Abstract
Organocatalytic asymmetric α-oxidation and amination reactions of carbonyl compounds are highly useful synthetic methodologies, especially in generating chiral building blocks that previously have not been easily accessible by traditional methods. The concept is relatively new and therefore the list of new catalysts, oxidizing and aminating reagents, as well as new substrates, are expanding at an amazing rate. The scope of this review includes new reactions and catalysts, mechanistic aspects and synthetic applications of α-oxidation, hydroxylation, aminoxylation, amination, hydrazination, hydroxyamination and related α-heteroatom functionalization of aldehydes, ketones and related active methylene compounds published during 2005–2009.
Keywords: organocatalyst, chiral catalyst, α-oxidation, α-amination, α-aminoxylation, nitrosoaldol reaction, aldehyde, ketone, active methylene compound
Outline
Introduction
-
α-Amination reactions
-
2.1. α-Amination with azodicarboxylate esters
2.1.1 Simple aldehydes and ketones
2.1.2 Other carbonyl substrates
2.1.3. Applications
2.2. α-Sulfamidation
2.3. α-Hydroxyamination
-
-
α-Aminoxylation reactions
-
3.1. α-Aminoxylation with nitrosobenzene
3.1.1. Substrates
3.1.2. Catalysts
3.1.3. Applications
3.1.4. Aminoxylation vs. nitrosoaldol reactions
3.2. α-Aminoxylation with TEMPO
-
-
α-Oxidation reactions
4.1. α-Oxybenzoylation
4.2. α-Oxidation with molecular oxygen
4.3. α-Oxidation with hydroperoxides
4.4. α-Aryloxylation with o-quinones
4.5. α-Oxidation with oxaziridine and iodosobenzene
4.6. α-Oxysulfonation catalyzed by iodoarenes
Conclusions and Outlook
1. Introduction
The rich chemistry of carbonyl compounds makes optically active α-heteroatom-functionalized carbonyl compounds important precursors for a large group of natural and non-natural compounds possessing a nitrogen or oxygen moiety attached to a stereogenic center. The direct introduction of a nitrogen or an oxygen atom to the α-position of the carbonyl group catalyzed by organocatalysts represents a powerful and promising methodology towards such optically active precursors (Figure 1).
Figure 1.
Asymmetric direct α-functionalization of carbonyl with oxygen and nitrogen electrophiles.
During the past decade, a number of impressive examples of organocatalytic enantioselective direct α-amination, α-aminoxylation, and α-oxidation reactions on carbonyl compounds to afford the corresponding α-amino and α-hydroxy carbonyl derivatives have been developed. Several reviews on the topic of organocatalyzed direct asymmetric α-aminoxylation [1,2], α-amination [2] as well as α-heterofunctionalization of carbonyl compounds in general [3,4,5,6] or some specific aspects [7,8,9,10,11] exist up to the year 2006. This review compiles a significant progress made up to the year 2009.
2. α-Amination Reactions
In 2002, Jørgensen [12] and List [13] independently reported the first successful proline-catalyzed enantioselective direct α-amination of aldehydes using azodicarboxylate esters as electrophiles. Since then, a number of α-aminations of simple and branched aliphatic aldehydes, as well as aliphatic cyclic and acyclic ketones catalyzed by proline and its derivatives have been reported. Early works have been covered in previous reviews on this subject [2,3,4,5]. Analogous reactions with other substrates such as 1,3-dicarbonyl compounds or α-substituted-α-cyanoacetates have been accomplished with different catalysts, including those derived from cinchona alkaloids [8], binaphthyl [14,15,16], or cyclohexanediamine derivatives [17]. Details of recent developments in the above mentioned topics will be described herein.
2.1. α-Amination with Azodicarboxylate Esters
2.1.1. Simple aldehydes and ketones
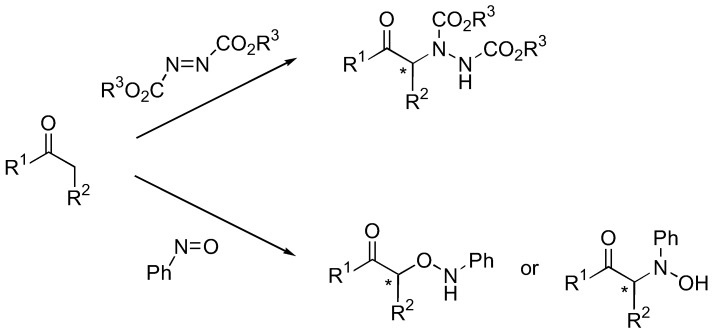
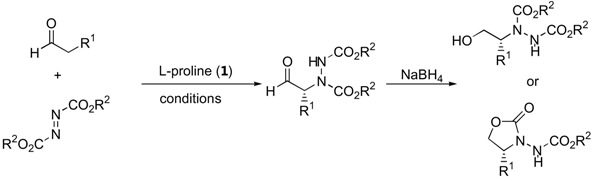
The α-amination of simple α-unbranched aldehydes with various azodicarboxylate esters proceeds very efficiently under catalysis by L-proline (1, Table 1). Since the α-aminated products are often chemically and configurationally unstable due to the presence of an acidic α-H, they are often reduced in situ to the corresponding aminoalcohols or, after subsequent cyclization under basic conditions, to N-aminooxazolidinones [12,13,14,18,19,20,14,18].
Table 1.
Proline catalyzed α-amination of unbranched aldehydes.
 |
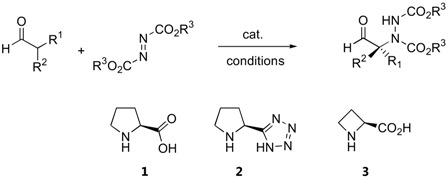
In later reports, the possibility of generating quaternary stereogenic centers at the α-position of α-branched aldehydes under catalysis by proline (1) [21,22], pyrrolidinyl tetrazole (2) [23,24] or L-azetidine-2-carboxylic acid (3) [21] were explored (Table 2). The observed enantioselectivities ranged from essentially none to >99%. Derivatives of 2-phenylpropanal gave better enantioselectivities than α,α-dialkyl substituted aldehydes.
Table 2.
α-Amination of α-branched aldehydes catalyzed by proline and derivatives.
 |
| R1 | R2 | R3 | Conditions | Yield (%) | ee (%) | Ref. |
|---|---|---|---|---|---|---|
| Et, n Bu, Ph, 2-Naph, | Me, | Et, | 50 mol% 1, | 17–87 | 4–28 (R1 = alkyl) | [21] |
| subst. Ph | Et | Bn | CH2Cl2, RT, | 76–85 (R1 = aryl) | ||
| 2.5–9 d | ||||||
| Et, Ph, 2-Naph, | Me, | Et, | 50 mol% 3, | N/A | 6 (R1 = alkyl) | [21] |
| subst. Ph | Et | Bn | CH2Cl2, RT, | 49–78 (R1 = aryl) | ||
| 2.5–9 d | ||||||
| Me, Et, Pr, Bu, Ph, | Me, | Et, | 50 mol% 1, | 19–99 | 0–39 (R1 = alkyl) | [22] |
| 2-Naph, subst. Ph, | Et | Bn | CH2Cl2, RT | 35–86 (R1 = aryl) | ||
| 2-thienyl | ||||||
| H, 2-Naph, | Me | Et | 50 mol% 1 or 2, | 54–97 | 52–91 | [24] |
| 4-subst. Ph | MeCN, microwave | (6–85)a | (24–84)a | |||
| 4-BrC6H4CH2 | Me | Bn, | 15 mol% 2, MeCN, | 95 | 80 | [23] |
| Et | RT, 3h | (90)b | (44)b | |||
 |
Bn | 20–30 mol% 1 or ent-1, MeCN, RT,1–4 h | 75–99 | >99 | [25] | |
a Without microwave irradiation b With 30 mol% L-proline (1) instead of 2 as catalyst.
Bräse and coworkers had employed microwave irradiation to accelerate the rate of proline-catalyzed amination, and found that yields as well as enantioselectivity had somewhat improved after shorter reaction times [24]. It appears that the pyrrolidinyl tetrazole 2 was a more effective catalyst for amination of 2-phenylpropanal derivatives than L-proline (1) [23,24]. Indane-1-carboxaldehydes are particularly good α-branched aldehyde substrates for L-proline- (1) or D-proline (ent-1)-catalyzed α-amination [25]. The corresponding amination products were obtained in 99% and >99% ee and were subsequently elaborated into the metabotropic glutamate receptor ligands, (S)-AIDA and (S)-APICA[25]. The possibilities of obtaining good yields and enantioselectivities from these inherently chiral aldehydes suggests that the original chirality of the aldehyde has no effect on the stereoselectivity, which is to be expected based on the proposed mechanism that proceeds through planar intermediates.
Jørgensen and coworkers also reported a proline-catalyzed enantioselective direct α-amination of ketones (Figure 2) [26]. The reaction of simple aliphatic ketones with diethyl azodicarboxylate (DEAD) was found to be fairly regioselective as the amination took place at the more highly substituted carbon atom (ratio = 76:24–91:9). The reaction also proceeded with excellent enantioselectivities. Subsequent reduction and cyclization could be carried out to afford the syn-α-amino alcohols or the N-amino-oxazolidinones [26].
Figure 2.
L-Proline-catalyzed α-amination of ketones [26].
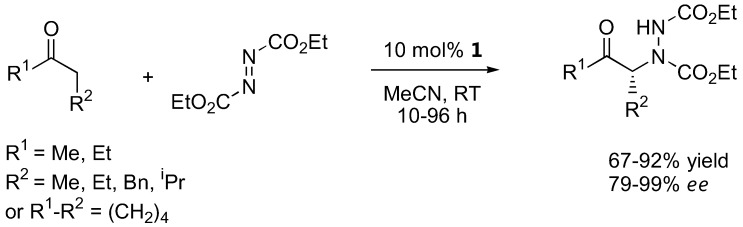
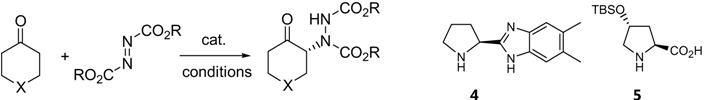
Cyclic ketones such as cyclohexanone and derivatives generally gave poor results with proline catalyst 1 (Table 3). The α-amination of cyclohexanone with DEAD or dibenzyl azodicarboxylate (DBAD) under L-azetidine-2-carboxylic acid (3) catalysis showed improved enantioselectivities of 88–90% [27]. The benzimidazole-pyrrolidine (BIP) catalyst 4 was comparable to L-proline in terms of enantioselectivity with cyclohexanone and 1-silacyclohexan-4-one as substrates [28]. In a comparison between the efficiency of L-proline (1) versus 4-silyloxyproline (5) in the α-amination of cyclohexanone and derivatives, the catalyst 5 provided higher yields and enantioselectivities than 1 [29].
Table 3.
α-Amination of cyclohexanone and derivatives.
 |
| ketone | R | cat. | conditions | yielda (%) | eeb (%) | ref. |
|---|---|---|---|---|---|---|
| X=CH2 | Bn | 3 | 20 mol% cat., CH2Cl2, 25 ºC , 24 h | 60 (56) | 90 (61) | [27] |
| X=CH2 | Bn | 4 | 10 mol% cat./TFA, CH2Cl2, 20 ºC, 24 h | 92 (88) | 66 (84) | [28] |
| X=SiPh2 | Bn | 4 | 20 mol% cat./TFA, CH2Cl2, 20 ºC, 24 h | 65 (85) | 71 (60) | [28] |
| X=CH2 | Et | 5 | 10 mol%, ClCH2CH2Cl, RT, 1.5–3 h | 89 (31) | 85 (85) | [29] |
| X=CH2 | Bn | 5 | 10 mol%, ClCH2CH2Cl, RT, 1.5–3 h | 86 (50) | 94 (75) | [29] |
a,b Numbers in parenthesis are results from L-proline catalyzed reactions under comparable conditions.
The silyloxyproline catalyst 5 proved to be a quite general catalyst for amination of ketones. In addition to cyclohexanone derivatives (Figure 3), the catalyst can also accept acyclic ketones and α-branched aldehydes as substrates (64–73% yield, 78–96% ee) [29].
Figure 3.
Examples of products from α-amination of cyclic ketones catalyzed by the silyloxyproline 5 [29].
Ionic liquids have been used as alternative solvent for L-proline-catalyzed α-amination of aldehydes and ketones [30]. Unfortunately, both the product yields and enantioselectivities were not comparable to those obtained from reactions in conventional solvents. The poor results may partly be due to side reactions between the ionic liquid [bmim]BF4 and the aminating agent (DEAD) used.
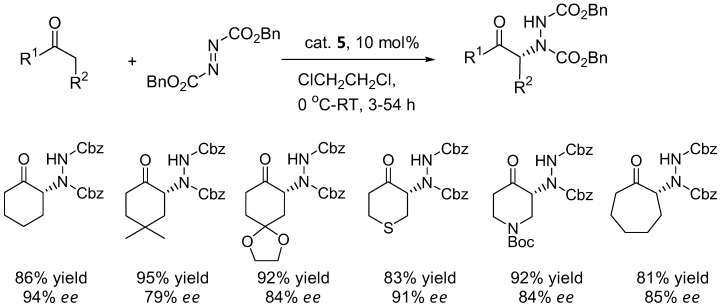
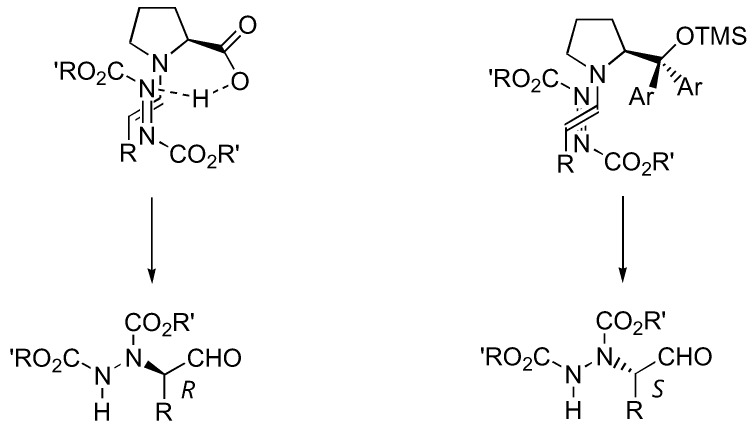
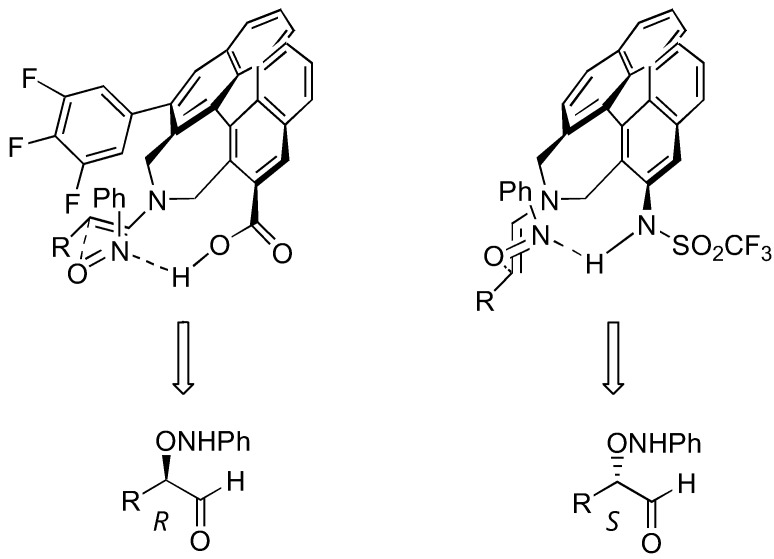
The aforementioned catalysts 1–5, as well as the sulfonamide catalyst 6 [18] (Figure 4), are based upon the proline skeleton with a hydrogen bond donating substituent. Such catalysts operate through the dual activation of both the aldehyde (by forming a more nucleophilic enamine) and the electrophile (by hydrogen bonding). It is generally believed that the approach of the electrophile (azodicarboxylate ester in this case) was controlled by hydrogen bonding with the carboxyl group of the catalyst. On the other hand, the presence of a hydrogen-bonding group was not prerequisite to high activities and enantioselectivities. Jørgensen et al. have developed a new silyl-protected diarylprolinol catalyst 7 that could not participate in hydrogen bonding [19] (Figure 4). The enantioselectivities observed with this catalyst are thus originated from controlling the geometry of the enamine and from steric bias of the two enamine faces by the large substituent on the pyrrolidine ring of the catalyst. This catalyst proved to be highly active, and α-amination of aldehydes with DEAD or di-isopropyl azodicarboxylate (DIAD) in the presence of 10 mol% of 7 was completed in 15 min at room temperature, giving the product in 73–88% yield and 90–97% ee [19]. Most importantly, despite the same absolute configuration of the catalyst 7 as that of proline, the reactions catalyzed by them afforded opposite enantiomers of the same product. This could be explained by two different transition state models shown in Figure 5. The origin of the stereoselectivity was further investigated by DFT calculations [31].
Figure 4.
Additional non-amino acid catalysts for α-amination of aldehydes.
Figure 5.
