Abstract
A series of new N1-(coumarin-7-yl)amidrazones incorporating N-piperazines and related congeners were synthesized by reacting the hydrazonoyl chloride derived from 7-amino-4-methylcoumarin with the appropriate piperazines. The chemical structures of the newly prepared compounds were supported by elemental analyses, 1H-NMR, 13C-NMR, and ESI-HRMS spectral data. The antitumor activity of the newly synthesized compounds was evaluated. Among all the compounds tested, 7-{2-[1-(4-(1-benzyl-2-ethyl-4-nitro-1H-imidazol-5-yl)piperazin-1-yl)-2-oxopropylidene]hydrazinyl}-4-methyl-2H-chromen-2-one (3n) was the most potent against MCF-7 and K562 cells, with IC50 values of 20.2 and 9.3 μM, respectively.
Keywords: 7-aminocoumarins, N-(coumarin-7-yl)hydrazonoyl chloride, 1-piperazinyl amidrazones, nitrileimine, antitumor activity
1. Introduction
Due to their structural and therapeutic diversity as pharmaceutical agents, along with their commercial availability, piperazine derivatives continue to capture the attention of synthetic and medicinal chemists. Piperazine-based compounds have been employed as antibacterial, antidepressant, and antitumor drugs, and as α-adrenoceptor antagonists, CCR5 receptor antagonists, 5-HT7 receptor antagonists, and adenosine A2a receptor antagonists [1]. Several piperazine derivatives have reached the stage of clinical application; among the known drugs that are used to treat anxiety is a pyrimidinyl piperazinyl compound (buspirone, BuSpar®) [2], while a 3-chlorophenyl piperazinyl drug (trazodone, Desyrel®) is used as an antidepressant [3]. Besides, several publications have dealt with the synthesis and evaluation of thrombin inhibitors that incorporate an amidrazone functionality as a structural motif [4,5,6], and there is a report pertaining to the inactivation of lipoxygenase-1 from soybeans by open-chain and cyclic amidrazones [7].
On the other hand, coumarin derivatives have drawn considerable attention from researchers due to their role in natural and synthetic organic chemistry, and their interesting biological activities. Compounds which contain a coumarin nucleus were found to exhibit various biological activities such as anticoagulant and antithrombotic properties [8]. Some derivatives have shown molluscicidal, anthelmintic [9], hypnotic, and insecticidal [10] activity, while others have served as antifungal [11], anti-inflammatory [12] and antiviral agents, including against human immunodeficiency virus [13], and anticoagulant properties [14]. In addition, coumarins have also been used as additives in food and cosmetics [15], and in the preparation of optical brighteners, dispersed fluorescent and laser dyes [16].
In view of the widespread interest in the activity spectrum and profile of coumarins [17,18,19,20], and in continuation of our work on the synthesis of new compounds of pharmacological and biological interest [1,21,22,23,24], we describe herein the preparation and spectroscopic characterization of some new piperazinyl amidrazones containing coumarin moieties and evaluation of their antitumor activity.
2. Results and Discussion
2.1. Chemistry
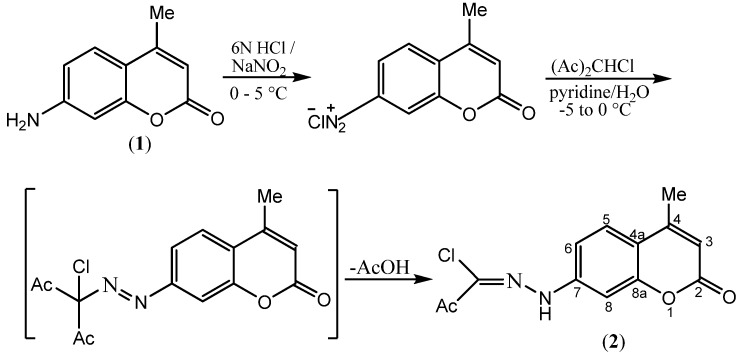
The hydrazonoyl chloride synthon 2 required in this study is prepared via direct coupling of 4-methylcoumarin-7-diazonium chloride with 3-chloropentane-2,4-dione in aqueous-ethanolic sodium acetate (Japp-Klingemann reaction) [25,26,27] (Scheme 1). An acidic solution of the former coumarin-7-diazonium chloride is freshly prepared by diazotization of 7-amino-4-methylcoumarin (suspended in 6N aq. HCl) which, in turn, is prepared from m-aminophenol according to a reported procedure [28,29].
Scheme 1.
Synthesis of N-(4-methyl-2-oxo-2H-chromen-7-yl)-2-oxopropanehydrazonoyl chloride (2).
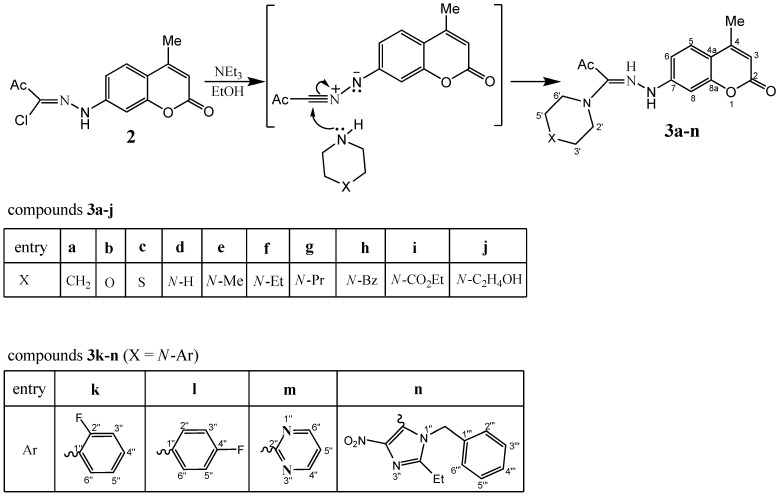
Piperazine, N-substituted piperazines and related cyclic secondary amine congeners, acting as nitrogen nucleophiles, are expected to add readily to N-(4-methylcoumarin-7-yl)nitrile imine (the reactive 1,3-dipolar species generated in situ from the corresponding hydrazonoyl chloride precursor 2 in the presence of triethylamine) to give the respective amidrazone adducts 3a-n (Scheme 2). This mode of nucleophilic addition reaction of various nucleophiles to 1,3-dipoles is well-documented [30,31,32,33,34,35,36,37,38] and several adducts related to 3 were obtained from the reaction of amines with hydrazonoyl chlorides.
Scheme 2.
Synthesis of 4-methyl-7-{2-[2-oxo-1-(substituted N-hexahydroazinyl)propylidene]-hydrazinyl}-2H-chromen-2-ones 3a-n.
