Abstract
A facile and efficient method for synthesis of fenamic acid hydrazides from their acids in one-step reaction under microwave irradiation and solvent-free conditions was developed. Compared with the two-step conventional heating method, the process was simple, the reaction time was very short and the yields were almost quantitative.
Keywords: fenamic acids, fenamic acid hydrazides, microwave irradiation, solvent-free reaction
1. Introduction
Flufenamic acid (FFA), N-(α,α,α-trifluoro-m-tolyl)anthranilic acid, mefenamic acid (MFA), N-(2,3-xylyl)anthranilic acid and meclofenamic acid (MCFA), N-(2,6-dichloro-3-methylphenyl) anthranilic acid are derivatives of N-phenylanthranilic acid (fenamates). They are non-steroidal anti-inflammatory drugs (NSAIDs) used as potent analgesic and anti-inflammatory agents in the treatment of osteoarthritis, rheumatoid arthritis and other painful musculosketal illnesses [1,2,3,4,5]. The fenamates exhibit pharmacologic actions similar to those of aspirin. They are potent inhibitors of cyclooxygenase, thereby inhibiting the release of prostaglandins [5]. Furthermore, in vitro and in vivo antimycobacterial activities of some NSAIDs such as diclofenac and its derivatives have been also reported [6]. Diclofenac is structurally related to fenamic acids suggesting that these NSAIDs may inhibit a new target in M. tuberculosis. On the other hand, hydrazides are important key intermediates in the synthesis of many series of biologically active heterocycles, and their synthesis has attracted significant attention due to their utility as building blocks [7,8,9,10,11] and aroused our interest in exploring the utility of hydrazides as versatile precursors for the synthesis of a variety of substituted heterocycles [12,13,14,15,16,17,18,19,20].
In view of the above facts and as a part of our ongoing project directed to develop new isatin hydrazone anti-TB agents using combinatorial chemistry and microwave-assisted synthesis technologies [21], an efficient, fast and high yielding method for preparing hydrazide building blocks for the design of combinatorial libraries is urgently needed. The current work describes a direct, microwave assisted, one-pot synthesis of some fenamic acid hydrazides from their corresponding fenamic acids.
2. Results and Discussion
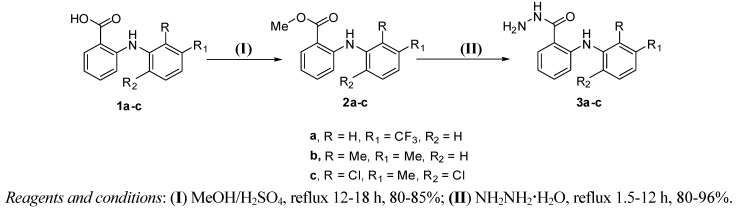
Over the last few years, there has been growing interest in the synthesis of organic compounds using green chemistry tools such as microwave irradiation because of increasing environmental consciousness. The feasibility of microwave assisted synthesis has been demonstrated in various transformations whose main features are enhanced reaction rates, greater selectivity and experimental ease of manipulation leading to efficient, environmentally friendly in addition to cost effective synthetic pathways to several compounds [22,23]. Moreover, the use of microwave irradiation in this regard is now a well-established procedure in MORE (microwave induced organic reaction enhancement) chemistry [24]. In the present study, a direct microwave-assisted one-step synthesis of some fenamic acid hydrazides from their corresponding acids was developed. The obtained results were compared with the conventional two-step method. In the latter, esters 2a-c are obtained in 80-85% yield by esterification of the corresponding fenamic acids, 1a-c, with methanol, in the presence of sulfuric acid, under reflux for 12-18 h [25,26,27,29]. The second step involves production in 80-96% yield of hydrazides 3a-c by the reaction of methyl esters 2a-c with hydrazine hydrate under reflux for 1.5-12 h. However, the combined reaction times of the latter two steps were 15-28 h, with overall yields being 64-86% [27,28,29] (Scheme 1, Table 1). It is worth mention that direct reaction of the fenamic acids, 1a-c, with hydrazine hydrate to synthesize the hydrazides 3a-c with the conventional heating method was unsuccessful.
Scheme 1.