Models to explain the opposite enantioselectivity of amination of aldehydes catalyzed by 1 and 7.
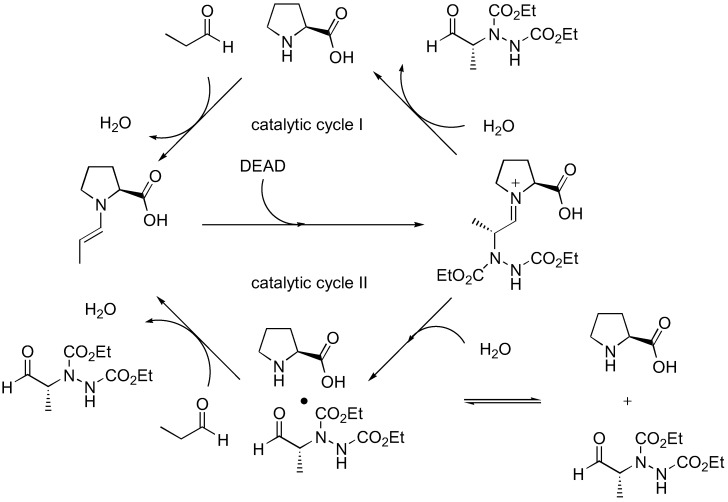
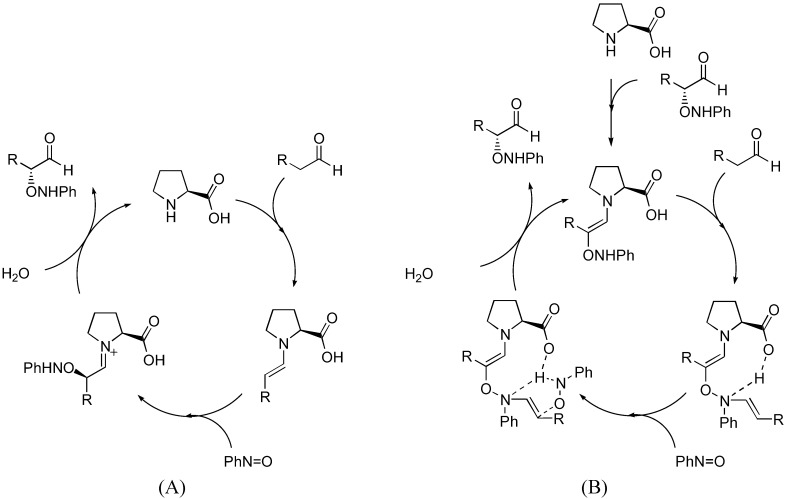
Although this simple model can satisfactorily explain the observed enantioselectivity of proline-catalyzed α-amination of aldehydes, it cannot account for the autoinductive behavior and asymmetric amplification observed upon detailed kinetic analysis by Blackmond et al. [32,33]. Such unusual kinetics for proline-catalyzed reactions are observed only in amination and aminoxylation (Section 3.1.2), but not aldol reactions. The possibility of rate acceleration by solubilization of the proline catalyst over time was ruled out since a proline derivative that is completely soluble also displays the same kinetic profile [34]. An alternative catalytic cycle involving product catalysis that is consistent with the kinetic data was proposed (Figure 6) [34].
Figure 6.
Catalytic cycles of proline-catalyzed amination of aldehydes as proposed by Blackmond et al. [34].
2.1.2. Other carbonyl substrates
Aromatic ketones
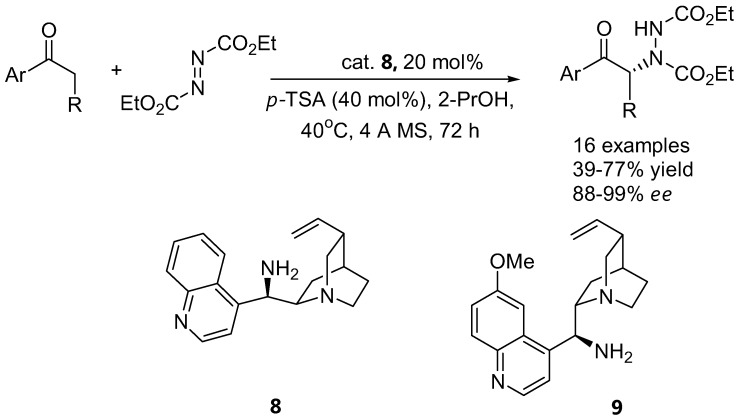
Proline and its derivatives are generally not effective catalysts for α-amination as well as other electrophilic reactions of aromatic ketones. Recently, a successful enantioselective direct α-amination of aromatic ketones catalyzed by primary amines derived from cinchona alkaloids has been reported [35].
In most cases, excellent enantioselectivities were observed for various aromatic ketones in the presence of the catalyst 9-amino-9-deoxyepicinchonine (ADC, 8) (Figure 7). The use of 9-amino-9-deoxyepiquinine (ADQ, 9) as catalyst afforded the opposite enantiomer of the α-aminated product in lower enantiomeric purity and lower yield. It was proposed that activation of the carbonyl with a primary amine would experience less steric interaction than with a secondary amine. In general the reaction was also found to be quite sensitive to steric groups on the azodicarboxylate electrophiles, for instance DEAD was found to give better enantiomeric excess than DBAD. A catalytic model of the reaction was also proposed to account for the observed stereochemistry of the product [35].
Figure 7.
Direct α-amination of aromatic ketones catalyzed by primary amines derived from cinchona alkaloids [35].
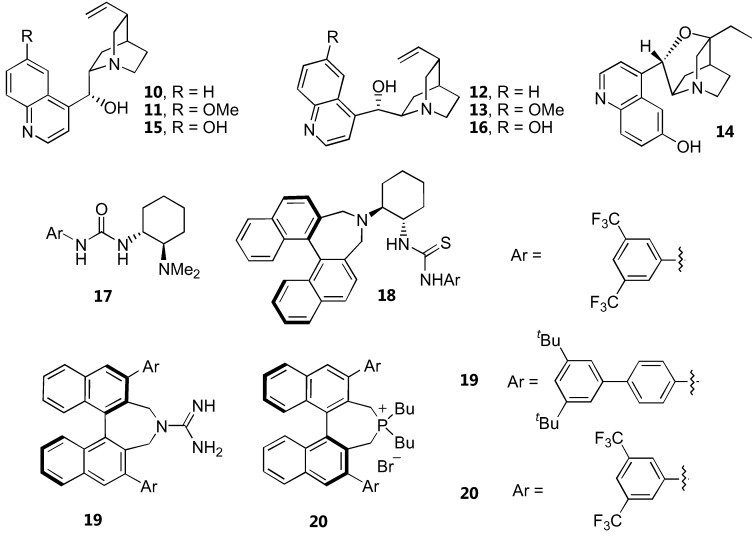
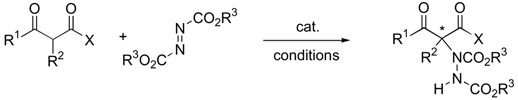
Dicarbonyl compounds
Structures of catalysts for α-amination of 1,3-dicarbonyl compounds are shown in Figure 8. The yields and enantioselectivities are summarized in Table 4. Simple unmodified cinchona alkaloids, cinchonidine (10) and cinchonine (12), have reportedly catalyzed α-amination of cyclic β-ketoesters in high yields and good enantioselectivity [36]. High reaction rates and good enantioselectivities were observed with five membered β-ketolactones and ketoesters. This was rationalized by the higher reactivity of the resulting enolate. In contrast, acyclic β-ketoesters required longer reaction time and gave only moderate to poor enantioselectivity (<40% ee). The structurally similar quinine (11) and quinidine (13) gave much lower enantioselectivities which could be attributed to the basicity or conformation difference.
Figure 8.
Various catalysts for direct α-amination of 1,3-dicarbonyl compounds.
Table 4.
Enantioselective α-amination of 1,3-dicarbonyl compounds with various catalysts.
 |
| Cat. | 1,3-Dicarbonylcompounds | Conditions | Yield(%) | ee(%) | Ref. |
|---|---|---|---|---|---|
| 10 | acyclic β-keto esters | 20 mol% cat., -25 ºC, 7 d | 72 | 47 | [36] |
| 12 | acyclic β-keto esters | 20 mol% cat., -25 ºC, 7 d | 72 | 27 | [36] |
| 10 | cyclic β-keto esters | 20 mol% cat., -25 ºC, 2 min – 48 h | 92–95 | 54–88 | [36] |
| 12 | cyclic β-keto esters | 20 mol% cat., -25 ºC, 5 min – 24 h | 81–95 | 77–87 | [36] |
| 10 | cyclic β-keto lactones | 20 mol% cat., -25 ºC, 2 min – 4 d | 68–91 | 49–60 | [36] |
| 12 | cyclic β-keto lactones | 20 mol% cat., -25 ºC, 2 min – 4 d | 51–96 | 42–57 | [36] |
| 14 | acyclic and cyclic β-keto esters | 5 mol% cat., -52ºC –RT, toluene, 16–143 h | 86–99 | 83–90 | [37] |
| 15 or 16 | acyclic β-keto thioesters | 0.5–10 mol% cat., -78 ºC to RT, 5 min–3 h | 91–95 | 90–>99 | [38] |
| 17 | cyclic β-keto esters | 10 mol% cat., toluene, -78 ºC to RT, 0.5–48 h | 84–98 | 50–92 | [17] |
| 18 | cyclic β-keto esters | 10 mol% cat., toluene, -70 to -30ºC | 85–95 | 93–99 | [14] |
| 19 | cyclic β-keto esters | 0.05–2 mol% cat., THF, -60 ºC, 0.5–24 h | >99 | 97–98 | [15] |
| 19 | acyclic β-keto esters | 2 mol% cat., THF, -60 ºC, 0.5–24 h | 54–>99 | 62–85 | [15] |
| 19 | cyclic β-diketones | 2 mol% cat., THF, -60 ºC, 0.5–24 h | ≥99 | 15–91 | [15] |
| 20 | cyclic β-keto esters | 3 mol% cat., K2CO3 or K2HPO4, toluene, -20 to -40 ºC, 2–96 h | 97–99 | 77–95 | [16] |
| 20 | tert-butyl α-fluoro-benzoylacetate | 3 mol% cat., K2HPO4, toluene, -20 ºC, 84 h | 99 | 73 | [16] |
| 20 | functionalized cyclic diketone | 5 mol% cat., K2HPO4, toluene, -20 ºC, 84 h | 75 | 88 | [16] |
Another compound in the cinchona alkaloid family, β-isocupreidine (β-ICD, 14), could also enantioselectively catalyze α-amination of 1,3-dicarbonyl compounds [37]. The reaction of α-substituted 1,3-dicarbonyl compounds including cyclic and acyclic β-ketoesters and 1,3-diketones with di-tert-butyl azodicarboxylate proceeded with high conversion and enantioselectivity.
Deng and coworkers [38] proposed that the low yields and poor enantioselectivities observed in the asymmetric α-amination of the less reactive acyclic β-ketoesters catalyzed by cinchona alkaloids may result from catalyst inactivation via a Friedel-Crafts type reaction with the diazocarboxylate esters. It was therefore envisaged that β-ketothioesters bearing a more acidic α-proton than simple β-ketoesters might be sufficiently reactive to undergo the amination before the catalyst inactivation. Gratifyingly, the α-amination reaction of various acyclic and cyclic β-ketothioesters proceeded very well in the presence of the catalysts 15 or 16 to afford the products with high yields and enantiomeric excesses.
A chiral bifunctional urea 17 derived from cyclohexanediamine had been proposed as alternative catalyst for α-amination of cyclic β-ketoesters [17]. The reactions of cyclic β-ketoesters gave excellent yields and high enantioselectivity. A related chiral diamine-thiourea derivative derived from a binaphthyl scaffold 18 has been developed as a catalyst for α-amination of β-ketoesters [14]. Although the α-amination of cyclic β-ketoesters proceeded efficiently giving high yields and very high enantioselectivity, the acyclic counterparts underwent the reaction very slowly, giving very low enantiomeric excess in the products. A transition state involving the electrophile activation by hydrogen bonding with the thiourea and the β-ketoester activation by the basic nitrogen atom in tertiary amine has been proposed for the catalyst 18. A DFT calculation confirmed that the catalyst 17 deprotonates the β-ketoester and binds the two substrates through hydrogen bonding with the urea and the protonated amino groups [39].
The axially chiral guanidine 19, which should possess a C2 symmetry when protonated, is another highly active catalyst for α-amination of various cyclic and acyclic 1,3-dicarbonyl compounds [15]. The chiral phosphonium salt 20 derived from the same binaphthyl skeleton was also a very effective catalyst for α-amination of a range of 1,3-dicarbonyl compounds. One particularly interesting aspect of this catalyst is that it can accept functionalized substrates such as α-fluorobenzoylacetate ester [16].
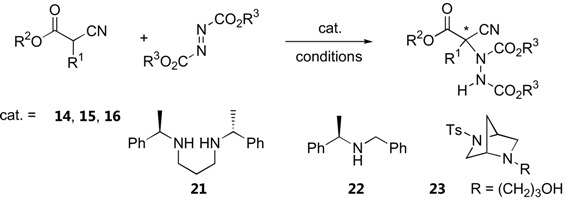
α-Substituted α-cyanoacetates and α-cyanoketones
In 2004, Jørgensen et al. reported the first organocatalytic α-amination of α-substituted α-cyanoacetates [37]. The cinchona alkaloid β-isocupreidine (14), which has been successfully used for α-amination of 1,3-diketones, was also an equally effective catalyst for α-amination of a range of α-aryl α-cyanoacetates [37]. The cinchona alkaloid-derived catalysts 15 and 16 were effective for α-aryl α-cyanoacetates [40] and α-alkyl α-cyanoacetates [38]. In the latter case, a more acidic 2,2,2-trifluoroethyl ester was required to achieve good enantioselectivity [38]. Other natural alkaloids and synthetic chiral amines such as 21–23 have also been reported as catalysts for α-amination of ethyl α-phenyl α-cyanoacetate [41,42]. However, the enantioselectivities were not as high as cinchona alkaloid derivatives and substrate generality has not been demonstrated. The results on direct α-amination of α-substituted α-cyanoacetates are summarized in Table 5.
Table 5.
Direct α-amination of α-substituted α-cyanoacetates.
 |
| Cat. | R1 | R2 | R3 | Conditions | Yield (%) | ee(%) | Ref. |
|---|---|---|---|---|---|---|---|
| 14 | Ph, subst. Ph, 2-Naph, 2-thienyl | tBu | tBu | 5 mol% cat., toluene, -50 to -78 °C, 16–20 h | 95–99 | 89–>98 | [37] |
| 15 | Ph, subst. Ph,1-Naph | Et | tBu, Bn | 5–10 mol% cat., -78 °C, toluene, 1 min–12 h | 72–98 | 87–99 | [40] |
| 16 | Ph, subst. Ph, 1-Naph | Et | tBu, Bn | 5–10 mol% cat., -78 °C, toluene, 1 min–12 h | 71–99 | 82–95 | [40] |
| 15 | Me | CF3CH2 | tBu | 10 mol% cat., toluene, -60 °C | 99(74)a | 91(23)a | [38,40] |
| 16 | Me | CF3CH2 | tBu | 10 mol% cat., toluene, -60 °C | 99(75)a | 81(35)a | [38,40] |
| 18 | Ph | Et | Et | 10 mol% cat., toluene, RT, 1 h | 86 | 65 | [43] |
| 21 | Ph | Et | tBu | 50 mol% cat., 100 °C, 1:1 toluene:hexane, 30 min | 91 | 84( R) | [41] |
| 22 | Ph | Et | tBu | 1–50 mol% cat., -100 °C 1:1 toluene:hexane, 0.5-20 h | 90–95 | 64–80( S) | [41] |
| 23 | Ph | Et | tBu | 25 mol% cat., toluene, -78 °C, 24 h | 76 | 40( R) | [42] |
a Yield and enantioselectivities obtained with ethyl α-methyl α-cyanoacetate (R2 = Et).
Amination of α-cyanoketones by di-tert-butyl azodicarboxylate has also been investigated. The thiourea catalyst 18 (1–10 mol%, toluene -78 °C to RT, 0.5 h–8 d) was found to give the best results. Various cyclic (five- and six-membered ring) and acyclic cyanoketones were excellent substrates for this reaction (14 examples, 45–95% yield, 87–99% ee) [43]. On the other hand, this catalyst gave only moderate enantioselectivity in direct amination of ethyl α-phenyl α-cyanoacetate (up to 65% ee) [43].
Miscellaneous substrates
Direct aminations of chiral protected β-hydroxyaldehydes under catalysis by proline have been recently reported by Greck [44]. The configuration of the newly formed stereogenic center at the α-carbon was mainly controlled by the catalyst, although some matched-mismatched effects have been observed. The 3- and 4-tert-butoxy derivatives of proline catalysts developed by the same group [45] gave comparable results to unmodified proline.
