Abstract
Three novel diterpene glycosides were isolated for the first time from the commercial extract of the leaves of Stevia rebaudiana, along with several known steviol glycosides, namely stevioside, rebaudiosides A-F, rubusoside and dulcoside A. The new compounds were identified as 13-[(2-O-β-d-glucopyranosyl-3-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy] ent-kaur-15-en-19-oic acid (1), 13-[(2-O-β-d-glucopyranosyl-3-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy]-16β-hydroxy-ent-kauran-19-oic acid (2) and 13-methyl-16-oxo-17-nor-ent-kauran-19-oic acid-β-d-glucopyranosyl ester (3) on the basis of extensive 2D NMR and MS spectroscopic data as well as chemical studies.
Keywords: Stevia rebaudiana, Compositae, Asteraceae, diterpenoid glycosides, spectral data, chemical studies
1. Introduction
Stevia rebaudiana (Bertoni) Bertoni is a perennial shrub belonging to the family of Asteraceae (Compositae) native to Brazil and Paraguay, but now grown commercially in a number of countries, particularly in Japan, Taiwan, Korea, Thailand and Indonesia [1-2]. Extracts of the leaves of S. rebaudiana have been used for decades to sweeten food and beverages in Japan, South America and China. The major constituents in the leaves of S. rebaudiana are the potently sweet diterpenoid glycosides stevioside, rebaudiosides A and D, and dulcoside A. These compounds, which are known as Stevia sweeteners, are the glycosides of the diterpene steviol, ent-13-hydroxykaur-16-en-19-oic acid [3]. As a part of our continuing research to discover natural sweeteners [4-5], we have collected commercial extracts of S. rebaudiana from various suppliers all over the World. This paper describes the isolation of three diterpene glycosides 1-3 isolated for the first time from the crude extract of the leaves of S. rebaudiana obtained from ShenZhen NII Natural Food Ingredient Co. Ltd, China and their identification based on extensive spectroscopic (NMR and MS) and chemical studies.
2. Results and Discussion
Purification of the commercial extract of S. rebaudiana obtained as Lot No: 20071003 from ShenZhen NII Natural Food Ingredient Co. Ltd, China, resulted in the isolation of three new diterpenoid glycosides 1-3, and the known steviol glycosides, stevioside, rebaudiosides A-F, rubusoside and dulcoside A (Figure 1). The structures of the known compounds were identified in comparison of their retention times with authentic standards using the HPLC-MS method as described previously [6] and the spectral data that were reported in the literature [7,8,9,10,11,12,13].
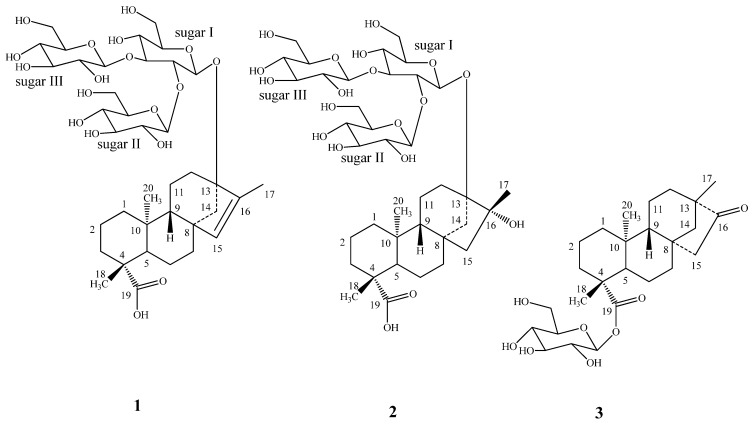
Figure 1.
Structures of 1-3.