Conventional synthesis of fenamic hydrazides 3a-c.
Table 1.
Reaction times and yield of conventional (2 steps) and microwave-assisted synthesis.
| Comp. | Conventional synthesis | MW-assisted synthesis | ||
|---|---|---|---|---|
| Time of 2 step route (h) | Overall yield (%) | Time (min) | Yield (%) | |
| 3a | 15 | 80 | 4 | 96 |
| 3b | 28 | 64 | 12 | 82 |
| 3c | 17 | 86 | 5 | 85 |
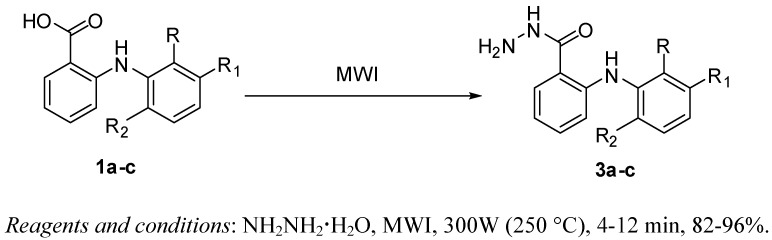
Interestingly, compounds 3a-c were obtained directly in excellent yields from the reaction of acids 1a-c with hydrazine hydrate in absence of organic solvents under microwave irradiation (300W, 250 °C) for 4-12 min (Scheme 2, Table 1). Spectral analyses of the synthesized hydrazides 3a-c are consistent with the proposed structures and with those reported [27,28,29].
Scheme 2.
Microwave-assisted one-step synthesis of fenamic hydrazides 3a-c.
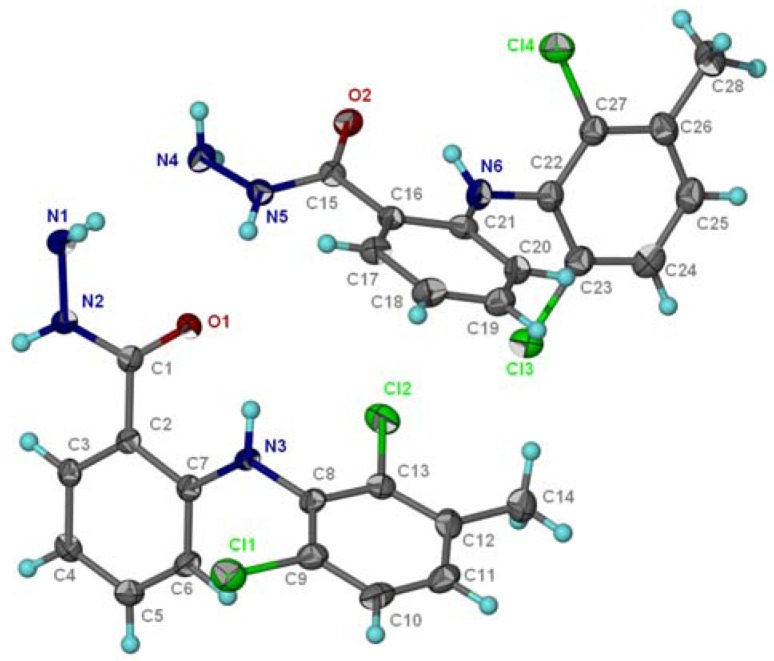
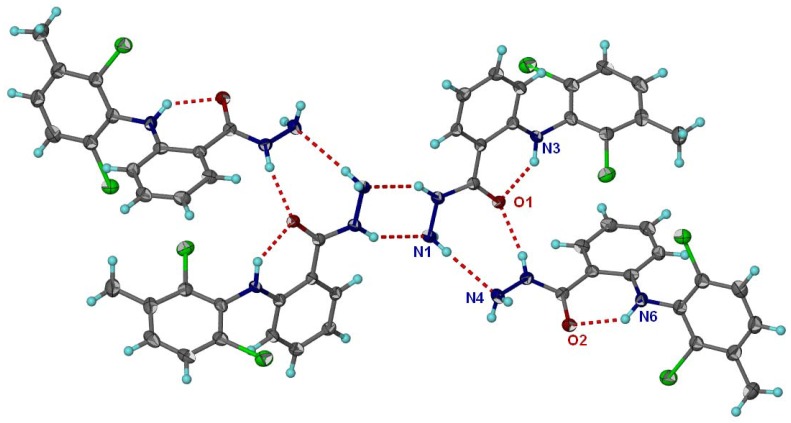
X-Ray diffraction of hydrazide 3c (Figure 1) [30] showed a network of hydrogen bonds. Two molecules of the dimmer are linked by intermolecular hydrogen bonds (N5-H5∙∙∙∙∙O1 and N1-H1∙∙∙∙∙N4). Besides the latter intermolecular H-bonds, there are two intramolecular H-bonds, one in each molecule of the dimmer, N3–H3∙∙∙∙∙O1 and N6–H6∙∙∙∙∙O2 (Figure 2).