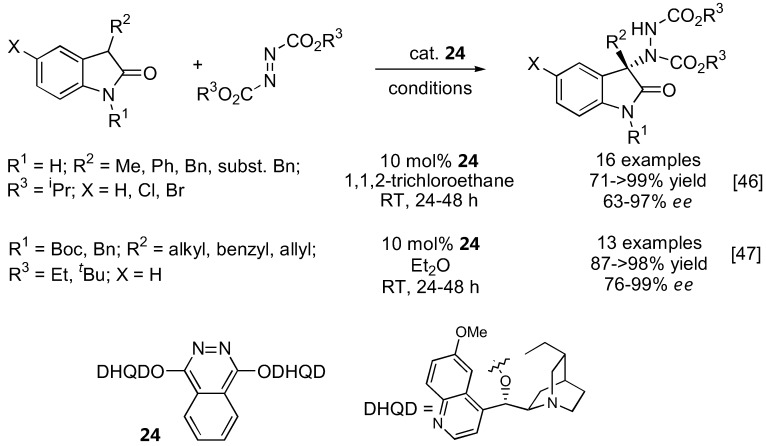
Two independent reports appeared in 2009 describing the first α-amination of 3-substituted 2-oxindoles under catalysis by a cinchona alkaloid-derived catalyst (Figure 9) [46,47]. Although the same (DHQD)2PHAL catalyst 24 was used, the substrate scopes are somewhat different, presumably due to the different conditions employed. The free indole NH was initially thought to be essential to the reactivity as well as enantioselectivity [46]. However, it was later demonstrated that a variety of N-substituted oxindoles also gave excellent yield and enantioselectivities [47].
Figure 9.
Direct α-amination of 3-substituted 2-oxindoles catalyzed by 24 [46,47].
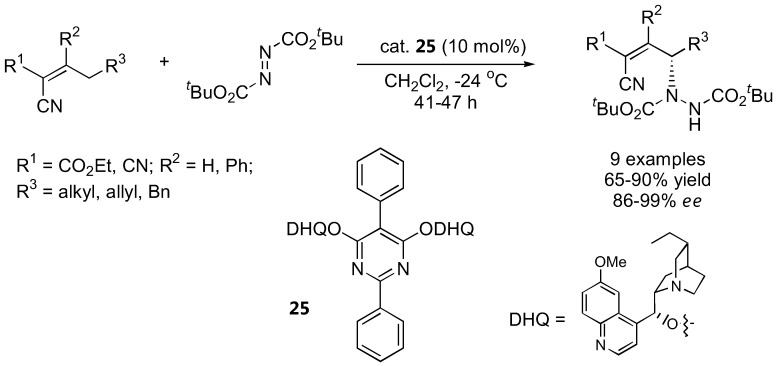
Although not strictly an α-amination of carbonyl compounds, it is worth including the two recently reported amination at the γ-positions of α,β-unsaturated aldehydes [48] and alkylidene cyanoacetates [49], both from the Jørgensen group. Cinchona alkaloids were employed in the allylic amination of alkylidene cyanoacetates (Figure 10) [49].
Figure 10.
Direct allylic amination of alkylidene cyanoacetates catalyzed by 25 [49].
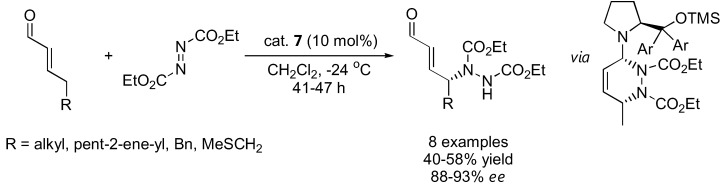
The (DHQ)2PYR catalyst 25 gave the best results. The highly functionalized γ-hydrazino alkylidene cyanoacetates could be further transformed into a variety of products [49]. For γ-amination of α,β-unsaturated aldehydes, the silyl-protected diarylprolinol (7) was more effective than proline and other derivatives (Figure 11) [48]. The stereochemistry of the γ-amination product was opposite to that of the α-amination of simple aldehydes using the same catalyst. A hetero-Diels-Alder reaction between the (E,s-cis,E)-dienamine intermediate and the azodicarboxylate ester was proposed based on DFT calculations to account for the unexpected stereochemical outcome [48].
Figure 11.
Direct γ-amination of α,β-unsaturated aldehydes catalyzed by 7 [48].
2.1.3. Applications
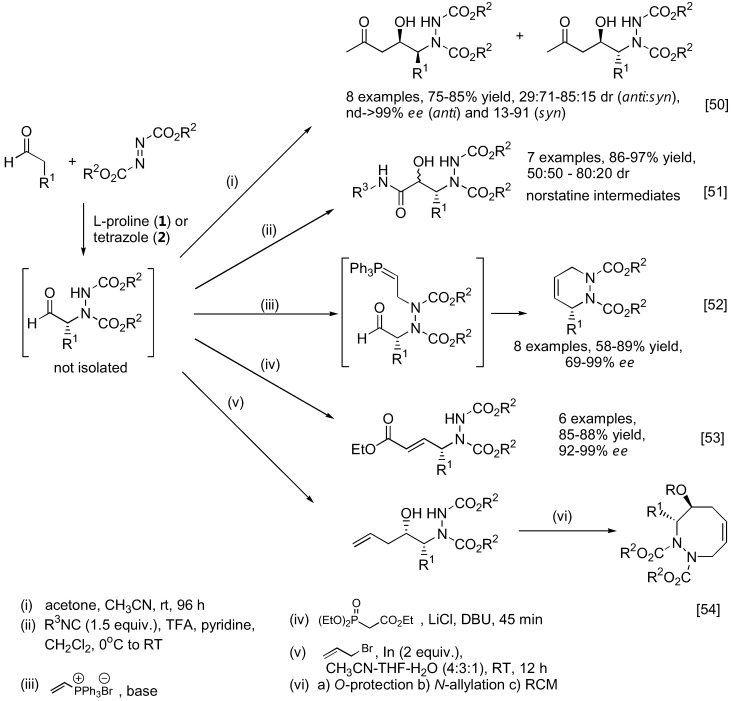
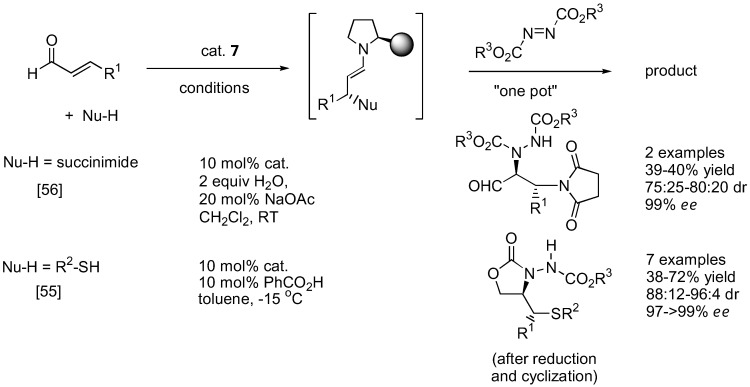
As mentioned earlier, the unstable α-hydrazinoaldehydes are often not isolated but rather subjected to further reactions such as reduction to yield a more stable amino alcohol or reduction-cyclization to afford the corresponding oxazolidinone. Other transformations are illustrated in Figure 12. For example, the so-formed α-hydrazinoaldehyde can further react with acetone under catalysis by 1 in a one-pot fashion to give optically active β-amino alcohols containing two stereogenic centers [50]. A subsequent Passerini reaction of the α-hydrazinoaldehyde may be performed by reacting the aldehyde with an isocyanide. Schmidt et al. had shown that this sequential reaction can provide rapid access to norstatine-based peptidomimetics [51]. A tandem α-amination followed by N-alkylation with a vinyl phosphonium salt gave a transient ylid intermediate which would then cyclize through an intramolecular Wittig reaction to furnish chiral 3,6-dihydropypridazines [52]. Alternatively, the α-hydrazino aldehyde can be trapped with the Horner-Wadsworth-Emmons (HWE) reagent (triethyl phosphonoacetate) under basic conditions to afford γ-hydrazino-α,β-unsaturated esters [53]. Applications of this one-pot α-amination-olefination of aldehydes in the synthesis of chiral 2-pyrrolidones have been demonstrated. Functionalization of the so-formed α-hydrazino aldehyde by a one-pot indium-promoted allylation followed by a ring-closing metathesis (RCM) would lead to an enantioselective synthesis of cyclic 8-membered ring hydrazines [54]. To further expand the scope of the α-amination reaction of aldehydes, it was demonstrated that β-functionalized aldehydes may be obtained from hetero-Michael type addition to α,β-unsaturated aldehydes under iminium catalysis by the silylprolinol catalyst 7 (Figure 13) [55,56]. The so-formed enamine intermediate may then undergo a subsequent α-amination to afford densely functionalized aldehydes bearing two stereogenic centers. Both sulfur [55] and nitrogen [56] heteroatom nucleophiles have been successfully used in combination with azodicarboxylate esters as the aminating agent in good yield, diastereo- and enantio-selectivities.
Figure 12.
Transformation of α-hydrazinoaldehydes to more complex products or intermediates.
Figure 13.
Tandem hetero-Michael addition-α-amination of α,β-unsaturated aldehydes [55,56].
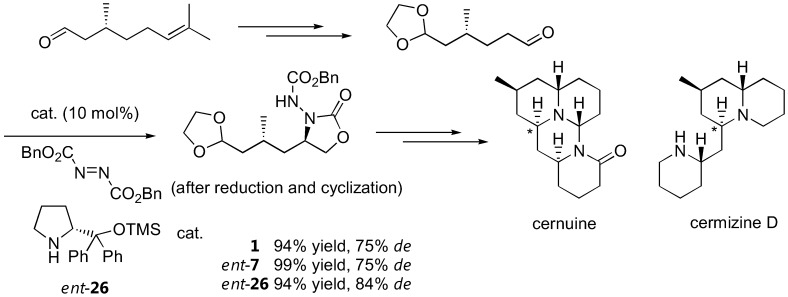
There are numerous applications of proline-catalyzed α-aminations in the synthesis of bioactive compounds. Parts of these have been previously reviewed [57]. For example, L-proline (1)-catalyzed α-amination has been shown as a key step in the synthesis of (R)-pipecolic acid and D-proline in moderate to good yields and good enantioselectivity [58]. The D-proline-derived catalyst ent-7 and its analogue ent-26 have also been reported to induce diasteroselectivity in the 75–84% range when applied to α-amination of a (+)-citronellal-derived aldehyde as part of the lycopodium alkaloids cernuine and cermizine D synthesis [20] (Figure 14). Another application of organocalalyzed enantioselective α-amination in the synthesis of biologically active molecules was elaborated by Barbas III [23]. As a key step in the synthesis of BIRT-377, a potent inhibitor of the interaction between intercellular adhesion molecule-1 (ICAM-1) and lymphocyte function-associated antigen-1 (LFA-1), the pyrrolidinyl tetrazole (2) was employed as the α-amination catalyst [23]. Two more recent applications include the syntheses of intermediates for (-)-anisomycin [59] and fumimycin [60], an antibacterial peptide deformylase inhibitor.
Figure 14.
Total synthesis of cernuine and cermizine D incorporating proline-catalyzed α-amination as a key step. The stereogenic centers created by the α-amination are marked by an asterisk [20].
2.2. α-Sulfamidation
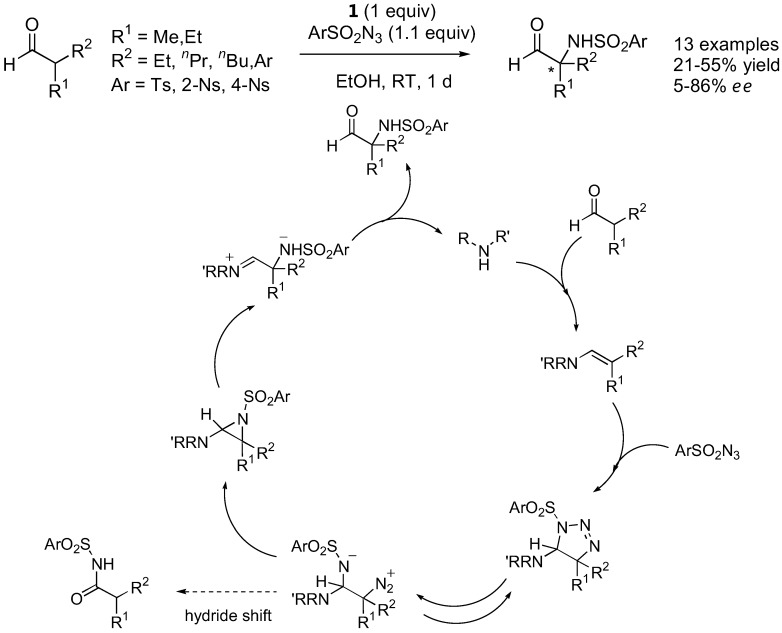
Tosyl and nosyl azides reacted with α-branched aldehydes under catalysis by L-proline (1) to give the configurationally stable α-sulfamidated aldehydes in ee ranging from poor to fairly good (up to 86%) [61]. Interestingly, α-unbranched aldehydes provided N-tosylamides instead of the desired sulfamidated products. The mechanism was proposed to involve a 1,3-dipolar cycloaddition pathway (Figure 15).
Figure 15.
α-Sulfamidation of α-branched aldehydes catalyzed by L-proline (1) [61].
2.3. α-Hydroxyamination
Organocatalytic hydroxyamination of aldehydes and ketones may take place using nitrosobenzene as the aminating agent. The selectivity between hydroxyamination (nitrosoaldol) and aminoxylation reactions is determined both by the substrate and the catalyst. For detailed discussion see Section 3.1.4 - α-Aminoxylation vs. nitrosoaldol reactions.
3. α-Aminoxylation Reactions
3.1. α-Aminoxylation with nitrosobenzene
3.1.1. Substrates
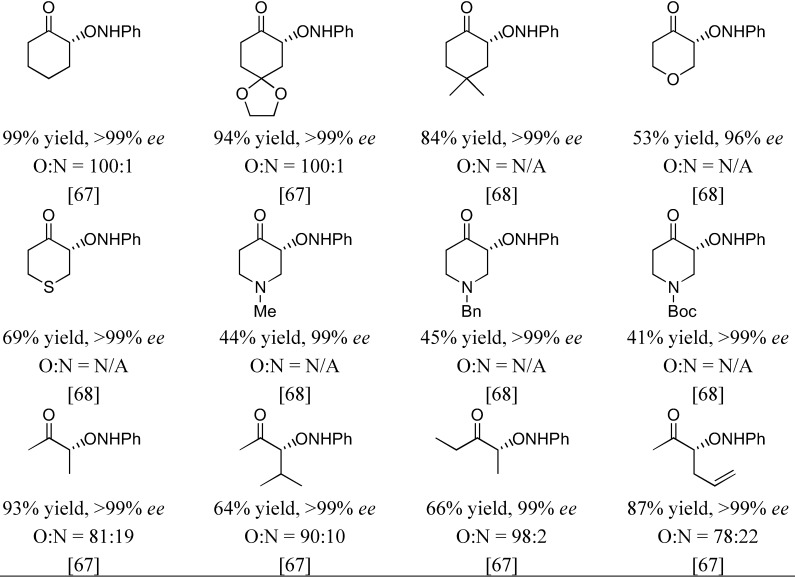
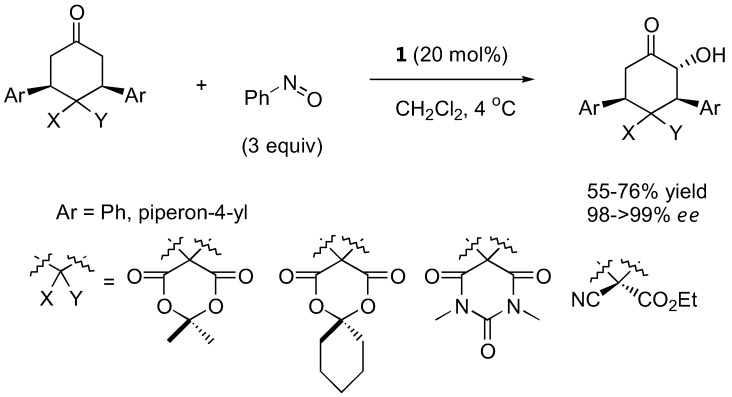
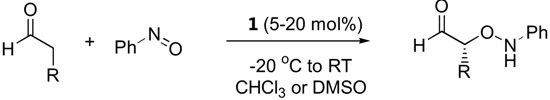
The first organocatalyzed enantioselective aminoxylation of aldehydes were independently reported by Hayashi [62], MacMillan [63], and Zhong [64] in 2003. Simple α-unbranched aliphatic aldehydes reacted with nitrosobenzene under proline catalysis by the attack at oxygen to give exclusively the α-aminoxylated aldehydes in excellent enantioselectivities (≥94% ee) (Table 6). α-Branched aliphatic aldehydes generally give mixture of products derived from both N and O attack (Section 3.1.4). Cyclohexanone and derivatives were also exclusively aminoxylated (O:N > 100:1) under catalysis by L-proline (1) [65,66,67,68] or its tetrazolyl derivative (2) [69] in excellent enantioselectivities (generally ≥99%) (Figure 16). Bis-aminoxylation may take place and could be suppressed by using large excess of the ketone (10 equiv) and slow addition of nitrosobenzene by a syringe pump. Racemic 3-substituted cyclohexanones and 4-substituted cyclohexanone derivatives give mixture of regio- (3-substituted cyclohexanones only) and diastereomeric aminoxylated products in high enantioselectivities [66,68].
