Abstract
A new 1,4-diazepine, callysponine (1), was isolated from a South China Sea Callyspongia sp. marine sponge, together with four known proline-based diketopiperazines: cyclo-(S-Pro-R-Leu) (2), cyclo-(S-Pro-R-Val) (3), cyclo-(S-Pro-R-Ala) (4), and cyclo-(S-Pro-R-Tyr) (5). The new structure was determined on the basis of NMR and MS analysis, and the absolute stereochemistry was defined by NOESY spectroscopy and optical rotation. The structures of the known compounds were identified by comparison of their spectroscopic data with those reported in the literature. Callysponine (1) did not inhibit the growth of HepG2 (hepatoma carcinoma cell), A549 (lung carcinoma cell), and HeLa (cervical cancer cell) cell lines.
Keywords: marine sponge, Callyspongia sp., diazepine, callysponine
1. Introduction
Marine sponges of the genus Callyspongia are known as common sources of structurally unique and biologically active natural products, such as polyacetylenes [1], peptides [2], terpenoids [3], alkaloids [4], fatty acids [5], polyketides [6], sterols [7], peroxides [8], and butenolides [9]. Some of these compounds possess antifouling [10], cytotoxic [11], anticancer [6], and antimicrobial [10] properties.
In our study of bioactive compounds from Callyspongia sp. marine sponges collected from the coast of Hainan, the new 1,4-diazepine 1, as well as proline-based diketopiperazines 2–5 were obtained. The structure of the new compound was elucidated by the aid of COSY, HSQC, HMBC, and MS experiments, while the absolute stereochemistry of 1 was defined by NOESY spectroscopy and optical rotation. Compounds 2–5 were identified as cyclo-(S-Pro-R-Leu) (2) [12], cyclo-(S-Pro-R-Val) (3) [12], cyclo-(S-Pro-R-Ala) (4) [13], and cyclo-(S-Pro-R-Tyr) (5) [14], respectively, by comparison of their spectroscopic data with those reported in the literature.
2. Results and Discussion
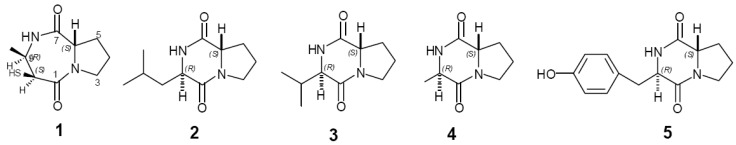
The aqueous EtOH extract of the Callyspongia sp. marine sponge was suspended in water, and partitioned successively with petroleum ether, EtOAc, and n-butanol. The EtOAc and n-butanol fractions were subjected to silica column, Sephadex LH-20, and ODS-HPLC, to yield compounds 1–5 (Figure 1).
Figure 1.
Structures of compounds 1–5.
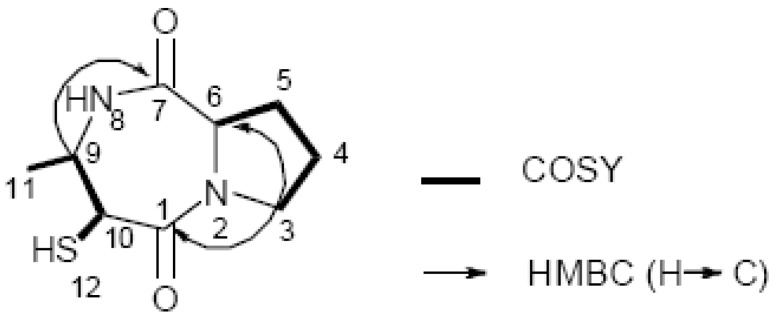
Compound 1 was obtained as yellow oil and had the molecular formula C9H14N2O2S, as deduced from the HREI mass spectrum (m/z 214.0774 [M]+; calc. for C9H14N2O2S, 214.0776) and NMR data (Table 1), the latter being unambiguously assigned by aid of COSY, HMQC, and HMBC experiments. 