Figure 1.
X-ray structure of hydrazide 3c.
Figure 2.
Crystal packing of hydrazide 3c.
3. Conclusions
A microwave-assisted one-step synthesis of some fenamic acid hydrazides by reaction of their corresponding acids with hydrazine hydrate under solvent-free conditions was developed and described. In this method, the reaction times were very short (4-12 min), the yields were excellent (82-96%) and the manipulations were simple compared to the conventional heating method.
4. Experimental
4.1. General
Fenamic acids were purchased from the Sigma-Aldrich Company (St. Louis, MO, USA). All other chemicals are of commercially available research-grade. Melting points were determined on a Gallenkamp melting point apparatus, and are uncorrected. NMR Spectra were scanned in DMSO-d6 on a Bruker NMR spectrophotometer operating at 500 MHz for 1H and 125.76 MHz for 13C at the Research Center, College of Pharmacy, King Saud University, Saudi Arabia. Chemical shifts are expressed in δ-values (ppm) relative to TMS as an internal standard and D2O was added to confirm the exchangeable protons. Mass spectra were measured on Agilent Triple Quadrupole 6410 QQQ LC/MS with ESI (Electrospray ionization) source. The X-ray diffraction measurements of compound 3c were done using Bruker SMART APEX diffractometer. The microwave irradiations were carried out using an Explorer-48 microwave reactor (CEM, USA).
4.2. Conventional Synthesis of Fenamic Acid Hydrazides 3a-c
4.2.1. Step 1
A solution of the appropriate fenamic acid 1a-c (10 mmol), absolute methanol (10 mL) and concentrated sulfuric acid (1 mL) was heated under reflux for the appropriate time (Table 1). The solvent was evaporated under reduced pressure, the remaining contents cooled to room temperature, neutralized with a concentrated solution of sodium carbonate, then the aqueous solution extracted with ether (3 times × 20 mL). The combined ether extracts were dried, and the solvent is removed under reduced pressure to yield the corresponding ester 2a-c in 80-85% yield (Table 1).
4.2.2. Step 2
A solution of hydrazine hydrate (99.9%, 5 mmol) and the appropriate methyl ester 2a-c (1 mmol) was brought to a gentle reflux for the appropriate time (Table 1), then cooled to room temperature, The solid formed was filtered (ice/water mixture was added in some cases to complete precipitation), washed with several portions of water and dried by suction. Crystallization from EtOH afforded the corresponding fenamic acid hydrazides 3a-c in 80-96% yield (Table 1). The reactions time of the latter two steps was 15-28 h, with overall yields of 64-86%
4.3. Microwave Irradiation Synthesis of Fenamic Acid Hydrazides 3a-c
A mixture of hydrazine hydrate (99.9%, 2.5 mmol) and the appropriate fenamic acid 1a-c(1 mmol) was irradiated, in closed vessel, under microwave irradiation at 300W and 250 °C, with 250 psi maximum pressure, for the appropriate time (Table 1). The reaction mixture was cooled; the separated solid was filtered, dried and crystallized from EtOH to give the corresponding hydrazides 3a-c in 82-96% yields (Table 1).
