Abstract
The title compounds, (4-trifluoromethoxyphenyl)-2,5-dimethyl-3-(2-R-thiazol-4-yl)-1H-pyrroles, were prepared in four steps starting from commercially available 4-trifluoromethoxyaniline. The pyrrole (second ring) was added in one step using the Paal-Knorr method. The thiazole (third ring) was added in three steps using chloroacylation with chloroacetonitrile followed by heterocyclization with thioamides/thioureas.
Keywords: pyrrole, fluorinated heterocycles, Paal-Knorr reaction, trifluoromethoxy group, chain heterocyclization
1. Introduction
In the course of our search for new anti-cancer compounds that can be used in chemotherapy of late androgen-independent stages of prostate cancer, we turned our attention to the specific class of fluoroheterocyclic systems containing three linked rings, A-B-C, where B is a heterocyclic ring, and A and C are either a heterocyclic or an aromatic ring. Typically, these heterocyclic systems are produced by connecting of one heterocyclic and two aromatic or heterocyclic fragments together using appropriate linking methods. Unfortunately, many common linking procedures cannot be used for direct ring connections. An alternative approach entails chain heterocyclization where heterocyclic moieties are being added in one-by-one fashion using appropriate alicyclic components. Here we report the results of successful application of this strategy for the synthesis of A-B-C fluoroheterocyclic systems with pyrrole as the central unit B.
The pyrrole ring is a part of many natural compounds [1,2,3] as well as synthetic biologically active molecules [4,5]. For example, dialkylaminoalkyl esters of N-(4-hydroxyphenyl)pyrrole exhibit anesthetic and hypotensive properties [6], N-(3-carboxyphenyl)pyrroles are known as antiphlogistic compounds [7], and N-(4-phenoxyphenyl)pyrroles have anticholesteremic properties [8]. Some derivatives of 4-(2,5-dimethylpyrrolyl)benzoic acid inhibit Anthrax Lethal Factor (LF) [9] and Serotonin N-Acetyltransferase [10]. The trifluoromethoxy group is known for its utility in synthesis [11] and usefulness in the optimization of biologically active compounds [12,13,14,15]. Moreover, heterocyclic systems bearing the СF3O group are especially attractive, given their presence in, among others, the insecticide indoxacarb16], the acaricide flufenerim [17], the plant growth regulator flurprimidol [18], and the neurologic drug riluzole [19]. At the same time, little is known about heterocyclic systems containing CF3O-group(s) and pyrrole rings. Our research in this area started with the synthesis of 1-(4-trifluoromethoxyphenyl)-2,5-dimethyl-3-chloroacetylpyrrole - a new building block for the synthesis of extended heterocyclic libraries containing the 1-(4-trifluoromethoxyphenyl)pyrrole fragment [20].
2. Results and Discussion
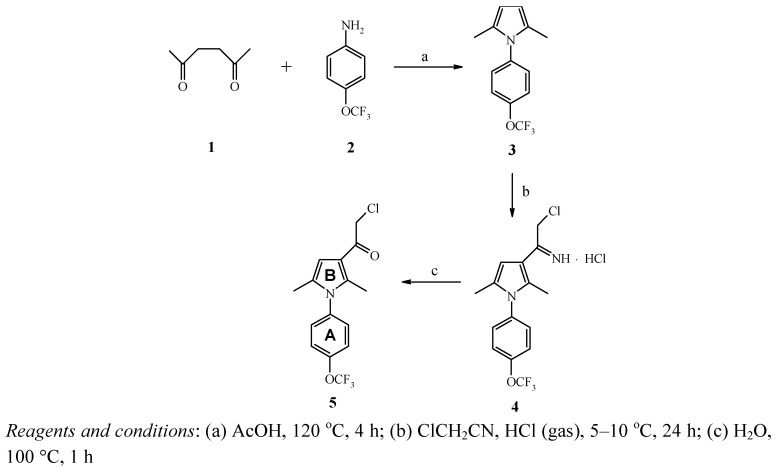
The Paal-Knorr method [21] (which entails condensation of 1,4-diketones with anilines) is the most frequently used method for the synthesis of 1-aryl-2,5-dialkyl(aryl) substituted pyrroles. For example, 1-(4-trifluoromethoxyphenyl)-2,5-dimethylpyrrole (3) can be prepared in 76% yield from readily available 2,5-hexanedione (1) and 4-trifluoromethoxyaniline (2) by this route. According to the literature data [22,23], Friedel-Crafts acylation of 1-phenyl-2,5-pyrrole with alkylcarboxylic acid chlorides yields bis-acylated products (3,4-diketones), whereas acylation with aryl carboxylic acid chlorides results in a mixture of mono- (3-acylated) and bis- (3,4-acylated) products. It appears that direct acylation with chloroacetyl acid chloride cannot be used for selective preparation of the desired 3-chloroacetyl-2,5-dimethylpyrrole (5).