Table 6.
Substrate scopes for L-proline-catalyzed α-aminoxylation of aldehydes with nitrosobenzene.
 |
| R | conditions | yield (%)a | ee (%)a | ref. |
|---|---|---|---|---|
| Me, Et, nPr, iPr, Ph, Bn | 30 mol% 1, MeCN, -20 ºC, 24 h | 62–>99 | 95–99 | [62] |
| Me, nBu, iPr, allyl, Bn, Ph, TIPSO(CH2)3, N-methylindol-3-ylmethyl | 5 mol% 1, CHCl3, 4 ºC, 4 h | 60–88 | 97–99 | [63] |
| Me, nPr, iPr, nBu, allyl, Bn, BnOCH2, BocNH(CH2)4 | 20 mol% 1, DMSO, RT, 10–20 min | 54–86 | 94–99 | [64] |
a Yield and ee were determined after NaBH4 reduction to the corresponding diols.
Figure 16.
Examples of aminoxylated ketones obtained from L-proline (1) catalysis [67,68].
High enantio- and diastereo-selectivities were also observed with meso-3,5-disubstituted spirocyclic ketones [70,71] (Figure 17). Simple acyclic ketones are more problematic substrates as they are prone to multiple aminoxylation and provide appreciable amounts of the hydroxyaminated product (O:N = 78:22–98:2, Section 3.1.4) [65,67,69]. Nevertheless, the aminoxylated products could be obtained in acceptable yield with high enantioselectivity (>99% ee) (Figure 16). The reaction took place regioselectively at the more substituted α-carbon of unsymmetrical ketones.
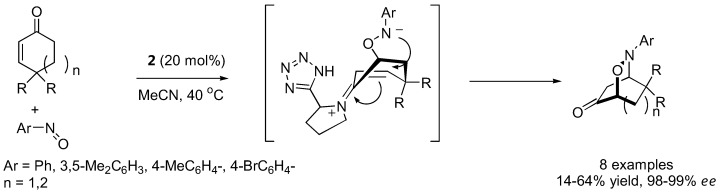
Figure 17.
L-Proline (1)-catalyzed α-aminoxylation of spirocyclic ketones followed by concomitant N-O bond heterolysis [70].
3.1.2. Catalysts
L-Proline (1) and its tetrazole analogue (2) were the two most widely used catalysts for α-aminoxylation. They are equally effective for simple aldehyde and ketone substrates both in terms of yields and enantioselectivities (usually >95%), although the catalyst loading required to achieve satisfactory results was lower for the latter (5–10 mol%) than the former (10–30 mol%) [69]. Variants to facilitate product isolation including polymer-supported proline catalyst [72]. Ionic liquid as the reaction medium had also been described [73,74].
Several models have been proposed to explain the regio- and stereoselectivity of proline catalyzed aminoxylation of aldehydes [62,63,64]. Detailed calculations by Houk [75] and by Córdova [67] suggested that the nitrosobenzene was protonated at the nitrogen atom by the carboxyl group and approached the (E)-anti-enamine intermediate from the Re-face (Figure 18). Houk also predicted that catalysts without hydrogen bonding capability should favor hydroxyamination [75] (Section 3.1.4).
Figure 18.
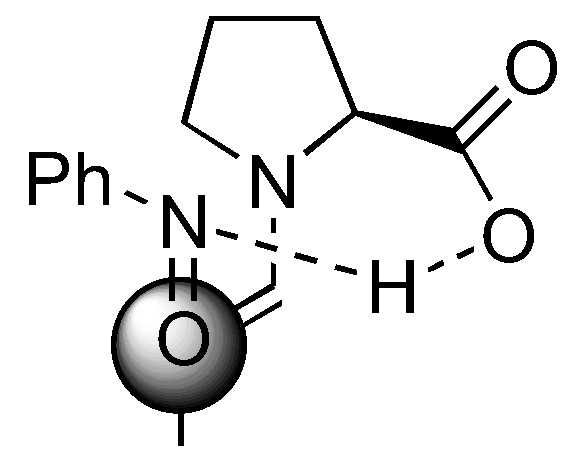
A transition structure involving reaction of proline enamine with nitrosobenzene according to Houk [75] and Córdova [67].
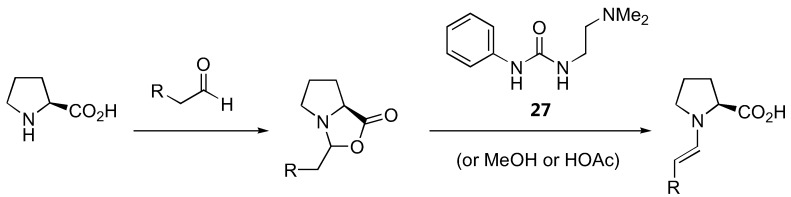
The generally accepted catalytic cycle [Figure 19 (A)] involving such enamine intermediate may be somewhat oversimplified. A detailed study of the reaction kinetics of proline-catalyzed aminoxylation of aldehydes by the Blackmond group revealed that the reaction is autocatalytic [76]. In addition to the rate enhancement, the enantiomeric excess gets improved during the course of the reaction [76]. A catalytic cycle involving an adduct of proline with the reaction product was proposed [Figure 19 (B)] to account for this unusual reaction kinetics not observed in other proline-catalyzed reactions except for α-amination [32,33] (see also Section 2.1.1). Addition of a bifunctional urea (27) was found to accelerate the rate of proline-catalyzed aminoxylation in nonpolar solvents, but in this case the reaction was no longer autocatalytic [77]. It was suggested that the urea additive might increase the reaction rate by promoting the formation of the enamine from proline via the oxazolidinone, which was believed to be the rate limiting step (Figure 20). It was subsequently observed that simple alcohols and acids such as methanol and acetic acid could also increase the reaction rate by promoting the enamine formation [78]. Such additive effect was also observed in the mechanistically related amination but not aldol reactions. The difference in kinetic behavior of these reactions was rationalized by proposing a different rate determining step for each reaction (enamine formation for amination and aminoxylation vs. electrophilic reaction of the enamine for aldol reaction) [78].
Figure 19.
(A) The generally accepted catalytic cycle of proline-catalyzed aminoxylation of aldehydes; (B) The proposed autocatalytic model by Blackmond et al. [76].
Figure 20.
The involvement of urea 27 in accelerating the rate of proline-catalyzed aminoxylation [77].
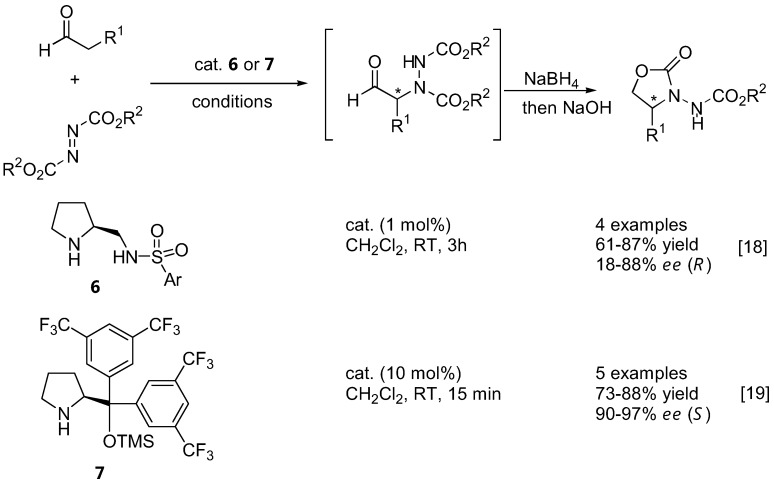
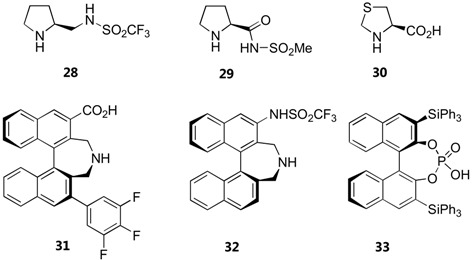
In addition to 1 and 2, other alternative catalysts for direct aminoxylation of aldehydes and ketones include 4-silyloxyproline (5) [79], pyrrolidine sulfonamide 28 [80], proline N-sulfonyl carboxamide 29 [82] and L-4-thiazolidinecarboxylic acid (L-thiaproline) 30 [81]. The latter was successfully employed for aminoxylation of aldehydes in aqueous media in the presence of Bu4NBr as a phase transfer catalyst. Yield and enantioselectivities in most cases are similar to those of unmodified proline, although the silyloxyproline 5 generally requires significantly shorter time and can be used with certain substrates that give poor results with proline such as cycloheptanone [79]. A summary of substrate scope and performance of these catalysts is shown in Table 7.
Table 7.
Summary of performance of other proline- and nonproline-based catalysts in α-aminoxylation of carbonyl compounds.
 |
| Cat. | Substrates a | Conditions | Yield (%) | ee (%) | Ref. |
|---|---|---|---|---|---|
| 5 | ketones (7) | 10 mol% cat., DMF, | 45–76 | 98–>99 | [79] |
| α-unbranched aldehydes (2) | 0 ºC, a few hours | ||||
| 28 | ketones (4) | 20 mol% cat., DMSO, RT | 66–94 | 97–>99 | [80] |
| α-unbranched aldehydes (5) | |||||
| 29 | cyclic ketones (4) | 10 mol% cat., DMSO, RT | 23–80 | 96–>99 | [82] |
| α-unbranched aldehydes (1) | |||||
| 30 | α-unbranched | 20 mol% cat., H2O, | 74–88 | 93–>99 | [81] |
| aldehydes (11) | 2 equiv Bu4NBr, 0 ºC–> RT | ||||
| 31 | α-unbranched aldehydes (3) | 5 mol% cat., toluene, 0 ºC | 69–89 | 86–88 | [83] |
| 32 | α-unbranched aldehydes (7) | 0.2–5 mol% cat., CHCl3, | 86–96 | 97–>98 | [84] |
| 0 ºC –> RT | |||||
| 33 | cyclic 1,3-diketones (5) | 1 mol% cat., benzene, 4 ºC | 49–88 | 68–>98 | [86] |
| cyclic β-ketoesters (11) |
a Number of examples shown in parentheses.
A few non-proline derivatives have been reported as effective organocatalysts for aminoxylation reactions. These include Maruoka's axially chiral secondary amino acid 31 [83] and triflamide 32 [84] (Table 7). The triflamide displayed a remarkable catalytic activity. As high as 76% yield and 98% ee could be obtained from α-aminoxylation of simple aldehydes at 0.2 mol% catalyst loading. The two catalysts, although having the same axial chirality, gave the aminoxylated products with opposite enantioselectivities. This is explained by the approach of the nitrosobenzene from the Re face of the s-trans-enamine (catalyst 31) or the Si face of the s-cis-enamine (catalyst 32) (Figure 21) [85].
Figure 21.
Transition state models to explain the opposite enantioselectivities observed with the catalysts 31 (left) and 32 (right) [85].
The chiral Brønsted binaphthylphosphoric acid catalyst 33 described by Zhong et al. catalyzes α-aminoxylation of cyclic β-dicarbonyl compounds in good yield and excellent regio- and enantioselectivity [86]. Interestingly, the N-O bond heterolysis took place to give the α-hydroxy-β-dicarbonyl compounds without requiring further chemical reduction.
3.1.3. Applications
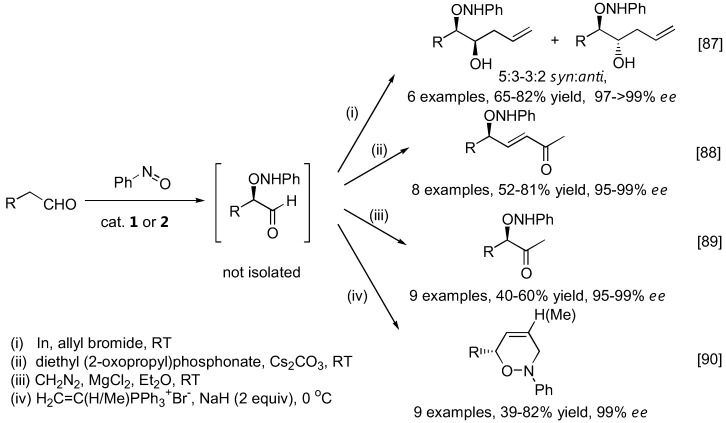
The α-aminoxylation products were generally isolated and characterized following NaBH4 reduction of the aldehyde group to give the more stable alcohol. Alternatively, the aminoxylated aldehydes could be transformed into other synthetically useful intermediates (Figure 22) such as substituted homoallylic alcohols [87], allylic alcohols [88] and methyl ketones [89]. In a more complex example, dihydro-1,2-oxazines were obtained from the aminoxylated aldehydes in a one-pot fashion by N-alkylation followed by an intramolecular Wittig-type reaction [90]. A similar transformation was also possible with cyclic ketones [91,92].
Figure 22.
Transformation of aminoxylated aldehydes to other synthetically useful intermediates.
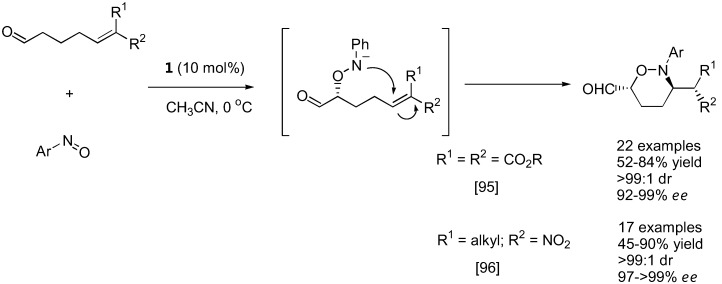
With strategically designed substrates, subsequent reactions may take place spontaneously following the initial aminoxylation. Tetrahydro-1,2-oxazines could be generated from cyclic enones [79,93,94] or 5-hexen-1-al derivatives carrying strong electron withdrawing groups at the 6 position [95,96] by intramolecular Michael-type addition (Figure 23 and Figure 24). An interesting dichotomy was observed in similar aldehyde substrates carrying just one carboxyalkyl instead of the nitro or malonate groups. In these cases, an intramolecular [3+2] cycloaddition between the in situ generated nitrone and the alkene was more favorable giving bicyclic isoxazolidines with up to five contiguous stereogenic centers in a one-pot fashion [97].
Figure 23.
Tandem aminoxylation-intramolecular Michael addition of aminoxylated products derived from unsaturated aldehydes [93,94].
Figure 24.
Tandem aminoxylation-intramolecular Michael addition of aminoxylated products derived from unsaturated aldehydes [95,96].
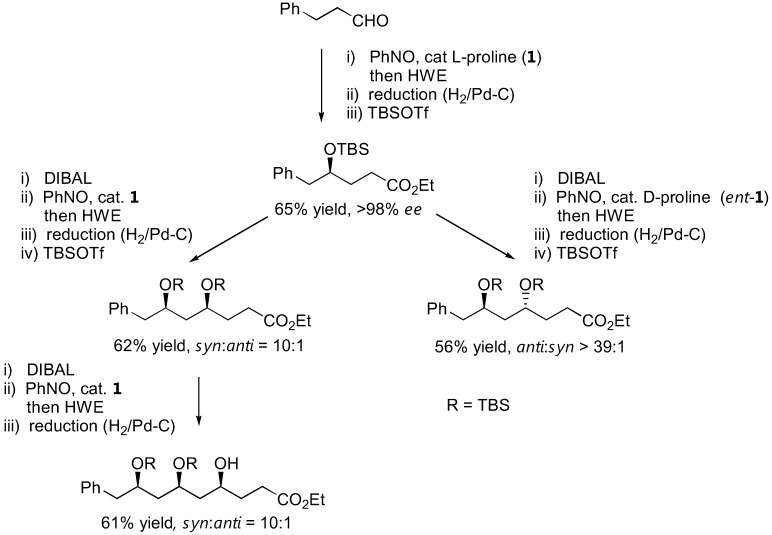
The N-O bond in the aminoxylated carbonyl compounds and their further products can be easily cleaved by a variety of methods, including catalytic amounts of CuSO4 in MeOH, Zn/H+ reduction or catalytic hydrogenolysis to afford the corresponding 1,2-diols (simple aminoxylated aldehydes), 1,4-aminoalcohols (1,2-oxazine derivatives) [91,92,94], or 1,3-aminoalcohols (bicyclic oxazolidine derivatives) [97] in good enantioselectivities. With excess of the nitrosobenzene in the reaction, N-O heterolysis took place spontaneously [70,86]. It was also possible to apply the aminoxylation-HWE olefination-reduction sequence to construct 1,3-polyol frameworks [98]. The absolute configuration of the newly added hydroxyl group could be reliably controlled by the appropriate choice of L- or D-proline catalyst. Most importantly, the reaction can be carried out in an iterative fashion to give longer 1,3-polyols in a stereocontrolled fashion (Figure 25).
Figure 25.
A strategy to synthesize 1,3-polyols by iterative aminoxylation-HWE olefination-reduction sequence [98].
