Abstract
Objective
To analyze the effects of melatonin on vascular endothelial growth factor A (VEGF-A) expression and follicle reserve in rat ovary.
Materials and Methods
A total of 45 female Wistar rats were used in the present study. Rats were divided into three groups: group 1 (control), group 2 (vehicle), and group 3 (melatonin). Rats in the melatonin group were treated with an intraperitoneal injection of melatonin at a dose of 50 mg/kg/day for 56 days. We investigated VEGF-A expression in rat ovary in all the groups using Western blot and reverse transcription-polymerase chain reaction. Histopathological parameters were evaluated using light microscopy.
Results
The number of atretic follicles was significantly lower in the melatonin treatment rats than in the control rats (p<0.05); however, the number of antral follicles was significantly higher in the former (p<0.05). Additionally, we observed a weak immunoblot stain in the melatonin group for VEGF-A protein. Interestingly, melatonin treatment induced a significant decrease in VEGF-A expression in the ovary of group 3 rats (p<0.05), whereas no such difference was observed between group 1 and group 2 rats (p>0.05).
Conclusion
The present study demonstrates that the protective effect of melatonin on the degeneration of follicles in rat ovary is reduced by decreasing the VEGF-A expression. These results suggest that melatonin is effective against follicular atresia and preserves antral follicles, thus, offering a therapeutic advantage in clinical use.
Keywords: Melatonin, antral follicle, atresia, vascular endothelial growth factor A (VEGF-A)
Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is an important lipophilic amine hormone that is secreted by the pineal gland and considered a powerful free radical scavenger. It plays a critical role in the physiology of the ovary in the reproductive system [1]. Some authors showed that the melatonin receptor is in the ovaries, suggesting that melatonin is of great importance to the reproductive system [2, 3]. Vascular endothelial growth factor (VEGF) is a chemokine that is the most active endogenous pro-angiogenic factor in humans [4, 5]. The VEGF system comprises ligands and receptors that play critical roles in tissue vascularization and endothelial cell growth [6]. It is a greater component of the family of angiogenic factors, which contains the placental growth factor, angiopoietin, basic fibroblast growth factor, and the VEGF family (A, B, C, D, and E) [7, 8]. Previous studies on cancer have shown that in other tissues, melatonin decreases VEGF secretion [9]. Melatonin may affect VEGF, and the relationship between melatonin and VEGF secretion in the ovary is unclear. Thus, we aimed to evaluate the effects of melatonin on the ovarian histology and VEGF expression and on the quantity of follicle selection in adult female rat ovaries.
Materials and Methods
Animals
In total, 45 female Wistar rats, aged between 8 and 10 weeks, with body weights ranging from 150 to 200 g were used. All animals were kept in a controlled environment with artificial light–dark cycle of 12 h lights on and 12 h lights off and received food and tap water ad libitum. The present study was approved by the Akdeniz University Institutional Animal Care and Use Ethical Committee. Rats were randomized into three experimental groups as follows: group 1: control, group 2: vehicle, and group 3: melatonin (Table 1).
Table 1.
Study groups
| Groups | Ethanol administered Melatonin-treated | Melatonin treatment | n |
|---|---|---|---|
| Group l: control | – | – | 15 |
| Group 2: vehicle | 10 mg/kg/day | – | 15 |
| Group 3: melatonin | – | 50 mg/kg/day | 15 |
Melatonin Exposure
Melatonin (M5250; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in absolute ethanol, and the final concentration was 50 mg/kg, which is the optimal dose that we had determined in our previous study [1]. Melatonin was intraperitoneally injected for 56 days in the melatonin group in the same light period (12:00 a.m.), whereas ethanol was administered in the vehicle-treated group (10% ethanol in saline). The melatonin and vehicle group animals continued to receive their usual treatments for 56 days until sacrificed by cervical dislocation.