The newly synthesized compounds 3a-n were characterized by elemental analyses, MS and NMR spectral data. These data, detailed in the experimental part, are consistent with the suggested structures. Thus, the mass spectra display the correct molecular ion peaks for which the measured high resolution (HRMS) data are in good agreement with the calculated values. DEPT and 2D (COSY, HMQC, HMBC) experiments showed correlations that helped in the 1H- and 13C-signal assignments to the different carbons and their attached, and/or neighboring hydrogens.
2.2. Antitumor Activity
The antitumor activities of compounds 3c-n were characterized by conducting cell viability assays using tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Cultures of MCF7 breast cancer cells and K562 human leukemia cells were treated first at one concentration of 50 μg/mL and the results are shown in Table 1.
Table 1.
Percentage cell survival of MCF-7 and K562 cells following 72 hours exposure to 50 μM f all compounds.
| Compound | MCF-7 % survival ± standard deviation | K562 % survival ± standard deviation |
|---|---|---|
| 3a | 81.8 ± 4.8 | 84.1 ± 3.8 |
| 3b | 81.7 ± 10.6 | 82.3 ± 4.4 |
| 3c | 89.8 ± 13.7 | 94.3 ± 6.3 |
| 3d | 86.4 ± 5.8 | 72.7 ± 1.5 |
| 3e | 89.7 ± 8.9 | 85.3 ± 3.0 |
| 3f | 91.4 ± 5.9 | 90.7 ± 2.3 |
| 3g | 81.5 ± 4.7 | 91.1 ± 3.5 |
| 3h | 80.6 ± 1.6 | 89.6 ± 3.1 |
| 3i | 46.8 ± 3.5 | 92.6 ± 12.2 |
| 3j | 85. 6 ± 2.8 | 86.5 ± 3.6 |
| 3k | 85.9 ± 6.0 | 96.4 ± 12.4 |
| 3l | 84.6 ± 11.0 | 93.4 ± 2.2 |
| 3m | 36.2 ± 5.7 | 101.3 ± 3.0 |
| 3n | 43. 3 ± 5.1 | 52.7 ± 6.1 |
Compounds 3i, 3m and 3n showed potential anti-MCF-7activity. These compounds were able to reduce the viability after 72 hours to less than 50%. With respect to K562 cells, only compound 3n showed considerable inhibition of cell proliferation. Furthermore, we explored the anti-tumor activity for 3i, 3m and 3n (compounds that showed potential activities) in two more cancer cells: breast cancer cell line ZR-75-1 and leukemia cell line HL60. We determined the IC50 values for compounds 3i, 3m and 3n on ZR-75-1 and HL60 additional cell lines, and on the MCF-7 and K526 cell lines as well (Table 2). Notably, compound 3n was the most potent against MCF-7 cells scoring an IC50 value of 20.2 μM and showing a very promising activity against the K562 cells with an IC50 of 9.3 μM (Table 2).
Table 2.
Effects of compounds 3i, 3m and 3n on MCF-7, ZR-75-1, K562 and HL60.
| Compound | IC50 MCF-7 (μM) | IC50 ZR-75-1 (μM) | IC50 K562 (μM) | IC50 HL60 (μM) |
|---|---|---|---|---|
| Doxorubicin | 0.31 ± 0.01 | 1.0 ± 0.01 | 3.54 ± 0.54 | 0.09 ± 0.005 |
| 3i | 47.8 ± 3.3 | >100 | >100 | >100 |
| 3m | 39.7 ± 1.7 | >100 | >100 | >100 |
| 3n | 20.2 ± 3.7 | >100 | 9.2 ± 2.8 | >100 |
From the structure-activity relationships point of view, the nature of substituents on the X position seems to play a critical role for the cytotoxic activity. For example, in case of MCF-7 breast cancer cells, compounds 3i, 3m, and 3n with the substituent N-CO2Et, N-(2-pyrimidyl), and N-(1-benzyl-2-ethyl-4-nitroimidazol-5-yl) appendages, respectively, exhibited cytotoxic IC50 values of 49, 38, and 20 μM. The shared characteristic feature between these three substituents, which are absent in all others, is the presence of the nucleophilic component next to position X. However, this belief cannot be generalized on the in anti-K562 cell activity, where compound 3n was the only active compound and no activity could be observed with compounds 3i and 3m. What make compound 3n active against K562 cells, giving a relatively low IC50 of 9 μM, may be linked with the bulky substituent that characterized this compound from all others tested. This bulky substituent on compound 3n may also be associated with the lowest IC50 value as exhibited by this compound against the MCF-7 cells.
3. Experimental
3.1. General
The following chemicals, used in this study, were purchased from Acros and were used as received: piperidine, morpholine, thiomorpholine, piperazine, N-alkylpiperazines, N-arylpiperazines, ethyl N-piperazinecarboxylate. 1-(1-Benzyl-2-ethyl-4-nitro-1H-imidazol-5-yl)piperazine was prepared according to a literature procedure [39]. Silica-gel for column chromatography was purchased from Macherey-Nagel GmbH & Co (Germany). Melting points (uncorrected) were determined on a Stuart scientific melting point apparatus in open capillary tubes. 1H- and 13C-NMR spectra were recorded on a 300 MHz spectrometer (Bruker DPX-300) with TMS as the internal standard. Chemical shifts are expressed in δ units; J-values for 1H-1H, 1H-F and 13C-F coupling constants are given in Hertz. High resolution mass spectra (HRMS) were acquired (in positive or negative mode) using electrospray ion trap (ESI) technique by collision-induced dissociation on a Bruker APEX-4 (7-Tesla) instrument. The samples were dissolved in acetonitrile, diluted in spray solution (methanol/water 1:1 v/v + 0.1% formic acid) and infused using a syringe pump with a flow rate of 2 µL/min. External calibration was conducted using arginine cluster in a mass range m/z 175-871.
3.2. 7-Amino-4-methylcoumarin (1)
This synthon, required in the present study, was prepared according to a literature procedure [28,29] which involves reaction of m-aminophenol with methoxycarbonyl chloride as the initial step; the resulting N-protected m-aminophenol underwent cyclocondensation upon reaction with ethyl acetoacetate and conc. sulpuric acid, followed by removal of the N-protecting group (via treatment with sodium hydroxide) to deliver the title compound 1; mp = 224-226 °C (lit. mp = 226-227 °C) [28,29].
