Abstract
Objective
In this study, we researched the availability of Echinococcus IgG ELISA in pulmonary hydatid cysts.
Materials and Methods
Between January 2008 and December 2015, 93 successive cases, which were studied in preoperative Echinococcus IgG and histopathologically found to have pulmonary hydatid cysts, were retrospectively analyzed. Age and sex of the cases and the cyst’s location, number, size, spread to other organs outside the lungs, and its condition as intact or ruptured were reviewed.
Results
Forty-seven (50.5%) patients were male and 46 (49.5%) patients were female; the mean age was 27.7±19.6 years. While in 56 (60.2%) cases, only lung cysts were detected, 32 (34.4%) cases presented with both lung and liver cysts. While lung cysts were single in 71 (76.3%), they were multiple in 22 (23.6%) cases (between 2 and 20 pieces). In 48 (51.6%) cases, cysts were in the right lung, and in 32 (34.4%) cases, they were in the left. In 13 (14%) cases, cysts presented in both the right and left lungs. The mean diameter of the pulmonary cysts was 6.4 cm (ranging from 2 to 19 cm). In 53 (57%) cases, hydatid cysts were ruptured, whereas in 40 (43%) cases, the cysts were intact. While general Echinococcus IgG was found to be positive in 53 (57%) cases, it was negative in 40 (43%) cases. There were 53 ruptured cases, and 48 (90.6%) of them were test-positive; however, the test was positive in only 5 (12.5%) out of the 40 cases where the cysts were intact (p<0.001). A statistically significant correlation has not been found between IgG and patient age, gender, cyst location, number of cysts, cyst diameter, and extrapulmonary involvement.
Conclusion
Our study demonstrated that the most important factor that affects the positivity of Echinococcus IgG is the rupture of cysts. When ruptured cysts become confusing, Echinococcus IgG can contribute toward a diagnosis.
Keywords: Hydatid cyst, lung, Echinococcus IgG
Introduction
A hydatid cyst is a tissue infestation caused by larval forms of E. granulosus. It may occur in any part of the body. Lung is the second most commonly affected organ [1]. Among serological tests, IgG ELISA and indirect hemagglutination assay (IHA) are two important tests often used in the diagnosis of hydatid cysts [2, 3].
Engvall and Perlmann developed the ELISA test in 1971, which is based on the principle of the conjunction of indirect enzyme labeled antihuman immunoglobulins on special antibodies, which are fastened to antigens molecules adsorbed by polystyrene plates and giving color by enzyme substrate in its composition [4]. The advantages of this method include sensitivity, reliability, easy application, yielding visible results by using an enzyme-marked chromogen substrate, and facilitating the study of a large amount of serum at the same time. Serological tests are very important for radiology in differential diagnoses for masses such as simple cysts, abscesses, and tumors, and for supporting diagnosis before the operation, healthy monitoring of recurrences after operation, and treatment success. Sensitivity and specificity of serological tests can vary depending on many factors such as type and preparation form of the antigen used, various positive criteria, cyst viability, cyst locations, and parasite strains [5].
Serology is the primary laboratory diagnosis for cystic echinococcosis, one of several such parasitic infections. No standard, highly sensitive, and specific serological test is available for the antibody detection of cystic echinococcosis. However, indirect hemagglutination test and ELISA are the most commonly used methods for the detection of anti-Echinococcus antibodies. It is reported that the most sensitive test is Echinococcus IgG ELISA in serological diagnosis in pulmonary hydatid cysts; IHA takes the second place. It has an important place for both diagnosing and monitoring the treatment. According to the preferred test method and other parameters, 10% patients with hepatic cysts and 40% with pulmonary cysts do not produce enough IgG antibodies to be detected in the serum and report false-negative results. For a reliable diagnosis of cystic echinococcosis and for the confirmation of a positive serological test result, imaging techniques should be used together with the clinical aspect [6–8].
There is no precise preoperative diagnostic laboratory test. The sensitivity of serological tests for pulmonary hydatid cysts is lower than that in the liver [2, 3]. In this study, we researched the availability of Echinococcus IgG in pulmonary hydatid cysts.