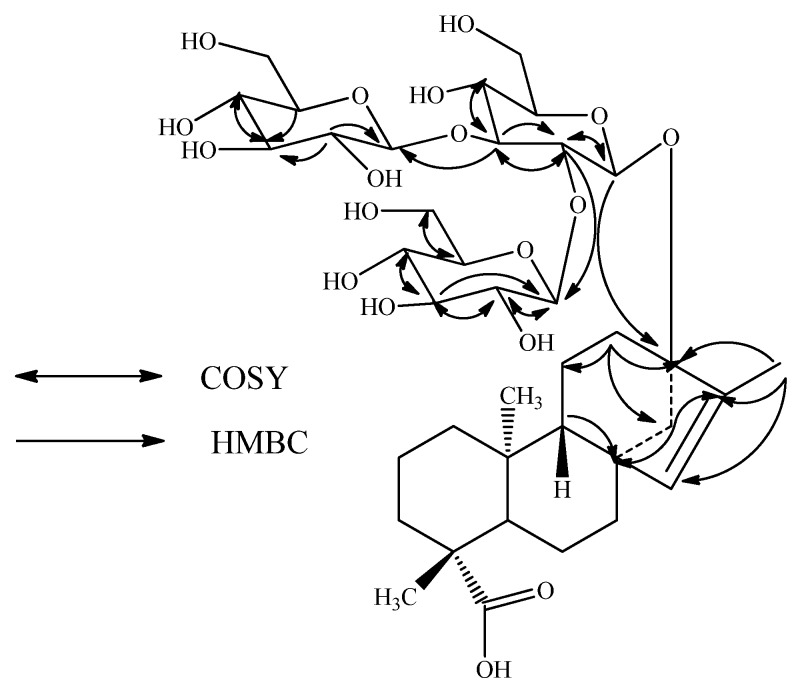
Compound 1 was obtained as a white powder and its molecular formula was assigned as C38H60O18 from its HRESI mass spectrum, which showed a (M + Na)+ ion at m/z 827.3661; this was supported by the 13C-NMR spectral data. The 1H-NMR spectrum of 1 (Table 1) showed the presence of three methyl singlets at δ 0.99, 1.17, and 1.72, eight methylene and two methine protons between δ 0.86-2.23, and a trisubstituted olefinic proton at δ 5.14, similar to ent-13-hydroxykaur-15-en-19-oic acid [15]. The basic skeleton of kaurane diterpenoids was supported by COSY (H-1/H-2; H-2/H-3; H-5/H-6; H-6/H-7; H-9/H-11; H-11/H-12) and HMBC (H-1/C-2, C-10; H-3/C-1, C-2, C-4, C-5, C-18, C-19; H-5/C-4, C-6, C-7, C-9, C-10, C-18, C-19, C-20; H-9/C-8, C-10, C-11, C-12; H-14/C-8, C-9, C-13, C-15, C-16 and H-17/C-13, C-15, C-16) correlations (Figure 2). In addition, the 1H-NMR spectrum of 1 also showed three anomeric protons as doublets at δ 4.66 (J = 7.8 Hz), 4.85 (J = 7.8 Hz), and 4.82 (J = 7.8 Hz) suggesting the presence in its structure of three sugar units, which was supported by the fragment ions observed at m/z 643, 481, and 319 in the ESI MS/MS spectrum corresponding to the successive loss of three hexose moieties from its [M + H]+ ion. Enzymatic hydrolysis of 1 furnished a compound which was found identical to ent-13-hydroxykaur-15-en-19-oic acid on the basis of NMR spectral data [13-14]. Acid hydrolysis of 1 afforded d-glucose that was identified by preparing the corresponding thiocarbamoyl-thiazolidine carboxylate derivative with L-cysteine methyl ester and O-tolyl isothiocyanate, and in comparison of its retention time with the standard sugars as described in the literature comparison [15].
Table 1.
1H-NMR chemical shift values for 1–3 isolated from Stevia rebaudiana recorded in CD3OD a.