3.3. N-(4-Methyl-2-oxo-2H-chromen-7-yl)-2-oxo-propanehydrazonoyl chloride (2)
Compound 1 (17.5 g, 0.10 mol) was dissolved in 17% aqueous hydrochloric acid (160 mL). To this solution was added drop-wise a solution of sodium nitrite (7.6 g, 0.11 mol) in water (15 mL) with efficient stirring at 0-5 °C. Stirring was continued for 20-30 min., and the resulting fresh cold 4-methyl-2-oxo-2H-chromene-7-diazonium chloride [also named as 7-(chlorodiazenyl)-4-methyl-coumarin] solution was poured onto cold solution (0 to -10 °C, ice-salt bath) of 3-chloropentan-2,4-dione (13.5 g, 0.1 mol) in ethanol/water (160 mL, 1:1 v/v) containing 30.0 g of sodium acetate with vigorous stirring. The resulting yellowish-colored mixture was further stirred until a solid precipitate was formed (5-10 min). The reaction mixture was then diluted with cold water (200 mL), the solid product was collected by suction filtration, washed several times with cold water, dried, and recrystallized from acetonitrile. Yield 25.4 g (91%); Mp = 271-274 °C. 1H-NMR (300 MHz, DMSO-d6): δ = 2.34 (d, J = 1.0 Hz, 3H, CH3-4), 2.46 (s, 3H, O=C-CH3), 6.17 (d, J =1.0 Hz, 1H, H-3), 7.27 (d, J = 1.8 Hz, 1H, H-8), 7.38 (dd, J = 8.7, 1.8 Hz, 1H, H-6), 7.67 (d, J = 8.7 Hz, 1H, H-5), 10.97 (s, 1H, N-H). 13C-NMR (75 MHz, DMSO-d6): δ = 18.6 (CH3-4), 26.0 (O=C-CH3), 101.9 (C-8), 111. 8 (C-6), 112.1 (C-3), 115.1 (C-4a), 127.1 (C-5), 146.0 (C-4), 146.2 (C-7), 153.8 (-C=N), 154.9 (C-8a), 160.5 (C-2), 188.6 (O=C-Me). Anal. Calcd. for C13H11ClN2O3 (278.69 g/mol): C, 56.03; H, 3.98; Cl, 12.72; N, 10.05. Found: C, 56.18; H, 4.05; Cl, 12.56; N, 9.93. ESI-HRMS m/z: Calcd. for C13H12ClN2O3 [M + H]+ 279.05364. Found: 279.05310.
3.4. General procedure for the preparation of 4-Methyl-7-{2-[2-oxo-1-(substituted N-hexahydro-azinyl)propylidene]hydrazinyl}-2H-chromen-2-ones 3a-n
To a cold suspension (0 to -10 °C) of compound 2 (0.5 g, 1.8 mmol) in ethanol (20 mL) was added, with stirring, a solution of the appropriate secondary amine (2.0 mmol) and triethylamine (3 mL) in ethanol (10 mL). Stirring was continued at 0-5 °C for 2-4 h, and then at ambient temperature for 10-12 h. The solvent was then removed under reduce pressure and the residue was treated with water (15 mL). The resulting crude solid product was collected by suction filtration, washed with water, dried and purified on preparative silica gel TLC plates.
4-Methyl-7-{2-[2-oxo-1-(piperidin-1-yl)propylidene]hydrazinyl}-2H-chromen-2-one (3a): Yield = 0.45 g (76.8%); Mp = 190-192 °C. 1H-NMR (300 MHz, DMSO-d6): δ = 1.50 (m, 2H, H2-4'), 1.63 (m, 4H, H2-3' + H2-5'), 2.34 (d, J = 1.0 Hz, 3H, CH3-4), 2.35 (s, 3H, O=C-CH3), 2.89 (m, 4H, H2-2' + H2-6' ), 6.11 (d, J = 1.0 Hz, 1H, H-3), 7.25 (d, J = 2.0 Hz, 1H, H-8), 7.34 (dd, J = 8.7, 2.0 Hz, 1H, H-6), 7.65 (d, J = 8.7 Hz, 1H, H-5), 9.83 (s, 1H, N-H). 13C-NMR (75 MHz, DMSO-d6): δ = 18.5 (CH3-4), 24.4 (C-4'), 25.7 (C-3'/C-5'), 26.6 (O=C-CH3), 49.1 (C-2'/C-6'), 100.9 (C-8), 111.6 (C-6), 111.1 (C-3), 113.8 (C-4a), 126.8 (C-5), 146.0 (C-4), 147.4 (C-7), 153.9 (-C=N), 155.1 (C-8a), 160.7 (C-2), 195.5 (O=C-Me). Anal. Calcd. for C18H21N3O3 (327.38 g/mol): C, 66.04; H, 6.47; N, 12.84. Found: C, 65.88; H, 6.41; N, 12.72. ESI-HRMS m/z: Calcd. for C18H20N3O3 [M − H]- 326.15047. Found: 326.14992.
4-Methyl-7-{2-[1-morpholino-2-oxopropylidene]hydrazinyl}-2H-chromen-2-one (3b): Yield = 0.41 g (69.2%); Mp = 213-215 °C. 1H-NMR (300 MHz, CDCl3): δ = 2.39 (d, J = 1.0 Hz, 3H, CH3-4), 2.42 (s, 3H, O=C-CH3), 3.07 (m, 4H, H2-2' + H2-6' ), 3.80 (m, 4H, H2-3' + H2-5' ), 6.12 (d, J = 1.0 Hz, 1H, H-3), 7.06 (dd, J = 8.7, 2.0 Hz, 1H, H-6), 7.19 (d, J = 2.0 Hz, 1H, H-8), 7.51 (d, J = 8.7 Hz, 1H, H-5), 9.32 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 18.5 (CH3-4), 26.6 (O=C-CH3), 66.5 (C-3'/C-5'), 48.2 (C-2'/C-6'), 101.2 (C-8), 111.3 (C-6), 111.7 (C-3), 113.9 (C-4a), 126.8 (C-5), 144.3 (C-4), 147.3 (C-7), 153.8 (-C=N), 155.1 (C-8a), 160.7 (C-2), 195.3 (O=C-Me). Anal. Calcd for C17H19N3O4 (329.35 g/mol): C, 62.00; H, 5.81; N, 12.76. Found: C, 62.14; H, 5.78; N, 12.65. ESI-HRMS m/z: Calcd for C17H18N3O4 [M − H]- 328.12973. Found: 328.12925.
4-Methyl-7-{2-[2-oxo-1-thiomorpholinopropylidene]hydrazinyl}-2H-chromen-2-one (3c): Yield = 0.45 g (72.4%); Mp = 236-238 °C. 1H-NMR (300 MHz, CDCl3): δ = 2.39 (d, J = 1.0 Hz, 3H, CH3-4), 2.41 (s, 3H, O=C-CH3), 2.75 (m, 4H, H2-3' + H2-5'), 3.26 (m, 4H, H2-2' + H2-6'), 6.14 (d, J = 1.0 Hz, 1H, H-3), 7.03 (dd, J = 8.7, 2.0 Hz, 1H, H-6), 7.18 (d, J = 2.0 Hz, 1H, H-8), 7.52 (d, J = 8.7 Hz, 1H, H-5), 9.12 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 18.7 (CH3-4), 25.9 (O=C-CH3), 28.5 (C-3'/C-5'), 50.3 (C-2'/C-6'), 101.6 (C-8), 110.7 (C-6), 112.3 (C-3), 114.7 (C-4a), 125.9 (C-5), 145.4 (C-4), 145.6 (C-7), 152.5 (-C=N), 155.3 (C-8a), 161.2 (C-2), 195.0 (O=C-Me). Anal. Calcd. for C17H19N3O3S (345.42 g/mol): C, 59.11; H, 5.54; N, 12.17. Found: C, 58.92; H, 5.51; N, 12.06. ESI-HRMS m/z: Calcd. for C17H19N3NaO3S [M + Na]+ 368.10448. Found: 368.10393.