We developed a new preparative method for the synthesis of 3-chloroacetylpyrroles using mild and selective chloroacetylimidoyl chloride [24] that can be prepared in situ from chloroacetonitrile and hydrogen chloride. The desired iminium salt precipitates directly from the reaction mixture in 87% yield. Subsequent hydrolysis gives the target product 5 in 69 % yield (Scheme 1). The presence of a reactive chloroacetyl group in pyrrole 5 makes this compound an attractive building block for the preparation of various nitrogen-, oxygen- and sulfur-containing compounds containing a 4-trifluoromethoxyphenyl fragment. For example, N-alkylation of imidazole, isothiazole, tetrazole derivatives; O-alkylation of hetarylcarboxylic and hetarylacetic acids; S-alkylation of thiazole, 1,3,4-oxadiazole and tetrazole derivatives proceed easily and in mild conditions in DMSO or DMF solutions. The use of thioamides and thioureas in reactions with chloroacetylpyrrole (5) allowed us to add the desired third heterocyclic ring (thiazole fragment) as the last step of chain heterocyclization. As a result, new 4-trifluoromethoxyphenyl substituted pyrroles 6a-c, 7a-c, 8a-c, 9a-g functionalized with a set of pharmacophoric heterocyclic groups were successfully prepared using this approach (Scheme 2, Table 1).
Scheme 1.
Synthesis of 3-chloroacetylpyrrole (5).
Scheme 2.
Synthesis of 4-trifluoromethoxyphenyl substituted pyrroles 6-9 (R1-R5: see Table 1).
Table 1.
Yields and melting points of the synthesized compounds.
| Product | R | Yield, % | Mp | Product | R | Yield, % | Mp |
|---|---|---|---|---|---|---|---|
| 6a |
 (R1R2N) (R1R2N) |
76 | 174-175 | 8c |
 (SR4) (SR4) |
92 | 106 |
| 6b |
 (R1R2N) (R1R2N) |
81 | 232-234 | 9a | Me (R5) | 62 | 134-135 |
| 6c |
 (R1R2N) (R1R2N) |
70 | 126-127 | 9b | 2-Thienyl (R5) | 65 | 115-116 |
| 7a |
 [OC(O)R3] [OC(O)R3] |
71 | 143-144 | 9c |
 (R5) (R5) |
69 | 131-132 |
| 7b |
 [OC(O)R3] [OC(O)R3] |
74 | 110-111 | 9d | NH2 (R5) | 71 | 126-127 |
| 7c |
 [OC(O)R3] [OC(O)R3] |
78 | 103 | 9e | MeNH (R5) | 64 | 166-167 |
| 8a |
 (SR4) (SR4) |
84 | 132-133 | 9f |
 (R5) (R5) |
70 | 129-130 |
| 8b |
 (SR4) (SR4) |
89 | 165-166 | 9g |
 (R5) (R5) |
65 | 108-109 |
3. Experimental
3.1. General
Melting points were determined with a Kofler micro hot-stage (Reichert, Wien) and were uncorrected. 1H- and 19F-NMR spectra were recorded in DMSO-d6 on a Varian-Gemini spectrometer at 299.94 and 188.14 MHz, respectively, with TMS (1H) and CCl3F (19F) as internal standards. 13C-NMR spectra were recorded on a Bruker Arano DRX-500 spectrometer (125.75 MHz), with TMS as internal standard. APCI MS data were obtained on an Agilent 1100\DAD\MSD VL G1965a instrument.
