Abstract
Reactive oxygen species (ROS) are well-known for playing a dual role as destructive and constructive species. Indeed, ROS are engaged in many redox-governing activities of the cells for the preservation of cellular homeostasis. However, its overproduction has been reported to result in oxidative stress, which is considered as a deleterious process, and is involved in the damage of cell structures that causes various diseased states. This review provides a concise view on some of the current research published in this topic for an improved understanding of the key roles of ROS in diverse conditions of health and disease. Previous research demonstrated that ROS perform as potential signaling molecules to control several normal physiological functions at the cellular level. Additionally, there is a growing body of evidence supporting the role of ROS in various pathological states. The binary nature of ROS with their profitable and injurious characteristics indicates the complexities of their specific roles at a biological compartment and the difficulties in establishing convenient intervention procedures to treat ROS-related diseases.
Keywords: Reactive oxygen species, oxidative stress, free radicals, infertility, cancer
Introduction
The “oxygen paradox” is the paradox faced by aerobic eukaryotes where organisms cannot survive without oxygen. Nevertheless, oxygen is naturally dangerous to their survival. The dark side of oxygen is directly related to its electronic distribution. Each oxygen atom has one unpaired electron in its exterior valence shell, whereas molecular oxygen has two unpaired electrons. This makes atomic oxygen a free radical and molecular oxygen a (free) bi-radical. The mitochondrial electron transport chain provides a safeguard tetravalent reduction of oxygen to produce water. However, the univalent reduction of oxygen produces reactive intermediates [1]. Considering the reductive cellular environment, abundant chances for oxygen to go through impromptu univalent reduction are present. Thus, the superoxide anion radical, hydrogen peroxide (H2O2), and the enormously reactive hydroxyl radical are regular products of aerobic respiration and oxidation processes in an aerobic environment [1].
Reactive oxygen species (ROS) are commonly used to define the reactive molecules and free radicals originating from molecular oxygen. ROS were previously thought to emerge almost exclusively from mitochondrial metabolism. Nevertheless, there is growing evidence that cellular enzymes known as nicotinamide adenine dinucleotide phosphate (NADPH) oxidases produce a considerable amount of ROS in humans [1]. Other cellular sources of ROS include neutrophils, monocytes, cardiomyocytes, endothelial cells, xanthine oxidases, cytochrome P450, lipoxygenases, and nitric oxide synthases [2, 3]. ROS are very reactive molecules that may undergo several reduction reactions to harm normal cells [4, 5]. Detoxification of ROS is fundamental for all cells to survive. Living organisms have evolved a variety of defense mechanisms to provide a balance between generation and elimination of ROS to endure the oxygen-rich cellular environment. The inequality between the systemic production of ROS and the ability of cells to instantly detoxify the reactive intermediates or to restore the resultant impairment is often called as “oxidative stress” (OS) [6].
Despite the enormous antioxidant and repair mechanisms that developed in biological systems, oxidative damage remains an inevitable outcome of aerobic life. Recently, OS has been associated with a broad range of degenerative processes, diseases, and syndromes [7, 8]. Conversely, ROS are not always considered as harmful metabolic byproducts; they play the role of intracellular signaling molecules when strictly regulated [9, 10]. In addition, at cellular levels, ROS contribute to complicated functions, such as blood pressure regulation [11], cognitive functions [12], and immune responses [13].
In this perspective, we review the well-known functions of ROS in human health and disease states. As evidence is accumulating for the various roles of ROS in many physiological and pathological processes, a comprehensive understanding and improved recognition of the fundamentals involved in ROS generation, regulation, and elimination will promote the search for preventive, protection, and therapeutic approaches associated with the antioxidant potential to reduce the redox stress.
Chemistry
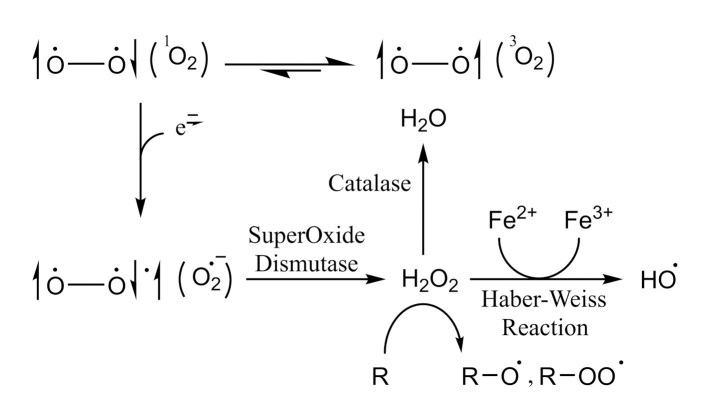
Except for oxygen, all known bi-atomic molecules, such as N2, Br2, Cl2, H2, and He2, have their electrons spin in an antiparallel manner, allowing the electrons from the two atoms to share a single orbital and, consequently, form a covalent bond [14]. Antiparallel spinning permits energy minimization according to the Pauli exclusion principle [15]. As each electron’s spin diminishes the energy of the other one, the total spin energy of electrons becomes zero in the orbital [16]. According to the molecular orbital theory, these paired electrons are in the singlet state of electron spin [17]. Nonetheless, and according to the restriction of the Pauli principle, if a bi-radical molecule has its electrons spin in a parallel rotation, then the energy of spinning cannot be dissipated by pairing in an orbital [18]. Each electron should remain oscillating separately as a radical leading to a total spin of one (2×(1/2)) [19]. The triplet state is the state of unpaired electrons [20]. Generally, this state is considered more energetic than the singlet state, except in oxygen, which has better stability in the unpaired state of electrons [21]. This discrepancy in oxygen behavior is responsible for the reactivity of ROS. The triplet state of the oxygen molecule (3O2) is more stable than the singlet state (1O2) [22]. Hence, the paired electrons of energetic oxygen are considered as strongly oxidative. The unpaired bi-radical state of oxygen dominates in normal conditions [23]. This form is quite stable compared with the singlet state because it is unlikely to find a species to interact with antiparallel electrons. However, one of these antiparallel electrons might be paired with an external electron transferred from an adjacent molecule to produce the superoxide radical (O·− 2) [24]. The superoxide radical does not easily cross cellular membranes, brief in action, local and momentary, but can be converted into a longer-lasting and membrane diffusible H2O2 by superoxide dismutase (SOD) [25].
In addition, the superoxide radical may interact with body metals through the Haber–Weiss reaction (Figure 1) [26, 27]. This reaction uses iron catalysis in generating hydroxyl radicals from superoxide and H2O2 [28, 29]. Other relevant ROS might be generated from peroxides, such as alkoxy radical (RO·) and peroxyl radical (ROO·) [30]. Interestingly, owing to its short life span (<1 ns) [31], mammalian cells have no defense mechanisms against the peroxide radical (OH·) except for the prevention of its formation in the first place [22]. On the other hand, nitric oxide (NO· or NO), which is considered as a second messenger ROS in the cytosol, is generated by a poorly understood mechanism involving nitrogen containing compounds, such as amino acids [32, 33]. The same procedure might be responsible for generating another group of oxidizing agents called reactive nitrogen species, such as peroxynitrite (ONOO−) and nitrogen dioxide (NO2) [32]. Interestingly, accumulating evidence suggested that the endoplasmic reticulum redox environment modulates the level of generated ROS, determining the outcome of proteins through redox signaling pathways [34].
Figure 1.
Chemistry of in vivo conversion of oxygen molecule to several ROS
Physiological Roles of ROS
ROS regulate aging
Aging is a multidimensional process that is gradual and complicated in nature. It involves the continuous deterioration of cells, tissues, organs, and the whole organism irreversibly [35]. Extensive attempts have been made to counter aging and limit its consequences. However, it remains to be ambiguous as its exact mechanism is yet to be elucidated [36]. Whereas some theories argue that aging is preprogrammed, others propose that it is entirely the sum of the effects related to the damage to proteins, lipids, and DNA [37].
In eukaryotes, aging is controlled by many factors, such as the “target of rapamycin”, a nutrient-perceiving protein kinase [38], and the “AMP-activated kinase”, a vastly conserved sensor of elevated adenosine monophosphate and adenosine diphosphate levels [39]. ROS delicately modulate the expression and activation of these two factors, in both physiological and pathological processes [40, 41].