Histomorphometric Analysis of Folliculogenesis and Follicle Count in the Ovaries
Histomorphometric analysis was performed by a histologist. The ovaries were preserved in Bouin’s fluid for 12 h, followed by immersion in 70% ethanol saturated with lithium carbonate to remove the excess picric acid. After processing, 5-μm thick serial cryosections were prepared using a cryostat to study the effect of melatonin on the number of follicles. Every fifth section was stained with routine hematoxylin and eosin, and the slides were mounted for further assessment. Approximately 15–20 slides (every fifth section) were evaluated for predictin g the follicle count of each ovary. Blind manual counting was exercised by a single observer owing to previous knowledge [10]. The follicles with clearly visible nucleus within the oocyte were classified as primordial, antral, and atretic follicles [11]. The slides were closely analyzed at 40× magnification, and the number of various follicles was individually noted for each animal.
Real-time Polymerase Chain Reaction
Total RNA was harvested from whole ovaries using reverse transcription-polymerase chain reaction (RT-PCR) (n=15). RNA was isolated from whole ovaries using TRIzol reagent (Invitrogen), followed by RNA extraction. Tissue cDNA was assayed in duplicate, and each experiment was repeated three times. RT-PCR for VEGF-A was performed in a 25-μl final reaction that included 2 μl of each RT sample within a reaction mix containing 1× Buffer D, 10 pmol of each primer, and Taq polymerase. RT-PCR was performed using the following parameters: 1 cycle at 94°C for 5 min (denaturation), followed by 35 cycles (amplification) at 94°C for 30 s, 59°C for 1 min, and 72°C for 45 s for all target genes and control β-actin. Table 2 shows a primer that was used for the amplification of VEGF-A gene.
Table 2.
Primer list
| Gene name | Sequence |
|---|---|
| VEGF-A (172 bp) | Sense: 5′ GCC CAT GAA GTG GTG AAG TT 3′ |
| Antisense: 5′ ACT CCA GGG CTT CAT CAT TG 3′ |
Western Blot
Briefly, 50 μg of protein lysates were loaded in each lane. Proteins carried out electrophoretically by sodium dodecyl sulfate polyacrylamide gel using 10% Tris–HCl gels. After electrophoresis, the proteins were electrotransferred to polyvinylidene fluoride membrane (Bio-Rad Laboratories). Cells were electrotransferred to Immobilon-P transfer membrane (Millipore, Billerica, MA, USA) and Tris-buffered saline that included 5% nonfat milk for 1 h at room temperature. Then, the primary antibody was added overnight at 4°C and washed, and the secondary antibody was used. The same procedure was repeated for β-actin that was used for internal control.
Statistical Analysis
Histological data were analyzed using analysis of variance (ANOVA; with post hoc Tukey’s test) or Kruskal–Wallis test (post hoc Dunnett’s test). RT-PCR results from each group were compared using one-way ANOVA followed by post hoc Holm–Sidak test. Statistical data were examined using SigmaStat for Windows version 3.0 (Jandel Scientific Corp., San Rafael, CA, USA). A p<0.05 was considered as significant.
Results
Morphological Evaluation
The melatonin, control, and vehicle groups were histologically normal. In the ovaries that appeared to have a normal histology, the follicles were found to have a normal histology at different stages of development, because there was a peripheral cortex, a medullary region displaying normal vessel network regions in the ovarian center (Figure 1).
Figure 1.