2,5-Dimethyl-1(4-(trifluoromethoxyphenyl)-1H-pyrrole (3). A mixture of 4-trifluoromethoxyaniline (30 g, 170 mmol) and 2,5-hexanedione (22.8 g, 200 mmol) in acetic acid (150 mL) was refluxed for 4 h. The reaction mixture was cooled and poured into water (500 mL), the precipitated solid was filtered and dried. Yield 32.9 g (76 %); mp 68 °C; 1H-NMR: δ 1.98 (s, 6H), 5.76 (s, 2H), 7.34 (d, 2H, J = 9.0 Hz), 7.41 (d, 2H, J = 9.0 Hz); 13C-NMR: δ 12.66 (2 CH3), 106.18 (pyrrole, 3,4-C), 120.57 (q, CF3, J = 254.6 Hz), 121.64 (4-CF3O-C6H4, 3,5-C), 127.60 (pyrrole, 2,5-C), 129.90 (4-CF3O-C6H4, 2,6-C), 137.32 (4-CF3O-C6H4, 1-C), 147.33 (4-CF3O-C6H4, 4-C); 19F-NMR: δ -57.75 (F3CO); MS: m/z 256.0 (M+1)+; Anal. Calcd. for C13H12CF3NO: C 61.18; H 4.74; N 5.49. Found: C 61.31; H 4.77; N 5.61.
2-Chloro-1-{2,5-dimethyl-1-[(4-trifluoromethoxy)phenyl]-1H-pyrrol-3yl}-ethanimine hydrochloride (4). Dry hydrogen chloridewas bubbled through a solution of 2,5-dimethyl-1[4-(trifluoromethoxy)phenyl]-1H-pyrrole (32.9 g, 130 mmol ) and chloroacetonitrile (15 g, 200 mmol) in diethyl ether (200 mL) for 2 hours with vigorous stirring at 5-10 оС. The reaction mixture was left for 12 hours at room temperature. The precipitated solid was collected by filtration, washed with diethyl ether and dried in air. Yield 41.4 g (87 %); mp 220o C; 1H-NMR: δ 2.23 (s, 3H), 2.36 (s, 3H), 5.11 (s, 2H), 7.015 (s, 1H), 7.44 (d, 2H, J = 9.0 Hz), 7.51 (d, 2H, J = 9.0 Hz), 11.62 (s, 1H), 11.96 (s, 1H); 19F-NMR: δ - 57.14 (F3CO); Anal. Calcd. for C15H15Cl2F3NO2: C 49.07; H 4.19; Cl 19.31. Found: C 49.23; H 4.08; Cl 19.17.
2-Chloro-1-{2,5-dimethyl-1-[4-(trifluoromethoxy)phenyl]-1H-pyrrol-3-yl}-ethanone (5). A suspension of 2-chloro-1-{2,5-dimethyl-1-[(4-trifluoromethoxy)phenyl]-1H-pyrrol-3yl}-ethanimine hydrochloride (4, 41.4 g, 110 mmol) in water (200 mL) was heated under reflux for 1 h. The reaction mixture was cooled to room temperature; the precipitated solid was collected, washed with water, and dried. The product was crystallized from methanol. Yield 25.8 g (69 %); mp 147 oC;IR (KBr, cm-1) 1685; 1H- NMR: δ 1.94 (s, 3H), 2.22 (s, 3H), 4.75 (s, 2H), 6.48 (s, 1H), 7.49 (d, 2H, J = 9.0 Hz), 7.56 (d, 2H, J = 9.0 Hz); 13C-NMR: δ 12.28 (CH3), 12.49 (CH3), 47.80 (CH2), 107.40 (pyrrole, 4-C), 117.04 (pyrrole, C-5), 121.45 (q, CF3, J =257.8 Hz), 123.19 (4-CF3O-C6H4, 3,5-C), 128.73 (pyrrole, 3-C), 130.02 (4-CF3O-C6H4, 2,6-C), 135.35 (4-CF3O-C6H4, 1-C), 136.09 (pyrrole, 2-C), 148.15 (q, 4-CF3O-C6H4 , 4-C, J = 1.3 Hz), 186.80 (C=O); 19F-NMR: δ -56.89 (F3CO); MS: m/z 332.0 (M+1)+; Anal. Calcd. for C15H13ClF3NO2: C 54.31; H 3.95; N 4.22. Found: C 54.20; H 3.87; N 4.11.