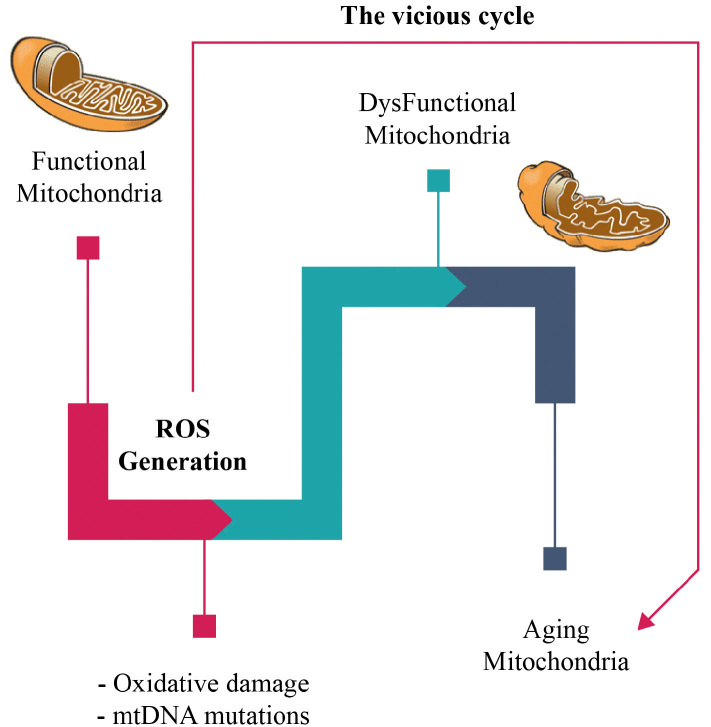
The “free radical theory of aging” was first suggested by Denham Harman in the 1950s to explain aging [42]. It states that free radicals, as byproducts of oxidative metabolism, initiate cumulative cellular impairment, leading to overall loss of cellular fitness over time. In the 1970s, the theory was extended into the Mitochondrial Free Radical Theory of Aging (MFRTA) [43]. The MFRTA proposes that aging is produced by the toxicity of ROS through a malicious cycle in which ROS injury to the mitochondrial elements precedes into the generation of more ROS (Figure 2) [44]. The MFRTA has been supported by the revelation of SODs and the remark that the mitochondria deliberately produce ROS. In addition, the generation of mitochondrial ROS was shown to be higher in isolated mitochondria from older animals [45]. More interestingly, oxidatively impaired protein, lipid, and DNA levels are highly correlated to aging [46]. Not surprisingly, a negative association between mitochondrial ROS production and lifetime can be perceived in several organisms [47].
Figure 2.
Schematic representation of the mitochondrial free radical theory
Yet, over the past two decades, paradoxical reports and reviews suggested that ROS are not the primary basis of aging. Therefore, it has been proposed that ROS harmonize the aging progression, mediating the stress response to age-dependent damage [48]. These studies demonstrated a lack of connection between the ROS production level and long life span in different species [49], detrimental contrary to positive outcomes on life expectancy from the administration of antioxidants in a variety of species from invertebrates to humans [50], failure to support the MFRTA by the outcomes of the suppression or overexpression of antioxidant activities in various genetically engineered organisms [46, 51], and the long-lived survival of mutants and species with elevated ROS production and extreme levels of oxidative damage [52–54].
In the light of these results and their impact on the MFRTA, the reliability and the validity of the theory have been the focus of recent reviews in the field [55, 56].
ROS augment the immune system
The immune system composes the organism’s defensive tools crucial to fight against environmental pathogens. There are two major types of immune responses: innate and acquired. They collaborate to provide the ultimate protection against foreign invaders [57]. Both types undergo delicate control of various regulatory mechanisms, including redox processes [58, 59].
Oxidative burst, one of the first lines of defense against environmental pathogens, is produced by activated phagocytes as part of the innate immune system [59]. As the phagocyte comes in contact with bacteria, it binds to the surface receptors, engulfs the bacteria, and traps the bacteria in a phagosome. The phagocyte then kills the microorganism using either oxygen-dependent or oxygen-independent intracellular killing [60]. In the oxygen-dependent pathway, consumption of oxygen by the phagocyte increases, leading to oxidative burst [61]. The resulting superoxide molecules are converted to H2O2 via SOD. In addition, superoxide and H2O2 react to provide hydroxyl radicals [62]. Furthermore, granules of neutrophils contain myeloperoxidase enzyme, creating a highly toxic compound, hypochlorite, by using H2O2 and chlorine [63].
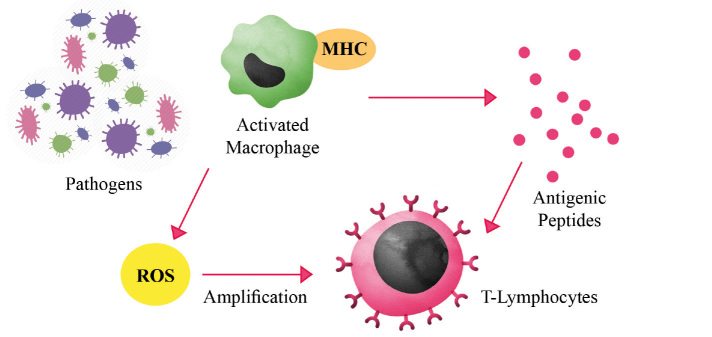
Phagocytosis and digestion of microorganisms by activated phagocytes are followed by antigen presentation [62]. Coupling of antigen to either major histocompatibility complex type I or type II results in recognition by CD8 T killer cells and CD4 T helper cells, respectively [64]. This allows for more specific and targeted response against the pathogen. ROS play a role in this specific response as they trigger the amplification of intracellular signal transduction cascades in the T lymphocytes (Figure 3) [58]. Another role of oxidative state in the immune system is the regulation of macrophages. Macrophages were shown to vary their release of prostaglandins, interleukin (IL)-6, and IL-12 according to the intracellular redox state [65]. A balance between reductive and oxidative macrophages is needed to control the ratio of type 1 and type 2 helper T cells [58].
Figure 3.
Functions of ROS in immunological response against environmental pathogens. Mass production of ROS by activated macrophages provides a first line of defense against environmental pathogens
In addition, the production of ROS was shown to regulate the initiation of inflammatory response. ROS are involved in the activation and assembly of inflammasomes [66, 67]. Several stimuli are responsible for the activation of NOD-like receptor 3 (NLRP3) inflammasome leading to ROS generation, most probably by the mitochondria [68, 69]. ROS in turn activate NLRP3 in a continuous cyclic pattern [70]. In chronic inflammatory state [58], excess ROS inhibit mitophagy, a special type of autophagy used to destroy malfunctioning mitochondria [71]. Thus, an increased level of ROS is generated activating more inflammasomes [69].
Genetic defect in a component of the NADPH oxidase multienzyme complex in the phagocytes makes it unable to produce ROS [72]. This results in chronic granulomatous disease. The lack of ROS renders the innate immune system inefficient [73]. Hence, patients suffer from recurrent and possibly life-threatening opportunistic infections associated with granuloma formation [74]. Surprisingly, the lack of ROS can also cause hyperinflammatory state manifested as autoimmune diseases [70].
ROS and cognitive function
In the context of the central nervous system (CNS), ROS have been always perceived as noxious species. However, recent studies reveal the various physiological roles of ROS in the cellular signaling mechanisms in the CNS. Neuronal communication forces a considerable metabolic strain in sustaining ionic gradients crucial for action potential firing and synaptic signaling. As it is well established that cellular metabolism functionally regulates excitatory neurotransmission [75–77], research was directed to the inhibitory signaling in the brain, which is mainly mediated by gamma-aminobutyric acid (GABA) receptors [78, 79].
High energy expenditure is observed in post-synaptic inhibitory signaling [80]. Thus, coupling of cellular metabolism and inhibitory signaling became an attractive zone to be investigated. Accardi et al. [81] examined the effect of mitochondrial ROS (mROS) on the strength of the inhibitory activity of GABA type A (GABAA) receptors. In short, post-synaptic GABAA signaling was studied in cerebellar stellate cells using antimycin A, a cytochrome c reductase inhibitor that disrupts the electron transport chain in the mitochondria, leading to the cessation of adenosine triphosphate (ATP) production [82]. In their study, the authors concluded that the generation of ROS in the mitochondria prompts the events that cause the strengthening of inhibitory synapses of cerebellar stellate cells [81]. Traditionally, ROS were commonly associated with neurodegenerative diseases [83]. However, evidence is emerging that mROS might be potential homeostatic signaling molecules that couple cellular metabolism to the strength of inhibitory transmission in the CNS.