4-Methyl-7-{2-[2-oxo-1-(piperazin-1-yl)propylidene]hydrazinyl}-2H-chromen-2-one (3d): Yield = 0.36 g (61%); Mp = 219-222 °C. 1H-NMR (300 MHz, CDCl3): δ = 1.65 (s, 1H, N(4')-H), 2.39 (d, J = 1.0 Hz, 3H, CH3-4), 2.41 (s, 3H, O=C-CH3), 2.98 (m, 4H, H2-2' + H2-6' ), 2.99 (m, 4H, H2-3' + H2-5' ), 6.13 (d, J = 1.0 Hz, 1H, H-3), 7.04 (dd, J = 8.7, 2.0 Hz, 1H, H-6), 7.16 (d, J = 2.0 Hz, 1H, H-8), 7.51 (d, J = 8.7 Hz, 1H, H-5), 9.29 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 18.7 (CH3-4), 26.0 (O=C-CH3), 46.6 (C-3'/C-5'), 49.3 (C-2'/C-6'), 101.5 (C-8), 110.7 (C-6), 112.1 (C-3), 114.5 (C-4a), 125.9 (C-5), 145.0 (C-4), 145.7 (C-7), 152.5 (-C=N), 155.3 (C-8a), 161.3 (C-2), 195.2 (O=C-Me). Anal. Calcd. for C17H20N4O3 (328.37 g/mol): C, 62.18; H, 6.14; N, 17.06. Found: C, 62.04; H, 6.08; N, 16.92. ESI-HRMS m/z: Calcd. for C17H21N4O3 [M + H]+ 329.16137. Found: 329.16083.
4-Methyl-7-{2-[1-(4-methylpiperazin-1-yl)-2-oxo-propylidene]hydrazinyl}-2H-chromen-2-one (3e): Yield = 0.50 g (81.2%); Mp = 182-183 °C. 1H-NMR (300 MHz, CDCl3): δ = 2.36 (d, J = 1.0 Hz, 3H, CH3-4), 2.40 (s, 3H, O=C-CH3), 2.43 (s, 3H, N-CH3), 2.63 (m, 4H, H2-3' + H2-5' ), 3.14 (m, 4H, H2-2' + H2-6' ), 6.12 (d, J = 1.0 Hz, 1H, H-3), 7.07 (dd, J = 8.7, 2.0 Hz, 1H, H-6), 7.16 (d, J = 2.0 Hz, 1H, H-8), 7.52 (d, J = 8.7 Hz, 1H, H-5), 9.23 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 18.7 (C-4), 25.9 (O=C-CH3), 46.4 (N-CH3), 47.9 (C-2'/C-6'), 55.7 (C-3'/C-5'), 101.4 (C-8), 110.7 (C-6), 112.0 (C-3), 114.4 (C-4a), 125.8 (C-5), 145.0 (C-4), 145.8 (C-7), 152.5 (-C=N), 155.2 (C-8a), 161.2 (C-2), 195.0 (O=C-Me). Anal. Calcd. for C18H22N4O3 (342.39 g/mol): C, 63.14; H, 6.48; N, 16.36. Found: C, 63.22; H, 6.51; N, 16.23. ESI-HRMS m/z: Calcd. for C18H23N4O3 [M + H]+ 343.17702. Found: 343.17652.
7-{2-[1-(4-Ethylpiperazin-1-yl)-2-oxopropylidene]hydrazinyl}4-methyl-2H-chromen-2-one (3f): Yield = 0.40 g (62.4%); Mp = 165-168 °C. 1H-NMR (300 MHz, CDCl3): δ = 1.12 (t, J = 7.2 Hz, 3H, CH3-CH2-), 2.50 (q, 2H, J = 7.2 Hz, CH3-CH2-N), 2.39 (d, J = 1.0 Hz, 3H, CH3-4), 2.42 (s, 3H, O=C-CH3), 2.58 (m, 4H, H2-3' + H2-5' ), 3.11 (m, 4H, H2-2' + H2-6' ), 6.12 (d, J = 1.0 Hz, 1H, H-3), 7.05 (dd, J = 8.7 Hz, 2.0 Hz, 1H, H-6), 7.15 (d, J = 2.0 Hz, 1H, H-8), 7.50 (d, J = 8.7 Hz, 1H, H-5), 9.19 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 12.0 (CH3-CH2), 18.7 (CH3-4), 26.0 (O=C-CH3), 47.8 (C-2'/C-6'), 53.3 (C-3'/C-5'), 52.5 (N-CH2), 101.4 (C-8), 110.7 (C-6), 112.0 (C-3), 114.5 (C-4a), 125.9 (C-5), 145.0 (C-4), 145.8 (C-7), 152.5 (-C=N), 155.3 (C-8a), 161.3 (C-2), 195.0 (O=C-Me). Anal. Calcd for C19H24N4O3 (356.42 g/mol): C, 64.03; H, 6.79; N, 15.72. Found: C, 63.86; H, 6.68; N, 15.54. ESI-HRMS m/z: Calcd for C19H25N4O3 [M + H]+ 357.19267. Found: 357.19212.
4-Methyl-7-{2-[2-oxo-1-(4-propylpiperazin-1-yl)propylidene]hydrazinyl}-2H-chromen-2-one (3g): Yield = 0.51g (76.5%); Mp = 178-181 °C. 1H-NMR (300 MHz, CDCl3): δ = 0.91 (t, J = 7.2 Hz, 3H, CH3CH2-), 1.53 (m, 2H, MeCH2-), 2.39 (d, J = 1.0 Hz, 3H, CH3-4), 2.41 (s, 3H, O=C-CH3), 2.48 (t, J = 7.2, 2H, N-CH2-), 2.56 (m, 4H, H2-3' + H2-5' ), 3.09 (m, 4H, H2-2' + H2-6' ), 6.11 (d, J = 1.0 Hz, 1H, H-3), 7.04 (dd, J = 8.7 Hz, 2.0 Hz, 1H, H-6), 7.15 (d, J = 2.0 Hz, 1H, H-8), 7.50 (d, J = 8.7 Hz, 1H, H-5), 9.20 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 12.0 (CH3CH2-), 18.8 (CH3-4), 20.0 (MeCH2-), 26.0 (O=C-CH3), 47.9 (C-2'/C-6'), 53.8 (C-3'/C-5'), 60.7 (N-CH2), 101.4 (C-8), 110.7 (C-6), 112.0 (C-3), 114.5 (C-4a), 125.8 (C-5), 145.0 (C-4), 145.8 (C-7), 152.6 (-C=N), 155.3 (C-8a), 161.4 (C-2), 195.2 (O=C-Me). Anal. Calcd. for C20H26N4O3 (370.45 g/mol): C, 64.84; H, 7.07; N, 15.12. Found: C, 64.63; H, 6.92; N, 15.05. ESI-HRMS m/z: Calcd. for C20H27N4O3 [M + H]+ 371.20832. Found: 371.20793.