| Position | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 0.86 (m, 1H), 1.86 (m, 1H) | 0.86 (m, 1H), 1.86 (m, 1H) | 0.97 (m, 1H), 1.71 (m, 1H) |
| 2 | 1.39 (m, 1H), 1.90 (m, 1H) | 1.42 (m, 1H), 1.94 (m, 1H) | 1.38 (m, 1H), 1.90 (m, 1H) |
| 3 | 1.02 (m, 1H), 2.10 (d, 11.9, 1H) | 1.00 (m, 1H), 2.12 (d, 12.6, 1H) | 1.08 (m, 1H), 2.18 (d, 13.7, 1H) |
| 5 | 1.08 (m, 1H) | 1.07 (m, 1H) | 1.22 (m, 1H) |
| 6 | 1.52 (m, 1H), 1.83 (m, 1H) | 1.82 (m, 1H), 1.97 (m, 1H) | 1.90 (m, 2H) |
| 7 | 1.50 (m, 1H), 1.58 (m, 1H) | 1.37 (m, 1H), 1.58 (m, 1H) | 1.49 (m, 1H), 1.68 (m, 1H) |
| 9 | 0.86 (m, 1H) | 0.93 (t, J = 7.8 Hz, 1H) | 1.24 (m, 1H) |
| 11 | 1.50 (m, 1H), 1.69 (m, 1H) | 1.65 (m, 1H), 1.85 (m, 1H) | 1.23 (m, 1H), 1.68 (m, 1H) |
| 12 | 1.63 (m, 1H), 1.67 (m, 1H) | 1.80 (m, 1H), 1.04 (m, 1H) | 1.43 (m, 1H), 1.53 (m, 1H) |
| 14 | 1.68 (m, 1H), 2.23 (d, 10.0, 1H) | 1.84 (m, 1H), 1.98 (d, 10.0, 1H) | 1.45 (m, 1H), 1.56 (m, 1H) |
| 15 | 5.14 (s, 1H) | 1.42 (d, 13.7, 1H), 1.60 (d, 13.7, 1H) | 1.84 (m, 1H), 2.63 (dd, 3.3, 18.9, 1H) |
| 17 | 1.72 (s, 3H) | 1.27 (s, 3H) | 0.81 (s, 3H) |
| 19 | 1.17 (s, 3H) | 1.16 (s, 3H) | 1.23 (s, 3H) |
| 20 | 0.99 (s, 3H) | 0.98 (s, 3H) | 0.94 (s, 3H) |
| 1′ | 4.66 (d, 7.8, 1H) | 4.66 (d, 8.2, 1H) | 5.39 (d, 8.2, 1H) |
| 2′ | 3.57 (m, 1H) | 3.56 (m, 1H) | 3.33 (dd, 7.5, 8.0, 1H) |
| 3′ | 3.77 (m, 1H) | 3.74 (m, 1H) | 3.43 (dd, 8.2, 8.9, 1H) |
| 4′ | 3.36 (m, 1H) | 3.34 (m, 1H) | 3.32 (dd, 8.4, 9.2, 1H) |
| 5′ | 3.28 (m, 1H) | 3.30 (m, 1H) | 3.36 (ddd, 8.4, 1.8, 6.9, 1H) |
| 6′ | 3.64 (m, 1H), 3.80 (m, 1H) | 3.67 (m, 1H), 3.88 (m, 1H) | 3.67 (dd, 2.1, 11.4, 1H), 3.80 (dd, 4.2, 12.2, 1H) |
| 1′′ | 4.85 (d, 7.8, 1H) | 4.89 (d, 7.8, 1H) | |
| 2′′ | 3.24 (m, 1H) | 3.25 (m, 1H) | |
| 3′′ | 3.32 (m, 1H) | 3.42 (m, 1H) | |
| 4′′ | 3.33 (m, 1H) | 3.32 (m, 1H) | |
| 5′′ | 3.33 (m, 1H) | 3.36 (m, 1H) | |
| 6′′ | 3.56 (m, 1H), 3.81 (m, 1H) | 3.60 (m, 1H), 3.80 (m, 1H) | |
| 1′′′ | 4.72 (d, 7.8, 1H) | 4.74 (d, 7.8, 1H) | |
| 2′′′ | 3.27 (m, 1H) | 3.25 (m, 1H) | |
| 3′′′ | 3.33 (m, 1H) | 3.32 (m, 1H) | |
| 4′′′ | 3.32 (m, 1H) | 3.32 (m, 1H) | |
| 5′′′ | 3.36 (m, 1H) | 3.36 (m, 1H) | |
| 6′′′ | 3.67 (m, 1H), 3.71 (m, 1H) | 3.65 (m, 1H), 3.80 (m, 1H) |
a assignments made on the basis of COSY, HSQC and HMBC correlations; b Chemical shift values are in δ (ppm); c Coupling constants are in Hz.
Figure 2.
Key COSY and HMBC correlations of 1.
The 1H- and 13C-NMR values for all the protons and carbons were assigned on the basis of COSY, HSQC and HMBC correlations (Figure 2) and are given in Table 1 and Table 2. A close comparison of the 1H- and 13C-NMR values of 1 with those of rebaudioside A suggested a 2,3-branched β-d-glucotriosyl substituent at C-13 as well as the absence of a glucosyl unit at C-19 and migration of the exocyclic double bond from C-16/C-17 to C-15/C-16. This was supported by the 13C-NMR values for the trisubstituted double bond between C-15 and C-16 which were observed at δ 136.1 and 142.6, respectively, and also from the HMBC correlations: H-12/C-9, C-11, C-13, C-14, C-16; H-15/C-8, C-9, C-14, C-16, C-17 and H-17/C-13, C-15, C-16. The large coupling constants observed for the three d-glucose anomeric protons suggested the β-orientation as reported for steviol glycosides [6,7,8,9,10,11,12,13]. Thus, structure of 1 was established as 13-[(2-O-β-d-glucopyranosyl-3-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy] ent-kaur-15-en-19-oic acid.