ROS and fertility
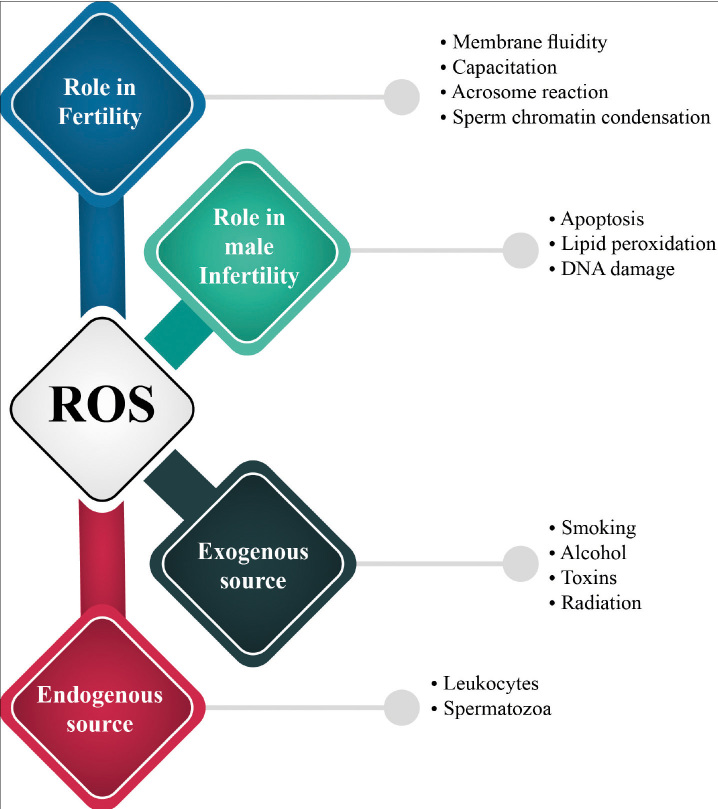
Sperm cells are considered as one of the key cellular supplies for ROS in the semen. Leukocytes are the other major sources of ROS in the semen, which, under normal conditions, yield up to 1000 times more ROS than the spermatozoa [84]. Tight control of the ROS levels during different stages of spermatogenesis and until fertilization appears to be crucial for healthy sperm parameters and successful conception [85]. From their primitive phases of development, male germ cells can produce limited quantities of ROS [86]. It has been shown that ROS are directly involved in the sperm chromatin condensation and regulate the count of germ cells by provocation of apoptosis or proliferation of spermatogonia [87, 88]. Additionally, in mature sperm, ROS considerably contribute to the capacitation by triggering a cascade of increased cyclic adenosine monophosphate production [85, 87, 89] that signals for tyrosine phosphorylation of fibrous sheath proteins, leading to the hyperactivation of the spermatozoa [90, 91]. Only hyperactivated sperms are capable of proceeding to the next stage of acrosome reaction [92]. Moreover, ROS were shown to increase membrane fluidity and the sperm–oocyte fusion rate through inhibition of phospholipase A2 deactivation, and thus enabling it to cleave to the membrane phospholipids resulting in increased membrane fluidity [93–95].
Interestingly, sperm cells with flawed morphology, mainly with cytoplasmic residues demonstrating their immaturity and diminished fertility potential, were found to deliver greater amounts of ROS than the spermatozoa with normal structure [96, 97]. In response to assorted intracellular and extracellular stimuli, the surplus production of ROS conquers the antioxidant defense system, resulting in deleterious effects on sperm functions [87, 96, 97]. Consequently, and as will be discussed later, overexposure to ROS leads to DNA-damaged spermatozoa, eventually jeopardizing either fertilization possibility or pregnancy rates.
In conclusion, accumulating evidence suggests that low and controlled production of ROS significantly modulates fertilization potential and signal transduction mechanisms [87, 89]. There are four critical steps that are involved in a successful fertilization process: sperm maturation, capacitation, hyperactivation, acrosome reaction, and sperm oocyte fusion [87, 98]. Interestingly, it has been established that ROS participate in almost all of these crucial phases [87, 99].
Pathological Roles of ROS
ROS and infertility
According to the World Health Organization (WHO), infertility is defined as the inability to achieve pregnancy for couples seeking conception within 12 months of regular sexual activity [100]. It can affect up to 20% of couples, and in approximately half of the cases, it is caused by the male factor [87]. Several parameters of healthy semen were established by the WHO, and lower reference values were assigned. These include volume, pH, total sperm number, sperm concentration, total sperm motility, morphology, and peroxidase-positive cells, among others [101].
OS is believed to play a foremost role in idiosyncratic male infertility [87]. In the United States, 30%–40% of infertile men were found to have high levels of ROS in their seminal plasma [102]. ROS in human seminal plasma can originate from endogenous and exogenous sources. Leukocytes (neutrophils and macrophages) and immature spermatozoa are the core endogenous sources of ROS (Figure 4). On the other hand, excessive alcohol intake and smoking along with other environmental factors, such as radiation and toxins, can elevate ROS levels in seminal plasma (Figure 4) [103–105].
Figure 4.
Roles of ROS in fertility and infertility
Infection and inflammation can stimulate peroxidase-positive leukocytes to produce up to 100 times higher levels of ROS [106, 107]. The WHO defines the presence of >1 million peroxidase-positive cells per milliliter of semen as leukocytospermia [108]. In this case, the extremely elevated levels of ROS in seminal plasma can lead to sperm damage. Several studies correlate decreased sperm function with abnormally high levels of ROS, IL-6, IL-8, and tumor necrosis factor [106, 109].
Spermatozoa were found to be greatly vulnerable to ROS, as they have cell membranes rich in polyunsaturated fatty acids (PUFAs). Lipid peroxidation (LPO) leads to the depletion of intracellular ATP, causing axonemal damage, low sperm viability, and increased defects in the midpiece of the sperms. Hence, decreased sperm motility is observed [103, 110]. Unfortunately, spermatozoa lack the repair enzymes needed to overcome the damage caused by ROS [111]. ROS are generated in the spermatozoa via two methods: either at the plasma membrane level by NADPH oxidase system or at the mitochondria level by NAD-dependent oxidoreductase reaction, which is the main source of ROS [87]. The majority of produced ROS in the spermatozoa are, which reacts with itself to produce H2O2, which in turn reacts with O2 in the presence of metals to generate the highly destructive hydroxyl radical [85, 112, 113]. The latter can disrupt the membrane fluidity of the sperms and result in loss of sperm function [87].
ROS levels in seminal plasma were associated with varicocele grade [114], which is defined as abnormal dilation of veins in the pampiniform plexus around the spermatic cord. It is found in approximately 40% of infertile males and considered to be the leading cause of male infertility [115].
On the other hand, an increased ROS production in seminal plasma can be attributed to exogenous sources. For example, radiation can disrupt the electron flow in the internal membranes of the cells, leading to organelle dysfunction [106]. Overall, the numbers, mobility, and vitality of sperm cells can be all decreased [116]. Toxins affect semen quality. Studies found that workers with frequent exposure to metals, such as cadmium, chromium, lead, mercury, and manganese, are more prone to low sperm quality, concentration, and volume [117]. Smoking manipulates the ratio between ROS and antioxidants in the semen. It causes impaired semen quality. The leukocyte concentration almost doubles, and ROS levels in semen increase by 107% [105]. Furthermore, the seminal plasma antioxidants, such as vitamins E and C, are reduced. Overall, prolonged tobacco smoking was found to increase DNA damage and apoptosis in sperms, leading to male infertility [87]. Finally, alcohol consumption can lead to a significant increase in ROS levels in seminal plasma, thus impairing semen quality [118, 119].
When levels of ROS exceed that of antioxidants, OS occurs and leads to several effects. First, the plasma membrane of the spermatozoa is highly susceptible to oxidation. It contains high amounts of PUFA. If these are oxidized by ROS, a self-catalytic cascade known as LPO is initiated [120]. Approximately 60% of PUFA can be lost, causing loss of membrane fluidity, increased permeability to ions, and deactivation of membrane-bound enzymes and receptors [121].