7-{2-[1-(4-Benzylpiperazin-1-yl)-2-oxopropylidene]hydrazinyl}-4-methyl-2H-chromen-2-one (3h): Yield = 0.46 g (61.1%); Mp = 199-201 °C. 1H-NMR (300 MHz, CDCl3): δ = 2.39 (d, J = 1.0 Hz, 3H, CH3-4), 2.41 (s, 3H, O=C-CH3), 2.55 (m, 4H, H2-3' + H2-5' ), 3.07 (m, 4H, H2-2' + H2-6' ), 3.57 (s, 2H, N-CH2-), 6.12 (d, J = 1.0 Hz, 1H, H-3), 7.04 (dd, J = 8.7 Hz, 2.0 Hz, 1H, H-6), 7.15 (d, J = 2.0 Hz, 1H, H-8), 7.26-7.33 (m, 5H, H-2''/H-3''/H-4''/H-5''/H-6''), 7.50 (d, J = 8.7 Hz, 1H, H-5), 9.19 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 18.7 (CH3-4), 26.0 (O=C-CH3), 48.0 (C-2'/C-6'), 53.7 (C-3'/C-5'), 63.2 (N-CH2), 101.4 (C-8), 110.7 (C-6), 112.1 (C-3), 114.5 (C-4a), 125.9 (C-5), 127.2 (C-4''), 128.3 (C-2''/C-6''), 129.1 (C-3''/C-5''), 137.9 (C-1''), 145.2 (C-4), 145.8 (C-7), 152.3 (-C=N), 155.3 (C-8a), 161.3 (C-2), 195.1 (O=C-Me). Anal. Calcd. for C24H26N4O3 (418.49 g/mol): C, 68.88; H, 6.26; N, 13.39. Found: C, 68.96; H, 6.18; N, 13.24. ESI-HRMS m/z: Calcd. for C24H25N4O3 [M − H]- 417.19267. Found: 417.19212.
Ethyl 4-{1-[2-(4-methyl-2-oxo-2H-chromen-7-yl)hydrazono]-2-oxopropyl}piperazine-1-carboxylate (3i): Yield = 0.52 g (72.2%); Mp = 221-223 °C. 1H-NMR (300 MHz, CDCl3): δ = 1.26 (t, J = 7.1 Hz, 3H, CH3-CH2), 2.39 (d, J = 1.0 Hz, 3H, CH3-4), 2.42 (s, 3H, O=C-CH3), 3.02 (m, 4H, H2-3' + H2-5' ), 3.58 (m, 4H, H2-2' + H2-6' ), 4.15 (q, J = 7.1 Hz, 2H, MeCH2 ), 6.13 (d, J = 1.0 Hz, 1H, H-3), 7.05 (dd, J = 8.7 Hz, 2.0 Hz, 1H, H-6), 7.19 (d, J = 2.0 Hz, 1H, H-8), 7.50 (d, J = 8.7 Hz, 1H, H-5), 9.27 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 14.7 (CH3CH2-), 18.7 (CH3-4), 25.9 (O=C-CH3), 44.3 (C-2'/C-6'), 47.9 (C-3'/C-5'), 61.7 (MeCH2-), 101.6 (C-8), 110.8 (C-6), 112.3 (C-3), 114.7 (C-4a), 125.9 (C-5), 144.5 (C-4), 145.5 (C-7), 152.5 (-C=N), 155.3 (C-8a), 155.5 (O=C-N), 161.2 (C-2), 195.1 (O=C-Me). Anal. Calcd. for C20H24N4O5 (400.43 g/mol): C, 59.99; H, 6.04; N, 13.99. Found: C, 60.12; H, 6.01; N, 13.78. ESI-HRMS m/z: Calcd. for C20H23N4O5 [M − H]- 399.16684. Found 399.16630.
7-{2-[1-(4-(2-Hydroxyethyl)piperazin-1-yl)-2-oxopropylidene]hydrazinyl}-4-methyl-2H-chromen-2-one (3j): Yield = 0.46 g (68.1%); Mp = 159-162 °C. 1H-NMR (300 MHz, CDCl3): δ = 2.39 (d, J = 1.0 Hz, 3H, CH3-4), 2.42 (s, 3H, O=C-CH3), 2.61 (t, J = 5.2 Hz, 2H, -NCH2CH2OH), 2.76 (m, 4H, H2-3' + H2-5'), 3.09 (m, 4H, H2-2' + H2-6'), 3.63 (t, J = 5.2 Hz, 2H, -NCH2CH2OH), 6.13 (d, J = 1.0 Hz, 1H, H-3), 7.05 (dd, J = 8.7, 2.0 Hz, 1H, H-6), 7.15 (d, J = 2.0 Hz, 1H, H-8), 7.51 (d, J = 8.7 Hz, 1H, H-5), 9.20 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 18.7 (CH3-4), 25.9 (O=C-CH3), 48.0 (C-2'/C-6'), 53.5 (C-3'/C-5'), 57.7 (-NCH2CH2OH), 59.5 (-NCH2CH2OH), 101.6 (C-8), 110.7 (C-6), 112.2 (C-3), 114.6 (C-4a), 125.9 (C-5), 144.9 (C-4), 145.7 (C-7), 152.5 (-C=N), 155.3 (C-8a), 161.3 (C-2), 195.2 (O=C-Me). Anal. Calcd. for C19H24N4O4 (372.42 g/mol): C, 61.28; H, 6.50; N, 15.04. Found: C, 61.02; H, 6.41; N, 14.88. ESI-HRMS m/z: Calcd. for C19H25N4O4 [M + H]+ 373.18758. Found: 373.18707.