Materials and Methods
Between January 2008 and December 2015, 93 successive cases, which were studied in preoperative Echinococcus IgG and histopathologically found to have pulmonary hydatid cysts, were retrospectively analyzed. All the patients had physical examinations after the completion of medical histories. Complete blood counts, biochemical parameters, and coagulation tests were performed in all the cases. The postero-anterior (PA) chest radiograph and chest computed tomography (CT) scan were used for diagnosis in all the cases. Additionally, magnetic resonance imaging (MRI) was used in 13 cases and positron emission tomography (PET) was used in 4 cases. Age and sex of the cases and the cyst’s location, number, size, spread to other organs outside the lungs, and condition as intact or ruptured were reviewed.
All the patients were operated. Ninety-three patients underwent a total of 106 surgical interventions. One hundred patients underwent a thoracotomy, and 6 patients underwent video-assisted thoracoscopic surgery (VATS). Only 1 patient underwent lobectomy. Cystotomy and capitonnage were performed on all the other patients. Histopathologically, hydatid cysts were detected in all the patients. Patients were postoperatively treated with 2 cycles of 15 mg/kg/day albendazole (using the drug for 2 weeks and not using the drug 10 days for each cycle).
Echinococcus IgG detection method
By centrifuging the preoperatively taken patient blood under sterile conditions at 1500 rpm/10 min, serums were obtained and were stored at −30°C until studying. The Echinococcus IgG ELISA method was studied in accordance with the commercial kit’s (NovaLisa, NovaTec Immundiagnostica GMBH, Germany) test procedure, which is a method that allows the detection of E. granulosus IgG antibodies in the serum samples with the Alisei system (Radim, Italy).
Statistical Analysis
Continuous variables were expressed as mean and standard deviation values. Categorical variables were expressed as frequencies and percentages. Numerical variables were tested for normal distribution by means of a histogram plot. Student’s t-test was used to assess the statistical significance of the difference between the two means. Chi-squared test was used to examine the relationship between categorical variables. A significance level of p<0.05 was used in all the tests. All the statistical procedures were carried out using Statistical Package for the Social Sciences version 18 (SPSS Inc., Chicago, IL, USA) for Windows.
Results
Forty-seven (50.5%) patients were male and 46 (49.5%) patients were female; the mean age was 27.7±19.6 (between 2 and 83) years. The most common symptoms were cough (76.3%), fever (31.2%), and chest pain (29.0%). Twelve patients were asymptomatic and their cysts were incidentally detected on a chest radiograph.
In 56 (60.2%) cases, only lung cysts were observed; in 32 (34.4%) cases, lung and liver cysts; in 3 cases, lung, liver, and diaphragm cysts; in 1 case, lung, liver, and pericardial cysts; and in 1 case, lung and kidney cysts. While lung cysts were single in 71 (76.3%), they were multiple in 22 (23.6%) cases (between 2 and 20 pieces). In 48 (51.6%) cases, cysts were in the right lung, and in 32 (34.4%) cases, they were in the left. Thirteen (14%) cases had cysts in both the right and left lungs. The mean diameter of the pulmonary cysts was 6.4 cm (range from 2 to 19 cm). In 12 cases (12.9%), giant pulmonary hydatid cysts (>10 cm) were present. In 53 (57%) cases, hydatid cysts were ruptured, whereas in 40 (43%) cases, the cysts were intact.
While general Echinococcus IgG was found to be positive in 53 (57%) cases, it was negative in 40 (43%) cases. There were 53 ruptured cases, and 48 (90.6%) of them were test-positive; however, the test was positive in only 5 (12.5%) out of the 40 cases where the cysts were intact (p<0.001). A statistically significant correlation has not been found between Echinococcus IgG and patient’s age and gender and the cyst’s location, number, and diameter, as well as extrapulmonary involvement (Tables 1–3 and Figures 1, 2).
Table 1.
Number and percentage of the entire categorical data in the study
| Echinococcus IgG test results | Nature of cyst | Extrapulmonary involvement | Location of the cyst | Gender | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| (+) | (−) | Intact | Ruptured | Exist | Absent | Right | Left | Bilateral | Male | Female | |
| n | 53 | 40 | 40 | 53 | 37 | 56 | 48 | 32 | 13 | 47 | 46 |
| % | 57 | 43 | 43 | 57 | 39.8 | 60.2 | 51.6 | 34.4 | 14 | 50.5 | 49.5 |
n: number
Table 2.