Chromatin is another target of ROS in the sperm nucleus, which is highly vulnerable to oxidation [122]. However, a complex compaction process occurs to protect DNA damage [123]. Nevertheless, in cases of poor or incomplete compaction, DNA becomes more vulnerable to OS, and mutations, cross-links, and rearrangements can happen [87]. If DNA damage is less, self-repair can be performed, and fertilization ability is regained [124]. Furthermore, the oocyte can repair sperm DNA damage. However, if the oocyte has poor repair machinery, the embryo may fail to develop or implant in the uterus [87].
OS and elevated levels of ROS were associated with impaired sperm parameters. The most important of which are sperm motility, concentration, and morphology. Reduced sperm motility was linked directly to H2O2 and ROS levels [105, 118, 125]. Another study found that radio frequency electromagnetic radiation emitted by mobile phones affects sperm motility, in addition to concentration and viability [126].
ROS were related with female infertility too [107]. Researchers found an association between ROS levels in the peritoneal fluid and the presence of idiopathic infertility [127]. A study by Attaran et al. [128] investigated the levels of ROS in the follicular fluid of women undergoing in vitro fertilization (IVF). They studied its effect on pregnancy outcome. They found that women who became pregnant had significantly lower levels of ROS. It might be hypothesized that a low concentration of ROS in follicular fluid may be a probable marker for predicting IVF success [128]. However, high levels of ROS in the follicular fluid could be deleterious to the embryo [107, 129, 130]. ROS levels play roles in fertilization and implantation as well [131].
Several studies investigated the effect of ROS on pregnancy complications. ROS due to reduced antioxidant capacity was correlated with an increased risk of spontaneous abortion in two retrospective studies [132]. In addition, it has been involved in the premature rupture of the fetal membranes [133]. It may also be associated with preeclampsia [134]. Smoking and alcohol intake negatively affect conception and birth outcomes. They affect both established pregnancy and time to pregnancy in couples seeking conception [135]. Interestingly, both tobacco smoke and alcohol intake have been found to significantly increase levels of ROS [136].
ROS in cancer
ROS signal transduction involves oxidation–reduction reactions, often causing reversible protein structure and function alterations that contrive a subsidiary cellular response. In cancer cells, the mitochondria exhibit accelerated metabolism demanding more ROS concentration, which amplifies the tumorigenic behavior and accelerates the piling up of additional mutations promoting metastasis.
As first proposed in the 1990s, studies brought the concept of ROS involvement in tumorigenesis. In particular, human SOD overexpression in murine sarcoma cells provided protection from radiation, but not chemically induced transformation, so as metastasis [137, 138]. In a recent study of the well-known highly malignant intrahepatic cholangiocarcinoma, mitochondrial dysfunction was found to trigger niche promoting cholangiocellular tumorigenesis and overgrowth. Additionally, antioxidant treatment has been shown to reduce cholangiocellular preneoplastic lesions [139].
Genetic modifications are considered to promote tumorigenesis either by acting as direct DNA mutagen [140–143] or by halting genomic stability through topoisomerase II activation [144]. Downregulation of the p53 tumor suppressor led to increased ROS production [145]. Interestingly, incubation of N-acetylcysteine (NAC) in drinking water prolonged survival and halted lymphoma formation in murine p53-deficient offspring [145]. The other ability of transcription factor in regulating intracellular redox environment is conceived as a crucial part of their tumor suppression potential. These comprise the Forkhead box O protein family, which upon trigging, leads to antioxidant enzyme stimulation, such as catalase and manganese superoxide dismutase [146]. Moreover, genetic ablation of breast cancer 1 (Brca1)-encoding gene increased ROS and mammary tumor formation frequency in p53-heterozygous mice [147]. Recently, it has been proposed that nuclear factor erythroid 2-related factor-2 mediates Brca1-dependent regulation of OS [148]. These findings support the concept of ROS-induced mutagenesis being a critical tumor origination and development promotion factor.
Contradictory evidence has brought to the surface the role of ROS in tumorigenesis. Even though ROS appear to promote cancer cell survival, angiogenesis, proliferation, and metastasis using human cell lines and mouse models [149], experimental studies have concluded that supplementation of NAC or vitamin E in the diet as antioxidants significantly accelerated development and mortality of tumor in mouse models of oncogenic Braf- and Kras-induced lung cancer [150].
Furthermore, folate dietary supplementation, a one-carbon cofactor of metabolism for NADPH production, has also appeared to promote breast cancer development and progression in murine experimental models [151, 152]. Finally, clinical trials have shown that antioxidant supplementation of NAC, vitamins A and E, or beta-carotene did not blunt the occurrence of lung, colorectal, head and neck, or prostate cancer. Instead, such treatments were observed to worsen the mortality and incidence of prostate and lung cancer [153, 154]. In congruence with previous findings, a study of melanoma cells that successfully metastasize underwent reversible metabolic changes during metastasis, making them more resistant to OS [155]. Furthermore, antioxidants have proven to stimulate distant metastasis for the same cells [155]. Thus, elevated intracellular reducing correspondents through metabolic variations enhance cancer cell survival, growth, and migration.
Conclusion
Reactive oxygen species (ROS) are typical byproducts of cellular metabolism, playing a role as secondary messengers and influencing different normal physiological functions of the body. Moreover, there is growing evidence supporting the role of ROS in numerous pathological conditions, that is, diseases. The paired character of ROS with their beneficial and detrimental characteristics indicates the sophistication of their specific roles at a biological compartment and the difficulties in attaining applicable procedures to treat ROS-related diseases. From principal science research to clinical trials, the biomedical scientific society has promptly progressed toward an improved interpretation of ROS-metabolizing systems and their impact on specific conditions.
Acknowledgements
Authors would like to thank the Deanship of Academic Research at the University of Jordan for financial support
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.K.B.; Design - S.K.B., M.G., M.A., A.I., H.A.A., R.M.B.; Supervision - S.K.B.; Data Collection and/or Processing - S.K.B., M.G., M.A., A.I., H.A.A., R.M.B.; Analysis and/or Interpretation - S.K.B., M.G., M.A., A.I., H.A.A., R.M.B.; Literature Search - S.K.B., M.G., M.A., A.I., H.A.A., R.M.B.; Writing Manuscript - S.K.B., M.G., M.A., A.I., H.A.A., R.M.B.; Critical Review - S.K.B., M.G., M.A., A.I., H.A.A., R.M.B.
Conflict of Interest: Authors have no conflicts of interest to declare.
Financial Disclosure: The authors declare that this study has received no financial support.