Numerous applications of organocatalyzed aminoxylation of aldehydes and ketones could be found in the literature [57]. Although a number of new catalysts have been described, the original L- and D-prolines (1 and ent-1) were the most widely used catalysts. Examples of complex target molecules successfully synthesized by using this strategy are shown in Figure 26.
Figure 26.
Examples of complex target molecules successfully synthesized by organocatalyzed aminoxylation of aldehydes. The position of the stereogenic center derived from the α-amination is marked by an asterisk.
Other target molecules which had been successfully synthesized using proline catalyzed α-aminoxylation as one of the key steps include halipeptin A [104], (+)-exo- and (–)-endo-brevicomins [105], linezolid and eperezolid [106], levetiracetam [107], (S,S)-ethambutol [108], (+)-harzialactone A and (R)-(+)-4-hexanolide [109], (R)-seleginine [110], (S)-propanolol and (S)-naftopidil [111], (+)-disparlure and its trans-isomer [112], (-)-anisomycin [59] and (1R,3S)-thysanone, a HRV 3C-protease inhibitor [113].
3.1.4. α-Aminoxylation vs. nitrosoaldol reactions
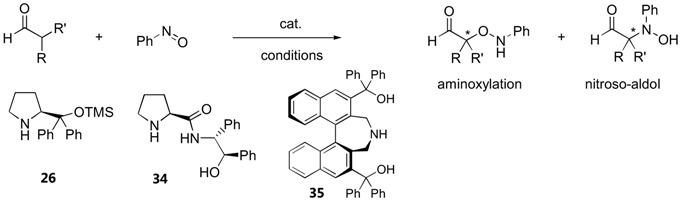
α-Unbranched aldehydes and cyclic ketones generally react with nitrosobenzene exclusively at the oxygen atom under catalysis by proline (Section 3.1.1). Significant amounts of products derived from the attack at the nitrogen atom were observed with acyclic ketones [67,69] and α-branched aliphatic aldehydes [114] (Table 8, see also Figure 16).
Table 8.
Comparison of catalysts in aminoxylation/nitrosoaldol reactions of aldehydes.
 |
| Cat. | Substratea | Conditions | Yield (%) | ee (%)b | Ref. |
|---|---|---|---|---|---|
| 1 | 2-methyl-3-propanal | 20 mol% cat., DMF, | 32 | 67 | [114] |
| 25 ºC, 24 h | (N:O = 1.5:1) | ||||
| 2 | α-branched aldehydes (10) | 20 mol% cat., DMF, | 55–96 | 5–90 | [114] |
| 0–25 ºC , 3–24 h | (N:O = 0.6:1–20:1) | ||||
| 34 | α-branched aldehydes (5) | 10 mol% cat., toluene, | 53–74 | 46–59 | [115] |
| -40 ºC, 2–3 d | (N:O = N/A) | ||||
| 26 | α-unbranched aldehydes (8) | 20 mol% cat., CH2Cl2, | 40–75 | 91–99 | [116] |
| 0 ºC | (N:O >99:1) | ||||
| 35 | α-unbranched aldehydes (6) | 10 mol% cat., THF, | 70–90 | 96–99 | [117] |
| 0 ºC, 1 h | (N:O >99:1) |
a Number of examples shown in parentheses bee of the N-nitrosoaldol product.
Steric effects between the substituent in the enamine intermediate and the phenyl group in nitrosobenzene had been proposed to facilitate the attack at nitrogen [114]. Despite this possibility of direct N-nitrosoaldol reaction, the N:O selectivity was not generally synthetically useful (0.6:1–1.7:1), except in two cases where the substrates were 2-phenylpropanal derivatives, whereby a very good N:O selectivity (10:1–20:1) could be obtained. Enantioselectivities for the nitrosoaldol products were only moderate to good (45–90%) for α-methyl substituted aldehydes and poor (5–25%) for the corresponding α-ethyl derivatives [114]. The L-prolinamide derived from aminoalcohol (34) was demonstrated to catalyze a selective nitrosoaldol reaction of α-branched aliphatic aldehydes in good yield (53–74%) with moderate ee (46–59%) at 10 mol% catalyst loading [115]. Under similar conditions, L-proline (1) gave only the aminoxylation product in low ee (37%). The preferential hydrogen bonding between the oxygen atom in nitrosobenzene and weakly acidic amide and hydroxyl protons was proposed to account for the high N-selectivity. In line with the prediction by Houk [75], the non-hydrogen bonding diphenylprolinol TMS ether (26) catalyzed the amination reaction between simple unbranched aliphatic aldehydes and nitrosobenzene to give exclusively the nitrosoaldol product in 40–75% yield with >99:1 N:O selectivity and excellent enantioselectivities (91–99%)[116]. The absolute configuration of the product was consistent with the approach of the nitrosobenzene from the Si face of the (E)-enamine, which is opposite to that of proline and its derivatives with hydrogen-bonding substituents. A DFT calculation supported the preferential attack on N over O by >3 kcal/mol [116].
A few non-proline derivatives have recently been demonstrated as effective catalysts for selective nitrosoaldol reactions of carbonyl compounds and related derivatives. The most general one is perhaps the axially chiral secondary aminoalcohol (35) developed by Maruoka et al. [117], which provide excellent N:O selectivity (>99:1) and enantioselectivities (≥96%) in nitrosoaldol reaction of simple α-unbranched aldehydes (Table 8).
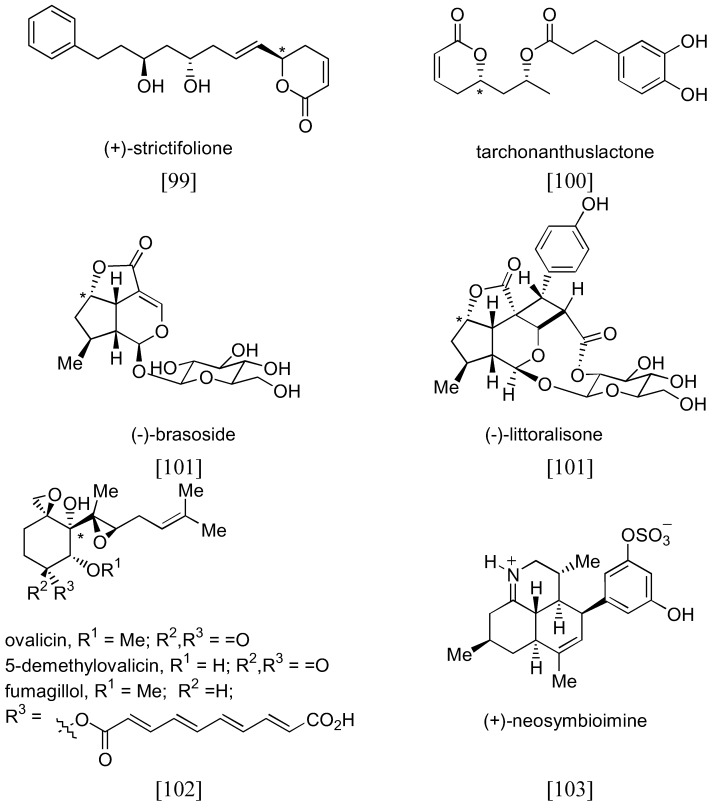
It should be noted that more strongly acidic substituents in place of the alcohol in the structurally related catalysts 31 and 32 provide preferentially O-attack products due to protonation of the nitrogen atom (Section 3.1.2) [83,84]. Other catalysts act on special substrates. The reaction between achiral morpholino-enamines derived from cyclohexanone and cycloheptanone derivatives with nitrosobenzene in the presence of a TADDOL derivative (36) (30 mol%) provided the nitrosoaldol product in good yields (63–91%) and enantioselectivities (65–91%) [118]. The N:O regioselectivity could be completely reversed using 1-naphthylglycolic acid (37) as a more strongly Brønsted acid catalyst. Good yields (63–91%) and enantioselectivities (70–93%) of the O-substituted products were also obtained (Figure 27).
Figure 27.
Regioselectivity control in reaction between a preformed enamine and nitrosobenzene by Brønsted acid catalysts [118].
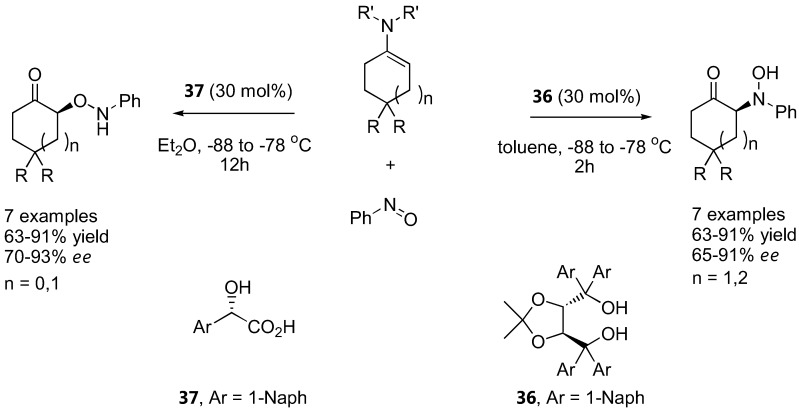
The binaphthol derivative 38 catalyzed regio- and enantio-selective hydroxyamination of cyclohexanone and cyclohexenone enamines [94]. With the cyclohexenone enamine, the reaction was followed by an intramolecular Michael addition to give a bicyclic ketone analogous to those obtained from α-aminoxylation of cyclohexenone with proline catalysis [93] (Section 3.1.3), but with complete reversal of regioselectivity (Figure 28).
Figure 28.
Binaphthol catalyzed tandem hydroxyamination-Michael addition of cyclohexenone enamine [94].
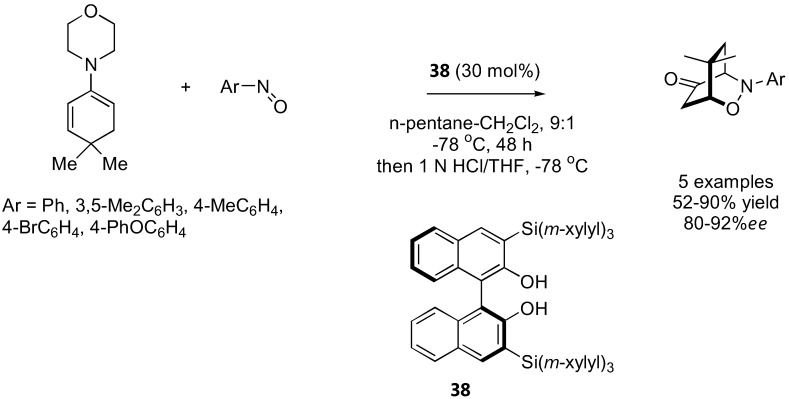
Under catalysis by quinine (11), α-aryl substituted cyanoacetate esters underwent exclusive nitrosoaldol reactions to give the chiral quaternary center in good yield (71–85%) and moderate ee (22–59%) (Figure 29) [119]. The enantioselectivity of the reaction is highly dependent to the solvent and concentration of the catalyst, which was explained by the different aggregation state of the catalyst.
Figure 29.
Nitrosoaldol reaction of α-aryl substituted cyanoacetate esters [119].
3.2. α-Aminoxylation reactions with TEMPO
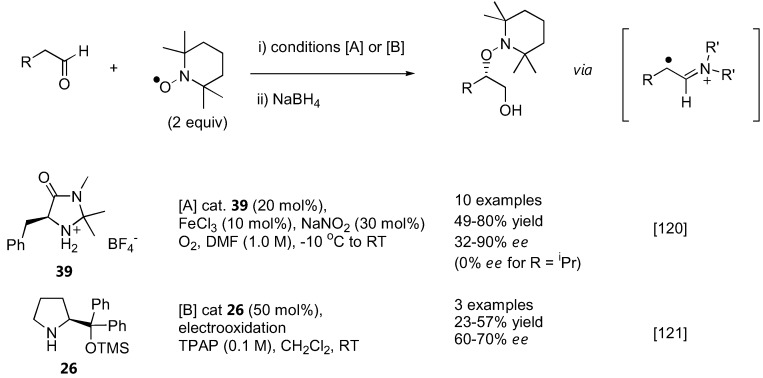
While all aminoxylation reactions described above used nitrosobenzene or substituted nitrosobenzenes as the aminoxylating agents, Sibi and Hasegawa have recently introduced a conceptually new approach to aminoxylate aldehydes using the stable radical TEMPO [120] (Figure 30). In this case, a radical intermediate was generated from a chiral secondary amine-derived enamine by an appropriate single electron transfer (SET) reagent. This radical can then be trapped with TEMPO, resulting in an overall α-aminoxylation of the carbonyl compound. A combination of FeCl3 (10 mol%) and NaNO2 (30 mol%) was found to be the best SET reagent. A MacMillan's imidazolinone catalyst 39 was more effective than proline and diphenylprolinol both in terms of yield and enantioselectivities. Only unbranched aldehydes with terminal π-system within 2–3 atoms from the reaction center such as 2-phenylpropanal and 4-penten-1-al gave optimal enantioselectivities (up to 90% ee) at 20 mol% catalyst loading. Simple aliphatic aldehydes such as isovaleraldehyde gave the product in 74% yield, but without enantioselectivity (0% ee). The chiral enamine radicals derived from 26 could also be generated by anodic oxidation, and subsequently trapped by TEMPO to give the aminoxylated aldehydes [121]. The yield and enantioselectivity was not high, but the substrates chosen in the latter [121] also gave poor results in the former reports [120].
Figure 30.
α-Aminoxylation reactions via radical intermediates with TEMPO [120,121].
4. α-Oxidation Reactions
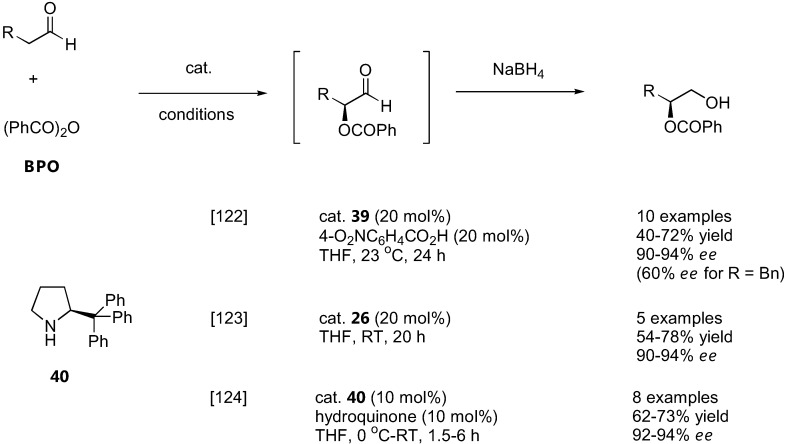
4.1. α-Oxybenzoylation
Three direct organocatalytic asymmetric α-oxybenzoylations of carbonyl compounds by benzoyl peroxide (BPO) have been independently reported in early 2009 by Tomkinson [122], Hayashi [123] and Maruoka [124] (Figure 31). Hayashi's group showed that the diphenylprolinol silyl ether 26 was an effective catalyst for this reaction [123].
Figure 31.
Organocatalyzed α-oxybenzoylation of aldehydes with benzoyl peroxide.
On the other hand, the Tomkinson's group employed a MacMillan's imidazolinone catalyst (39) and the Maruoka's group used a tritylpyrrolidine catalyst (40), both of which were thought to be more stable to oxidation by BPO than simple proline derivatives [122,124]. In all cases, similar substrate scopes (simple α-unbranched aliphatic aldehydes, some carrying inert functional groups such as aromatic, alkenyl, ether or phthalimido), yield (40–78%) and ee (90–94%) were reported. The stereochemical outcome of the reaction was consistent with the simple electrophilic attack of the E-enamine intermediate from the less hindered face. The resulting α-oxybenzoylated aldehydes were successfully transformed into a variety of products by standard chemical manipulation of the aldehyde group [122,124].
4.2. α-Oxidation with molecular oxygen
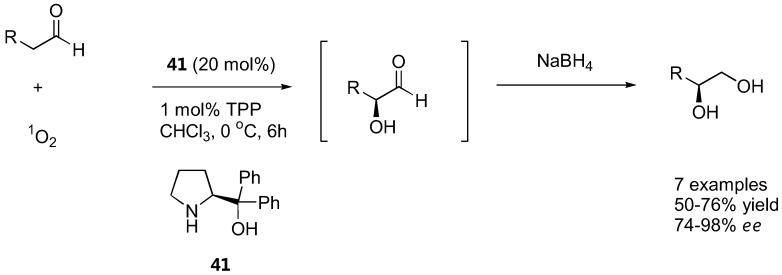
Following their 2004 report of L-α-methylproline-catalyzed oxidation of aldehydes by singlet oxygen to give directly the optically active hydroxyaldehydes and diols (after reduction) in up to 64% ee [126], the Córdova group has again shown in 2006 that diphenylprolinol (41) was a more effective for direct α-oxidation of α-unbranched aldehydes with singlet oxygen [125] (Figure 32). The singlet oxygen was photochemically generated from oxygen or air in the presence of a catalytic amount of tetraphenylphorphine (TPP). The reaction was carried out in the presence of the diphenylprolinol catalyst (10–20 mol%) at 0 ºC. The intermediate α-hydroperoxyaldehydes was not isolated, but further reduced by NaBH4 to afford the corresponding diols in moderate to good yield (50–76%). The ee was moderate with simple aliphatic aldehydes (propanal: 74% ee, 1-hexanal: 75% ee) but was dramatically improved with 2-phenylpropanal and its derivatives (up to 98% ee).