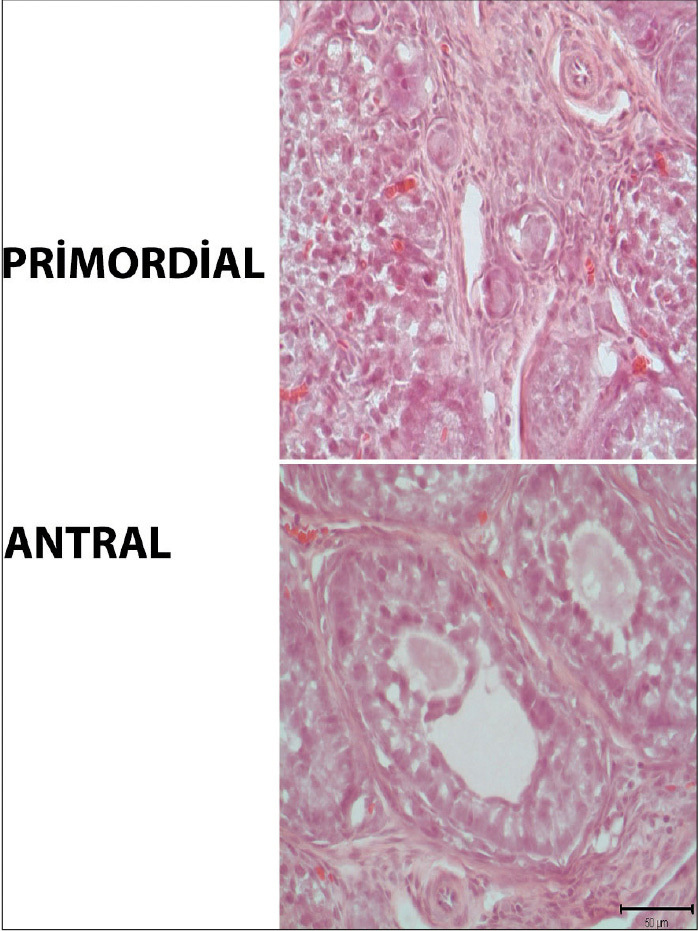
Histomorphometric analysis
Histomorphometric assessment of the antral and primordial follicles in rat ovary. Scale bars represent 50 μm.
Effect of Melatonin Treatment on Antral and Atretic Follicles
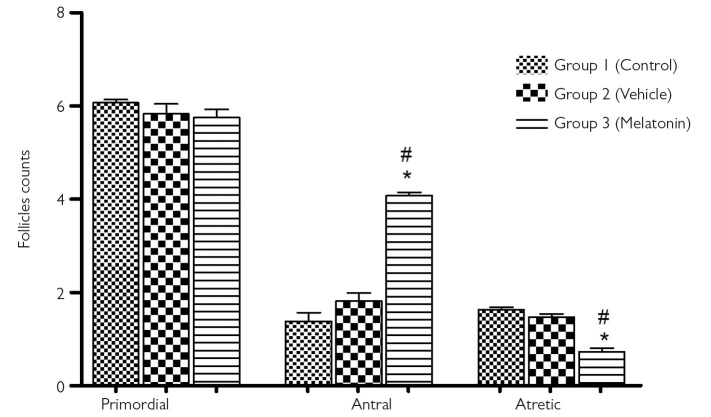
Figure 2 shows the number of antral, primordial, and atretic follicles in the ovaries of all groups. There was no significant difference between the three groups regarding the primordial follicle (p>0.05). The evaluation of atretic and antral follicle counts revealed significant differences between the groups (p<0.05). The melatonin group had the highest antral and the lowest atretic follicle count. Furthermore, the number of atretic follicles in the melatonin group was significantly lower than that in the control group. In addition, the percentage of atretic follicles in the melatonin group was 2.23 fold less than that in the control group, indicating that melatonin treatment decreases follicular degeneration reserve (atretic, 1.9±0.4 in group 1 vs. 0.85±0.1 in group 3) (p<0.05) (Figure 2). We observed that the percentage of antral follicles was 2.55 fold higher in the melatonin group than in the control group; it was also estimated that melatonin treatment supported antral follicle development, preserving the follicular reserve (antral, 1.38±0.2 in group 1 vs. 4.08±0.8 in group 3) (p<0.05).
Figure 2.
Follicle count
Comparison of the number of primordial, antral, and atretic follicles between the three groups. Data are shown as mean±S.E.M.; n=15 rats/group. *, p<0.05 indicates significance from respective vehicle values. #, p<0.05 indicates significance from respective control values.