Relationship between Echinococcus IgG test results with patient’s age, cyst diameter, and number of cysts in the lung
| Echinococcus IgG results | n | Mean | Std. Deviation | p | |
|---|---|---|---|---|---|
| Age | Positive | 53 | 28.87 | 19.731 | 0.509 |
| Negative | 40 | 26.13 | 19.643 | ||
| Cyst diameter (cm) | Positive | 53 | 6.30 | 2.431 | 0.609 |
| Negative | 40 | 6.59 | 3.076 | ||
| Number of cysts in the lung | Positive | 53 | 2.02 | 1.858 | 0.495 |
| Negative | 40 | 1.67 | 3.081 |
Table 3.
Relationship between Echinococcus IgG test results with patient’s age, nature and location of the cyst, and extrapulmonary involvement
| Echinococcus IgG results | p |
|---|---|
| Age | 0.071 |
| Nature of cyst | <0.000 |
| Location of the cyst | 0.545 |
| Extrapulmonary involvement | 0.053 |
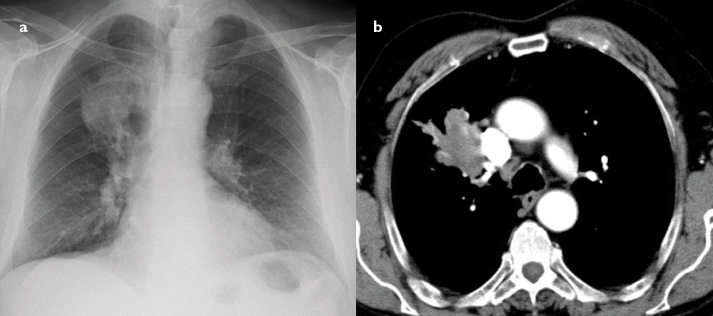
Figure 1. a, b.
Plain radiography shows a relatively heterogeneous density with a sharp margin in the lower left zone (a). Contrast enhancement axial CT scans show a heterogeneous hypodense peripheral lung mass with necrotic foci (b). Echinococcus IgG test was positive. Hemoptysis could not be controlled with medical treatment and progressed. Therefore, the patient was surgically treated by means of left lower lobectomy. It was diagnosed as hydatid disease by pathology.
Figure 2. a, b.
Plain radiography shows a round homogenous density with a sharp margin in the right perihilar area (a). Axial CT scans show a large (4×5 cm) heterogeneous hypodense lobulated central lung mass with necrotic foci. A mass located at the upper lobe anterior segment of the right lung is invading the adjacent superior vena cava (b). Echinococcus IgG test was positive. The patient underwent cystotomy and capitonnage operation.
Discussion
Echinococcus IgG was generally found positive in 57% pulmonary hydatid cyst patients in our study. It has been seen that cysts rupture is the only factor that affects Echinococcus IgG positivity. In addition, it is found that Echinococcus IgG positivity is very low in intact cysts; further, the number of cysts in the lung, cyst diameter, cyst location, extrapulmonary involvement, age, and gender do not affect Echinococcus IgG positivity.
On radiographs, intact cysts are seen as sharply demarcated and homogeneous round radiopacity. Ruptured cysts often become infected and show abscess formation. Complicated cysts may show meniscus sign or air crescent sign, cumbo sign or onion peel sign, water-lily sign, or consolidation adjacent to the cyst (ruptured cyst) on radiography. These findings are not pathognomonic for hydatid cysts. Fungus ball or hematoma in tuberculosis cavities and lung gangrene can exhibit similar characteristics. In addition, chronic infected cysts could not be distinguished from cystic pyogenic abscess. Therefore, the density of the fluid, appearance of inner wall, and daughter cysts can help in the diagnosis [9]. Therefore, ruptured hydatid cysts, particularly in adult patients, may be indistinguishable from lung cancer based on clinical and radiological findings. In addition, complicated pulmonary hydatid cysts can show increased FDG metabolic activity like lung cancer and could not be distinguished from malignancy [10].
