sugimoto C, Bowers LC, Khan IA, Kuroda MJ. Immune correlates of aging in outdoor-housed captive rhesus macaques (Macaca mulatta) Immun Ageing. 2012;9:25. doi: 10.1186/1742-4933-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