3.2. General method for synthesis of 2,5-dimethyl-3-(2-N-substituted-1-oxoethyl)-1-[4-(trifluoro-methoxy)phenyl]-1H-pyrroles 6a-c
An appropriate heterocyclic compound (5 mmol) and triethylamine (1.0 mL, 7.5 mmol) were added to a solution of 2-сhloro-1-{2,5-dimethyl-1-[4-(trifluoromethoxy)phenyl]-1H-pyrrol-3-yl}-ethanone (5, 1.66 g, 5 mmol) in DMF (50 mL). The reaction mixture was heated at 75-80 °C with stirring for 2 h, and then it was cooled and poured into water (100 mL). The precipitated solid was filtrated and crystallized from dioxane.
1-{2-[2,5-Dimethyl-1(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-oxoethyl}-3-methylinidazolidine-2,4,5-trione (6a). 1H-NMR: δ 1.99 (s, 3H), 2.23 (s, 3H), 3.07 (s, 3H), 4.81 (s, 2H), 6.63 (s, 1H), 7.48 (d, 2H, J = 9.0 Hz), 7.54 (d, 2H, J = 9.0 Hz); 19F-NMR: δ - 57.47 (F3CO); MS: m/z 424.0 (M+1)+; Anal. Calcd. for C19H16F3N3O5: C 55.93; H 3.81; N 9.93. Found: C 56.04; H 3.88; N 9.80.
2-{2-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-oxoethyl}-1,1-dioxo-1,2-dihydro-1λ6-benzo[d]isothiazol-3-one (6b). 1H-NMR: δ 2.01 (s, 3H), 2.24 (s, 3H), 4.97 (s, 2H), 6.67 (s, 1H), 7.47 (d, 2H, J = 7.7 Hz), 7.53 (d, 2H, J = 8.8 Hz), 8.05 (m, 3H), 8.38 (d, 1H, J = 8.8 Hz); 19F-NMR: δ ‑ 57.44 (F3CO). MS: m/z 479.0 (M+1)+; Anal. Calcd. for C22H17F3N2O5S: C 55.23; H 3.58; N 5.86. Found: C 55.35; H 3.68; N 5.97.
1-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-[5-(3-methoxyphenyl)-tetrazol-2-yl]-ethanone (6c). 1H-NMR: δ 2.02 (s, 3H), 2.25 (s, 3H), 3.86 (s, 3H), 6.19 (s, 2H), 6.65 (s, 1H), 7.09 (dd, 1H, J = 7.4 Hz, J = 2.3 Hz), 7.59 (m, 7H). 19F-NMR: δ - 57.49 (F3CO); MS: m/z 472.0 (M+1)+; Anal. Calcd. for C23H20F3N5O3: C 58.60; H 4.28; N 14.86. Found: C 58.93; H 4.09; N 14.64.
3.3. General method for synthesis of2,5-dimethyl-3-(2-O-substituted 1-oxoethyl)-1-[4-(trifluoromethoxy)-phenyl]-1H-pyrroles 7 a-c
An appropriate acid (5 mmol) and potassium сarbonate (1.4 g, 10 mmol) were added to a solution of 2-сhloro-1-{2,5-dimethyl-1-[4-(trifluoromethoxy)phenyl]-1H-pyrrol-3-yl}-ethanone (5, 1.66 g, 5 mmol) in DMSO (50 mL). The reaction mixture was heated at 45-50оС with stirring for 4 h. The reaction mixture was cooled and poured into water (100 mL). The precipitated solid was collected, dried and recrystallized from 2-propanol.
3-Methylisoxazole-5-carboxylic acid 2-[2,5-dimethyl-1(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-oxoethyl ester (7a). IR (KBr, cm-1) 1700, 1775; 1H-NMR: δ 1.99 (s, 3H), 2.25 (s, 3H), 2.36 (s, 3H), 5.41 (s, 2H), 6.50 (s, 1H), 7.21 (s, 1H), 7.49 (d, 2H, J = 9.0 Hz), 7.54 (d, 2H, J = 9.0 Hz); 19F-NMR: - 57.47 (F3CO); MS: m/z 423.0 (M+1)+; Anal. Calcd. for C20H17F3N2O5: C 56.88; H 4.06; N 6.63. Found: C 56.79; H 4.10; N 6.59.