4.3.1. 2-(3-(Trifluoromethyl)phenylamino)benzohydrazide (3a)
White fibers; Mp: 136-138 °C (reported [28] 134-136 °C). 1H-NMR δ: 4.59 (br. S, D2O exch., 2H, NH2), 6.94-7.21 (m, 2H, ArH), 7.29-7.47 (m, 5H, ArH), 7.62-7.63 (m, 1H, ArH), 9.56 (s, D2O exch., 1H, NH), 9.91 (s, D2O exch., 1H, NH). 13C-NMR δ: 113.80, 116.72, 116.91, 119.93, 120.84, 121.29, 123.01, 125.18, 128.58, 129.80-130.55 (m, -CF3), 131.55, 142.15, 143.10, 167.71. MS m/z (%): 295 (M+-1).
4.3.2. 2-(2,3-Dimethylphenylamino)benzohydrazide (3b)
White powder; Mp: 118-120 °C (reported [27] 118-120°C). 1H-NMR δ: 2.13 (s, 3H, CH3), 2.27 (s, 3H, CH3), 4.38 (br. S, D2O exch., 2H, NH2), 6.72-6.91 (m, 3H, ArH), 7.10-7.23 (m, 3H, ArH), 6.62-7.63 (m, 1H, ArH), 9.53 (s, D2O exch., 1H, NH), 9.88 (s, D2O exch., 1H, NH). 13C-NMR δ: 13.47, 20.24, 113.87, 116.04, 116.86, 119.47, 124.97, 125.80, 128.17, 129.22, 131.73, 137.62, 139.28, 145.91, 168.45. MS m/z (%): 255 (M+-1).
4.3.3. 2-(2,6-Dichloro-3-methylphenylamino)benzohydrazide (3c)
White fibers; Mp: 155-157 °C (reported [29] 158-160 °C). 1H-NMR δ: 2.38 (s, 3H, CH3), 4.57 (s, D2O exch., 2H, NH2), 6.21 (d, 1H, J = 8 Hz, ArH), 6.77 (t, 1H, J = 7 Hz, ArH), 7.22 (t, 1H, J = 7 Hz, ArH), 7.29 (d, 1H, J = 8 Hz, ArH), 7.48 (d, 1H, J = 8 Hz, ArH), 7.62 (d, 1H, J = 8 Hz, ArH), 9.74 (s, D2O exch., 1H, NH), 9.89 (s, D2O exch., 1H, NH). 13C NMR δ: 20.13, 113.29, 115.82, 117.62, 127.87, 128.06, 128.34, 129.27, 131.60, 132.36, 135.21, 136.39, 144.65, 168.16. MS m/z (%): 310 (M+).
Acknowledgements
This research project is supported by “NPST program by King Saud University”, Project Number MED598-02-08.
Footnotes
Sample Availability: Samples of the compounds 2a-c and 3a-c are available from Tarek Aboul-Fadl, Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia.
References and Notes
- 1.Syed M.M., Parekh A.B., Tomita T. Receptors involved in mechanical responses to catecholamines in the circular muscle of guinea-pig stomach treated with meclofenamate. Brit. J. Pharm. 1990;101:809–814. doi: 10.1111/j.1476-5381.1990.tb14162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belsole S.C. Meclofenamic acid topical pharmaceutical composition. US Patent 4602040. 1986 [Google Scholar]
- 3.Martindale J.E.F. In: The extra pharmacopoeia. 31st. Reynolds, editor. The Pharmaceutical Press; London, UK: 1996. [Google Scholar]
- 4.Metz G. Method of producing 2-(2-hydroxyethoxy)-ethanol ester of flufenamic acid. US Patent 498. 1990
- 5.Insel P.A., Gilman A.G., Rall T.W., Nies A.S., Taylor P. Goodman and Gilman’s:The Pharmacological Basis of Therapeutics. 8th. McGraw-Hill; New York, NY, USA: 1990. [Google Scholar]
- 6.Sriram D., Yogeeswari P., Devakaram R.V. Synthesis, in vitro and in vivo antimycobacterial activities of diclofenac acid hydrazones and amides. Bioorg. Med. Chem. 2006;14:3113–3118. doi: 10.1016/j.bmc.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Koz’minykh V.O. Synthesis and biological activity of substituted amides and hydrazides of 1,4-dicarboxylic acids. Pharm. Chem. J. 2006;40:8–17. doi: 10.1007/s11094-006-0048-0. [DOI] [Google Scholar]
- 8.Kidawi M., Misra P., Kumma R., Saxena R.K., Gupta R., Bardoo S. Microwave assisted synthesis and antibacterial activity of new quinolone derivatives. Monatsh. Chem. 1998;129:961–965. [Google Scholar]