References
- 1.Lasségue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–90. doi: 10.1161/CIRCRESAHA.111.243972. https://doi.org/10.1161/CIRCRESAHA.111.243972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izyumov D, Domnina L, Nepryakhina O, et al. Mitochondria as source of reactive oxygen species under oxidative stress. Study with novel mitochondria-targeted antioxidants—the “Skulachev-ion” derivatives. Biochemistry (Mosc) 2010;75:123–9. doi: 10.1134/s000629791002001x. https://doi.org/10.1134/S000629791002001X [DOI] [PubMed] [Google Scholar]
- 3.Cubero FJ, Nieto N. Arachidonic acid stimulates TNFα production in Kupffer cells via a reactive oxygen species-pERK1/2-Egr1-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;303:G228–G39. doi: 10.1152/ajpgi.00465.2011. https://doi.org/10.1152/ajpgi.00465.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies BW, Kohanski MA, Simmons LA, Winkler JA, Collins JJ, Walker GC. Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Mol Cell. 2009;36:845–60. doi: 10.1016/j.molcel.2009.11.024. https://doi.org/10.1016/j.molcel.2009.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishikawa T, F Sato E, Choudhury T, et al. Effect of nitric oxide on the oxygen metabolism and growth of E. faecalis. J Clin Biochem Nutr. 2009;44:178. doi: 10.3164/jcbn.08-235. https://doi.org/10.3164/jcbn.08-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin C, Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. 2006;132:108–13. doi: 10.1111/j.1365-2141.2005.05834.x. https://doi.org/10.1111/j.1365-2141.2005.05834.x [DOI] [PubMed] [Google Scholar]
- 7.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. https://doi.org/10.1186/2046-2395-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/1245049. https://doi.org/10.1155/2016/1245049 1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–90. doi: 10.1016/j.cellsig.2012.01.008. https://doi.org/10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scandalios JG. The rise of ROS. Trends Biochem Sci. 2002;27:483–6. doi: 10.1016/s0968-0004(02)02170-9. https://doi.org/10.1016/S0968-0004(02)02170-9 [DOI] [PubMed] [Google Scholar]
- 11.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. https://doi.org/10.1097/00004872-200018060-00002 [DOI] [PubMed] [Google Scholar]
- 12.Qin B, Cartier L, Dubois-Dauphin M, Li B, Serrander L, Krause KH. A key role for the microglial NADPH oxidase in APP-dependent killing of neurons. Neurobiol Aging. 2006;27:1577–87. doi: 10.1016/j.neurobiolaging.2005.09.036. https://doi.org/10.1016/j.neurobiolaging.2005.09.036 [DOI] [PubMed] [Google Scholar]
- 13.Krause K-H, Bedard K, editors. NOX enzymes in immuno-inflammatory pathologies. Semin Immunopathol. 2008;30:193–4. doi: 10.1007/s00281-008-0127-2. https://doi.org/10.1007/s00281-008-0127-2 [DOI] [PubMed] [Google Scholar]
- 14.Breher F. Stretching bonds in main group element compounds-Borderlines between biradicals and closed-shell species. Coord Chem Rev. 2007;251:1007–43. https://doi.org/10.1016/j.ccr.2006.09.007 [Google Scholar]
- 15.Pines D. Solid state physics. Elsevier; 1955. Electron interaction in metals; pp. 367–450. [Google Scholar]
- 16.Wagner M, Merkt U, Chaplik A. Spin-singlet–spin-triplet oscillations in quantum dots. Physical Review B. 1992;45:1951. doi: 10.1103/physrevb.45.1951. https://doi.org/10.1103/PhysRevB.45.1951 [DOI] [PubMed] [Google Scholar]
- 17.Dougherty DA. Spin control in organic molecules. Acc Chem Res. 1991;24:88–94. https://doi.org/10.1021/ar00003a005 [Google Scholar]
- 18.Fletcher L, Hudson H. Impulsive phase flare energy transport by large-scale Alfvén waves and the electron acceleration problem. Astrophys J. 2008;675:1645. https://doi.org/10.1086/527044 [Google Scholar]
- 19.Ishida K, Mukuda H, Kitaoka Y, et al. Spin-triplet superconductivity in Sr2RuO4 identified by 17O Knight shift. Nature. 1998;396:658–60. https://doi.org/10.1038/25315 [Google Scholar]
- 20.Wasielewski MR, O’Neil MP, Lykke KR, Pellin MJ, Gruen DM. Triplet states of fullerenes C60 and C70. Electron paramagnetic resonance spectra, photophysics, and electronic structures. J Am Chem Soc. 1991;113:2774–6. https://doi.org/10.1021/ja00007a074 [Google Scholar]
- 21.Harvey JN, Aschi M, Schwarz H, Koch W. The singlet and triplet states of phenyl cation. A hybrid approach for locating minimum energy crossing points between non-interacting potential energy surfaces. Theor Chem Acc. 1998;99:95–9. https://doi.org/10.1007/s002140050309 [Google Scholar]
- 22.Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays. 2006;28:1091–101. doi: 10.1002/bies.20493. https://doi.org/10.1002/bies.20493 [DOI] [PubMed] [Google Scholar]
- 23.Raballand V, Benedikt J, Wunderlich J, Von Keudell A. Inactivation of Bacillus atrophaeus and of Aspergillus niger using beams of argon ions, of oxygen molecules and of oxygen atoms. J Phys D Appl Phys. 2008;41:115207. https://doi.org/10.1088/0022-3727/41/11/115207 [Google Scholar]
- 24.Kellogg EW, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975;250:8812–7. [PubMed] [Google Scholar]
- 25.Guzik TJ, West NE, Black E, et al. Vascular super-oxide production by NAD (P) H oxidase association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86:E85–90. doi: 10.1161/01.res.86.9.e85. https://doi.org/10.1161/01.RES.86.9.1008 [DOI] [PubMed] [Google Scholar]
- 26.Kładna A, Aboul-Enein HY, Kruk I, Lichszteld K, Michalska T. Scavenging of reactive oxygen species by some nonsteroidal anti-inflammatory drugs and fenofibrate. Biopolymers. 2006;82:99–105. doi: 10.1002/bip.20402. https://doi.org/10.1002/bip.20402 [DOI] [PubMed] [Google Scholar]
- 27.Kandaz M, Ertekin MV, Erdemci B, et al. The effects of zinc sulfate on the levels of some elements and oxidative stress occurring in lenses of rats exposed to total cranium radiotherapy. Eurasian J Med. 2009;41:110–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung CY, McCartney SJ, Anseth KS. Synthesis of polymerizable superoxide dismutase mimetics to reduce reactive oxygen species damage in transplanted biomedical devices. Adv Funct Mater. 2008;18:3119–26. https://doi.org/10.1002/adfm.200800566 [Google Scholar]
- 29.Wan J, Winn LM. In utero–initiated cancer: The role of reactive oxygen species. Birth Defects Res C Embryo Today. 2006;78:326–32. doi: 10.1002/bdrc.20080. https://doi.org/10.1002/bdrc.20080 [DOI] [PubMed] [Google Scholar]
- 30.Aboul-Enein HY, Kruk I, Kładna A, Lichszteld K, Michalska T. Scavenging effects of phenolic compounds on reactive oxygen species. Biopolymers. 2007;86:222–30. doi: 10.1002/bip.20725. https://doi.org/10.1002/bip.20725 [DOI] [PubMed] [Google Scholar]
- 31.Fantel AG. Reactive oxygen species in developmental toxicity: review and hypothesis. Teratology. 1996;53:196–217. doi: 10.1002/(SICI)1096-9926(199603)53:3<196::AID-TERA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Jimenez-Del-Rio M, Suarez-Cede-o G, Velez-Pardo C. Using paraquat to generate anion free radicals and hydrogen peroxide in in vitro: Antioxidant effect of vitamin E. Biochem Mol Biol Educ. 2010;38:104–9. doi: 10.1002/bmb.20349. https://doi.org/10.1002/bmb.20349 [DOI] [PubMed] [Google Scholar]
- 33.Yildirim S, Yildirim A, Dane S, Aliyev E, Yigitoglu R. Dose-dependent protective effect of L-carnitineon oxidative stress in the livers of hyperthyroid rats. Eurasian J Med. 2013;45:1–6. doi: 10.5152/eajm.2013.01. https://doi.org/10.5152/eajm.2013.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic Reticulum Stress and Associated ROS. Int J Mol Sci. 2016;17:327. doi: 10.3390/ijms17030327. https://doi.org/10.3390/ijms17030327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed A, Tollefsbol T. Telomeres and telomerase: basic science implications for aging. J Am Geriatr Soc. 2001;49:1105–9. doi: 10.1046/j.1532-5415.2001.49217.x. https://doi.org/10.1046/j.1532-5415.2001.49217.x [DOI] [PubMed] [Google Scholar]
- 36.Ortuño-Sahagún D, Pallàs M, Rojas-Mayorquín AE. Oxidative Stress in Aging: Advances in Proteomic Approaches. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/573208. 573208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin K. Modern Biological Theories of Aging. Aging Dis. 2010;1:72–4. [PMC free article] [PubMed] [Google Scholar]