Table 2.
13C NMR chemical shift values for 1–3 isolated from Stevia rebaudiana recorded in CD3OD a.
| Position | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 40.9 | 42.0 | 39.7 |
| 2 | 19.1 | 19.0 | 19.2 |
| 3 | 37.9 | 38.0 | 38.1 |
| 4 | 43.5 | 43.5 | 43.8 |
| 5 | 56.5 | 55.3 | 57.4 |
| 6 | 20.9 | 21.2 | 20.8 |
| 7 | 39.6 | 39.3 | 41.3 |
| 8 | 48.3 | 41.8 | 48.6 |
| 9 | 48.0 | 55.2 | 54.0 |
| 10 | 39.8 | 40.6 | 37.8 |
| 11 | 20.6 | 20.2 | 21.2 |
| 12 | 30.4 | 37.2 | 38.7 |
| 13 | 91.4 | 86.8 | 94.4 |
| 14 | 48.1 | 43.4 | 54.7 |
| 15 | 136.1 | 56.9 | 48.1 |
| 16 | 142.6 | 76.8 | 224.0 |
| 17 | 11.4 | 21.8 | 12.9 |
| 18 | 180.4 | 180.3 | 176.9 |
| 19 | 29.0 | 28.3 | 27.8 |
| 20 | 15.3 | 15.1 | 19.0 |
| 1′ | 96.6 | 95.8 | 95.3 |
| 2′ | 80.1 | 79.8 | 73.8 |
| 3′ | 87.5 | 86.8 | 77.4 |
| 4′ | 70.2 | 69.8 | 69.9 |
| 5′ | 77.3 | 77.2 | 77.5 |
| 6′ | 61.4 | 61.5 | 61.2 |
| 1′′ | 103.1 | 103.0 | |
| 2′′ | 74.2 | 74.0 | |
| 3′′ | 77.3 | 77.4 | |
| 4′′ | 70.3 | 70.2 | |
| 5′′ | 77.1 | 77.2 | |
| 6′′ | 61.4 | 61.3 | |
| 1′′′ | 102.5 | 102.6 | |
| 2′′′ | 73.8 | 74.6 | |
| 3′′′ | 77.1 | 77.0 | |
| 4′′′ | 70.3 | 70.4 | |
| 5′′′ | 77.4 | 77.2 | |
| 6′′′ | 61.5 | 61.3 |
a assignments made on the basis of HSQC and HMBC correlations; b Chemical shift values are in δ (ppm).
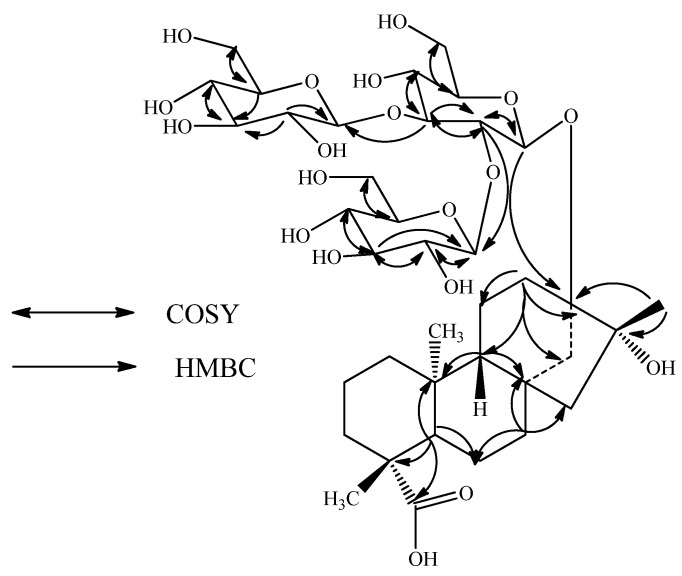
The molecular formula of compound 2 was determined to be C38H62O19 from the [M + H]+ ion at m/z 823, together with an [M + Na]+ adduct at m/z 845 in the positive ESI mass spectrum, which was confirmed by the HRMS data. The 1H-NMR spectrum of 2 (Table 1) showed the presence of three methyl singlets, nine methylene and two methine protons. In the absence of any unsaturated protons together with the appearance of a methyl group at δ 1.27 indicated that the structure of 2 should be similar to ent-13,16-dihydroxykauran-19-oic acid [13-14] which was supported by the δC 76.8 corresponding to the tertiary hydroxyl at C-16. The presence of ent-13,16-dihydroxykaurane skeleton in 2 was supported by the key HMBC correlations: H-12/C-11, C-13, C-14, C-16; H-14/C-8, C-12, C-13, C-15, C-16; H-17/C-13, C-15, C-16 (Figure 3). The ESI MS/MS spectrum of 2 showed the fragment ions at m/z 661, 499, and 337 corresponding to the successive loss of three hexose moieties from its [M + H]+ ion and this was supported by the three anomeric protons observed at δ 4.66, 4.74 and 4.89 in its 1H-NMR spectral data. Acid hydrolysis of 2 performed as in 1 afforded d-glucose confirming the three sugar molecules present in 2 as glucosyl units. The 1H- and 13C-NMR values for all the protons and carbons were assigned on the basis of COSY, HMQC and HMBC correlations (Table 1 and Table 2).