7-{2-[1-(4-(2-Fluorophenyl)piperazin-1-yl) -2-oxopropylidene]hydrazinyl}-4-methyl-2H-chromen-2-one (3k): Yield = 0.42 g (55.3%); Mp = 256-258 °C. 1H-NMR (300 MHz, CDCl3): δ = 2.36 (d, J = 1.0 Hz, 3H, CH3-4), 2.44 (s, 3H, O=C-CH3), 3.21 (m, 4H, H2-3' + H2-5' ), 3.24 (m, 4H, H2-2' + H2-6' ), 6.13 (d, J = 1.0 Hz, 1H, H-3), 6.95-7.00 (m, 4H, H-3'' + H-4'' + H-5'' + H-6''), 7.05 (dd, J = 8.7 Hz, 2.1 Hz, 1H, H-6), 7.18 (d, J = 2.1 Hz, 1H, H-8), 7.51 (d, J = 8.7 Hz, 1H, H-5), 9.25 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 18.7 (CH3-4), 26.0 (O=C-CH3), 48.2 (C-2'/C-6'), 51.3 (d, J = 3.2 Hz, C-3'/ C-5'), 101.5 (C-8), 110.7 (C-6), 112.2 (C-3), 114.6 (C-4a), 116.3 (d, 2JC-F = 20.6 Hz, C-3''), 119.2 (d, 4JC-F = 2.7 Hz, C-5''), 122.9 (d, 3JC-F = 7.9 Hz, C-4''), 124.4 (d, 3JC-F = 3.5 Hz, C-6''), 125.9 (C-5), 139.5 (d, 2JC-F = 19.7 Hz, C-1''), 144.9 (C-4), 145.7 (C-7), 152.4 (-C=N), 155.3 (C-8a), 155.9 (d, 1JC-F = 245 Hz, C-2''), 161.2 (C-2), 195.1 (O=C-Me). Anal. Calcd. for C23H23FN4O3 (422.45 g/mol): C, 65.39; H, 5.49; N, 13.26. Found: C, 65.18; H, 5.40; N, 13.15. ESI-HRMS m/z: Calcd. for C23H22FN4O3 [M − H]- 421.16759. Found: 421.16814.
7-{2-[1-(4-(4-Fluorophenyl)piperazin-1-yl)-2-oxopropylidene]hydrazinyl}-4-methyl-2H-chromen-2-one (3l): Yield = 0.45 g (59.3%); Mp = 271-273 °C. 1H-NMR (300 MHz, CDCl3): δ = 2.40 (d, J = 1.0 Hz, 3H, CH3-4), 2.45 (s, 3H, O=C-CH3), 3.21 (m, 4H, H2-3' + H2-5' ), 3.22 (m, 4H, H2-2' + H2-6' ), 6.14 (d, J = 1.0 Hz, 1H, H-3), 6.89-6.98 (m, 4H, H-2'' + H-3'' + H-5'' + H-6''), 7.03 (dd, J = 8.7 Hz, 2.1 Hz, 1H, H-6), 7.18 (d, J = 2.1 Hz, 1H, H-8), 7.51 (d, J = 8.7 Hz, 1H, H-5), 9.24 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 18.7 (CH3-4), 26.0 (O=C-CH3), 48.2 (C-2'/C-6'), 51.3 (C-3'/C-5'), 101.6 (C-8), 110.7 (C-6), 112.2 (C-3), 114.7 (C-4a), 115.7 (d, 2JC-F = 22 Hz, C-3''/C-5''), 118.3 (d, 3JC-F = 7.7 Hz, C-2''/C-6''), 157.2 (d, 1JC-F = 240 Hz, C-4''), 125.9 (C-5), 147.3 (d, 4JC-F = 2.2 Hz, C-1''), 144.8 (C-4), 145.6 (C-7), 152.5 (-C=N), 155.3 (C-8a), 161.2 (C-2), 195.2 (O=C-Me). Anal. Calcd. for C23H23FN4O3 (422.45 g/mol): C, 65.39; H, 5.49; N, 13.26. Found: C, 65.22; H, 5.45; N, 13.18. ESI-HRMS m/z: Calcd. for C23H24FN4O3 [M + H]+ 423.18324. Found: 423.18270.
4-Methyl-7-(2-(2-oxo-1-(4-(pyrimidin-2-yl)piperazin-1-yl)propylidene)hydrazinyl)-2H-chromen-2-one (3m): Yield = 0.55 g (75.2%); Mp = 236-238 °C. 1H-NMR (300 MHz, CDCl3): δ = 2.39 (d, J = 1.0 Hz, 3H, CH3-4), 2.43 (s, 3H, O=C-CH3), 3.12 (m, 4H, H2-3' + H2-5' ), 3.94 (m, 4H, H2-2' + H2-6' ), 6.12 (d, J = 1.0 Hz, 1H, H-3), 6.52 (t, J = 4.8 Hz, 1H, H-5''), 7.06 (dd, J = 8.7, 2.0 Hz, 1H, H-6), 7.20 (d, J = 2.0 Hz, 1H, H-8), 7.52 (d, J = 8.7 Hz, 1H, H-5), 8.32 (d, J = 4.8 Hz, 2H, H-4'' + H-6''), 9.35 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 18.7 (CH3-4), 26.0 (O=C-CH3), 44.4 (C-2'/C-6'), 47.9 (C-3'/C-5'), 101.6 (C-8), 110.3 (C-5''), 110.8 (C-6), 112.2 (C-3), 114.7 (C-4a), 125.9 (C-5), 144.8 (C-4), 145.7 (C-7), 152.5 (-C=N), 155.3 (C-8a), 157.8 (C-4'' + C-6''), 159.8 (C-2''), 161.2 (C-2), 195.2 (O=C-Me). Anal. Calcd. for C21H22N6O3 (406.44 g/mol): C, 62.06; H, 5.46; N, 20.68. Found: C, 61.87; H, 5.38; N, 20.52. ESI-HRMS m/z: Calcd. for C21H21N6O3 [M − H]- 405.16751. Found: 405.16696.
7-{2-[1-(4-(1-benzyl-2-ethyl-4-nitro-1H-imidazol-5-yl)piperazin-1-yl)-2-oxopropylidene]hydrazinyl}-4-methyl-2H-chromen-2-one (3n): Yield = 0.89 g (88.7%); Mp = 193-195 °C. 1H-NMR (300 MHz, CDCl3): δ = 1.28 (t, J = 7.5 Hz, 3H, CH2-CH3), 2.39 (d, J = 1.0 Hz, 3H, CH3-4), 2.41 (s, 3H, O=C-CH3), 2.61 (q, J = 7.5 Hz, 2H, CH2-Me), 2.68 (m, 4H, H2-3' + H2-5' ), 3.36 (m, 4H, H2-2' + H2-6' ), 5.17 (s, 2H, CH2-Ph), 6.12 (d, J = 1.0 Hz, 1H, H-3), 7.00 (dd, J = 8.7 Hz, 2.0 Hz, 1H, H-6), 7.02 (d, J = 7.5 Hz, 2H, H-2'''/ H-6'''), 7.30 (d, J = 2.0 Hz, 1H, H-8), 7.32-7.37 (m, 3H, H-3''' + H-4''' + H-5'''), 7.51 (d, J = 8.7 Hz, 1H, H-5), 9.34 (s, 1H, N-H). 13C-NMR (75 MHz, CDCl3): δ = 11.3 (-CH2-CH3), 18.8 (CH3-4), 21.2 (-CH2-Me), 25.9 (O=C-CH3), 46.2 (CH2-Ph), 48.4 (C-2'/C-6'), 49.6 (C-3'/C-5'), 101.6 (C-8), 111.1 (C-6), 112.2 (C-3), 114.8 (C-4a), 125.9 (C-5), 126.0 (C-2'''/C-6'''), 128.3 (C-4'''), 129.3 (C-3'''/C-5'''), 135.4 (C-1'''), 139.1 (C-1''), 140.0 (C-5''), 144.5 (C-4), 145.3 (C-4''), 145.5 (C-7), 152.5 (Ac-C=N), 155.3 (C-8a), 161.3 (C-2), 195.4 (O=C-Me). Anal. Calcd. for C29H31N7O5 (557.60 g/mol): C, 62.47; H, 5.60; N, 17.58. Found: C, 62.54; H, 5.64; N, 17.42. ESI-HRMS m/z: Calcd. for C29H31N7NaO5 [M + Na]+ 580.22844. Found: 580.22789.