Figure 32.
Organocatalyzed α-oxidation with molecular oxygen [125].
4.3. α-Oxidation with hydroperoxides
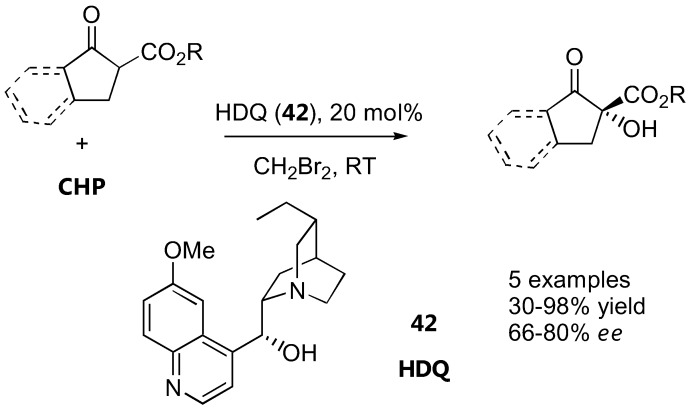
Alkyl cyclopentanone 2-carboxylate and its benzo-derivatives were oxidized with cumyl hydroperoxide (CHP) in the presence of catalytic amounts of dihydroquinine (HDQ, 42) to give α-hydroxy-β-keto esters in 66–80% ee (Figure 33). Subsequent borane reduction gave the anti-diol in an excellent diastereoselectivity (99:1) [127].
Figure 33.
Organocatalyzed α-oxidation of cyclic β-ketoesters with hydroperoxides [127].
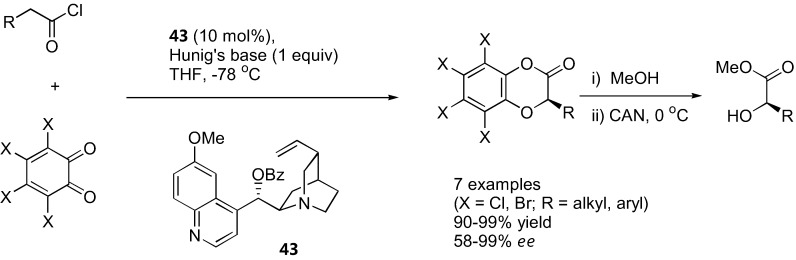
4.4. α-Aryloxylation with o-quinones
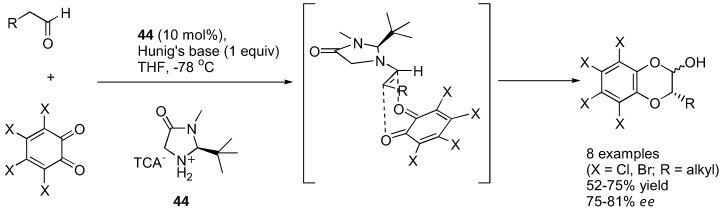
Lectka's group showed that o-chloranil and o-bromonil can react enantioselectively with ketene enolates in a [4+2] cycloaddition fashion to give 1,4-benzodioxane-2-one under catalysis by a cinchona alkaloid derivative 43 [128] (Figure 34). The ketene enolates were generated from their corresponding acid chloride in the presence of Hünig's base. Excellent yields and good enantioselectivities were obtained in most cases. The cycloadducts could be converted into the corresponding α-hydroxy esters by methanolysis followed by ceric ammonium nitrate (CAN) treatment. Similarly, α-unbranched aliphatic aldehydes reacted with o-chloranil or o-bromonil under organocatalysis to give the α-aryloxylated aldehydes in their hemiacetal form [129] (Figure 35). The MacMillan type catalyst (44) provided superior results in aryloxylation of propionaldehyde (>70% yield and >80% ee) than proline and its simple derivatives (36–53% yield, 33–40% ee). A range of α-unbranched aliphatic aldehydes were acceptable substrates, providing ee in the range of 75–81%. The hemiacetal products were successfully converted to alcohols and 1,4-benzodioxanes without loss of optical purities.
Figure 34.
Organocatalyzed α-aryloxylation of carboxylic acid derivatives via ketene enolates [128].
Figure 35.
Organocatalyzed α-aryloxylation of aldehydes by o-quinones [129].
4.5. α-Oxidation with oxaziridine and iodosobenzene
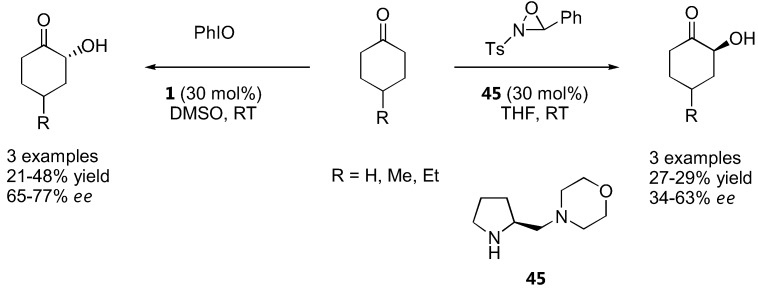
Cyclohexanones could be oxidized by an N-sulfonyloxaziridine in the presence of proline (1) or its derivatives to the corresponding α-hydroxyketones in poor to moderate yield and enantioselectivity. The best result was obtained with the diamine 45 (up to 63% ee) [130] (Figure 36).
Figure 36.
Proline and diamine 45 catalyzed α-oxidation of ketones with oxaziridine and iodosobenzene [130].
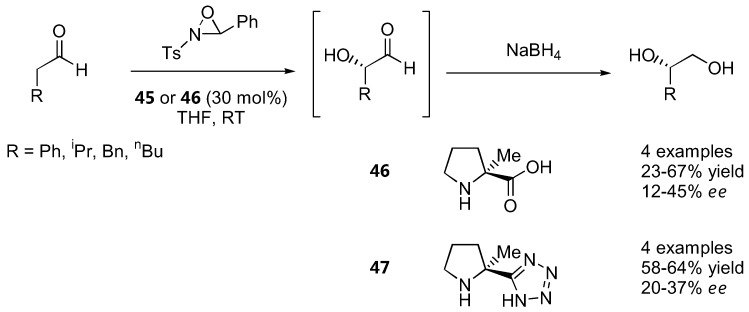
Iodosobenzene was a superior oxidant for cyclohexanone derivatives with unmodified L-proline (1) as catalyst and ee in the range of 65–77% were obtained [130] (Figure 36). The absolute configurations of the oxidation products derived from proline-catalyzed reactions were opposite to that of the diamine-catalyzed reactions regardless of the oxidant used, which could be explained by their different transition states (see 2.1.1). Simple α-unbranched aldehydes could also be oxidized to the corresponding α-hydroxyaldehydes by N-sulfonyloxaziridine with α-methylproline (46) or its tetrazole analogue 47 as catalysts. Interestingly, the (S)-product predominated instead of the expected (R)-product in analogy to the amination and aminoxylation reactions, but the ee was rather poor (<50%) [131] (Figure 37).
Figure 37.
α-Methylproline (46) and tetrazole (47) catalyzed α-oxidation of aldehydes with oxaziridine [131].
4.6. α-Oxysulfonation catalyzed by iodoarenes
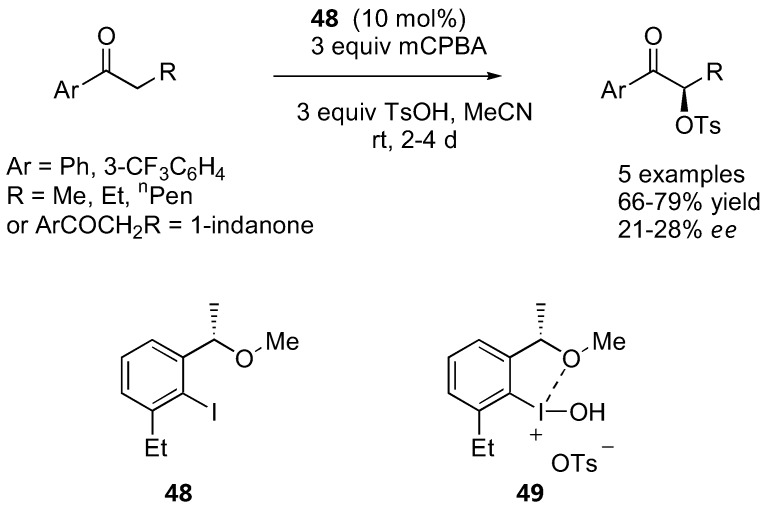
While aryl ketones are generally unreactive towards enamine catalysis by proline derivatives (see also Section 2.1.2), propiophenone derivatives have been successfully oxidized to the corresponding α-tosyloxy derivatives by chiral Koser-type reagents catalytically generated in situ from the corresponding iodoarenes, m-CPBA and p-toluenesulfonic acid at room temperature [132,133] (Figure 38).
Figure 38.
α-Oxysulfonation of aromatic ketones catalyzed by iodoarenes [132,133].
Extensive screening of the iodoarenes revealed that the best catalyst precursor was 48, which was presumably converted into the active oxidizing agent 49in situ. Although conceptually unique, the ee was not particularly good (21–28%) and no improvement could be made by changing the steric bulk of the sulfonic acids used [133].
5. Conclusions and Outlook
During the past few years an exciting advancement in the field of organocatalyzed asymmetric α-amination, α-aminoxylation, and related α-oxidation process has been witnessed. With high and predictable stereoselectivity, operational simplicity, and catalyst availability in both enantiomers with a touch of "green" aspect, the proline catalyzed α-aminoxylation and α-amination of carbonyl compounds have become a well established tool for constructing complex chiral molecules. Nevertheless, certain limitations still exist with respect to the low catalyst turnover numbers and frequency, which results in a typically high catalyst loading (≥10 mol%) and long reaction times (several hours to several days). These limitations, together with the rather limited substrate scope, prompted the design of new alternative catalysts that are more active, provide higher selectivity, and accept a broader substrate range. It is also important to find new catalysts that can catalyze new reactions, or can accept certain "difficult" substrates that proline cannot – most notably α-branched or aromatic substrates. Many of such new catalysts, such as those derived from cinchona alkaloids or other chiral amines as the source of chirality, are presently discovered by trial-and-error. With an emerging understanding as to how the catalysts work, it will clearly lead to a more rational design of new catalysts with such properties in the future. Finally, creative combination of new catalysts and new reaction sequences will further broaden the scope and applicability of these organocatalyzed reactions. It remains to be seen when it will be possible to design an organocatalyst that can catalyze a specific reaction or reaction sequence with enzyme-like efficiency at will. By that time, the traditional use of highly reactive and environmentally unfriendly metal-based reagents and catalysts may become obsolete!
Acknowledgements
We thank Chalotorn Boonlua and Woraluk Mansawat for their assistance in obtaining some references.
References and Notes
- 1.Merino P., Tejero T. Organocatalyzed asymmetric α-aminoxylation of aldehydes and ketones - An efficient access to enantiomerically pure α-hydroxycarbonyl compounds, diols, and even amino alcohols. Angew. Chem. Int. Ed. 2004;43:2995–2997. doi: 10.1002/anie.200301760. [DOI] [PubMed] [Google Scholar]
- 2.Janey J.M. Recent advances in catalytic, enantioselective α aminations and α oxygenations of carbonyl compounds. Angew. Chem. Int. Ed. 2005;44:4292–4300. doi: 10.1002/anie.200462314. [DOI] [PubMed] [Google Scholar]
- 3.Marigo M., Jørgensen K.A. Organocatalytic direct asymmetric α-heteroatom functionalization of aldehydes and ketones. Chem. Commun. 2006:2001–2011. doi: 10.1039/b517090g. [DOI] [PubMed] [Google Scholar]
- 4.Guillena G., Ramón D.J. Enantioselective α-heterofunctionalisation of carbonyl compounds: organocatalysis is the simplest approach. Tetrahedron-Asymmetr. 2006;17:1465–1492. doi: 10.1016/j.tetasy.2006.05.020. [DOI] [Google Scholar]
- 5.Marigo M., Jørgensen K.A. Enamine Catalysis. In: Dalko P.I., editor. Enantioselective Organocatalysis: Reactions and Experimental Procedures. Wiley VCH Verlag; Weinheim, Germany: 2007. pp. 56–67. [Google Scholar]
- 6.Kotsuki H., Ikishima H., Okuyama A. Organocatalytic asymmetric synthesis using proline and related molecules. Part 2. Heterocycles. 2008;75:757–797. doi: 10.3987/REV-07-621. [DOI] [Google Scholar]
- 7.Kano T., Maruoka K. Design of chiral bifunctional secondary amine catalysts for asymmetric enamine catalysis. Chem. Commun. 2008:5465–6473. doi: 10.1039/b809301f. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y.-C. The development of asymmetric primary amine catalysts based on cinchona alkaloids. Synlett. 2008:1919–1930. doi: 10.1055/s-2008-1078524. [DOI] [Google Scholar]
- 9.Yamamoto H., Momiyama N. Rich chemistry of nitroso compounds. Chem. Commun. 2005:3514–3525. doi: 10.1039/b503212c. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto H., Kawasaki M. Nitroso and azo compounds in modern organic synthesis: Late blooming but very rich. Bull. Chem. Soc. Jpn. 2007;80:595–607. doi: 10.1246/bcsj.80.595. [DOI] [Google Scholar]
- 11.Enders D., Grondal C., Hüttl R.M. Asymmetric organocatalytic domino reactions. Angew. Chem. Int. Ed. 2007;46:1570–1581. doi: 10.1002/anie.200603129. [DOI] [PubMed] [Google Scholar]
- 12.Bøgevig A., Juhl K., Kumaragurubaran N., Zhuang W., Jørgensen K.A. Direct organo-catalytic asymmetric α-amination of aldehydes–A simple approach to optically active α-amino aldehydes, α-amino alcohols, and α-amino acids. Angew. Chem. Int. Ed. 2002;41:1790–1793. doi: 10.1002/1521-3773(20020517)41:10<1790::AID-ANIE1790>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.List B. Direct catalytic asymmetric α-amination of aldehydes. J. Am. Chem. Soc. 2002;124:5656–5657. doi: 10.1021/ja0261325. [DOI] [PubMed] [Google Scholar]
- 14.Jung S.H., Kim D.Y. Catalytic enantioselective electrophilic α-hydrazination of β-ketoesters using bifunctional organocatalysts. Tetrahedron Lett. 2008;49:5527–5530. doi: 10.1016/j.tetlet.2008.07.041. [DOI] [Google Scholar]
- 15.Terada M., Nakano M., Ube H. Axially chiral guanidine as highly active and enantioselective catalyst for electrophilic amination of unsymmetrically substituted 1,3-dicarbonyl compounds. J. Am. Chem. Soc. 2006;128:16044–16045. doi: 10.1021/ja066808m. [DOI] [PubMed] [Google Scholar]
- 16.He R., Wang X., Hashimoto T., Maruoka K. Binaphthyl-modified quaternary phosphonium salts as chiral phase-transfer catalysts: Asymmetric amination of β-keto esters. Angew. Chem. Int. Ed. 2008;47:9466–9468. doi: 10.1002/anie.200804140. [DOI] [PubMed] [Google Scholar]
- 17.Xu X., Yabuta T., Yuan P., Takemoto Y. Organocatalytic enantioselective hydrazination of 1,3-dicarbonyl compounds: Asymmetric synthesis of α,α-disubstituted α-amino acids. Synlett. 2006:137–140. [Google Scholar]
- 18.Dahlin N., Bøgevig A., Adolfsson H. N-Arenesulfonyl-2-aminomethylpyrrolidines - Novel modular ligands and organocatalysts for asymmetric catalysis. Adv. Synth. Catal. 2004;346:1101–1105. doi: 10.1002/adsc.200404098. [DOI] [Google Scholar]
- 19.Franzén J., Marigo M., Fielenbach D., Wabnitz T., Kjærsgaard A., Jørgensen K.A. A general organocatalyst for direct α-functionalization of aldehydes: Stereoselective C-C, C-N, C-F, C-Br, and C-S bond-forming reactions. Scope and mechanistic insights. J. Am. Chem. Soc. 2005;127:18296–18304. doi: 10.1021/ja056120u. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa Y., Kitajima M., Takayama H. First asymmetric total syntheses of cernuane-type Lycopodium alkaloids, cernuine, and cermizine D. Org. Lett. 2008;10:1987–1990. doi: 10.1021/ol800574v. [DOI] [PubMed] [Google Scholar]
- 21.Vogt H., Vanderheiden S., Bräse S. Proline-catalysed asymmetric amination of α,α-disubstituted aldehydes: synthesis of configurationally stable enantioenriched α-aminoaldehydes. Chem. Commun. 2003:2448–2449. doi: 10.1039/b305465a. [DOI] [PubMed] [Google Scholar]
- 22.Baumann T., Vogt H., Bräse S. The proline-catalyzed asymmetric amination of branched aldehydes. Eur. J. Org. Chem. 2007:266–282. [Google Scholar]
- 23.Chowdari N.S., Barbas C.F. III, Total synthesis of LFA-1 antagonist BIRT-377 via organocatalytic asymmetric construction of a quaternary stereocenter. Org. Lett. 2005;7:867–870. doi: 10.1021/ol047368b. [DOI] [PubMed] [Google Scholar]
- 24.Baumann T., Bächle M., Hartmann C., Bräse S. Thermal effects in the organocatalytic asymmetric α-amination of disubstituted aldehydes with azodicarboxylates: A high-temperature organocatalysis. Eur. J. Org. Chem. 2008:2207–2212. [Google Scholar]
- 25.Suri J.T., Steiner D., Barbas C.F. III Organocatalytic enantioselective synthesis of metabotropic glutamate receptor ligands. Org. Lett. 2005;7:3885–3888. doi: 10.1021/ol0512942. [DOI] [PubMed] [Google Scholar]
- 26.Kumaragurubaran N., Juhl K., Zhuang W., Bøgevig A., Jørgensen K.A. Direct L-proline-catalyzed asymmetric α-amination of ketones. J. Am. Chem. Soc. 2002;124:6254–6255. doi: 10.1021/ja026412k. [DOI] [PubMed] [Google Scholar]
- 27.Thomassigny C., Prim D., Greck C. Amino acid-catalyzed asymmetric α-amination of carbonyls. Tetrahedron Lett. 2006;47:1117–1119. doi: 10.1016/j.tetlet.2005.12.028. [DOI] [Google Scholar]
- 28.Lacoste E., Vaique E., Berlande M., Pianet I., Vincent J.-M., Landais Y. Benzimidazole-pyrrolidine/H+ (BIP/H+), a highly reactive organocatalyst for asymmetric processes. Eur. J. Org. Chem. 2007:167–177. [Google Scholar]