Detectable immune responses have been associated with the physical location, integrity, and vitality of larval cysts. A cyst in the liver is more likely to cause antibody response than cysts in the lung; regardless of location, the sensitivity of serodiagnostic reactivity is less in patients with intact hyaline cysts. Cysts located in the lungs, brain, and spleen cause less antibody response. However, cysts located in the bone invoke detectable antibodies more regularly. The integrity of the cysts affects the stimulation of antibodies. Fissuration or rupture of a cyst causes abrupt stimulation of antibodies, whereas senescent, calcified, or dead cysts generally cause less antibody response [11]. Age can also affect the serodiagnostic reactivity. In particular, children aged 3–15 years may exhibit minimal antibody response [12].
The recovery of disease serological titrations gradually decreases, but may remain positive for years. The increase of titration serology may indicate the formation of new cysts [2, 13]. False positivity can occur in Taenia solium, Taenia saginata, Ascaris lumbricoides, Fasciola hepatica, Toxoplasma gondii, and plasmodium infections because of common antigens with Echinococcus species [8].
The best results in the diagnosis of pulmonary hydatid disease are obtained by the combination of serology and radiology. However, serology is more specific, but its sensitivity is lower than imaging [14]. Aydin et al. [15] have found 35.5% IgG positivity rate in their study, which have included 186 cases. In gender terms, they have not found statistically significant difference between ELISA IgG positivity and sex and age of the patient and the location of hydatid cysts. In our study, 57% Echinococcus IgG positivity in overall pulmonary hydatid cyst patients is similar to that in the literature. However, it is important that the test is highly positive, i.e., 90.6%, in ruptured cysts and has lower rates, i.e., 12.5%, in intact cysts. We did not find any study that evaluated IgG results in ruptured cysts and intact cysts in the literature. In general, positivity rates in lung hydatid cysts are emphasized. In our study, it seems that IgG antibody response against E. granulosus antigens significantly increased in ruptured cysts. We do not know the exact reason for this. However, when hydatid cysts are ruptured, interleukin is released and associated antibody formations can be seen [16].
A hydatid cyst is considered an endemic in our region. The majority of cases are clinically and radiologically diagnosed, and surgical treatments are performed. In our practice, serological tests are not required for the diagnosis of type-1 hydatid cyst. However, there can be diagnosing difficulties for complicated cases involving ruptured hydatid cysts. In our study, it is seen that Echinococcus IgG provides an important contribution toward diagnoses, particularly in ruptured and hard-to-diagnose cases.
In conclusion, our study demonstrated that the most important factor that affects the positivity of Echinococcus IgG test is cyst rupture. Despite the fact that a pulmonary hydatid cyst is benign pathology, its ruptured or complicated forms can radiologically mimic lung cancer. Clinical, radiological, and bronchoscopic findings of pulmonary hydatid cysts cannot be distinguished from lung cancer, particularly in elderly patients. In these cases, positive Echinococcus IgG test supports the diagnosis of hydatid cysts.
Footnotes
Ethics Committee Approval: Ethical Committee approval is not required for this type of study.
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Y.A.; Design - Y.A., B.A.; Supervision - A.E.; Resources - Y.A., B.A., A.K., A.B.U., M.H.U.; Materials - Y.A., A.K., M.H.U.; Data Collection and/or Processing - Y.A., B.A.; Analysis and/or Interpretation - Y.A., A.K., M.H.U.; Literature Search - Y.A., A.E.; Writing Manuscript - Y.A., B.A., A.K., A.B.U., M.H.U., A.E.; Critical Review - Y.A, A.E.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Aydin Y, Dostbil A, Araz O, et al. Pre-school children with hydatid lung disease. Acta Chir Belg. 2013;113:340–5. https://doi.org/10.1080/00015458.2013.11680941 [PubMed] [Google Scholar]