- 38.Kapahi P, Chen D, Rogers AN, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–65. doi: 10.1016/j.cmet.2010.05.001. https://doi.org/10.1016/j.cmet.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–41. doi: 10.1016/j.arr.2011.12.005. https://doi.org/10.1016/j.arr.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 40.Park I, Hwang J, Kim Y, Ha J, Park O. Differential modulation of AMPK signaling pathways by low or high levels of exogenous reactive oxygen species in colon cancer cells. Ann N Y Acad Sci. 2006;1091:102–9. doi: 10.1196/annals.1378.059. https://doi.org/10.1196/annals.1378.059 [DOI] [PubMed] [Google Scholar]
- 41.Sandström ME, Zhang SJ, Bruton J, et al. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol. 2006;575:251–62. doi: 10.1113/jphysiol.2006.110601. https://doi.org/10.1113/jphysiol.2006.110601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. https://doi.org/10.1093/geronj/11.3.298 [DOI] [PubMed] [Google Scholar]
- 43.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–7. doi: 10.1111/j.1532-5415.1972.tb00787.x. https://doi.org/10.1111/j.1532-5415.1972.tb00787.x [DOI] [PubMed] [Google Scholar]
- 44.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. https://doi.org/10.1016/j.cell.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 45.Sohal RS, Sohal BH. Hydrogen peroxide release by mitochondria increases during aging. Mech Ageing Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-w. https://doi.org/10.1016/0047-6374(91)90034-W [DOI] [PubMed] [Google Scholar]
- 46.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. https://doi.org/10.1016/j.freeradbiomed.2007.03.034 [DOI] [PubMed] [Google Scholar]
- 47.Lambert AJ, Boysen HM, Buckingham JA, et al. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–18. doi: 10.1111/j.1474-9726.2007.00312.x. https://doi.org/10.1111/j.1474-9726.2007.00312.x [DOI] [PubMed] [Google Scholar]
- 48.Hekimi SLJ, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–76. doi: 10.1016/j.tcb.2011.06.008. https://doi.org/10.1016/j.tcb.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen JH, Hales CN, Ozanne SE. DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res. 2007;35:7417–28. doi: 10.1093/nar/gkm681. https://doi.org/10.1093/nar/gkm681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howes RM. The free radical fantasy. Ann N Y Acad Sci. 2006;1067:22–6. doi: 10.1196/annals.1354.004. https://doi.org/10.1196/annals.1354.004 [DOI] [PubMed] [Google Scholar]
- 51.Pérez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–5. doi: 10.1111/j.1474-9726.2008.00449.x. https://doi.org/10.1111/j.1474-9726.2008.00449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andziak B, O’Connor TP, Qi W, et al. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–71. doi: 10.1111/j.1474-9726.2006.00237.x. https://doi.org/10.1111/j.1474-9726.2006.00237.x [DOI] [PubMed] [Google Scholar]
- 53.Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/-mice. J Biol Chem. 2008;283:26217–27. doi: 10.1074/jbc.M803287200. https://doi.org/10.1074/jbc.M803287200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. https://doi.org/10.1371/journal.pbio.1000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Raamsdonk JM, Hekimi S. Reactive oxygen species and aging in Caenorhabditis elegans: causal or casual relationship? Antioxid Redox Signal. 2010;13:1911–53. doi: 10.1089/ars.2010.3215. https://doi.org/10.1089/ars.2010.3215 [DOI] [PubMed] [Google Scholar]
- 56.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–67. doi: 10.1016/j.molcel.2012.09.025. https://doi.org/10.1016/j.molcel.2012.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. https://doi.org/10.1038/nature06246 [DOI] [PubMed] [Google Scholar]
- 58.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. https://doi.org/10.1152/physrev.00018.2001 [DOI] [PubMed] [Google Scholar]
- 59.Alfadda AA, Sallam RM. Reactive Oxygen Species in Health and Disease. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/936486. 936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–32. doi: 10.1038/nrmicro1004. https://doi.org/10.1038/nrmicro1004 [DOI] [PubMed] [Google Scholar]
- 61.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. https://doi.org/10.1016/S0022-1759(99)00146-5 [DOI] [PubMed] [Google Scholar]
- 62.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–45. doi: 10.1182/blood-2007-12-077917. https://doi.org/10.1182/blood-2007-12-077917 [DOI] [PubMed] [Google Scholar]
- 63.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. https://doi.org/10.1189/jlb.1204697 [DOI] [PubMed] [Google Scholar]
- 64.Janeway CA, Travers P, Walport M, Shlomchik MJ. The major histocompatibility complex and its functions. New York: Garland Science; 2001. [Google Scholar]
- 65.Hamuro J, Murata Y, Suzuki M, Takatsuki F, Suga T. The triggering and healing of tumor stromal inflammatory reactions regulated by oxidative and reductive macrophages. Gann Monograph on Cancer Research. 1999;48:153–64. [Google Scholar]
- 66.Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011;21:558. doi: 10.1038/cr.2011.20. https://doi.org/10.1038/cr.2011.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci. 2012;69:2999–3013. doi: 10.1007/s00018-012-0962-0. https://doi.org/10.1007/s00018-012-0962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tschopp J. Mitochondria: sovereign of inflammation? Eur J Immunol. 2011;41:1196–202. doi: 10.1002/eji.201141436. https://doi.org/10.1002/eji.201141436 [DOI] [PubMed] [Google Scholar]
- 69.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5. doi: 10.1038/nature09663. https://doi.org/10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- 70.Brieger K, Schiavone S, Miller FJ, Jr, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659. doi: 10.4414/smw.2012.13659. https://doi.org/10.4414/smw.2012.13659 [DOI] [PubMed] [Google Scholar]
- 71.Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 2012;4:166. doi: 10.18632/aging.100444. https://doi.org/10.18632/aging.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vignais P. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–59. doi: 10.1007/s00018-002-8520-9. https://doi.org/10.1007/s00018-002-8520-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–84. doi: 10.1016/s0952-7915(03)00109-2. https://doi.org/10.1016/S0952-7915(03)00109-2 [DOI] [PubMed] [Google Scholar]
- 74.Mauch L, Lun A, O’Gorman MR, et al. Chronic granulomatous disease (CGD) and complete myeloperoxidase deficiency both yield strongly reduced dihydrorhodamine 123 test signals but can be easily discerned in routine testing for CGD. Clin Chem. 2007;53:890–6. doi: 10.1373/clinchem.2006.083444. https://doi.org/10.1373/clinchem.2006.083444 [DOI] [PubMed] [Google Scholar]
- 75.Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43. doi: 10.1038/nature09613. https://doi.org/10.1038/nature09613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci. 2005;6:841. doi: 10.1038/nrn1784. https://doi.org/10.1038/nrn1784 [DOI] [PubMed] [Google Scholar]
- 77.Allaman I, Bélanger M, Magistretti PJ. Astrocyte–neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. https://doi.org/10.1016/j.tins.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 78.Campagna-Slater V, Weaver DF. Molecular modelling of the GABA A ion channel protein. J Mol Graph Model. 2007;25:721–30. doi: 10.1016/j.jmgm.2006.06.001. https://doi.org/10.1016/j.jmgm.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 79.Bardaweel SK, Alzweiri M, Ishaqat AA. D-Serine in neurobiology: CNS neurotransmission and neuromodulation. Can J Neurol Sci. 2014;41:164–76. doi: 10.1017/s031716710001653x. https://doi.org/10.1017/S031716710001653X [DOI] [PubMed] [Google Scholar]