- 29.Hayashi Y., Aratake S., Imai Y., Hibino K., Chen Q.-Y., Yamaguchi J., Uchimaru T. Direct asymmetric α-amination of cyclic ketones catalyzed by siloxyproline. Chem. Asian J. 2008;3:225–232. doi: 10.1002/asia.200700307. [DOI] [PubMed] [Google Scholar]
- 30.Kotrusz P., Alemayehu S., Toma Š., Schmalz H.-G., Adler A. Enantioselective organocatalysis in ionic liquids: Addition of aliphatic aldehydes and ketones to diethyl azodicarboxylate. Eur. J. Org. Chem. 2005:4904–4911. [Google Scholar]
- 31.Dinér P., Kjærsgaard A., Lie M.A., Jørgensen K.A. On the origin of the stereoselectivity in organocatalysed reactions with trimethylsilyl-protected diarylprolinol. Chem. Eur. J. 2008;14:122–127. doi: 10.1002/chem.200701244. [DOI] [PubMed] [Google Scholar]
- 32.Iwamura H., Methew H.P., Blackmond D.G. In situ catalyst improvement in the proline-mediated α-amination of aldehydes. J. Am. Chem. Soc. 2004;126:11770–11771. doi: 10.1021/ja046258x. [DOI] [PubMed] [Google Scholar]
- 33.Iwamura H., Wells D.H., Mathew S.P., Klussmann M., Armstrong A., Blackmond D.G. Probing the active catalyst in product-accelerated proline-mediated reactions. J. Am. Chem. Soc. 2004;126:16312–16313. doi: 10.1021/ja0444177. [DOI] [PubMed] [Google Scholar]
- 34.Mathew S.P., Klussmann M., Iwamara H., Wells D.H., Jr., Armstrong A., Blackmond D.G. A mechanistic rationalization of unusual kinetic behavior in proline-mediated C-O and C-N bond-forming reactions. Chem. Commun. 2006:4291–4293. doi: 10.1039/b609926b. [DOI] [PubMed] [Google Scholar]
- 35.Liu T.-Y., Cui H.-L., Zhang Y., Jiang K., Du W., He Z.-Q., Chen Y.C. Organocatalytic and highly enantioselective direct α-amination of aromatic ketones. Org. Lett. 2007;9:3671–3674. doi: 10.1021/ol701648x. [DOI] [PubMed] [Google Scholar]
- 36.Pihko P.M., Pohjakallio A. Enantioselective organocatalytic Diels aminations: α-Aminations of cyclic β-keto esters and β-keto lactones with cinchonidine and cinchonine. Synlett. 2004:2115–2118. [Google Scholar]
- 37.Saaby S., Bella M., Jørgensen K.A. Asymmetric construction of quaternary stereocenters by direct organocatalytic amination of α-substituted α-cyanoacetates and β-dicarbonyl compounds. J. Am. Chem. Soc. 2004;126:8120–8121. doi: 10.1021/ja047704j. [DOI] [PubMed] [Google Scholar]
- 38.Liu X., Sun B., Deng L. Catalytic enantioselective electrophilic aminations of acyclic α-alkyl β-carbonyl nucleophiles. Synlett. 2009:1685–1689. doi: 10.1055/s-0029-1217334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu R., Zhang D., Wu J., Liu C. Theoretical study on the enantioselective α-amination reaction of 1,3-dicarbonyl compounds catalyzed by a bifunctional-urea. Tetrahedron Asymmetry. 2007;18:1655–1662. doi: 10.1016/j.tetasy.2007.07.009. [DOI] [Google Scholar]
- 40.Liu X., Li H., Deng L. Highly enantioselective amination of α-substituted α-cyanoacetates with chiral catalysts accessible from both quinine and quinidine. Org. Lett. 2005;7:167–169. doi: 10.1021/ol048190w. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y., Melgar-Fernández Juaristi., E. Enantioselective amination of α-phenyl-α-cyanoacetate catalyzed by chiral amines incorporating the α-phenylethyl auxiliary. Eur. J. Org. Chem. 2007:1522–1525. doi: 10.1021/jo0622633. [DOI] [PubMed] [Google Scholar]
- 42.Melgar-Fernández R., González-Olvera R., Olivares-Romero J.L., González-López V., Romero-Ponce L., Ramírez-Zárate M.R., Demare P., Regla I., Juaristi E. Synthesis of novel derivatives of (1S,4S)-2,5-diazabicyclo[2.2.1]heptane and their evaluation as potential ligands in asymmetric catalysis. Eur. J. Org. Chem. 2008:655–672. [Google Scholar]
- 43.Kim S.M., Lee J.H., Kim D.Y. Enantioselective Direct amination of α-cyanoketones catalyzed by bifunctional organocatalysts. Synlett. 2008:2659–2662. [Google Scholar]
- 44.Ait-Youcef R., Sbargoud K., Moreau X., Greck C. Asymmetric α-amination of chiral protected β-hydroxyaldehydes catalyzed by proline. Synlett. 2009:3007–3010. [Google Scholar]
- 45.Ait-Youcef R., Kalch D., Moreau X., Greck C. Asymmetric α-amination of aldehydes and ketones catalyzed by tert-butoxy-L-proline. Lett. Org. Chem. 2009;6:377–380. doi: 10.2174/157017809788681293. [DOI] [Google Scholar]
- 46.Cheng L., Liu L., Wang D., Chen Y.-J. Highly enantioselective and organocatalytic α-amination of 2-oxindoles. Org. Lett. 2009;11:3874–3877. doi: 10.1021/ol901405r. [DOI] [PubMed] [Google Scholar]
- 47.Bui T., Borregan M., Barbas C.F., III J. Expanding the scope of cinchona alkaloid-catalyzed enantioselective α-aminations of oxindoles: A versatile approach to optically active 3-amino-2-oxindole derivatives. J. Org. Chem. 2009;74:8935–8938. doi: 10.1021/jo902039a. [DOI] [PubMed] [Google Scholar]
- 48.Bertelsen S., Marigo M., Brandes S., Dinér P., Jørgensen K.A. Dienamine catalysis: Organocatalytic asymmetric γ-amination of α,β unsaturated aldehydes. J. Am. Chem. Soc. 2006;128:12973–12980. doi: 10.1021/ja064637f. [DOI] [PubMed] [Google Scholar]
- 49.Poulsen T.B., Alemparte C., Jørgensen K.A. Enantioselective organocatalytic allylic amination. J. Am. Chem. Soc. 2005;127:11614–11615. doi: 10.1021/ja0539847. [DOI] [PubMed] [Google Scholar]
- 50.Chowdari N.S., Ramachary D.B., Barbas III C.F. Organocatalytic asymmetric assembly reactions: One-pot synthesis of functionalized β-amino alcohols from aldehydes, ketones, and azodicarboxylates. Org. Lett. 2003;5:1685–1688. doi: 10.1021/ol034333n. [DOI] [PubMed] [Google Scholar]
- 51.Umbreen S., Brockhaus M., Ehrenberg H., Schmidt B. Norstatines from aldehydes by sequential organocatalytic α-amination and Passerini reaction. Eur. J. Org. Chem. 2006:4585–4595. [Google Scholar]
- 52.Oelke A.J., Kumarn S., Longbottom D.A., Ley S.V. An enantioselective organocatalytic route to chiral 3,6-dihydropyridazines from aldehydes. Synlett. 2006:2548–2552. [Google Scholar]
- 53.Kotkar S.P., Chavan V.B., Sudalai A. Organocatalytic sequential α-amination-Horner-Wadsworth-Emmons olefination of aldehydes: Enantioselective synthesis of γ-amino-α,β-unsaturated esters. Org. Lett. 2007;9:1001–1004. doi: 10.1021/ol063012j. [DOI] [PubMed] [Google Scholar]
- 54.Lim A., Choi J.H., Tae J. Organocatalytic α-amination-allylation-RCM strategy: enantioselective synthesis of cyclic hydrazines. Tetrahedron Lett. 2008;49:4882–4885. doi: 10.1016/j.tetlet.2008.06.004. [DOI] [Google Scholar]
- 55.Marigo M., Schulte T., Franzén, Jørgensen K.A. Asymmetric multicomponent domino reactions and highly enantioselective conjugated addition of thiols to α,β-unsaturated aldehydes. J. Am. Chem. Soc. 2005;127:15710–15711. doi: 10.1021/ja055291w. [DOI] [PubMed] [Google Scholar]
- 56.Jiang H., Nielsen J.B., Nielsen M., Jørgensen K.A. Organocatalysed asymmetric β-amination and multicomponent syn-selective diamination of α,β-unsaturated aldehydes. Chem. Eur. J. 2007;13:9068–9075. doi: 10.1002/chem.200700696. [DOI] [PubMed] [Google Scholar]
- 57.de Figueiredo R.M., Christmann M. Organocatalytic synthesis of drugs and bioactive natural products. Eur. J. Org. Chem. 2007:2575–2600. [Google Scholar]
- 58.Kalch D., De Rycke N., Mareau X., Greck C. Efficient syntheses of enantioenriched (R)-pipecolic acid and (R)-proline via electrophilic organocatalytic amination. Tetrahedron Lett. 2009;50:492–494. doi: 10.1016/j.tetlet.2008.11.054. [DOI] [Google Scholar]
- 59.Chouthaiwale P.V., Kotkar S.P., Sudalai A. Formal synthesis of (-)anisomycin via organocatalysis. ARKIVOC. 2009;ii:88–94. [Google Scholar]
- 60.Hartmann C.E., Gross P.J., Nieger M., Bräse S. Towards an asymmetric synthesis of the bacterial peptide deformylase (PDF) inhibitor fumimycin. Org. Biomol. Chem. 2009;7:5059–5062. doi: 10.1039/b916372g. [DOI] [PubMed] [Google Scholar]
- 61.Vogt H., Baumann R., Nieger M., Bräse S. Direct asymmetric α-sulfamidation of α-branched aldehydes: A novel approach to enamine catalysis. Eur. J. Org. Chem. 2006:5315–5338. [Google Scholar]
- 62.Hayashi Y., Yamaguchi J., Hibino K., Shoji M. Direct proline catalyzed asymmetric α-aminooxylation of aldehydes. Tetrahedron Lett. 2003;44:8293–8296. [Google Scholar]
- 63.Brown S.P., Brochu M.P., Sinz C.J., MacMillan D.W.C. The direct and enantioselective organocatalytic α-oxidation of aldehydes. J. Am. Chem. Soc. 2003;125:10808–10809. doi: 10.1021/ja037096s. [DOI] [PubMed] [Google Scholar]
- 64.Zhong G. A facile and rapid route to highly enantiopure 1,2-diols by novel catalytic asymmetric α-aminoxylation of aldehydes. Angew. Chem. Int. Ed. 2003;42:4247–4250. doi: 10.1002/anie.200352097. [DOI] [PubMed] [Google Scholar]
- 65.Bøgevig A., Sundén H., Córdova A. Direct catalytic enantioselective α-aminoxylation of ketones: A stereoselective synthesis of α-hydroxy and α,α'-dihydroxy ketones. Angew. Chem. Int. Ed. 2004;43:1109–1112. doi: 10.1002/anie.200353018. [DOI] [PubMed] [Google Scholar]
- 66.Hayashi Y., Yamaguchi J., Sumiya T., Shoji M. Direct proline-catalyzed asymmetric α-aminoxylation of ketones. Angew. Chem. Int. Ed. 2004;43:1112–1115. doi: 10.1002/anie.200353085. [DOI] [PubMed] [Google Scholar]
- 67.Córdova A., Sundén H., Bøgevig A., Johannson M., Himo F. The direct catalytic asymmetric α-aminooxylation reaction: Development of stereoselective routes to 1,2-diols and 1,2-amino alcohols and density functional calculations. Chem. Eur. J. 2004;10:3673–3684. doi: 10.1002/chem.200400137. [DOI] [PubMed] [Google Scholar]
- 68.Hayashi Y., Yamaguchi J., Sumiya T., Hibino K., Shoji M. Direct proline-catalyzed asymmetric α-aminoxylation of aldehydes and ketones. J. Org. Chem. 2004;69:5966–5973. doi: 10.1021/jo049338s. [DOI] [PubMed] [Google Scholar]
- 69.Momiyama N., Torii H., Yamamoto H. O-Nitroso aldol synthesis: Catalytic enantioselective route to α-aminooxy carbonyl compounds via enamine intermediate. Proc. Natl. Acad. Sci. USA. 2004;101:5374–5378. doi: 10.1073/pnas.0307785101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramachary D.B., Barbas C.F. III Direct amino acid-catalyzed asymmetric desymmetrization of meso-compounds: Tandem aminoxylation/O-N bond heterolysis reactions. Org. Lett. 2005;7:1577–1580. doi: 10.1021/ol050246e. [DOI] [PubMed] [Google Scholar]
- 71.Joseph J., Ramachary D.B., Jemmis E.D. Electrostatic repulsion as an additional selectivity factor in asymmetric proline catalysis. Org. Biomol. Chem. 2006;4:2685–2689. doi: 10.1039/b606996g. [DOI] [PubMed] [Google Scholar]
- 72.Font D., Bastero A., Sayalero S., Jimeno C., Pericàs M.A. Highly enantioselective α-aminoxylation of aldehydes and ketones with a polymer-supported organocatalyst. Org. Lett. 2007;9:1943–1946. doi: 10.1021/ol070526p. [DOI] [PubMed] [Google Scholar]
- 73.Huang K., Huang Z.-Z., Li X.-L. Highly enantioselective α-aminoxylation of aldehydes and ketones in ionic liquids. J. Org. Chem. 2006;71:8320–8323. doi: 10.1021/jo061507g. [DOI] [PubMed] [Google Scholar]
- 74.Guo H.-M., Niu H.-Y., Xue M.-X., Guo Q.-X., Cun L.-F., Mi A.-Q., Jiang Y.-Z., Wang J.-J. L-Proline in an ionic liquid as an efficient and reusable catalyst for direct asymmetric α-aminoxylation of aldehydes and ketones. Green Chem. 2006;8:682–684. doi: 10.1039/b604191d. [DOI] [Google Scholar]
- 75.Cheong P. H.-Y., Houk K.N. Origins of selectivities in proline-catalyzed α-aminoxylations. J. Am. Chem. Soc. 2004;126:13912–13913. doi: 10.1021/ja0464746. [DOI] [PubMed] [Google Scholar]
- 76.Mathew S.P., Iwamura H., Blackmond D.G. Amplification of enantiomeric excess in a proline-mediated reaction. Angew. Chem. Int. Ed. 2004;43:3317–3321. doi: 10.1002/anie.200453997. [DOI] [PubMed] [Google Scholar]
- 77.Poe S.L., Bogdan A.R., Mason B.P., Steinbacher J.L., Opalka S.M., McQuade D.T. Use of bifunctional ureas to increase the rate of proline-catalyzed α-aminoxylations. J. Org. Chem. 2009;74:1574–1580. doi: 10.1021/jo802461w. [DOI] [PubMed] [Google Scholar]
- 78.Zotova N., Moran A., Armstrong A., Blackmond D.G. A coherent mechanistic rationale for additive effects and autoinductive behaviour in proline-mediated reactions. Adv. Synth. Catal. 2009;351:2765–2769. doi: 10.1002/adsc.200900665. [DOI] [Google Scholar]
- 79.Hayashi Y., Yamaguchi J., Hibino K., Sumiya T., Urushima T., Shoji M., Hashizume D., Koshino H. A highly active 4-siloxyproline catalyst for asymmetric synthesis. Adv. Synth. Catal. 2004;346:1435–1439. doi: 10.1002/adsc.200404166. [DOI] [Google Scholar]
- 80.Wang W., Wang J., Li H., Liao L. An amine sulfonamide organocatalyst for promoting direct, highly enantioselective α-aminoxylation reactions of aldehydes and ketones. Tetrahedron Lett. 2004;45:7235–7238. doi: 10.1016/j.tetlet.2004.08.029. [DOI] [Google Scholar]
- 81.Chua P.J., Tan B., Zhong G. Highly enantioselective L-thiaproline catalyzed α-aminoxylation of aldehydes in aqueous media. Green Chem. 2009;11:543–547. doi: 10.1039/b817950f. [DOI] [Google Scholar]