Western Blot
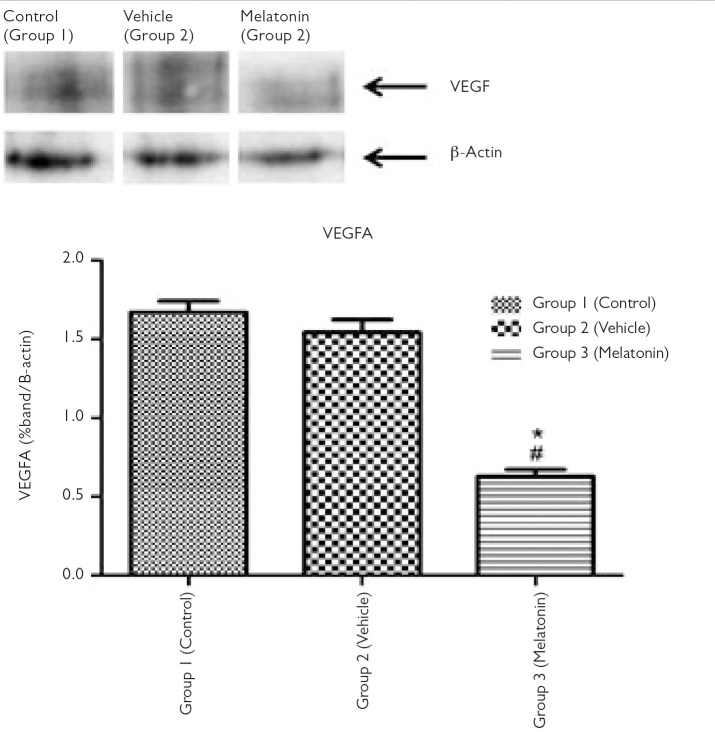
No difference in VEGF-A expression was found in the control and vehicle groups. On examining the level of VEGF-A protein expression in the control and melatonin groups, a dramatic decrease in the expression was observed in the melatonin group (Figure 3).
Figure 3.
VEGF-A protein expression
VEGF-A protein expression in group 1 (control), group 2 (vehicle), and group 3 (melatonin). Data are shown as mean±S.E.M.; n=15 rats/group. *, p<0.05 indicates significance from respective control values. #, p<0.05 indicates significance from respective vehicle values.
Real-time Polymerase Chain Reaction
RT-PCR analysis revealed that VEGF-A, the key molecule of angiogenesis, was expressed in all three groups. However, VEGF-A showed a significantly lower protein expression in the melatonin group than in the other groups (p<0.05).
Discussion
The incidence of infertility is gradually increasing worldwide [12]. Female infertility can be induced by various processes involving folliculogenesis, ovulation, early embryogenesis, and implantation in most cases. However, a lack of follicle quantity or number with defects in follicular development in the ovary has become the principal cause of female fertility, affecting approximately 1% of women aged under 40 years [13–15]. Kandemir et al. [1] showed that melatonin treatment may be effective in increasing the female follicular reserve. Melatonin has been actively used against oxidative stress and inflammation and to renovate multiple functions of many tissues, such as regulation of fertility [16–18]. For example, ovarian activity promotes estrous cyclicity and gonadal atrophy depending on photoperiodic conditions as stimulated by melatonin [19]. Previous studies reported that melatonin ensures a protective effect on premature ovarian insufficiency in ovarian and increasing ovarian follicles, decreasing follicular atresia [20–22]. Chan et al. [23] established that reactive oxygen species and the endoplasmic reticulum are responsible for the increased loss of ovarian reserve. Melatonin dose administered in this study was based on our previous study on the effect of melatonin on mammalian target of rapamycin expression in rat ovary [1]. In the present study, we also showed that melatonin treatment causes an increase in the number of antral follicles and a decrease in the number of atretic follicles, suggesting that melatonin may inhibit follicular atresia.