(5-Methylisoxazol-3-yloxy)acetic acid 2-[2,5-dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-oxoethyl ester (7b). IR (KBr, cm-1) 1695, 1770; 1H-NMR: δ 1.97 (s, 3H), 2.24 (s, 3H), 2.33 (s, 3H), 4.95 (s, 2H), 5.21 (s, 2H), 6.03 (s, 1H), 6.46 (s, 1H), 7.47 (d, 2H, J = 8.7 Hz), 7.52 (d, 2H, J = 8.7 Hz); 19F-NMR: - 57.50 (F3CO); MS: m/z 453.0 (M+1)+; Anal. Calcd. for C21H19F3N2O6: C 55.76; H 4.23; N 6.19. Found: C 55.65; H 4.24; N 6.12.
(2-Oxo-2H-pyridin-1-yl)acetic acid 2-[2,5-dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-oxoethyl ester (7c). IR (KBr, cm-1) 1670, 1695, 1770;1H-NMR: δ 1.96 (s, 3H), 2.23 (s, 3H), 4.86 (s, 2H), 5.22 (s, 2H), 6.27 (t, 1H, J = 5.4 Hz), 6.42 (d, 1H, J = 9.0 Hz), 6.49 (s, 1H), 7.46 (dd, 1H, J = 6.3 Hz, J = 2.1 Hz), 7.50 (d, 2H, J = 8.8 Hz), 7.57 (d, 2H, J = 8.8 Hz), 7.73 (dd, 1H, J = 5.1 Hz, J = 1.9 Hz); 19F-NMR: - 57.16 (F3CO); MS: m/z 449.2 (M+1)+; Anal. Calcd for C22H19F3N2O5: C 58.93; H 4.27; N 6.25. Found: C 58.82; H 4.24; N 6.16.
3.4. General method for synthesis of 2,5-dimethyl-3-(2-S-substituted 1-oxoethyl)-1-[4-(trifluoromethoxy)phenyl]-1H-pyrroles 8 a-c
An appropriate thione (5 mmol) and potassium сarbonate (1.4 g, 10 mmol) were added to a solution of2-сhloro-1-{2,5-dimethyl-1-[4-(trifluoromethoxy)phenyl]-1H-pyrrol-3-yl}-ethanone (5, 1.66 g, 5 mmol) in DMF (50 mL). The reaction mixture was heated at 65-70 °C with stirring for 2 h, and then it was cooled and poured into water (100 mL). The precipitated solid was collected, dried and recrystallized from ethanol/DMF 4:1.
1-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-([1,3,4]thiadiazol-2-ylsulfanyl)-ethanone (8a). 1H-NMR: δ 1.99 (s, 3H), 2.25 (s, 3H), 4.77 (s, 2H), 6.57 (s, 1H), 7.45 (d, 2H, J =7.6 Hz), 7.50 (d, 2H, J = 7.6 Hz), 9.50 (s,1H) 19F-NMR: - 57.50 (F3CO); MS: m/z 414.0 (M+1)+; Anal. Calcd. for C17H14F3N2O2S2: C 59.45; H 4.99; N 12.23 Found: C 59.58; H 4.91; N 12.32.
1-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-(5-[1,2,4]triazol-1-ylmethyl-[1,3,4]-oxadiazol-2-ylsulfanyl)ethanone (8b). 1H-NMR: δ 1.99 (s, 3H), 2.24 (s, 3H), 4.73 (s, 2H), 5.80 (s, 2H), 6.52 (s, 1H), 7.49 (d, 2H, J = 9.0 Hz), 7.52 (d, 2H, J = 9.0 Hz), 7.95 (s, 1H), 8.60 (s, 1H). 19F-NMR: - 57.47 (F3CO); MS: m/z 479.0 (M+1)+; Anal. Calcd. for C20H17F3N6O3S: C 50.21; H 3.58; N 17.56. Found: C 50.32; H 3.52; N 17.63.
1-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-(1-methyl-1H-tetrazol-5-ylsulfanyl)ethanone (8c). 1H NMR (DMSO-d6): δ 1.99 (s, 3H), 2.24 (s, 3H), 4.00 (s, 3H), 4.75 (s, 2H), 6.54 (s, 1H), 7.47 (d, 2H, J = 9.0 Hz), 7.53 (d, 2H, J = 9.0 Hz). 19F NMR (DMSO-d6): - 57.47 (F3CO). MS: m/z 412.0 (M+1)+. Anal. Calcd. for C17H16F3N5O2S: C 49.63; H 3.92; N 17.02. Found: C 49.75; H 3.82; N 17.13.