- 80.Howarth C, GPa, AD Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32:1222–32. doi: 10.1038/jcbfm.2012.35. https://doi.org/10.1038/jcbfm.2012.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Accardi MV, Daniels BA, Brown PM, Fritschy JM, Tyagarajan SK, Bowie D. Mitochondrial reactive oxygen species regulate the strength of inhibitory GABA-mediated synaptic transmission. Nat Commun. 2014;5:3168. doi: 10.1038/ncomms4168. https://doi.org/10.1038/ncomms4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith RAHR, Cochemé HM, Murphy MP. Mitochondrial pharmacology. Trends Pharmacol Sci. 2012;33:341–52. doi: 10.1016/j.tips.2012.03.010. https://doi.org/10.1016/j.tips.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 83.Jones QR, Warford J, Rupasinghe HV, Robertson GS. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol Sci. 2012;33:602–10. doi: 10.1016/j.tips.2012.08.002. https://doi.org/10.1016/j.tips.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 84.Plante M, De Lamirande E, Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil Steril. 1994;62:387–93. doi: 10.1016/s0015-0282(16)56895-2. https://doi.org/10.1016/S0015-0282(16)56895-2 [DOI] [PubMed] [Google Scholar]
- 85.Chen SJAJ, Duan YG, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013;288:191–9. doi: 10.1007/s00404-013-2801-4. https://doi.org/10.1007/s00404-013-2801-4 [DOI] [PubMed] [Google Scholar]
- 86.Fisher HM, Aitken RJ. Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J Exp Zool. 1997;277:390–400. doi: 10.1002/(sici)1097-010x(19970401)277:5<390::aid-jez5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 87.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. https://doi.org/10.5534/wjmh.2014.32.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aitken RJ. The human spermatozoon–a cell in crisis? J Reprod Fertil. 1999;115:1–7. doi: 10.1530/jrf.0.1150001. https://doi.org/10.1530/jrf.0.1150001 [DOI] [PubMed] [Google Scholar]
- 89.de Lamirande E, Lamothe G, Villemure M. Control of superoxide and nitric oxide formation during human sperm capacitation. Free Radic Biol Med. 2009;46:1420–7. doi: 10.1016/j.freeradbiomed.2009.02.022. https://doi.org/10.1016/j.freeradbiomed.2009.02.022 [DOI] [PubMed] [Google Scholar]
- 90.O’Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med. 2006;41:528–40. doi: 10.1016/j.freeradbiomed.2006.04.027. https://doi.org/10.1016/j.freeradbiomed.2006.04.027 [DOI] [PubMed] [Google Scholar]
- 91.de Lamirande E, O’Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta. 2008;1784:106–15. doi: 10.1016/j.bbapap.2007.08.024. https://doi.org/10.1016/j.bbapap.2007.08.024 [DOI] [PubMed] [Google Scholar]
- 92.Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14:647–57. doi: 10.1093/humupd/dmn029. https://doi.org/10.1093/humupd/dmn029 [DOI] [PubMed] [Google Scholar]
- 93.Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77:190–201. doi: 10.1095/biolreprod.107.060558. https://doi.org/10.1095/biolreprod.107.060558 [DOI] [PubMed] [Google Scholar]
- 94.Calamera JBM, Ollero M, Alvarez J, Doncel GF. Superoxide dismutase content and fatty acid composition in subsets of human spermatozoa from normozoospermic, asthenozoospermic, and polyzoospermic semen samples. Mol Reprod Dev. 2003;66:422–30. doi: 10.1002/mrd.10368. https://doi.org/10.1002/mrd.10368 [DOI] [PubMed] [Google Scholar]
- 95.Khosrowbeygi A, Zarghami N. Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins Leukot Essent Fatty Acids. 2007;77:117–21. doi: 10.1016/j.plefa.2007.08.003. https://doi.org/10.1016/j.plefa.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 96.Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, Aitken RJ. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl. 1996;17:276–87. [PubMed] [Google Scholar]
- 97.Aziz N, Saleh RA, Sharma RK, et al. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil Steril. 2004;81:349–54. doi: 10.1016/j.fertnstert.2003.06.026. https://doi.org/10.1016/j.fertnstert.2003.06.026 [DOI] [PubMed] [Google Scholar]
- 98.Sánchez R, Sepúlveda C, Risopatrón J, Villegas J, Giojalas LC. Human sperm chemotaxis depends on critical levels of reactive oxygen species. Fertil Steril. 2010;93:150–3. doi: 10.1016/j.fertnstert.2008.09.049. https://doi.org/10.1016/j.fertnstert.2008.09.049 [DOI] [PubMed] [Google Scholar]
- 99.Ford WC. Regulation of sperm function by reactive oxygen species. Hum Reprod Update. 2004;10:387–99. doi: 10.1093/humupd/dmh034. https://doi.org/10.1093/humupd/dmh034 [DOI] [PubMed] [Google Scholar]
- 100.Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66:60–7. doi: 10.5173/ceju.2013.01.art19. https://doi.org/10.5173/ceju.2013.01.art19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Safarinejad MR. Infertility among couples in a population-based study in Iran: prevalence and associated risk factors. Int J Androl. 2008;31:303–14. doi: 10.1111/j.1365-2605.2007.00764.x. https://doi.org/10.1111/j.1365-2605.2007.00764.x [DOI] [PubMed] [Google Scholar]
- 102.Lanzafame FM, La Vignera S, Vicari E, Calogero AE. Oxidative stress and medical antioxidant treatment in male infertility. Reprod Biomed Online. 2009;19:638–59. doi: 10.1016/j.rbmo.2009.09.014. https://doi.org/10.1016/j.rbmo.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 103.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26:1628–40. doi: 10.1093/humrep/der132. https://doi.org/10.1093/humrep/der132 [DOI] [PubMed] [Google Scholar]
- 104.Choudhary R, Chawala V, Soni N, Kumar J, Vyas R. Oxidative stress and role of antioxidants in male infertility. Pak J Physiol. 2010;6:54–9. [Google Scholar]
- 105.Saleh RA, Agarwal A, Sharma RK, Nelson DR, Thomas AJ. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril. 2002;78:491–9. doi: 10.1016/s0015-0282(02)03294-6. https://doi.org/10.1016/S0015-0282(02)03294-6 [DOI] [PubMed] [Google Scholar]
- 106.Lavranos G, Balla M, Tzortzopoulou A, Syriou V, Angelopoulou R. Investigating ROS sources in male infertility: a common end for numerous pathways. Reprod Toxicol. 2012;34:298–307. doi: 10.1016/j.reprotox.2012.06.007. https://doi.org/10.1016/j.reprotox.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 107.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–43. doi: 10.1016/s0015-0282(02)04948-8. https://doi.org/10.1016/S0015-0282(02)04948-8 [DOI] [PubMed] [Google Scholar]
- 108.World Health Organization, Department of Reproductive Health and Research. WHO laboratory manual for the examination and processing of human semen. 2010. p. 271. [Google Scholar]
- 109.Nandipati K, Pasqualotto F, Thomas A, Agarwal A. Relationship of interleukin-6 with semen characteristics and oxidative stress in vasectomy reversal patients. Andrologia. 2005;37:131–4. doi: 10.1111/j.1439-0272.2005.00668.x. https://doi.org/10.1111/j.1439-0272.2005.00668.x [DOI] [PubMed] [Google Scholar]
- 110.Bansal AK, Bilaspuri GS. Impacts of Oxidative Stress and Antioxidants on Semen Functions. Vet Med Int. 2011;2011:7. doi: 10.4061/2011/686137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saleh R, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002;23:737–52. [PubMed] [Google Scholar]
- 112.Hazout A, Menezo Y, Madelenat P, Yazbeck C, Selva J, Cohen-Bacrie P. [Causes and clinical implications of sperm DNA damages]. Gynecol Obstet Fertil. 2008;36:1109–17. doi: 10.1016/j.gyobfe.2008.07.017. https://doi.org/10.1016/j.gyobfe.2008.07.017 [DOI] [PubMed] [Google Scholar]
- 113.Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. 2001;8:851–62. doi: 10.2174/0929867013373039. https://doi.org/10.2174/0929867013373039 [DOI] [PubMed] [Google Scholar]
- 114.Shiraishi K, Matsuyama H, Takihara H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int J Urol. 2012;19:538–50. doi: 10.1111/j.1442-2042.2012.02982.x. https://doi.org/10.1111/j.1442-2042.2012.02982.x [DOI] [PubMed] [Google Scholar]
- 115.Will MASJ, Fode M, Sonksen J, Christman GM, Ohl D. The great debate: varicocele treatment and impact on fertility. Fertil Steril. 2011;95:841–52. doi: 10.1016/j.fertnstert.2011.01.002. https://doi.org/10.1016/j.fertnstert.2011.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Iuliis GN, Newey RJ, King BV, Aitken RJ. Mobile Phone Radiation Induces Reactive Oxygen Species Production and DNA Damage in Human Spermatozoa In Vitro. PLoS One. 2009;4:e6446. doi: 10.1371/journal.pone.0006446. https://doi.org/10.1371/journal.pone.0006446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jurasović J, Cvitković P, Pizent A, Čolak B, Telišman S. Semen quality and reproductive endocrine function with regard to blood cadmiumin Croatian male subjects. Biometals. 2004;17:735–43. doi: 10.1007/s10534-004-1689-7. https://doi.org/10.1007/s10534-004-1689-7 [DOI] [PubMed] [Google Scholar]