Figure 3.
Key COSY and HMBC correlation of 2.
Enzymatic hydrolysis of 2 furnished a compound which was found identical to ent-13, 16β-dihydroxykauran-19-oic acid on the basis of NMR spectral data comparisons [13-14]. A close comparison of the 1H- and 13C-NMR values of 2 with those of 1 suggested the presence of three glucose units attached as a 2,3-branched β-d-glucotriosyl substituent at C-13 hydroxyl of ent-13, 16β-dihydroxykauran-19-oic acid. The large coupling constants observed for the three anomeric protons of the glucose moieties at δ 4.66 (d, J = 8.2 Hz), 4.74 (d, J = 7.8 Hz), and 4.89 (d, J = 7.8 Hz), suggested the β-orientation as in 1. Considering the stereochemistry for the 16-hydroxyl group in 2 as β on the basis of the acid obtained by enzymatic hydrolysis, the structure was thus deduced to be13-[(2-O-β-d-glucopyranosyl-3-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy]-16β-hydroxy ent-kauran-19-oic acid.
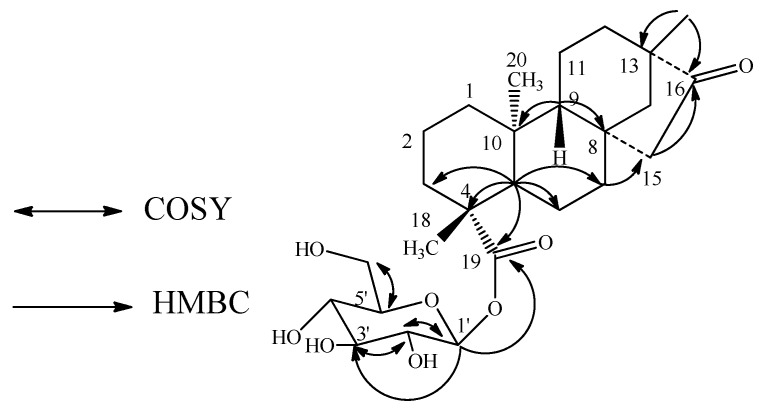
The molecular formula of compound 3 was deduced as C26H40O8 from the [M + H]+ ion observed at m/z 481, together with an [M + Na]+ adduct at m/z 503 in the positive ESI mass spectrum and this was supported by the HRMS data. The 1H-NMR spectrum of 3 (Table 1) also showed the presence of three methyl singlets at δ 0.81, 0.94 and 1.23; nine methylene and two methine protons, similar to 2. Enzymatic hydrolysis of 3 furnished a compound which was found identical to isosteviol on the basis of its NMR spectral data, identical to the reported values in the literature [14]. The presence of isosteviol skeleton was supported by the key HMBC correlations: H-12/C-9, C-11, C-13, C-14, C-16, C-17; H-14/C-8, C-12, C-13, C-15, C-16, C-17; H-15/C-8, C-14, C-16; H-17/C-13, C-14, C-16. The 1H NMR spectrum also showed an anomeric proton as doublet at δ 5.39 (J = 8.2 Hz), suggesting the presence of one sugar residue in its structure. Acid hydrolysis of 3 afforded d-glucose, confirming the sugar unit present in 3 as glucose as in 1 and 2. The placement of the glucose moiety in 3 was assigned to be at C-19 based on the key COSY and HMBC correlations shown in Figure 4. The 1H and 13C NMR values for all the protons and carbons were assigned on the basis of COSY, HMQC and HMBC correlations (Table 1 and Table 2). Based on the results from chemical and spectral studies, 3 was assigned as 13-methyl-16-oxo-17-nor-ent-kauran-19-oic acid-β-d-glucopyranosyl ester.