3.5. General method for synthesis of 4-[2,5-dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]thiazoles 9a-g
A mixture of 2-сhloro-1-{2,5-dimethyl-1-[4-(trifluoromethoxy)phenyl]-1H-pyrrol-3-yl}-ethanone (5, 1.66 g, 5 mmol) and thioamide/thiourea (5 mmol) in ethanol (40 mL) was refluxed for 5 h. The solid separated on cooling was collected by filtration, dried and recrystallized from ethanol.
4-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-methylthiazole (9a). 1H-NMR: δ 2.00 (s, 3H), 2.25 (s, 3H), 2.67 (s, 3H), 6.24 (s, 1H), 7.15 (s, 1H), 7.44 (d, 2H, J = 9.0 Hz), 7.50 (d, 2H, J = 9.0 Hz); 19F-NMR: - 57.43 (F3CO); MS: m/z 353.0 (M+1)+; Anal. Calcd. for C17H15F3N2OS: C 57.95; H 4.29; N 7.95. Found: C 57.86; H 4.21; N 8.04.
4-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]-2-thiophen-2-ylthiazole (9b). 1H-NMR: δ 2.03 (s, 3H), 2.32 (s, 3H), 6.35 (s, 1H), 7.12 (t, 1H, J = 5.6 Hz), 7.20 (s, 1H), 7.42 (d, 2H, J = 8.7 Hz), 7.47 (d, 2H, J = 8.7 Hz), 7.70 (m, 2H); 19F-NMR (DMSO-d6): - 57.69 (F3CO); MS: m/z 421.0 (M+1)+; Anal. Calcd. for C20H15F3N2OS2: C 57.13, H 3.60; N 6.66. Found: C 57.19; H 3.55; N 6.74.
2-{4-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]thiazol-2-yl}-N-phenylacetamide (9c). 1H-NMR: δ 1.99 (s, 3H), 2.24 (s, 3H), 4.25 (s, 2H), 6.28 (s, 1H), 7.03 (t, 1H, J =7.6 Hz), 7.31 (m,2H) 7.41 (s, 1H), 7.48 (d, 2H, J =9.0 Hz), 7.52 (m, 2H), 7.64 (d, 2H, J =9.0 Hz), 10.55 (s,1H); 19F-NMR: - 57.43 (F3CO). MS: m/z 472.0 (M+1)+; Anal. Calcd. for C24H20F3N3O2S: C 61.14, H 4.28; N 8.91. Found: C 61.29; H 4.21; N 8.84.
4-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]thiazol-2-ylamine (9d). 1H-NMR: δ 2.02 (s, 3H), 2.12 (s, 3H), 6.27 (s, 1H), 6.49 (s, 1H), 7.43 (d, 2H, J = 8.7 Hz), 7.48 (d, 2H, J = 8.7 Hz), 9.10 (br s, 2H). 19F-NMR: - 57.70 (F3CO). MS: m/z 354.0 (M+1)+; Anal. Calcd. for C16H14F3N3OS: C 54.38; H 3.99; N 11.89. Found: C 54.46; H 4.07; N 11.80.
{4-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]thiazol-2-yl}methylamine (9e). 1H- NMR: δ 1.90 (s, 3H), 2.25 (s, 3H), 3.00 (s, 3H), 6.24 (s, 1H), 6.65 (s, 1H), 7.45 (d, 2H, J = 9.0 Hz), 7.51 (d, 2H, J = 9.0 Hz), 9.34 (br s, 1H); 19F-NMR: - 57.43 (F3CO). MS: m/z 368.0 (M+1)+; Anal. Calcd. for C17H16F3N3OS: C 55.58; H 4.39; N 11.44. Found: C 55.67; H 4.31; N 11.50.
{4-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]thiazol-2-yl}-(2-methoxyethyl)amine (9f). 1H-NMR: δ 1.99 (s, 3H), 2.24 (s, 3H), 3.29 (s, 3H), 3.43 (t, 2H, J = 5.4 Hz), 3.52 (t, 2H, J = 5.4 Hz), 6.12 (s, 1H), 6.23 (s, 1H), 7.40 (d, 2H, J = 9.0 Hz), 7.48 (d, 2H, J =9.0 Hz); 19F-NMR: - 57.70 (F3CO); MS: m/z 412.0 (M+1)+; Anal. Calcd for C19H20F3N3O2S: C 55.47; H 4.80; N 10.21. Found: C 55.58; H 4.79; N 10.09.