Many endogenous activators or inhibitors can affect physiological angiogenesis balance. VEGF-A is the most significant angiogenesis activator that acts through binding of VEGFRs and primary VEGFR-2, which is the most prominent receptor associated with angiogenesis [24]. A previous study evaluated the effects of melatonin on angiogenesis and showed that melatonin can significantly decrease VEGFR-2 expression in mice [25]. The results of the present study showed a remarkable relationship involving VEGF-A and melatonin in rat ovary. We found that melatonin significantly protected the follicles via the angiogenic factor VEGF-A, which is closely related to the angiogenesis process in the follicle. Additionally, VEGF-A is linked to several cytokines that could be related to the accelerated degeneration of the corpus luteum, leading to attenuated progesterone production [26]. Several studies have shown that melatonin decreases VEGF-A and proliferating cell nuclear antigen expression in the epithelial and stromal cells in rat ovary [26, 27]. In the present study, melatonin mitigated VEGF-A levels. Our observed loss of VEGF-A expression may be indicative of an increased protection from atresia in the melatonin group. It can be concluded that a decrease in atresia and an increase in the number of antral follicles can be attributed to diminishing VEGF-A levels.
Acknowledgements
My special thanks go to Berna Sözen (Department of Histology, Akdeniz University School of Medicine, Antalya, Turkey.), for her valuable contribution to this study. The author is also grateful to the staff of the animal care laboratory for their technical assistance.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Akdeniz University Institutional Animal Care and Use Committee Policies for Animal Use (No:2018.01.24).
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Y.B.K.; Design – E.K.; Supervision - M.B.; Materials – E.K.; Data Collection and/or Processing – Y.B.K.; Analysis and/or Interpretation – M.B.; Literature Search – M.S.; Writing Manuscript – M.S.; Critical Review – Y.B.K.
Conflict of Interest: Authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Behram Kandemir Y, Aydin C, Gorgisen G. The effects of melatonin on oxidative stress and prevention of primordial follicle loss via activation of mTOR pathway in the rat ovary. Cell Mol Biol (Noisy-le-grand) 2017;63:100–6. doi: 10.14715/cmb/2017.63.2.16. https://doi.org/10.14715/cmb/2017.63.2.16. [DOI] [PubMed] [Google Scholar]
- 2.Inkster CF, Ng SG, Leatherbarrow B. Primary banked scleral patch graft in the prevention of exposure of hydroxyapatite orbital implants. Ophthalmology. 2002;109:389–92. doi: 10.1016/s0161-6420(01)00904-6. https://doi.org/10.1016/S0161-6420(01)00904-6 [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Liu T, Qu J. The effect of orbital implantation on peripheral blood melatonin and sex hormone levels in child patients with congenital eyeball dysplasia. Exp Ther Med. 2017;14:2211–5. doi: 10.3892/etm.2017.4719. https://doi.org/10.3892/etm.2017.4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol. 2002;39:225–37. doi: 10.1016/s1537-1891(03)00011-9. https://doi.org/10.1016/S1537-1891(03)00011-9 [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Qin C, Sin JE, et al. Discovery of novel dual VEGFR2 and Src inhibitors using a multistep virtual screening approach. Future Med Chem. 2017;9:7–24. doi: 10.4155/fmc-2016-0162. https://doi.org/10.4155/fmc-2016-0162 [DOI] [PubMed] [Google Scholar]
- 6.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti-and Pro-Angiogenic Therapies. Genes Cancer. 2011;2:1097–105. doi: 10.1177/1947601911423031. https://doi.org/10.1177/1947601911423031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–54. [PubMed] [Google Scholar]
- 8.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–94. doi: 10.1016/S0968-0004(03)00193-2. https://doi.org/10.1016/S0968-0004(03)00193-2 [DOI] [PubMed] [Google Scholar]
- 9.Lissoni P, Rovelli F, Malugani F, Bucovec R, Conti A, Maestroni GJ. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuro Endocrinol Lett. 2001;22:45–7. [PubMed] [Google Scholar]
- 10.Tilly JL. Ovarian follicle counts--not as simple as 1, 2, 3. Reprod Biol Endocrinol. 2003;1:11. doi: 10.1186/1477-7827-1-11. https://doi.org/10.1186/1477-7827-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–50. doi: 10.1126/science.1103974. https://doi.org/10.1126/science.1103974 [DOI] [PubMed] [Google Scholar]
- 12.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–10. doi: 10.1200/JCO.2013.49.2678. https://doi.org/10.1200/JCO.2013.49.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goswami D, Conway GS. Premature ovarian failure. Horm Res. 2007;68:196–202. doi: 10.1159/000102537. https://doi.org/10.1159/000102537 [DOI] [PubMed] [Google Scholar]
- 14.Reddy P, Liu L, Adhikari D, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–3. doi: 10.1126/science.1152257. https://doi.org/10.1126/science.1152257 [DOI] [PubMed] [Google Scholar]
- 15.Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology. 2008;149:6207–12. doi: 10.1210/en.2008-0294. https://doi.org/10.1210/en.2008-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Navarro A, Gonzalez-Puga C, Escames G, et al. Cellular mechanisms involved in the melatonin inhibition of HT-29 human colon cancer cell proliferation in culture. J Pineal Res. 2007;43:195–205. doi: 10.1111/j.1600-079X.2007.00463.x. https://doi.org/10.1111/j.1600-079X.2007.00463.x [DOI] [PubMed] [Google Scholar]
- 17.Maldonado MD, Siu AW, Sanchez-Hidalgo M, Acuna-Castroviejo D, Escames G. Melatonin and lipid uptake by murine fibroblasts: clinical implications. Neuro Endocrinol Lett. 2006;27:601–8. [PubMed] [Google Scholar]
- 18.Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–78. doi: 10.1111/jpi.12360. https://doi.org/10.1111/jpi.12360 [DOI] [PubMed] [Google Scholar]
- 19.Horton TH, Yellon SM. Aging, reproduction, and the melatonin rhythm in the Siberian hamster. J Biol Rhythms. 2001;16:243–53. doi: 10.1177/074873040101600307. https://doi.org/10.1177/074873001129001953 [DOI] [PubMed] [Google Scholar]
- 20.Ma M, Chen XY, Li B, Li XT. Melatonin protects premature ovarian insufficiency induced by tripterygium glycosides: role of SIRT1. Am J Transl Res. 2017;9:1580–602. [PMC free article] [PubMed] [Google Scholar]
- 21.Chen XY, Gu C, Ma M, et al. A mouse model of premature ovarian insufficiency induced by tripterygium glycoside via subcutaneous injection. Int J Clin Exp Pathol. 2014;7:144–51. [PMC free article] [PubMed] [Google Scholar]
- 22.Chen XY, Xia HX, Guan HY, Li B, Zhang W. Follicle Loss and Apoptosis in Cyclophosphamide-Treated Mice: What’s the Matter? Int J Mol Sci. 2016 May 30;17:E836. doi: 10.3390/ijms17060836. https://doi.org/10.3390/ijms17060836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan KA, Tsoulis MW, Sloboda DM. Early-life nutritional effects on the female reproductive system. J Endocrinol. 2015;224:R45–62. doi: 10.1530/JOE-14-0469. https://doi.org/10.1530/JOE-14-0469 [DOI] [PubMed] [Google Scholar]
- 24.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. https://doi.org/10.1038/nrc1093 [DOI] [PubMed] [Google Scholar]
- 25.Jardim-Perassi BV, Arbab AS, Ferreira LC, et al. Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS One. 2014;9:e85311. doi: 10.1371/journal.pone.0085311. https://doi.org/10.1371/journal.pone.0085311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romeu LR, da Motta EL, Maganhin CC, et al. Effects of melatonin on histomorphology and on the expression of steroid receptors, VEGF, and PCNA in ovaries of pinealectomized female rats. Fertil Steril. 2011;95:1379–84. doi: 10.1016/j.fertnstert.2010.04.042. https://doi.org/10.1016/j.fertnstert.2010.04.042 [DOI] [PubMed] [Google Scholar]
- 27.Greenaway J, Connor K, Pedersen HG, Coomber BL, LaMarre J, Petrik J. Vascular endothelial growth factor and its receptor, Flk-1/KDR, are cytoprotective in the extravascular compartment of the ovarian follicle. Endocrinology. 2004;145:2896–905. doi: 10.1210/en.2003-1620. https://doi.org/10.1210/en.2003-1620 [DOI] [PubMed] [Google Scholar]