1H-NMR (Table 1) chemical shifts of two α-methine protons at δH 4.36 and 4.11, and 13C-NMR (Table 1) chemical shifts of two carbonyl carbons at δC 170.2 and 165.4, supported the presence of a peptide fragment. The fact that compound 1 was negative to the ninhydrin test, suggested a cyclic or a N-terminus-blocked peptide [15]. The 1H-NMR spectrum of 1 showed the presence of one methyl signal at δH 1.34 (d, J = 6.5 Hz, 3H), one thiol proton at δH 1.89 (m, 1H), three consecutive methylene signals (δH = 2.0–3.7), and three methine signals at δH 3.97 (br d, J = 3.0 Hz, 1H), 4.11 (t, J = 7.5 Hz, 1H), and 4.36 (dt, J = 6.5, 3.0 Hz, 1H), and one acid amide proton at δH 6.73 (s, 1H). The 13C-NMR spectrum of 1 showed the presence of two carbonyl carbons (δC 170.2 and 165.4), two N-methine carbons (δC 65.6 and 59.0), one N-methylene carbon (δC 45.3), one S-methine carbon (δC 59.4), and one methyl carbon (δC 18.9). The remaining carbons were assigned to two methylene carbons (δC 28.1 and 22.6). The COSY spectra (Figure 2) indicated three consecutive methylenes (δH 2.0–3.7) characteristic of a proline residue [16], and showed correlations of H-9 with H-10 and H-11. The assignments of a remaining C3H6S fragment, and one site of unsaturation were carried out by 2D NMR experiments. Key HMBC correlations H-9/C-7, H-3a/C-1, H-3a/C-6, H-3b/C-1, H-3b/C-6, H-11/C-9, and H-11/C-10, as well as COSY connectivities (Figure 2), were indicative of a pyrrolidine-fused seven-membered diazepine ring. The placement of a methyl group at C-9, and a thiol group at C-10, were deduced from the COSY correlations H-11/H-9, and SH-12/H-10, respectively.
Table 1.
1H- (500 MHz) and 13C-NMR (125 MHz) data of compound 1 (in CDCl3, δ in ppm, J in Hz).
| No. | δ c | δ H | HMBC (H to C) |
|---|---|---|---|
| 1 | 165.4 | – | – |
| 2 | – | – | – |
| 3 | 45.3 | 3.51 (m) | C-1, 4, −5, −6 |
| 3.61 (m) | C-1, 4, −5, −6 | ||
| 4 | 22.6 | 2.00 (m) | C-3, −5, −6 |
| 2.06 (m) | C-3, −5, −6 | ||
| 5 | 28.1 | 2.34 (m) | C-4, −6, −7 |
| C-4, −6, −7 | |||
| 6 | 59.0 | 4.11 (t, 7.5) | C-4, −5, −7 |
| 7 | 170.2 | – | – |
| 8 | – | 6.73 (s) | – |
| 9 | 65.6 | 4.36 (dt, 6.5, 3.0) | C-7, −11 |
| 10 | 59.4 | 3.97 (d, 3.0) | C-1, −9, −11 |
| 11 | 18.9 | 1.34 (d, 6.5) | C-9, −10 |
| 12 | – | 1.89 (m) | – |
Figure 2.
Key HMBC and COSY correlations of 1.
The absolute configuration of compound 1 was determined by the optical rotation, NOESY spectrum and analysis of the coupling constants (J), along with inspection of the molecular model. In the NOESY spectrum (Figure 3), the key NOE correlations of H-9/H-10 and H-6/H-11 showed that H-9 and H-10, H-6 and H-11 were on the same face, so the relative stereochemistry was determined. The coupling patterns of the H-9 (dt, J = 3.0 and 6.5 Hz) and H-10 (d, J = 3.0 Hz) led to confirmation of the cis-orientation of H-9/H-10 and possessed β-orientation [17], and thus the configuration of C-9 and C-10 were determined as R* and S*, respectively. The sign of [α]D for proline-containing DKPs is either negative or positive, depending only on the absolute configuration of Pro [12]. On the basis of the sign of [α]20D (−52.6) and by comparison of the NMR data of the proline residue with those of proline-containing DKPs [12,13,14], which suggested C-6 has (S)-configuration, Pro in 1 has therefore (S)-configuration, and the above data approved the absolute configuration of compound 1 as the new (6S, 9R, 10S)-1,4-diazepine.
Figure 3.
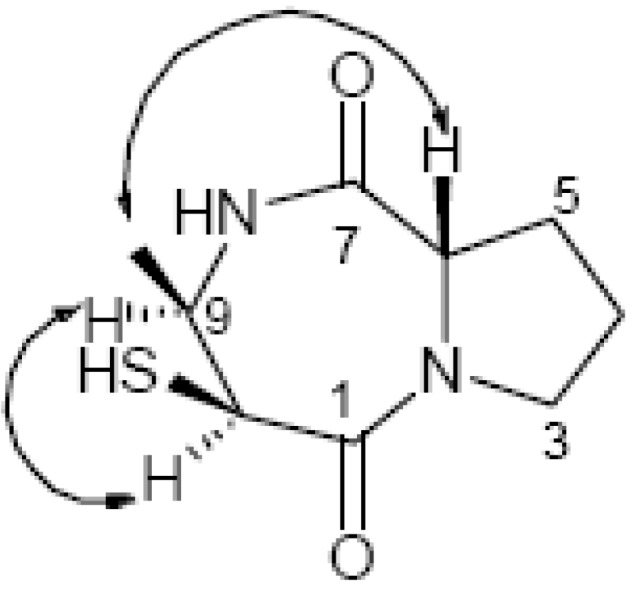
Key NOE correlations of compound 1.
The structures of known compounds 2–5 were confirmed by detailed NMR data comparison with those in the literature [12,13,14]. The absolute configuration of 2–5 were determined by comparison with reported optical rotation values [12,13,14].
Compound 1 was evaluated for cytotoxicity by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method [18], and showed a marginal activity against a small panel of three human tumour HepG2 (hepatoma carcinoma cell), A549 (lung carcinoma cell), and HeLa (cervical cancer cell) cell lines, their inhibition ratio were lower than 10% at concentration of 100 μg/mL with the IC50 values of the positive control compound 5-Fu 13.70, 2.13, and 3.83 μg/mL, respectively.
3. Experimental
3.1. General
NMR spectra were recorded on a Bruker AC 500 NMR spectrometer with TMS as an internal standard. ESI-MS data were measured on a Agilent 1200 LC-MS spectrometer. HREIMS data were obtained from MAT 95XP (Thermo) mass spectrometer. The silica gel GF254 plates used for TLC were supplied by the Qingdao Marine Chemical Factory (Qingdao, China). Analytical HPLC was performed on a Hitachi L-2400 HPLC system, using a YMC ODS-H80 column (250 × 4.6 mm i.d., 4 μm) coupled to an Alltech ELSD 800 detector; semi-preparative HPLC was performed on a Hitachi L-2400 HPLC system, using a YMC ODS-H80 column (250 × 10 mm i.d., 4 μm) coupled to an Alltech ELSD 800 detector with flow-splitter valve (Parker: NS) set at a split ratio of 20:1 (collector: detector). Optical rotation data were measured by Perkin-Elmer Model 341 polarimeter. Spots were detected on TLC under UV light or by heating after spraying with 5% H2SO4 in EtOH (v/v).
3.2. Animal Material
The sponge is an undescribed species of Callyspongia (order Haplosclerida, family Callyspongiidae) collected off the coast of Hainan Island, South China Sea, in January, 2007. The specimen was identified by Dr. Kyung Jin Lee. A voucher specimen (No. 20070101) was deposited in Natural History Museum, Hannam University, Daejon, Korea and Key Laboratory of Marine Bio-resources Sustainable Utilization, South China Sea Institute of Oceanology, Chinese Academy of Sciences, China.