{4-[2,5-Dimethyl-1-(4-trifluoromethoxyphenyl)-1H-pyrrol-3-yl]thiazol-2-yl}-(tetrahydrofuran-2-yl-methyl)amine (9g). 1H-NMR: δ 1.62 (m, 1H), 1.73 (m,2H), 1.85 (m, 1H), 1.96 (s, 3H), 2.23 (s, 3H), 3.25 (s,2H), 3.64 (m, 1H), 3.77 (m, 1H), 4.03 (m, 1H), 6.13 (s, 1H), 6.27 (s, 1H), 7.41 (d, 2H, J = 8.7 Hz), 7.47 (d, 2H, J = 8.7 Hz), 8.56 (m, 1H); 19F-NMR: - 57.47 (F3CO); MS: m/z 438.0 (M+1)+; Anal. Calcd for C21H22F3N3O2S: C 57.66; H 4.5.07; N 9.60. Found: C 57.79; H 5.09; N 9.49.
4. Conclusions
In summary, we developed a successful chain heterocyclization strategy for the synthesis of A‑B‑C fluoroheterocyclic systems with pyrrole as the central unit B. These compounds were prepared in four steps starting from commercially available 4-trifluoromethoxyaniline. The pyrrole ring was added in one step using Paal-Knorr method, and the third thiazole ring was added in three steps using chloroacylation with cloroacetonitrile followed by heterocyclization with thioamide/thiourea. We are currently evaluating biological profiles of these new fluorinated heterocyclic systems.
Acknowledgements
This research was supported by the Global IPP program through the Science and Technology Center in Ukraine (STCU). Oak Ridge National Laboratory is managed and operated by UT-Battelle, LLC, under U.S. Department of Energy contract DE-AC05-00OR22725. This paper is a contribution from the Discovery Chemistry Project.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Fürstner A. Chemistry and biology of roseophilin and the prodigiosin alkaloids: A survey of the last 2500 years. Angew. Chem. Int. Ed. 2003;42:3582–3603. doi: 10.1002/anie.200300582. [DOI] [PubMed] [Google Scholar]
- 2.Tsukamoto S., Tane K., Ohta T., Matsunaga S., Fusetani N., van Soest R.W.M. Four new bioactive pyrrole-derived alkaloids from the marine sponge Axinella brevistyla. J. Nat. Prod. 2001;64:1576–1978. doi: 10.1021/np010280b. [DOI] [PubMed] [Google Scholar]
- 3.Grube A., Köck M. Oxocyclostylidol, an intramolecular cyclized oroidin derivative from the marine sponge Stylissa caribica. J. Nat. Prod. 2006;69:1212–1214. doi: 10.1021/np050408f. [DOI] [PubMed] [Google Scholar]
- 4.Bellina F., Rossi R. Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl group on adjacent positions. Tetrahedron. 2006;62:7213–7256. doi: 10.1016/j.tet.2006.05.024. [DOI] [Google Scholar]
- 5.La Regina G., Silvestri R., Artico M., Lavecchia A., Novellino E., Befani O., Turini P., Agostinelli E. New pyrrole inhibitors of monoamine oxidase: Synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. J. Med. Chem. 2007;50:922–931. doi: 10.1021/jm060882y. [DOI] [PubMed] [Google Scholar]
- 6.Rips R., Buu-Hoï N.P. Pyrrole compounds. 2986564. U.S. Patent. 1961
- 7.Pons A.L., Robba M.F., Marcy R.H., Duval D.J.C. 1-Phenylpyrrole. 1938904. Ger. Patent. 1970
- 8.Vollenberg W., Beckmann R. Neue pyrrolderivate und verfahren zu ihrer herstellung. 2427614. Ger. Patent. 1976
- 9.Schepetkin I.A., Khlebnikov A.I., Kirpotina L.N., Quinn M.T. Novel small-molecule inhibitors of Anthrax lethal factor identified by high-throughput screening. J. Med. Chem. 2006;49:5232–5244. doi: 10.1021/jm0605132. [DOI] [PubMed] [Google Scholar]