3.5. Cell Lines and Cell Culture
Materials and Methods
Human breast cancer cell line MCF-7, was a gift from Drs. Prakash and Mitzi Nagarkatti (University of South Carolina, School of Medicine, Columbia, SC, USA). Human leukemia HL-60-Acute Myelocytic Leukemia (AML) and K5626 were gifts from Salem Akel at Hashemite University, Zarqa, Jordan. The three cell lines were maintained in complete RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin (all from Gibco), 50 μM 2-mercaptoethanol (Research Organics, USA), 10 mM HEPES buffer (AppliChem, Germany), and gentamicin sulfate at 0.05 mg/mL. Cells were maintained under standard culture conditions at 37 °C in a water-saturated atmosphere of 5% CO2 in air. ZR-75-1 breast cancer cells (obtained from ATCC) were cultured in DMEM supplemented with 2 mM glutamine and 10% Fetal Bovine Serum (FBS, Gibco Life Technologies).
3.2.2 Cell Proliferation Assay
MCF-7 and K562 cells were seeded at a density of 1 × 104 and 4 × 104 per well in 96-well plates in appropriate medium, then treated with 50 μM concentrations of the tested compounds. For the IC50 determination, the cells were treated with increasing concentrations of the tested compound (1.56-100 μM). In all assays, the drugs were dissolved in DMSO immediately before the addition to cell cultures and equal amounts of the solvent were added to control cells; the final concentration of DMSO did not exceed 1%. Cell viability was assessed, after 3 days of treatment, with tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), obtained from Sigma (Dorset, UK). IC50 concentrations were obtained from the dose-response curves using Graph Pad Prism Software 5 (San Diego, CA, USA, www.graphpad.com), and doxorubicin as positive control.
Acknowledgements
We wish to thank the Deanship of Scientific Research at the University of Jordan, Amman, Jordan, for financial support.
Footnotes
Sample availability: Contact the authors.
References and Notes
- 1.Abdel-Jalil R.J., El Momani E.Q., Hamad M., Voelter W., Mubarak M.S., Smith B.H., Peters D.G. Synthesis, antitumor activity, and electrochemical behavior of some piperazinyl amidrazones. Monatsh. Chem. 2010;141:251–258. doi: 10.1007/s00706-009-0241-4. and references cited therein. [DOI] [Google Scholar]
- 2.Tollefson G.D., Lancaster S.P., Montague-Clouse J. The association of buspirone and its metabolite 1-pyrimidinylpiperazine in the remission of comorbid anxiety with depressive features and alcohol dependency. Psychopharmacol. Bull. 1991;27:163–170. [PubMed] [Google Scholar]
- 3.Rotzinger S., Fang J., Baker G.B. Trazodone is metabolized to m-chlorophenyl-piperazine by CYP3A4 from human sources. Drug Metab. Dispos. 1998;26:572–575. [PubMed] [Google Scholar]
- 4.Oh Y.S., Yun M., Hwang S.Y., Hong S., Shin Y., Lee K., Yoon K.H., Yoo Y.J., Kim D.S., Lee S.H., et al. Discovery of LB30057, a benzamidrazone-based selective oral thrombin inhibitor. Bioorg. Med. Chem. Lett. 1998;8:631–634. doi: 10.1016/S0960-894X(98)00079-1. [DOI] [PubMed] [Google Scholar]
- 5.Lee K., Hwang S.Y., Hong S., Hong C.Y., Lee C.-S., Shin Y., Kim S., Yun M., Yoo Y.J., Kang M., et al. Structural modification of an orally active thrombin inhibitor, LB30057: replacement of the D-pocket-binding naphthyl moiety. Bioorg. Med. Chem. 1998;6:869–876. doi: 10.1016/s0968-0896(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 6.Lee K., Jung W.-H., Park C.W., Park H.D., Lee S.H., Kwon O.H. Noncovalent tripeptidic thrombin inhibitors incorporating amidrazone, amine and amidine functions at P1. Bioorg. Med. Chem. Lett. 2002;12:1017–1022. doi: 10.1016/S0960-894X(02)00093-8. [DOI] [PubMed] [Google Scholar]
- 7.Clemens F., Drutkowski G., Wiese M., Frohberg P. The inactivation of lipoxygenase-1 from soybeans by amidrazones. Biochim. Biophys. Acta. 2001;1549:88–98. doi: 10.1016/s0167-4838(01)00248-5. [DOI] [PubMed] [Google Scholar]
- 8.Murray R.D.H., Mendenz J., Bouwn S.A. The Natural Coumarins. Wiley & Sons; New York, NY, USA: 1982. [Google Scholar]
- 9.Schonberg A., Latif N. Furochromones and coumarins. XI. The molluscicidal activity of bergapten, isopimpinellin, and xanthotoxin. J. Am. Chem. Soc. 1954;76:6208. doi: 10.1021/ja01652a112. [DOI] [Google Scholar]
- 10.Mitra A.K., Misra S.K., Patra A. New synthesis of 3-alkyl coumarins. Synth. Commun. 1980;10:915–919. doi: 10.1080/00397918008061851. [DOI] [Google Scholar]
- 11.Sardari S., Mori Y., Horita K., Micetich R.G., Nishibe S., Daneshtalab M. Synthesis and antifungal activity of coumarins and angular furanocoumarins. Bioorg. Med. Chem. 1999;7:1933–1940. doi: 10.1016/s0968-0896(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 12.Khan M.S.Y., Sharma P. Synthesis of new α-pyronoflavones and related products. Part II. Indian J. Chem. 1993;32:817–821. [Google Scholar]
- 13.Xie L., Takeuchi Y., Cosentino L.M., MacPhail A.T., Lee H.K. Anti-AIDS agents. 42. Synthesis and anti-HIV activity of disubstituted (3'R,4'R)-3',4'-di-O-(S)-camphanoyl-(+)-cis-khellactone analogues. J. Med. Chem. 2001;44:664–671. doi: 10.1021/jm000070g. [DOI] [PubMed] [Google Scholar]