3.3. Extraction and Isolation
The wet sponges (10 kg) were extracted three times with EtOH/H2O (90:10, 20 L). The aqueous EtOH extract was concentrated under vacuum. The combined extract was partitioned between EtOAc and H2O. The EtOAc soluble portion (28 g) was partitioned between petroleum ether and EtOH/H2O (7:3, 500 mL). The EtOH/H2O soluble portion (8.0 g) was chromatographed on a silica gel column (80 g) eluted with petroleum ether (500 mL, fraction A), petroleum ether/EtOAc (8:2, 500 mL, fraction B), petroleum ether/EtOAc (1:1, 500 mL, fraction C), petroleum ether/EtOAc (3:7, 500 mL, fraction D), petroleum ether/ EtOAc (1:9, 500 mL, fraction E), EtOAc (500 mL, fraction F), EtOAc/acetone (1:1, 500 mL, fraction G), and MeOH (500 mL, fraction H), to give eight fractions. Fraction D (1.06 g) was subjected to column chromatography (CC) with gradient EtOAc/acetone (10:0, 250 mL; 8:2, 250 mL; 7:3, 250 mL; 5:5, 250 mL) to give four subfractions (D1–D4). Fraction D1 (45.2 mg) was purified by Sephadex LH-20 (CHCl3/MeOH, 2:8, 250 mL) to afford 3 (13.3 mg). Fraction D4 (90.1 mg) was purified by Sephadex LH-20 (CHCl3/MeOH, 2:8, 250 mL) to yield 2 (23.4 mg). Fraction G3-2 (112.3 mg) was purified by Sephadex LH-20 (CHCl3/MeOH, 2:8, 250 mL) to yield fraction G3-2-1 (80.2 mg). Fraction G3-2-1 was further subjected to CC with CHCl3/MeOH (98:2, 150 mL) to give 4 (4.3 mg). Fraction G6 (300.1 mg) was subjected to CC with CHCl3/MeOH (9:1, 250 mL) to give two subfractions (G6-1, G6-2). Fraction G6-1 (122.6 mg) was further purified by Sephadex LH-20 (CHCl3/MeOH, 2:8, 150 mL) to afford fraction G6-2-1 (68.1 mg). Fraction G6-2-1 was further purified by reverse-phase HPLC (ODS, MeOH/H2O, 5:5, 180 mL;) to yield 5 (7.2 mg). The n-butanol soluble portion (80 g) was chromatographed on ODS column eluted with MeOH/H2O (0:100, 2,000 mL; 10:90, 2,000 mL; 20:80, 2,000 mL; 40:60, 2,000 mL; 60:40, 2,000 mL; 100:0, 2,000 mL) to give six fractions I, J, K, L, M, N. Fraction J (20 g) was subjected to CC with gradient CHCl3/MeOH (100:0, 500 mL; 90:10, 500 mL; 80:20, 500 mL; 60:40, 500 mL; 40:60, 500 mL; 0:100, 500 mL;) to give six fractions (J1–J6). Fraction J1 was further purified by reverse-phase HPLC (ODS, MeOH/H2O, 5:95, 150 mL) to yield 1 (6.9 mg, 2.8 × 10−3 % of the total extractum 250.0 g).
Callysponine (1). White amorphous powder; [α]20D = −52.6 (c = 0.41, MeOH); NMR data (CD3OD), see Table 1; ESI-MS (m/z): 237 [M+Na]+, 213 [M-H]−; HREIMS (m/z): 214.0774 [M]+ (calc. for C9H14N2O2S, 214.0776).
Cyclo-(S-Pro-R-Leu) (2). White crystals; [α]20D = −78.3 (c = 0.03, MeOH).
Cyclo-(S-Pro-R-Val) (3). White crystals; [α]20D = −102.6 (c = 1.36, MeOH).
Cyclo-(S-Pro-R-Ala) (4). White solid; [α]20D = −40.4 (c = 0.45, MeOH).
Cyclo-(S-Pro-R-Tyr) (5). Viscous oil; [α]20D = −9.3 (c = 0.91, MeOH).