Figure 4.
Key COSY and HMBC correlations of 3.
This is the first report of the occurrence of the diterpene glycosides 1-3 from S. rebaudiana in nature, which were reported earlier as rebaudioside A derivative/degradation products [16-17]. Also, this is the first report of their complete 1H- and 13C-NMR spectral assignments that were made on the basis of spectral (COSY, HSQC, HMBC, and MS/MS) and chemical studies.
3. Experimental
3.1. General
Melting points were measured using a SRS Optimelt MPA 100 instrument and are uncorrected. Optical rotations were recorded using a Rudolph Autopol V at 25 °C and NMR spectra were acquired on Bruker Avance DRX 500 MHz and Varian Unity Plus 600 MHz instruments using standard pulse sequences. The spectra were referenced to the residual solvent signal (δH 3.30, δC 49.0 for CD3OD), chemical shifts are given in δ (ppm), and coupling constants are reported in Hz. MS and MS/MS data were generated with a Waters Premier Quadrupole Time-of-Flight (Q-Tof) mass spectrometer equipped with an electrospray ionization source operated in the positive-ion mode and Thermo Fisher Discovery OrbiTrap in the positive mode electrospray. Samples were diluted with water: acetonitrile (1:1) containing 0.1% formic acid and introduced via infusion using the onboard syringe pump. Preparative HPLC was performed on an Agilent 1100 system using a Phenomenex Prodigy ODS (3) column (250 × 21.2 mm, 5 μm).
3.2. Plant Material
A commercial Stevia extract with Lot No.: 20071003 was obtained from ShenZhen NII Natural Food Ingredient Co. Ltd, China. A voucher specimen is deposited at The Coca-Cola Company, No. VSPC-3166-153.
3.3. Isolation
Preliminary separation of the crude extract from the leaves of S. rebaudiana SRA 40, Lot No. 20071003 consisting of a mixture of various diterpenoid glycosides including 40-60% rebaudioside A was carried out via preparative HPLC on an Agilent 1100 system. The HPLC method A involved using Phenomenex Prodigy Column C18 (250 × 10 mm, 10 μm); UV Detection: 220 nm; Mobile Phase A: H2O (0.1% TFA); Mobile Phase B: Acetonitrile; Flow Rate: 5.0 mL/min; Injection volume: 400 μL. The gradient increased from 95: 5 (A: B) to 0: 100 (A: B) over 30 min, and remained at 0: 100 (A: B) for 5 min. Removal of the dominant rebaudioside A (tR 17.13 min) using the above HPLC method and subsequent collection of the peak eluting at tR 14.60 min over multiple runs furnished 1 (4.6 mg). The peak eluting at tR 12.86 min contained a mixture of 2 along with several other compounds. Since 2 was only partially resolved under the first HPLC method, a second round of purification was applied to isolate the pure compound. This was carried out using HPLC method B which was a modification to HPLC method A using the gradient of 80: 20 (A: B) to 70: 30 (A: B) applied for 40 min. The peak eluted at tR 32.4 min was confirmed to correspond to 2 (3 mg). Similar to 1, removal of rebaudioside A and combining the peak eluted at tR 18.2 min using HPLC method A over multiple runs and concentration under vacuum furnished 3 (4.2 mg). All the known compounds were identified using HPLC-MS in comparison with authentic standards as described previously [6] and the spectral data that were reported in the literature [7,8,9,10,11,12,13].
13-[(2-O-β-d-glucopyranosyl-3-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy] ent-kaur-15-en-19-oic acid (1). White powder, mp 254.5-254.7 °C, [α]D25 -49.56 (c 1.0, MeOH); 1H-NMR (500 MHz, CD3OD, δ ppm) and 13C-NMR (125 MHz, CD3OD, δ ppm) spectroscopic data see Table 1 and Table 2; HRMS (M + Na)+ m/z 827.3661 (calcd. for C38H60O18Na: 827.3677)
Enzymatic hydrolysis of 1. A solution of 1 (250 μg) was dissolved in 0.1 M sodium acetate buffer, pH 4.5 (2.5 mL) and crude pectinase from Aspergillus niger (50 μL, Sigma-Aldrich, P2736) was added. The mixture was stirred at 50 °C for 48 hr. The product precipitated out during the reaction and was filtered and then crystallized from methanol (MeOH). The resulting product was identical to ent-13-hydroxykaur-15-en-19-oic acid by comparison of their 1H-NMR spectral data [13-14].