- 9.Fuquang L.I.U., Palmer D.C., Sorgi K.L. Diethoxyphosphinyl acetic acid hydrazide: A uniquely versatile reagent for the preparation of fused [5,5]-, [5,6]-, and [5,7]-3-[(E)-2-(arylvinyl)]-1,2,4-triazole. Tetrahedron Lett. 2004;45:1877–1880. doi: 10.1016/j.tetlet.2004.01.015. [DOI] [Google Scholar]
- 10.Demirbas N., Ugurluoglu R., Demirbas A. Synthesis of 3-alkyl(aryl)-4-alkylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-ones and 3-alkyl-4-alkylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones as antitumor agents. Bioorg. Med. Chem. 2002;10:3717–3723. doi: 10.1016/S0968-0896(02)00420-0. [DOI] [PubMed] [Google Scholar]
- 11.Holla B.S., Akberali P.M., Shivananda M.K. Studies on nitrophenylfuran derivatives-Part XII. Synthesis, characterization, antibacterial and antiviral activities of some nitrophenylfurfurylidene-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines. Farmaco. 2001;56:919–927. doi: 10.1016/S0014-827X(01)01124-7. [DOI] [PubMed] [Google Scholar]
- 12.Aboul-Fadl T., Mohammed F.A., Hassan E.A. Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff Bases derived from 1-alkylisatin and isonicotinic acid hydrazide (INH) Arch. Pharm. Res. 2003;26:778–784. doi: 10.1007/BF02980020. [DOI] [PubMed] [Google Scholar]
- 13.Hussein M.A., Aboul-Fadl T., Hussein A. Synthesis and antitubercular activity of some Mannich bases derived from isatin isonicotinic acid hydrazone. Bull. Pharm. Sci. Assiut Univ. 2005;28:131–136. [Google Scholar]
- 14.Abdel-Aziz H.A., Hamdy N.A., Farag A.M., Fakhr I.M.I. Synthesis and reactions of 3-methylthiazolo[3,2-a]benzimidazole-2-carboxylic acid hydrazide: Synthesis of some new pyrazole, 1,3-thiazoline, 1,2,4-triazole and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazine derivatives pendant to thiazolo[3,2-a]benzimidazole moiety. J. Chin. Chem. Soc. 2007;54:1573–1582. [Google Scholar]
- 15.Abdel-Aziz H.A., Gamal-Eldeen A.M., Hamdy N.A., Fakhr I.M.I. Immunomodulatory and anti-cancer activity of some novel 2-substituted-6-bromo-3-methylthiazolo[3,2-a]benzimidazole derivatives. Arch. Pharm. 2009;342:230–237. doi: 10.1002/ardp.200800189. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Aziz H.A., Abdel-Wahab B.F., Badria F.A. Stereoselective synthesis and antiviral activity of (1E,2Z,3E)-1-(piperidin-1-yl)-1-(arylhydrazono)-2-[(benzoyl/benzothiazol-2-oyl)-hydrazono]-4-(aryl1)but-3-enes. Arch. Pharm. 2010;343:152–159. doi: 10.1002/ardp.200900195. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Aziz H.A., Mekawey A.A.I. Stereoselective synthesis and antimicrobial activity of benzofuran-based (1E)-1-(piperidin-1-yl)-N2-arylamidrazones. Eur. J. Med. Chem. 2009;44:3985–4997. doi: 10.1016/j.ejmech.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Aziz H.A., Mekawey A.A.I., Dawood K.M. Convenient synthesis and antimicrobial evaluation of some novel 2-substituted-3-methylbenzofuran derivatives. Eur. J. Med. Chem. 2009;44:3637–3644. doi: 10.1016/j.ejmech.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Wahab B.F., Abdel-Aziz H.A., Ahmed E.M. Synthesis and antimicrobial evaluation of some new 1,3-thiazole, 1,3,4-thiadiazole, 1,2,4-triazole and [1,2,4]triazolo[3,4-b]-[1,3,4]thiadiazine derivatives including 5-(benzofuran-2-yl)-1-phenyl-pyrazole moiety. Monatsh. Chem. 2009;140:601–605. doi: 10.1007/s00706-008-0099-x. [DOI] [Google Scholar]