4. Conclusions
A new 1,4-diazepine, callysponine (1), was isolated from South China Sea Callyspongia sp. marine sponge, together with four known proline-based diketopiperazines. Callysponine did not show activities against HepG2, A549, and HeLa cell lines.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 40706046, 30973679, and 20902094), Knowledge Innovation Program of Chinese Academy of Sciences (LYQY 200703, SQ200904), the National Key Basic Research Program of China (973)’s Project (2010CB833800), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, and LMB (091002) Foundation.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Diaa T.A.Y., Yoshida W.Y., Kelly M., Scheuer P.J. Polyacetylenes from a Red Sea sponge Callyspongia species. J. Nat. Prod. 2000;63:1406–1410. doi: 10.1021/np0000668. [DOI] [PubMed] [Google Scholar]
- 2.Berer N., Rudi A., Goldberg I., Benayahu Y., Kashman Y. Callynormine A, a new marine cyclic peptide of a novel class. Org. Lett. 2004;6:2543–2545. doi: 10.1021/ol0491787. [DOI] [PubMed] [Google Scholar]
- 3.Gray C.A., de Lira S.P., Silva M., Pimenta E.F., Thiemann O.H., Oliva G., Hajdu E., Andersen R.J., Berlinc R.G.S. Sulfated meroterpenoids from the Brazilian sponge Callyspongia sp. are inhibitors of the antileishmaniasis target adenosine phosphoribosyl transferase. J. Org. Chem. 2006;71:8685–8690. doi: 10.1021/jo060295k. [DOI] [PubMed] [Google Scholar]
- 4.Davies-Coleman M.T., Faulkner D.J., Dubowchik G.M., Roth G.P., Polson C., Fairchild C. A new EGF-active polymeric pyridinium alkaloid from the sponge Callyspongia fibrosa. J. Org. Chem. 1993;58:5925–5930. [Google Scholar]
- 5.Carballeria N.M., Pagán M. New methoxylated fatty acids from the Caribbean sponge Callyspongia fallax. J. Nat. Prod. 2001;64:620–623. doi: 10.1021/np000537q. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M., Higuchi K., Murakami N., Tajima H., Aoki S. Callystatin A, a potent cytotoxic polyketide from the marine sponge, Callyspongia truncate. Tetrahedron Lett. 1997;38:2859–2862. doi: 10.1016/S0040-4039(97)00482-6. [DOI] [Google Scholar]
- 7.Theobald N., Shoolery J.N., Djerassi C., Erdman T.R., Scheuer P.J. Minor and trace sterols in marine invertebrates. 24-ethyl-.delta.5,24(28)-cholestatrien-3.beta.-ol-a naturally occurring allenic marine sterol. J. Am. Chem. Soc. 1978;100:5571–5575. [Google Scholar]
- 8.Toth S.I., Schmitz J.F. Two new cytotoxi peroxide-containing acids from a new Guinea sponge, Callyspongia sp. J. Nat. Prod. 1994;57:123–127. doi: 10.1021/np50103a017. [DOI] [PubMed] [Google Scholar]
- 9.Layne T.H., Tinto W.F. A butenolide from the marine sponge Callyspongia vaginalis. Heterocycles. 2006;68:2161–2164. doi: 10.3987/COM-06-10823. [DOI] [Google Scholar]
- 10.Qian P.Y., Dobretsov S., Dahms H.U., Pawlik J. Antifouling activity and microbial diversity of two congeneric sponges Callyspongia spp. from Hong Kong and the Bahamas. Mar. Ecol. Prog. Ser. 2006;324:151–165. doi: 10.3354/meps324151. [DOI] [Google Scholar]
- 11.Murakami N., Sugimoto M., Nakajima T., Kawanishi M., Tsutsui Y., Kobayashi M. Participation of the conjugated diene part of for potent cytotoxicity of callystatin A, a spongean polyketide. Bioorg. Med. Chem. 2000;8:2651–2661. doi: 10.1016/S0968-0896(00)00199-1. [DOI] [PubMed] [Google Scholar]
- 12.Adamczeski M., Reed A.R., Crews P. New and known diketopiperazines from the Caribbean sponge, Calyx cf. podatypa. J. Nat. Prod. 1995;58:201–208. doi: 10.1021/np50116a007. [DOI] [PubMed] [Google Scholar]
- 13.Gautschi M., Schmid J.P., Peppard T.L., Ryan T.P., Tuorto R.M., Yang X.G. Chemical characterization of diketopiperaxines in beer. J. Agric. FoodChem. 1997;45:3183–3189. [Google Scholar]
- 14.Jayatilake G.S., Thornton M.P., Leonard A.C., Grimwade J.E., Baker B.J. Metabolites from an Antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996;59:293–296. doi: 10.1021/np960095b. [DOI] [PubMed] [Google Scholar]
- 15.Shigemori H., Tenma M., Shimazaki K., Kobayashi J. Three new metabolites from the marine yeast Aureobasidium pullulans. J. Nat. Prod. 1998;61:696–698. doi: 10.1021/np980011u. [DOI] [PubMed] [Google Scholar]
- 16.Hecher S.J., Werner K.M. Total synthesis of (±)-leuhistin. J. Org. Chem. 1993;58:762–1765. [Google Scholar]
- 17.Wang Y.L., Li Z.L., Zhang H.L., Sha Y., Pei Y.H., Hua H.M. New germacrane sequiterpenes from Salvia chinensis. Chem. Pharm. Bull. 2008;56:843–846. doi: 10.1248/cpb.56.843. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]