- 118.Agarwal A, Prabakaran SA. Mechanism, measurement and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43:963. [PubMed] [Google Scholar]
- 119.Saalu L. The incriminating role of reactive oxygen species in idiopathic male infertility: an evidence based evaluation. Pak J Biol Sci. 2010;13:413–22. doi: 10.3923/pjbs.2010.413.422. https://doi.org/10.3923/pjbs.2010.413.422 [DOI] [PubMed] [Google Scholar]
- 120.Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;1297:357–67. [PubMed] [Google Scholar]
- 121.Tremellen K. Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. https://doi.org/10.1093/humupd/dmn004 [DOI] [PubMed] [Google Scholar]
- 122.Zribi N, Chakroun NF, Elleuch H, et al. Sperm DNA fragmentation and oxidation are independent of malondialdheyde. Reprod Biol Endocrinol. 2011;9:47. doi: 10.1186/1477-7827-9-47. https://doi.org/10.1186/1477-7827-9-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schulte RT, Ohl DA, Sigman M, Smith GD. Sperm DNA damage in male infertility: etiologies, assays, and outcomes. J Assist Reprod Genet. 2010;27:3–12. doi: 10.1007/s10815-009-9359-x. https://doi.org/10.1007/s10815-009-9359-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. https://doi.org/10.1038/aja.2010.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Du Plessis S, McAllister D, Luu A, Savia J, Agarwal A, Lampiao F. Effects of H2O2 exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels. Andrologia. 2010;42:206–10. doi: 10.1111/j.1439-0272.2009.00980.x. https://doi.org/10.1111/j.1439-0272.2009.00980.x [DOI] [PubMed] [Google Scholar]
- 126.Vignera S, Condorelli RA, Vicari E, D’Agata R, Calogero AE. Effects of the exposure to mobile phones on male reproduction: a review of the literature. J Androl. 2012;33:350–6. doi: 10.2164/jandrol.111.014373. https://doi.org/10.2164/jandrol.111.014373 [DOI] [PubMed] [Google Scholar]
- 127.Wang Y, Sharma RK, Falcone T, Goldberg J, Agarwal A. Importance of reactive oxygen species in the peritoneal fluid of women with endometriosis or idiopathic infertility. Fertil Steril. 1997;68:826–30. doi: 10.1016/s0015-0282(97)00343-9. https://doi.org/10.1016/S0015-0282(97)00343-9 [DOI] [PubMed] [Google Scholar]
- 128.Attaran M, Pasqualotto E, Falcone T, et al. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 1999;45:314–20. [PubMed] [Google Scholar]
- 129.Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–89. doi: 10.1093/humupd/7.2.175. https://doi.org/10.1093/humupd/7.2.175 [DOI] [PubMed] [Google Scholar]
- 130.Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early embryo development. Reproduction. 2002;123:479–86. doi: 10.1530/rep.0.1230479. https://doi.org/10.1530/rep.0.1230479 [DOI] [PubMed] [Google Scholar]
- 131.Sharma RK, Agarwal A. Role of reactive oxygen species in gynecologic diseases. Reprod Med Biol. 2004;3:177–99. doi: 10.1111/j.1447-0578.2004.00068.x. https://doi.org/10.1111/j.1447-0578.2004.00068.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vural P, Akgül C, Yildirim A, Canbaz M. Antioxidant defence in recurrent abortion. Clin Chim Acta. 2000;295:169–77. doi: 10.1016/s0009-8981(99)00255-7. https://doi.org/10.1016/S0009-8981(99)00255-7 [DOI] [PubMed] [Google Scholar]
- 133.Woods JR, Plessinger MA, Miller RK. Vitamins C and E: missing links in preventing preterm premature rupture of membranes? Am J Obstet Gynecol. 2001;185:5–10. doi: 10.1067/mob.2001.115868. https://doi.org/10.1067/mob.2001.115868 [DOI] [PubMed] [Google Scholar]
- 134.Bilodeau JF, Hubel CA. Current concepts in the use of antioxidants for the treatment of preeclampsia. J Obstet Gynaecol Can. 2003;25:742–50. doi: 10.1016/s1701-2163(16)31003-9. https://doi.org/10.1016/S1701-2163(16)31003-9 [DOI] [PubMed] [Google Scholar]
- 135.Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol. 2009;21:219. doi: 10.1097/gco.0b013e32832924ba. https://doi.org/10.1097/GCO.0b013e32832924ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Augood C, Duckitt K, Templeton A. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13:1532–9. doi: 10.1093/humrep/13.6.1532. https://doi.org/10.1093/humrep/13.6.1532 [DOI] [PubMed] [Google Scholar]
- 137.Safford SE, Oberley TD, Urano M, St Clair DK. Suppression of fibrosarcoma metastasis by elevated expression of manganese superoxide dismutase. Cancer Res. 1994;54:4261–5. [PubMed] [Google Scholar]
- 138.St Clair DK, Wan XS, Oberley TD, Muse KE, St Clair WH. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol Carcinog. 1992;6:238–42. doi: 10.1002/mc.2940060404. https://doi.org/10.1002/mc.2940060404 [DOI] [PubMed] [Google Scholar]
- 139.Yuan D, Huang S, Berger E, et al. Kupffer Cell-Derived Tnf Triggers Cholangiocellular Tumorigenesis through JNK due to Chronic Mitochondrial Dysfunction and ROS. Cancer Cell. 2017;31:771–89. doi: 10.1016/j.ccell.2017.05.006. https://doi.org/10.1016/j.ccell.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang JQ, Li S, Domann FE, Buettner GR, Oberley LW. Superoxide generation in v-Ha-ras-transduced human keratinocyte HaCaT cells. Mol Carcinog. 1999;26:180–8. [PubMed] [Google Scholar]
- 141.Irani K, Xia Y, Zweier JL, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–52. doi: 10.1126/science.275.5306.1649. https://doi.org/10.1126/science.275.5306.1649 [DOI] [PubMed] [Google Scholar]
- 142.Sohn J, Rudolph J. Catalytic and chemical competence of regulation of cdc25 phosphatase by oxidation/reduction. Biochemistry. 2003;42:10060–70. doi: 10.1021/bi0345081. https://doi.org/10.1021/bi0345081 [DOI] [PubMed] [Google Scholar]
- 143.Ogrunc M, Di Micco R, Liontos M, et al. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014;21:998–1012. doi: 10.1038/cdd.2014.16. https://doi.org/10.1038/cdd.2014.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li TK, Chen AY, Yu C, Mao Y, Wang H, Liu LF. Activation of topoisomerase II-mediated excision of chromosomal DNA loops during oxidative stress. Genes Dev. 1999;13:1553–60. doi: 10.1101/gad.13.12.1553. https://doi.org/10.1101/gad.13.12.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–13. doi: 10.1038/nm1320. https://doi.org/10.1038/nm1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Greer EL, Brunet A. FOXO transcription factors in ageing and cancer. Acta Physiol (Oxf) 2008;192:19–28. doi: 10.1111/j.1748-1716.2007.01780.x. https://doi.org/10.1111/j.1748-1716.2007.01780.x [DOI] [PubMed] [Google Scholar]
- 147.Cao L, Xu X, Cao LL, et al. Absence of full-length Brca1 sensitizes mice to oxidative stress and carcinogen-induced tumorigenesis in the esophagus and forestomach. Carcinogenesis. 2007;28:1401–7. doi: 10.1093/carcin/bgm060. https://doi.org/10.1093/carcin/bgm060 [DOI] [PubMed] [Google Scholar]
- 148.Gorrini C, Baniasadi PS, Harris IS, et al. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J Exp Med. 2013;210:1529–44. doi: 10.1084/jem.20121337. https://doi.org/10.1084/jem.20121337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–21. doi: 10.1038/nrc3803. https://doi.org/10.1038/nrc3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221ra15. doi: 10.1126/scitranslmed.3007653. https://doi.org/10.1126/scitranslmed.3007653 [DOI] [PubMed] [Google Scholar]
- 151.Deghan Manshadi S, Ishiguro L, Sohn KJ, et al. Folic acid supplementation promotes mammary tumor progression in a rat model. PLoS One. 2014;9:e84635. doi: 10.1371/journal.pone.0084635. https://doi.org/10.1371/journal.pone.0084635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ebbing M, Bonaa KH, Nygard O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119–26. doi: 10.1001/jama.2009.1622. https://doi.org/10.1001/jama.2009.1622 [DOI] [PubMed] [Google Scholar]
- 153.Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96:1743–50. doi: 10.1093/jnci/djh320. https://doi.org/10.1093/jnci/djh320 [DOI] [PubMed] [Google Scholar]
- 154.Klein EA, Thompson IM, Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial(SELECT) JAMA. 2011;306:1549–56. doi: 10.1001/jama.2011.1437. https://doi.org/10.1001/jama.2011.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Piskounova E, Agathocleous M, Murphy MM, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–91. doi: 10.1038/nature15726. https://doi.org/10.1038/nature15726 [DOI] [PMC free article] [PubMed] [Google Scholar]