Acid Hydrolysis of 1. Compound 1 (500 μg) was hydrolyzed with 0.5 M HCl (0.5 mL) for 1.5 h. After cooling, the mixture was passed through an Amberlite IRA400 column and the eluate was lyophilized. The residue was dissolved in pyridine (0.25 mL) and heated with L-cysteine methyl ester HCl (2.5 mg) at 60 °C for 1.5 h, and then O-tolyl isothiocyanate (12.5 μL) was added to the mixture and heated at 60 °C for an additional 1.5 h. The reaction mixture was analyzed by HPLC: column Phenomenex Luna C18, 150 × 4.6 mm (5 u); 25% acetonitrile-0.2% TFA water, 1 mL/min; UV detection at 250 nm. The sugar was identified as d-glucose (tR, 12.26 min) [authentic samples, d-glucose (tR, 12.35) and L-glucose (tR, 11.12 min)] [15].
13-[(2-O-β-d-glucopyranosyl-3-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy]-16β-hydroxy-ent-kauran-19-oic acid (2). White powder, mp 220.4-221.2 °C, [α]D25 -32.60 (c 1.0, MeOH); 1H-NMR (500 MHz, CD3OD, δ ppm) and 13C-NMR (125 MHz, CD3OD, δ ppm) spectroscopic data see Table 1 and Table 2; HRMS (M + Na)+ m/z 845.3765 (calcd. for C38H62O19Na: 845.3783)
Enzymatic hydrolysis of 2. Enzymatic hydrolysis of 2 (500 μg) as described above furnished ent-13, 16β-dihydroxykauran-19-oic acid which was confirmed on the basis of 1H NMR spectral data [13,14].
Acid Hydrolysis of 2. Hydrolysis of 2 (500 μg) as described above furnished d-glucose [15].
13-methyl-16-oxo-17-nor-ent-kauran-19-oic acid-β-d-glucopyranosyl ester (3). White powder, mp 172.5 °C, [α]D25 -55.83 (c 1.0, EtOH); 1H-NMR (500 MHz, CD3OD, δ ppm) and 13C-NMR (125 MHz, CD3OD, δ ppm) spectroscopic data see Table 1 and Table 2; HRMS (M + Na)+ m/z 503.2608 (calcd. for C26H40O8Na: 503.2621)
Enzymatic hydrolysis of 3. Enzymatic hydrolysis of 3 (500 μg) as described above furnished isosteviol which was confirmed on the basis of 1H-NMR spectral data [14].
Acid Hydrolysis of 3. Hydrolysis of 2 (500 μg) as described above furnished d-glucose [15].
4. Conclusions
Three diterpenoid glycosides 1-3 were isolated from a commercial extract of the leaves of S. rebaudiana obtained from ShenZhen NII Natural Food Ingredient Co. Ltd, China, along with the known steviol glycosides stevioside, rebaudiosides A-F, rubusoside and dulcoside A. The new compounds were identified as 13-[(2-O-β-d-glucopyranosyl-3-O-β-d-glucopyranosyl-β-d-gluco-pyranosyl)oxy] ent-kaur-15-en-19-oic acid, 13-[(2-O-β-d-glucopyranosyl-3-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy]-16β-hydroxy-ent-kauran-19-oic acid, 13-methyl-16-oxo-17-nor-ent-kauran-19-oic acid-β-d-glucopyranosyl ester on the basis of spectroscopic and chemical studies. This is the first report of the isolation of these three diterpene glycosides from S. rebaudiana in nature. The discovery of these compounds is an important addition in expanding our understanding of the diversity of the diterpenoid glycosides present in the S. rebaudiana.
Acknowledgements
We wish to thank ShenZhen NII Natural Food Ingredient Co. Ltd, China for providing the Stevia extract.
Footnotes
Sample Availability: Samples of the new diterpene glycoside 1-3, and the known steviol glycosides stevioside, rebaudiosides A-F, rubusoside and dulcoside A are available from the authors.