- 14.Singer L.A., Kong N.P. Vinyl radicals. Stereoselectivity in hydrogen atom transfer to equilibrated isomeric vinyl radicals. J. Am. Chem. Soc. 1966;88:5213–5219. doi: 10.1021/ja00974a033. [DOI] [Google Scholar]
- 15.O’Kennedy R., Thornes R.D. Coumarins: Biology, Application and Mode of Action. Wiley & Sons; Chichester, UK: 1997. [Google Scholar]
- 16.Zahradnik M. The Production and Application of Florescent Brightening Agents. Wiley & Sons; New York, NY, USA: 1992. [Google Scholar]
- 17.Brzozowski Z., Saczewski F., Slawinski J., Bednarski P., Grunert R., Gdaniec M. Synthesis, structural characterization, and in vitro antitumor activity of novel N-(6-chloro-1,1-dioxo-1,4,2-benzodithiazin-3-yl)arylsulfonamides. Bioorg. Med. Chem. 2007;15:2560–2572. doi: 10.1016/j.bmc.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 18.Burbuliene M.M., Maldutyte E., Vainilavicius P. Synthesis of S- and O-alkanoic acid derivatives of 6-phenyl-2-sulfanyl-4(3H)-pyrimidinone. Chemija. 2005;16:39–42. [Google Scholar]
- 19.Kontogiorgis C., Hadjipavlou-Litina D. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidant agents. J. Enz. Inhib. Med. Chem. 2003;18:63–69. doi: 10.1080/1475636031000069291. [DOI] [PubMed] [Google Scholar]
- 20.Satyanarayana V.S.V., Sreevani P., Sivakumar A., Vijayakumar V. Synthesis and antimicrobial activity of new Schiff bases containing coumarin moiety and their spectral characterization. ARKIVOC. 2008;17:221–233. [Google Scholar]
- 21.Al-Zghoul K.H.A., Salih K.S.M., Ayoub M.T., Mubarak M.S. A convenient procedure for the synthesis of substituted 4-methylaminocoumarins. Heterocycles. 2005;65:2937–2947. [Google Scholar]
- 22.Al-Soud Y.A., Al-Sa’doni H.H., Amajaour H.A.S., Salih K.S.M., Mubarak M.S., Al-Masoudi N.A., Jaber I.H. Synthesis, characterization and anti-HIV and antitumor activities of new coumarin derivatives. Z. Naturforsch. 2008;63b:83–89. [Google Scholar]
- 23.Salih K.S.M., Ayoub M.T., Saadeh H.A., Al-Masoudi N.A., Mubarak M.S. Synthesis, characterization, and biological activities of new benzofuran derivatives. Hetrocycles. 2007;71:1577–1587. [Google Scholar]
- 24.Al-Rifai A.A., Ayoub M.T., Shakya A.K., Abu Safieh K.A., Mubarak M.S. Synthesis, characterization, and antimicrobial activity of some new coumarin derivatives. Med. Chem. Res. 2011;20 (in press). [Google Scholar]
- 25.Phillips R.R. The Japp-Klingemann Reaction. Org. React. 1959;10:143–178. [Google Scholar]
- 26.Yao H.-C., Resnick P. Azo-hydrazone conversion. I. The Japp-Klingemann reaction. J. Am. Chem. Soc. 1962;84:3514–3517. doi: 10.1021/ja00877a018. [DOI] [Google Scholar]
- 27.Barrett G.C., El-Abadelah M.M., Hargreaves M.K. Cleavage of 2-acetyl-2-phenylazopropionanilide and related compounds by boron trifluoride. New Japp-Klingemann reactions. J. Chem. Soc. (C) 1970:1986–1989. [Google Scholar]
- 28.Pozdnev V.F. An improved method of synthesis of 7-amino-4-methylcoumarin. Khim. Geterotsikl. Soed. 1990;3:312–314. [Google Scholar]
- 29.Ronad P., Dharbamalla S., Hunshal R., Maddi V. Synthesis of novel substituted 7-(benzylideneamino)-4-methyl-2H-chromen-2-one derivatives as anti-inflammatory and analgesic agents. Arch. Pharm. 2008;341:696–700. doi: 10.1002/ardp.200800057. [DOI] [PubMed] [Google Scholar]
- 30.Butler R.N., Scott F.L. Versatile reactive intermediates: Hydrazidic halides. Chem. Ind. (London) 1970:1216–1221. [Google Scholar]
- 31.Ulrich H. The Chemistry of Imidoyl Halides. Chapter 7. Plenum Press; New York, NY, USA: 1968. pp. 174–192. [Google Scholar]
- 32.Hegarty A.F., Aylward J.B., Scott F.L. Synthesis and rearrangement of hydrazidic azides. J. Chem. Soc. (C) 1967:2587–2593. [Google Scholar]
- 33.Dalla Corce P., Del Buttero P., Licandro E., Maiorana S. Synthesis of (arylazomethylene)triphenylphosphoranes from arylhydrazonoyl chlorides (via nitrilimines in situ) and triphenylphosphine. Synthesis. 1979:299–300. [Google Scholar]
- 34.Heubach G. Synthesis of new 2,5-dihydro-1,2,3,5-thiatriazole 1-oxides and 3,4-dihydro-2H-1,2,4,3-triazaphosphole 3-oxides. Liebigs. Ann. Chem. 1980;1980:1376–1383. doi: 10.1002/jlac.198019800905. [DOI] [Google Scholar]
- 35.Hassaneen H.M., Mousa H.A.H., Abed N.M. Chemistry of C-heteroarylhydrazidoyl halides. Synthesis and reactions of N-(p-nitrophenyl)-C-(2-thienyl)formohydrazidoyl halides. Heterocycles. 1988;27:695–706. doi: 10.3987/COM-87-4381. [DOI] [Google Scholar]
- 36.Benincori T., Sannicoló F. New access to 2-(arylazo)-, 2-(arylhydrazo)-, and 2-aminoindoles, -benzofurans, and -thianaphthenes. J. Org. Chem. 1988;53:1309–1312. doi: 10.1021/jo00241a035. [DOI] [Google Scholar]
- 37.Galishev V.A., Chistokletov V.N., Petrov A.A. 1,3-Dipolar addition to unsaturated compounds. XXXIV. Reactions of diphenylmethyleneaminodiphenylphosphine with nitrilimines. Zh. Obshch. Khim. 1975;45:1695–1697. [Google Scholar]
- 38.Shawali A.S., Párkányi C. Hydrazidoyl halides in the synthesis of heterocycles. J. Heterocycl. Chem. 1980;17:833–854. [Google Scholar]
- 39.Al-Soud Y.A., Al-Sa'doni H., Amajaour H.A.S., Al-Masoudi N.A. Nitroimidazoles. Part 3. Synthesis and anti-HIV activity of new N-alkyl-4-nitroimidazoles bearing benzothiazole and benzoxazole backbones. Z. Naturforsch. 2007;62:523–528. [Google Scholar]