- 2.Eris FN, Akisu C, Aksoy U. Evaluation of two ELISA and two indirect hemagglutination tests for serodiagnosis of pulmonary hydatid disease. Korean J Parasitol. 2009;47:427–9. doi: 10.3347/kjp.2009.47.4.427. https://doi.org/10.3347/kjp.2009.47.4.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyhan YE, Babür C, Mungan M, Taylan Özkan A. Evaluation of cystic echinococcosis suspected patients applied to national parasitology reference laboratory of public health institution of Turkey between 2009–2013. Turkiye Parazitol Derg. 2015;39:17–21. doi: 10.5152/tpd.2015.3646. https://doi.org/10.5152/tpd.2015.3646 [DOI] [PubMed] [Google Scholar]
- 4.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–4. doi: 10.1016/0019-2791(71)90454-x. https://doi.org/10.1016/0019-2791(71)90454-X [DOI] [PubMed] [Google Scholar]
- 5.Karadağ A, Yanık K, Ünal N, Odabaşı H, Hökelek M. Evaluation of materials sent due to suspected cystic echinococcosis to the parasitology laboratory of Ondokuz Mayıs University Medical School between the Years 2005–2011. Turkiye Parazitol Derg. 2013;37:28–31. doi: 10.5152/tpd.2013.07. https://doi.org/10.5152/tpd.2013.07 [DOI] [PubMed] [Google Scholar]
- 6.Siracusano A, Buttari B, Delunardo F, et al. Critical points in the immunodiagnosis of cystic echinococcosis in humans. Parassitologia. 2004;46:401–3. [PubMed] [Google Scholar]
- 7.Eşgin M, Aktaş M, Coşkun S. The investigation of antibody presence in the sera of patients with a suspicion of cystic echinococcosis by using indirect hemaglutination test (IHA) Turkiye Parazitol Derg. 2007;31:283–7. [PubMed] [Google Scholar]
- 8.Wuestenberg J, Gruener B, Oeztuerk S, et al. Diagnostics in cystic echinococcosis: serology versus ultrasonography. Turk J Gastroenterol. 2014;25:398–404. doi: 10.5152/tjg.2014.7112. https://doi.org/10.5152/tjg.2014.7112 [DOI] [PubMed] [Google Scholar]
- 9.Erdem CZ, Erdem LO. Radiological characteristics of pulmonary hydatid disease in children: Less common radiological appearances. Eur J Radiol. 2003;45:123–8. doi: 10.1016/s0720-048x(02)00054-2. https://doi.org/10.1016/S0720-048X(02)00054-2 [DOI] [PubMed] [Google Scholar]
- 10.Long NM, Smith CS. Causes and imaging features of false positives and false negatives on F-PET/CT in oncologic imaging. Insights Imaging. 2011;6:679–98. doi: 10.1007/s13244-010-0062-3. https://doi.org/10.1007/s13244-010-0062-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lightowlers MW, Gottstein B. Echinococcosis/hydatidosis: antigens, immunological and molecular diagnosis. In: Thompson RCA, Lymbery AJ, editors. Echinococcus and hydatid disease. Wallingford, UK: CAB International; 1995. pp. 355–410. [Google Scholar]
- 12.Ben Nouir N, Nuñez S, Gianinazzi C, et al. Assessment of Echinococcus granulosus somatic protoscolex antigens for serological follow-up of young patients surgically treated for cystic echinococcosis. J Clin Microbiol. 2008;46:1631–40. doi: 10.1128/JCM.01689-07. https://doi.org/10.1128/JCM.01689-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okuş A, Sevinç B, Ay S, Karahan Ö, Eryılmaz MA, Er C. Relation between serology and grow-up time in atypically localized hydatic cysts. Turkiye Parazitol Derg. 2013;37:257–61. doi: 10.5152/tpd.2013.3056. https://doi.org/10.5152/tpd.2013.3056 [DOI] [PubMed] [Google Scholar]
- 14.Babba H, Messedi A, Masmoudi S, et al. Diagnosis of human hydatidosis: Comparison between imagery and six serologic techniques. Am J Trop Med Hyg. 1994;50:64–68. doi: 10.4269/ajtmh.1994.50.64. https://doi.org/10.4269/ajtmh.1994.50.64 [DOI] [PubMed] [Google Scholar]
- 15.Aydın M, Adıyaman G, Doğruman-Al F, Kuştimur S, Ozkan S. Determination of anti-echinococcus IgG antibodies by ELISA in patients with suspected hydatid cyst. Turkiye Parazitol Derg. 2012;36:61–4. doi: 10.5152/tpd.2012.16. https://doi.org/10.5152/tpd.2012.16 [DOI] [PubMed] [Google Scholar]
- 16.Chan SY, De Bruyne LA, Goodman RE, et al. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation. 1995;59:1155–61. https://doi.org/10.1097/00007890-199504270-00014 [PubMed] [Google Scholar]