References and Notes
- 1.Mosettig E., Nes W.R. Stevioside. II. The structure of the aglucon. J. Org. Chem. 1955;20:884–899. doi: 10.1021/jo01125a013. [DOI] [Google Scholar]
- 2.Mosettig E., Beglinger U., Dolder F., Lichiti H., Quitt P., Waters J.A. The absolute configuration of steviol and isosteviol. J. Am. Chem. Soc. 1963;85:2305. doi: 10.1021/ja00898a025. [DOI] [Google Scholar]
- 3.Brandle J.E., Starrratt A.N., Gijen M. Stevia rebaudiana: Its agricultural, biological and chemical properties. Can. J. Plant Sci. 1998;78:527–536. doi: 10.4141/P97-114. [DOI] [Google Scholar]
- 4.Chaturvedula V.S.P., Rhea J., Milanowski D., Mocek U., Prakash I. Two minor diterpene glycosides from the leaves of Stevia rebaudiana. Nat. Prod. Commun. 2011;6:175–178. [PubMed] [Google Scholar]
- 5.Chaturvedula V.S.P., Clos J.F., Rhea J., Milanowski D., Mocek U., DuBois G.E., Prakash I. Minor diterpenoid glycosides from the leaves of Stevia rebaudiana. Phytochemistry Lett. 2011 doi: 10.1016/j.phytol.2011.01.002. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Clos J.F., DuBois G.E., Prakash I. Photostability of rebaudioside a and stevioside in beverages. J. Agric. Food Chem. 2008;56:8507–8513. doi: 10.1021/jf801343e. [DOI] [PubMed] [Google Scholar]
- 7.Kohda H., Kasai R., Yamsaki K., Murakami K., Tanaka O. New sweet diterpene glucosides from Stevia rebaudiana. Phytochemistry. 1976;15:981–983. doi: 10.1016/S0031-9422(00)84384-8. [DOI] [Google Scholar]
- 8.Kobayashi M., Horikawa S., Degrandi I.H., Ueno J., Mitsuhashi H. Dulcosides A and B, new diterpene glycosides from Stevia rebaudiana. Phytochemistry. 1977;16:1405–1408. doi: 10.1016/S0031-9422(00)88792-0. [DOI] [Google Scholar]
- 9.Starratt A.N., Kirby C.W., Pocs R., Brandle J.E. Rebaudioside F, a diterpene glycoside from Stevia rebaudiana. Phytochemistry. 2002;59:367–370. doi: 10.1016/S0031-9422(01)00416-2. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto I., Yamasaki K., Tanaka O. Application of 13C NMR spectroscopy to the chemistry of natural glycosides: rebaudioside C, a new sweet diterpene glycoside of Stevia rebaudiana. Chem. Pharm. Bull. 1977;25:844–846. doi: 10.1248/cpb.25.844. [DOI] [Google Scholar]
- 11.Sakamoto I., Yamasaki K., Tanaka O. Application of 13C NMR spectroscopy to chemistry of plant glycosides: rebaudiosides D and E, new sweet diterpene glucosides of Stevia rebaudiana Bertoni. Chem. Pharm. Bull. 1977;25:3437–3439. doi: 10.1248/cpb.25.3437. [DOI] [Google Scholar]
- 12.Ohta M., Sasa S., Inoue A., Tamai T., Fujita I., Morita K., Matsuura F. Characterization of novel steviol glycosides from leaves of Stevia rebaudiana Morita. J. Appl. Glycosci. 2010;57:199–209. doi: 10.5458/jag.57.199. [DOI] [Google Scholar]
- 13.Ohtani K., Aikawa Y., Kasai R., Chou W., Yamasaki K., Tanaka O. Minor diterpene glycosides from sweet leaves of Rubus suavissimus. Phytochemistry. 1992;31:1553–1559. doi: 10.1016/0031-9422(92)83105-8. [DOI] [Google Scholar]
- 14.Avent A.G., Hanson J.R., de Oliviera B.H. Hydrolysis of the diterpenoid glycoside, Stevioside. Phytochemistry. 1990;29:2712–2715. doi: 10.1016/0031-9422(90)85225-5. [DOI] [Google Scholar]
- 15.Tanaka T., Nakashima T., Ueda T., Tomii K., Kouno I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007;55:899–901. doi: 10.1248/cpb.55.899. [DOI] [PubMed] [Google Scholar]
- 16.Prakash I., DuBois G.E., San Miguel R.I., Clos J.F. Rebaudioside A derivatives and methods for Making. 108680 A2. PCT Int. Appl. 2009 66 pp.(or pp. 66.)
- 17.Lee T. Steviol glycoside isomers for use as sweeteners in food and beverage products. 2009038978 A2. PCT Int. Appl. 2009 58 pp. (or pp. 58.)