- 10.Szewczuk L.M., Saldanha S.A., Ganguly S., Bowers E.M., Javoroncov M., Karanam B., Culhane J.C., Holbert M.A., Klein D.C., Abagyan R., Cole P.A. De novo discovery of serotonin N-acetyltransferase inhibitors. J. Med. Chem. 2007;50:5330–5338. doi: 10.1021/jm0706463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leroux F., Jeschke P., Schlosser M. α-Fluorinated ethers, thioethers, and amines: Anomerically biased specie. Chem. Rev. 2005;105:827–856. doi: 10.1021/cr040075b. [DOI] [PubMed] [Google Scholar]
- 12.Riess J.G. Blood substitutes and other potential biomedical applications of fluorinated colloids. J. Fluor. Chem. 2002;114:119–126. doi: 10.1016/S0022-1139(02)00017-9. [DOI] [Google Scholar]
- 13.Riess J.G. Fluorous micro- and nanophases with a biomedical perspective. Tetrahedron. 2002;58:4113–4131. doi: 10.1016/S0040-4020(02)00262-4. [DOI] [Google Scholar]
- 14.Liu K., Black R.M., Acton J.J., Mosley R., Debenham S., Abola R., Yang M., Tschirret-Guth R., Colwell L., Liu C., Wu M., Wang C.F., MacNaul K.L., McCann M.E., Moller D.E., Berger J.P., Meinke P.T., Jones A.B, Wood H.B. Selective PPARγ modulators with improved pharmacological profiles. Bioorg. Med. Chem. Lett. 2005;15:2437–2440. doi: 10.1016/j.bmcl.2005.03.092. [DOI] [PubMed] [Google Scholar]
- 15.Vovk M.V., Pinchuk O.M., Sukach V.A., Tolmachov A.O., Gakh A.A. Trifluoromethoxy Containing Azoles and Azines: Synthesis and Biological Activity. In: Gakh A.A., Kirk K.L., editors. Fluorinated Heterocycles. American Chemical Society; Washington, DC, USA: 2009. pp. 307–345. [Google Scholar]
- 16.Harder H.H., Riley S.R., McCann S.F., Irving S.N. DPX-MP062: A novel, broad-spectrum, environmentally-soft, insect control compound. Brighton Crop Prot. Conf. Pests. Dis. 1996;2:449–454. [Google Scholar]
- 17.Obata T., Fujii K., Ooka A., Yamanaka Y. 4-Phenethylamino pyrimidine derivative, process for preparing the same and agricultural and horticultural chemical for controlling noxious organisms containing the same. EP0665225. Eur. Patent. 1995
- 18.Rademacher W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;51:501–537. doi: 10.1146/annurev.arplant.51.1.501. [DOI] [PubMed] [Google Scholar]
- 19.Bensimon G., Lacomblez L., Meininger V. A controlled trial of Riluzole in amyotrophic lateral sclerosis. New Engl. J. Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 20.Dolle R.E., Le Bourdonnec B., Goodman A.J., Morales G.A., Salvino J.M., Zhang W. Comprehensive survey of chemical libraries for drug discovery and chemical biology. 2006. J. Comb. Chem. 2007;9:855–902. doi: 10.1021/cc700111e. [DOI] [PubMed] [Google Scholar]
- 21.Broadbent H.S., Burnham W.S., Olsen R.K., Sheeley R.M. Sterically-crowded pyrroles. J. Heterocycl. Chem. 1968;5:757–767. doi: 10.1002/jhet.5570050604. [DOI] [Google Scholar]
- 22.Rips R., Buu-Hoï N.P. Friedel-Crafts acylations of 1-phenyl-2,5-dimethylpyrrole and 1,2-diphenyl-5-methylpyrrole. J. Org. Chem. 1959;24:551–554. doi: 10.1021/jo01086a027. [DOI] [Google Scholar]
- 23.Bishop W. S. New 2,5-dimethylpyrrole derivatives. J. Am. Chem. Soc. 1945;67:2261–2262. doi: 10.1021/ja01228a502. [DOI] [Google Scholar]
- 24.Schmidt A. Imidiumazid-hexachloroantimonate (V) mesomeriestabilisierte azide. Chem. Ber. 1967;100:3319–3325. doi: 10.1002/cber.19671001021. [DOI] [Google Scholar]