- 82.Sundén H., Dahlin N., Ibrahem I., Adolfsson H., Córdova A. Novel organic catalysts for the direct enantioselective α-oxidation of carbonyl compounds. Tetrahedron Lett. 2005;46:3385–3389. doi: 10.1016/j.tetlet.2005.03.085. [DOI] [Google Scholar]
- 83.Kano T., Yamamoto A., Mii H., Takai J., Shirakawa S., Maruoka K. Direct asymmetric aminoxylation reaction catalyzed by axially chiral amino acids. Chem. Lett. 2008;37:250–251. doi: 10.1246/cl.2008.250. [DOI] [Google Scholar]
- 84.Kano T., Yamamoto A., Maruoka K. Direct asymmetric aminoxylation reaction catalyzed by a binaphthyl-based chiral amino sulfonamide with high catalytic performance. Tetrahedron Lett. 2008;49:5369–5371. doi: 10.1016/j.tetlet.2008.06.093. [DOI] [Google Scholar]
- 85.Kano T., Yamamoto A., Shirozu F., Maruoka K. Enantioselectivity switch in direct asymmetric aminoxylation catalyzed by binaphthyl-based chiral secondary amines. Synthesis. 2009:1557–1563. [Google Scholar]
- 86.Lu M., Lu Y., Zeng X., Tan B., Xu Z., Zhong G. Chiral Brønsted acid-catalyzed enantioselective α-hydroxylation of β-dicarbonyl compounds. J. Am. Chem. Soc. 2009;131:4562–4563. doi: 10.1021/ja8088907. [DOI] [PubMed] [Google Scholar]
- 87.Zhong G. Tandem aminoxylation-allylation reactions: a rapid, asymmetric conversion of aldehydes to mono-substituted 1,2-diols. Chem. Commun. 2004:606–607. doi: 10.1039/b314356b. [DOI] [PubMed] [Google Scholar]
- 88.Zhong G., Yu Y. Enantioselective synthesis of allylic alcohols by the sequential aminoxylation-olefination reactions of aldehydes under ambient conditions. Org. Lett. 2004;6:1637–1639. doi: 10.1021/ol049524k. [DOI] [PubMed] [Google Scholar]
- 89.Yang L., Liu R.-H., Wang B., Weng L.-L., Zheng H. Asymmetric synthesis of 3-hydroxyl-2-alkanones via tandem organocatalytic aminoxylation of aldehydes and chemoselective diazomethane homologation. Tetrahedron Lett. 2009;50:2628–2631. doi: 10.1016/j.tetlet.2009.03.057. [DOI] [Google Scholar]
- 90.Kumarn S., Shaw D.M., Longbottom D.A., Ley S.V. A highly selective, organocatalytic route to chiral dihydro-1,2-oxazines. Org. Lett. 2005;7:4189–4191. doi: 10.1021/ol051577u. [DOI] [PubMed] [Google Scholar]
- 91.Kumarn S., Shaw D.M., Ley S.V. A highly selective, organocatalytic route to chiral 1,2-oxazines from ketones. Chem. Comm. 2006:3211–3213. doi: 10.1039/b606338a. [DOI] [PubMed] [Google Scholar]
- 92.Kumarn S., Oelke A.J., Shaw D.M., Longbottom D.A., Ley S.V. A sequential enantioselective, organocatalytic route to chiral 1,2-oxazines and chiral pyridazines. Org. Biomol. Chem. 2007;5:2678–2689. doi: 10.1039/b708646f. [DOI] [PubMed] [Google Scholar]
- 93.Yamamoto Y., Momiyama N., Yamamoto H. Enantioselective tandem O-nitroso aldol/Michael reaction. J. Am. Chem. Soc. 2004;126:5962–5963. doi: 10.1021/ja049741g. [DOI] [PubMed] [Google Scholar]
- 94.Momiyama N., Yamamoto Y., Yamamoto H. Diastereo- and enantioselective synthesis of nitroso Diels-Alder-type bicycloketones using dienamine: Mechanistic insight into sequential nitroso aldol/Michael reaction and application for optically pure 1-amino-3,4-diol synthesis. J. Am. Chem. Soc. 2007;129:1190–1195. doi: 10.1021/ja066037m. [DOI] [PubMed] [Google Scholar]
- 95.Zhu D., Lu M., Chua P.J., Tan B., Wang F., Yang X., Zhong G. A highly stereoselective organocatalytic tandem aminoxylation/aza-Michael reaction for the synthesis of tetrahydro-1,2-oxazines. Org. Lett. 2008;10:4585–4588. doi: 10.1021/ol801864c. [DOI] [PubMed] [Google Scholar]
- 96.Lu M., Zhu D., Lu Y., Hou Y., Tan B., Zhong G. Organocatalytic asymmetric α-aminoxylation/aza-Michael reactions for the synthesis of functionalized tetrahydro-1,2-oxazines. Angew. Chem. Int. Ed. 2008;47:10187–10191. doi: 10.1002/anie.200803731. [DOI] [PubMed] [Google Scholar]
- 97.Zhu D., Lu M., Dai L., Zhong G. Highly stereoselective one-pot synthesis of bicyclic isoxazolidines with five stereogenic centers by an organocatalytic process. Angew. Chem. Int. Ed. 2009;48:6089–6092. doi: 10.1002/anie.200901249. [DOI] [PubMed] [Google Scholar]
- 98.Kondekar N.B., Kumar P. Iterative approach to enantiopure syn/anti-1,3-polyols via proline-catalyzed sequential α-aminoxylation and Horner-Wadsworth-Emmons olefination of aldehydes. Org. Lett. 2009;11:2611–2614. doi: 10.1021/ol900868v. [DOI] [PubMed] [Google Scholar]
- 99.Enders D., Müller M. Efficient asymmetric syntheses of (+)-strictifolione. Synthesis. 2004:1486–1496. doi: 10.1055/s-2004-822387. [DOI] [Google Scholar]
- 100.George S., Sudalai A. Enantioselective synthesis of tarchonanthuslactone using proline-catalyzed asymmetric α-aminooxylation. Tetrahedron Asymmetry. 2007;18:975–981. doi: 10.1016/j.tetasy.2007.04.008. [DOI] [Google Scholar]
- 101.Mangion I.K., MacMillan D.W.C. Total synthesis of brasoside and littoralisone. J. Am. Chem. Soc. 2005;127:3696–3697. doi: 10.1021/ja050064f. [DOI] [PubMed] [Google Scholar]
- 102.Yamaguchi J., Toyoshima M., Shoji M., Kakeya H., Osada H., Hayashi Y. Concise enantio- and diastereoselective total syntheses of fumagillol, RK-805, FR65814, ovalicin, and 5-demethylovalicin. Angew. Chem. Int. Ed. 2006;45:789–793. doi: 10.1002/anie.200502826. [DOI] [PubMed] [Google Scholar]
- 103.Varseev G.N., Maier M.E. Enantioselective total synthesis of (+)-neosymbioimine. Org. Lett. 2007;9:1461–1464. doi: 10.1021/ol070049a. [DOI] [PubMed] [Google Scholar]
- 104.Hara S., Makino K., Hamada Y. Total synthesis of halipeptin A, a potent anti-inflammatory cyclodepsipeptide from a marine sponge. Tetrahedron Lett. 2006;47:1081–1085. doi: 10.1016/j.tetlet.2005.12.026. [DOI] [Google Scholar]
- 105.Kim S.-G., Park T.-H., Kim B. J. Efficient total synthesis of (+)-exo-, (-)-endo-brevicomin and their derivatives via asymmetric organocatalysis and olefin cross-metathesis. Tetrahedron Lett. 2006;47:6369–6372. doi: 10.1016/j.tetlet.2006.06.170. [DOI] [Google Scholar]
- 106.Narina S.V., Sudalai A. Short and practical enantioselective synthesis of linezolid and eperezolid via proline-catalyzed asymmetric α-aminooxylation. Tetrahedron Lett. 2006;47:6799–6802. doi: 10.1016/j.tetlet.2006.07.065. [DOI] [Google Scholar]
- 107.Kotkar S.P., Sudalai A. A short enantioselective synthesis of the antiepileptic agent, levetiracetam based on proline-catalyzed asymmetric α-aminooxylation. Tetrahedron Lett. 2006;47:6813–6815. doi: 10.1016/j.tetlet.2006.07.061. [DOI] [Google Scholar]
- 108.Kotkar S.P., Sudalai A. Enantioselective synthesis of (S,S)-ethambutol using proline-catalyzed asymmetric α-aminooxylation and α-amination. Tetrahedron Asymmetry. 2006;17:1738–1742. doi: 10.1016/j.tetasy.2006.06.011. [DOI] [Google Scholar]
- 109.Kotkar S.P., Suryavanshi G.S., Sudalai A. A short synthesis of (+)-harzialactone A and (R)-(+)-4-hexanolide via proline-catalyzed sequential α-aminooxylation and Horner-Wadsworth-Emmons olefination of aldehydes. Tetrahedron-Asymmetr. 2007;18:1795–1798. doi: 10.1016/j.tetasy.2007.07.031. [DOI] [Google Scholar]
- 110.Talluri S.K., Sudalai A. An organo-catalytic approach to the enantioselective synthesis of (R)-selegiline. Tetrahedron. 2007;63:9758–9763. doi: 10.1016/j.tet.2007.07.012. [DOI] [Google Scholar]
- 111.Panchgalle S.P., Gore R.G., Chavan S.P., Kalkote U.R. Organocatalytic enantioselective synthesis of β-blockers: (S)-propranolol and (S)-naftopidil. Tetrahedron-Asymmetr. 2009;20:1767–1770. doi: 10.1016/j.tetasy.2009.07.002. [DOI] [Google Scholar]
- 112.Kim S.-G. Concise total synthesis of (+)-disparlure and its trans-isomer using asymmetric organocatalysis. Synthesis. 2009:2418–2422. doi: 10.1055/s-0029-1216855. [DOI] [Google Scholar]
- 113.Sawant R.T., Waghmode S.B. Organocatalytic enantioselective formal synthesis of HRV 3C-protease inhibitor (1R,3S)-thysanone. Tetrahedron. 2009;65:1599–1602. doi: 10.1016/j.tet.2008.12.060. [DOI] [Google Scholar]
- 114.Kim S.-G., Park T.-H. Organocatalyzed asymmetric α-hydroxyamination of α-branched aldehydes: Asymmetric synthesis of optically active N-protected α,α-disubstituted amino aldehydes and amino alcohols. Tetrahedron Lett. 2006;47:9067–9071. doi: 10.1016/j.tetlet.2006.10.081. [DOI] [Google Scholar]
- 115.Guo H.-M., Cheng L., Cun L.-F., Gong L.-Z., Mi A.-Q., Jiang Y.-Z. L-Prolinamide-catalyzed direct nitroso aldol reactions of α-branched aldehydes: A distinct regioselectivity from that with L-proline. Chem. Commun. 2006:429–431. doi: 10.1039/b514194j. [DOI] [PubMed] [Google Scholar]
- 116.Palomo C., Vera S., Velilla I., Mielgo A., Gómez-Bengoa E. Regio- and enantioselective direct oxyamination reaction of aldehydes catalyzed by α,α-diphenylprolinol trimethylsilyl ether. Angew. Chem. Int. Ed. 2007;46:8054–8056. doi: 10.1002/anie.200703001. [DOI] [PubMed] [Google Scholar]
- 117.Kano T., Ueda M., Takai J., Maruoka K. Direct asymmetric hydroxyamination reaction catalyzed by an axially chiral secondary amine catalyst. J. Am. Chem. Soc. 2006;128:6046–6047. doi: 10.1021/ja0604515. [DOI] [PubMed] [Google Scholar]
- 118.Momiyama N., Yamamoto H. Brønsted acid catalysis of achiral enamine for regio- and enantioselective nitroso aldol synthesis. J. Am. Chem. Soc. 2005;127:1080–1081. doi: 10.1021/ja0444637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.López-Cantarero J., Cid M.B., Poulsen T.B., Bella M., Ruano J.L.G., Jørgensen K.A. Intriguing behavior of cinchona alkaloids in the enantioselective organocatalytic hydroxyamination of α-substituted-α-cyanoacetates. J. Org. Chem. 2007;72:7062–7065. doi: 10.1021/jo071186o. [DOI] [PubMed] [Google Scholar]
- 120.Sibi M.P., Hasegawa M. Organocatalysis in radical chemistry. Enantioselective α-oxyamination of aldehydes. J. Am. Chem. Soc. 2007;129:4124–4125. doi: 10.1021/ja069245n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bui N.-N., Ho X.-H., Mho S.I., Jang H.-Y. Organocatalyzed α-oxyamination of aldehydes using anodic oxidation. Eur. J. Org. Chem. 2009:5309–5312. [Google Scholar]
- 122.Vaismaa M.J.P., Yau S.C.Y., Tomkinson N.C.O. Organocatalytic α-oxybenzoylation of aldehydes. Tetrahedron Lett. 2009;50:3625–3627. doi: 10.1016/j.tetlet.2009.03.082. [DOI] [Google Scholar]
- 123.Gotoh H., Hayashi Y. Diphenylprolinol silyl ether as a catalyst in an asymmetric, catalytic and direct α-benzoyloxylation of aldehydes. Chem. Commun. 2009:3083–3085. doi: 10.1039/b902287b. [DOI] [PubMed] [Google Scholar]
- 124.Kano T., Mii H., Maruoka K. Direct asymmetric benzoyloxylation of aldehyde catalyzed by 2-tritylpyrrolidine. J. Am. Chem. Soc. 2009;131:3450–3451. doi: 10.1021/ja809963s. [DOI] [PubMed] [Google Scholar]
- 125.Ibrahem I., Zhao G.-L., Sundén H., Córdova A. A route to 1,2-diols by enantioselective organocatalytic α-oxidation with molecular oxygen. Tetrahedron Lett. 2006;47:4659–4663. doi: 10.1016/j.tetlet.2006.04.133. [DOI] [Google Scholar]
- 126.Córdova A., Sundén H., Engqvist M., Ibrahem I., Casas J. The direct amino acid-catalyzed asymmetric incorporation of molecular oxygen to organic compounds. J. Am. Chem. Soc. 2004;126:8914–8915. doi: 10.1021/ja047930t. [DOI] [PubMed] [Google Scholar]
- 127.Acocella M.R., Mancheño O.G., Bella M., Jørgensen K.A. Organocatalytic asymmetric hydroxylation of β-keto esters: Metal-free synthesis of optically active anti-diols. J. Org. Chem. 2004;69:8165–8167. doi: 10.1021/jo048655w. [DOI] [PubMed] [Google Scholar]
- 128.Bekele T., Shah M.H., Wolfer J., Abraham C.J., Weatherwax A., Lectka T. Catalytic, enantioselective [4+2]-cycloadditions of ketene enolates and o-quinones: efficient entry to chiral, α-oxygenated carboxylic acid derivatives. J. Am. Chem. Soc. 2006;128:1810–1811. doi: 10.1021/ja058077g. [DOI] [PubMed] [Google Scholar]
- 129.Hernandez-Juan F.A., Cockfield D.M., Dixon D.J. Enantioselective organocatalytic aryloxylation of aldehydes with o-quinones. Tetrahedron Lett. 2007;48:1605–1608. doi: 10.1016/j.tetlet.2006.12.140. [DOI] [Google Scholar]
- 130.Engqvist M., Casas J., Sundén H., Ibrahem I., Córdova A. Direct organocatalytic asymmetric α-oxidation of ketones with iodosobenzene and N-sulfonyloxaziridines. Tetrahedron Lett. 2005;46:2053–2057. doi: 10.1016/j.tetlet.2005.01.167. [DOI] [Google Scholar]
- 131.Tong A.-T., Brimble M.A., Barker D. Influence of α-methyl substitution of proline-based organocatalysts on the asymmetric α-oxidation of aldehydes. Tetrahedron. 2009;65:4801–4807. doi: 10.1016/j.tet.2009.04.060. [DOI] [Google Scholar]
- 132.Richardson R.D., Page T.K., Altermann S., Paradine S.M., French A.N., Wirth T. Enantioselective α-oxytosylation of ketones catalysed by iodoarenes. Synlett. 2007:538–542. [Google Scholar]
- 133.Altermann S.M., Richardson R.D., Page T.K., Schmidt R.K., Holland E., Mohammed U., Paradine S.M., French A.N., Richter C., Bahar A.M., Witulski B., Wirth T. Catalytic enantioselective α-oxysulfonation of ketones mediated by iodoarenes. Eur. J. Org. Chem. 2008:5315–5328. [Google Scholar]