- 20.Abdel-Wahab B.F., Abdel-Aziz H.A., Ahmed E.M. Convenient synthesis and antimicrobial activity of some new 3-substituted-5-(benzofuran-2-yl)-pyrazole derivatives. Arch. Pharm. 2008;341:734–739. doi: 10.1002/ardp.200800119. [DOI] [PubMed] [Google Scholar]
- 21.Aboul-Fadl T., Abdel-Aziz H.A., Khadi A., Darwish I., Al-Samani T., Ahmad P., Bari A., Al-Hajoj S. Design and Synthesis of a Combinatorial Library of Indoline-2,3-dione Schiff Bases with Potential Anti-tubercular Activity; Proceedings of The 46th International Conference on Medicinal Chemistry, Interfacing Chemistry, Biology and Drug Discovery; Reims, France. June 30-July 2, 2010; Abstract No. P009. [Google Scholar]
- 22.Suman A., Poonam R., Ambati N.R., Kumaran G., Ramesh C.M. Synthesis, characterization and spectral studies of various newer long chain aliphatic acid (2-hydroxy benzylidene and 1H-indol-3-ylmethylene) hydrazides as mosquito para-pheromones. J. Kor. Chem. Soc. 2007;51:506–512. doi: 10.5012/jkcs.2007.51.6.506. [DOI] [Google Scholar]
- 23.Saha A., Kumar R., Kumar R., Devakumar C. Development and assessment of green synthesis of hydrazides. Indian J. Chem. 2010;49B:526–531. [Google Scholar]
- 24.Bose A.K., Manhas M.S., Banik B.K., Robb E.W. Microwave-induced organic reaction enhancement (more) chemistry: Techniques for rapid, safe and inexpensive synthesis. Res. Chem. Intermed. 1994;20:1–11. [Google Scholar]
- 25.Lehmann J., Kraft G. Amphiphile Verbindungen, 1. Mitt. Zur Synthese von 1-Aryl-, 1-Aroyl- und 1-benzyl-2,3,4-5-tetrahydro-1H-1,4-benzodiazepinen. Arch. Pharm. 1984;317:595–606. doi: 10.1002/ardp.19843170705. [DOI] [Google Scholar]
- 26.Narsinghani T., Chaturvedi S.C. QSAR analysis of meclofenamic acid analogues as selective COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2006;16:461–468. doi: 10.1016/j.bmcl.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 27.Reddy L.V., Suman A., Beevi S.S., Mangamoori L.N., Mukkanti K., Pal S. Design and synthesis of 1-aroyl-2-ylidene hydrazines under conventional and microwave irradiation conditions and their cytotoxic activities. J. Braz. Chem. Soc. 2010;21:98–104. doi: 10.1590/S0103-50532010000100015. [DOI] [Google Scholar]
- 28.Onnis V., Cocco M.T., Fadda R., Congiu C. Synthesis and evaluation of anticancer activity of 2-arylamino-6-trifluoromethyl-3-(hydrazonocarbonyl)pyridines. Bioorg. Med. Chem. 2009;17:6158–6165. doi: 10.1016/j.bmc.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 29.Boschelli D.H., Connor D.T., Flynn D.L., Sircar J.C., Hoefle M.L. Triazole derivatives of fenamates as antiinflammatory agents. US Patent 496. 1990
- 30.A white single crystal of hydrazide 3c was crystallized from ethanol by slow evaporation at room temperature. Crystallographic data for the structure 3c has been deposited with the Cambridge Crystallographic Data Center (CCDC) under the deposition number 813246. Copies of the data can be obtained, free of charge, on application to CCDC 12 Union Road, Cambridge CB2 1EZ, UK [Fax: +44-1223-336033; Email: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk]