Abstract
Background
Cystatin C seems promising for evaluating the risk of cardiovascular events and mortality.
Objective
To evaluate the association between high levels of cystatin C and the development of cardiovascular events or mortality.
Methods
The articles were selected in the Medline/PubMed, Web of Science, and Scielo databases. The eligibility criteria were prospective cohort observational trials that assessed the association of high serum levels of cystatin C with the development of cardiovascular events or mortality in individuals with normal renal function. Only studies that evaluated the mortality outcome compared the fourth with the first quartile of cystatin C and performed multivariate Cox’s proportional hazard regression analysis were included in the meta-analysis. A p value < 0,05 was considered significant.
Results
Among the 647 articles found, 12 were included in the systematic review and two in the meta-analysis. The risk of development of adverse outcomes was assessed by eight studies using the hazard ratio. Among them, six studies found an increased risk of cardiovascular events or mortality. The multivariate regression analysis was performed by six studies, and the risk of developing adverse outcomes remained significant after the analysis in four of these studies. The result of the meta-analysis [HR = 2.28 (1.70-3.05), p < 0.001] indicated that there is a significant association between high levels of cystatin C and the risk of mortality in individuals with normal renal function.
Conclusion
There is a significant association between high levels of cystatin C and the development of cardiovascular events or mortality in individuals with normal renal function.
Keywords: Cardiovascular Diseases/mortality; Cystatin C; Coronary Artery Disease; Myocardial Infarction; Renal Insufficiency, Chronic; Meta-Analysis as Topic
Introduction
Cardiovascular diseases are the leading cause of death in the world, accounting for 31% of all deaths. In 2015, an estimated 17.7 million people died from cardiovascular diseases, mainly coronary heart disease, cerebrovascular disease, and peripheral arterial disease.1 In addition to high mortality, cardiovascular diseases are also associated with high morbidity, contributing to a significant share of public expenditure on health.2
Chronic kidney disease is an important risk factor for the development of cardiovascular events, and is also responsible for increased morbidity and mortality in patients with cardiovascular disease3. Cystatin C consists of a marker of renal dysfunction that has been shown to be more sensitive than serum creatinine to assess the early stages of renal failure4. It consists of a relatively stable cysteine protease inhibitor, produced in all nucleated cells at a constant rate.5
Because of the greater sensitivity of cystatin C for detecting the early and milder stages of renal dysfunction, the evaluation of serum levels has been shown to be promising for assessing the risk of cardiovascular events and mortality in individuals with apparently normal renal function. In recent years, some studies have demonstrated an association between serum cystatin C levels and the development of AMI.6 In addition, cystatin C has been shown to be useful for prognostic stratification in patients with ACS.7
However, there is a divergence between the results of studies performed to date on the clinical utility of cystatin C to assess the risk of cardiovascular events and mortality in individuals with normal renal function.3,7,8 Although some meta-analyses.9-12 have been published on the subject, the population of the studies selected did not consist only of patients with normal renal function. Therefore, the objective of this systematic review and meta-analysis was to evaluate the association between high levels of cystatin C and the development of cardiovascular events or mortality in subjects with normal renal function.
Methods
This systematic review followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.13
Articles Selection
The articles selection was performed through the data bases Medline (PubMed) and Web of Science, using the descriptors “cystatin C”, “post-gamma-globulin”, “post-gamma globulin”, “neuroendocrine basic polypeptide”, “basic polypeptide, neuroendocrine”, “cystatin 3”, “gamma-trace”, “gamma trace”, combined with the descriptors “acute coronary syndrome”, “acute coronary syndromes”, “coronary syndrome, acute”, “coronary syndromes, acute”, “syndrome, acute coronary”, “syndromes, acute coronary”, “myocardial infarction”, “infarction, myocardial”, “infarctions, myocardial”, “myocardial infarctions”, “cardiovascular stroke”, “cardiovascular strokes”, “stroke, cardiovascular”, “strokes, cardiovascular”, “heart attack”, “heart attacks”, “myocardial infarct”, “infarct, myocardial”, “infarcts, myocardial”, “myocardial infarcts”, “myocardial ischemia”, “ischemia, myocardial”, “ischemias, myocardial”, “myocardial ischemias”, “ischemic heart disease”, “heart disease, ischemic”, “disease, ischemic heart”, “diseases, ischemic heart”, “heart diseases, ischemic”, “ischemic heart diseases”, using the connector “AND” between the terms. The Medical Subject Headings (MeSH) was used to define these descriptors.
The selection of the articles was also performed in Scielo database, using the descriptors “cystatin C” with the Boolean operators “acute coronary syndrome”, “coronary disease”, “coronary heart disease”, “myocardial infarction”, “heart attack”, “cardiac attack”, “myocardial ischemia”, “heart disease, ischemic”, “ischemia, myocardial” and “ischemic heart disease” using AND connector between the terms. The Descriptors in Health Sciences (DeCS) was used to define these descriptors.
Eligibility criteria
The eligibility criteria were established according to the PRISMA recommendation,13 and consist of prospective cohort observational studies written in English, Portuguese or Spanish evaluating the association between high levels of cystatin C, and the development of cardiovascular events or mortality in individuals with normal renal function. There was no restriction of the period of publication of articles in the research. PECOS strategy was used to elaborate the research question:
Population of interest: Individuals with normal renal function.
Exposure: High levels of cystatin C.
Outcome: Cardiovascular events or mortality.
Study Design: Prospective cohort.
Extracting data from selected articles
The following data were obtained from the studies that met the eligibility criteria: method used for measuring serum levels of cystatin C, patient group size, patient follow-up time, patient age range, criterion used to define normal renal function, outcome obtained in the study, outcome assessed, study population, patient classification, and parameters included in Cox proportional hazards multivariate regression analysis.
Quality of the selected articles
The methodological quality evaluation process of the studies included in the review was carried out by two reviewers using the Newcastle-Ottawa Scale (NOS)14 questionnaire for cohort studies, which contains the following categories of evaluation: cohort selection; comparability of the cohort and outcome. The quality of the study is indicated with a maximum of nine stars, with only one star being allowed to be assigned in the selection and outcome categories, and two stars in the comparability category. The articles reaching a score of five to six stars were considered as articles of good methodological quality, and those with seven or more stars were considered articles of excellent methodological quality.
Meta-Analysis
The meta-analysis included only those studies that assessed the outcome all-cause mortality comparing the fourth quartile of cystatin C with the first quartile and that conducted multivariate regression analysis of Cox proportional hazards. The hazard ratio value and the 95% confidence interval adjusted by the multivariate regression analysis were used in the meta-analysis and the I2 test was used to assess the heterogeneity among the studies. The studies were considered heterogeneous when I2 > 50% and p < 0.10. When there was homogeneity, the hazard ratio was calculated using the fixed effect model. The distribution of the studies included in the meta-analysis was analyzed by a funnel plot. The statistical software Review Manager version 5.3 was used to perform the statistical analysis. The p value < 0.05 was considered significant.
Results
Literature search
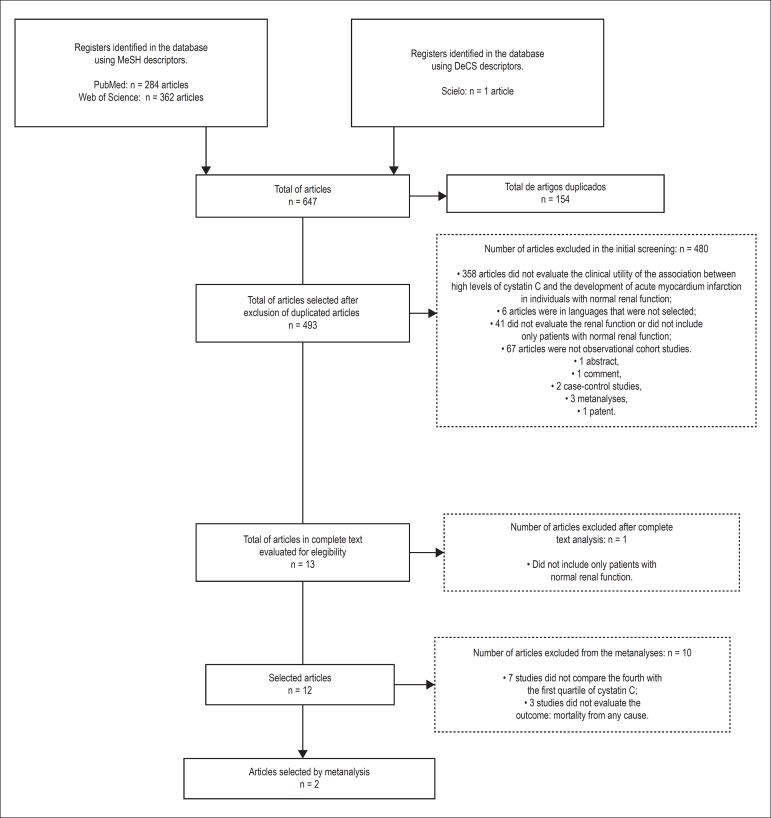
The initial search through the descriptors in the electronic databases resulted in a total of 647 articles. After completing the selection steps, 12 articles were included in the systematic review, and two were included in the meta-analysis. The flow chart for the selection of articles according to the eligibility criteria is presented in Figure 1.
Figure 1.
Flow chart of the articles selected for review, according to the elegibility criteria used in the study.
Characteristics and results of selected articles
The studies that met the eligibility criteria were published between 2007 and 2016 and their characteristics are found in Table 1.
Table 1.
Characteristics of selected studies
| Author/Year | Number of patients/ Age group |
Study population | Patient follow-up time | Evaluated outcome |
|---|---|---|---|---|
| Sai et al., 201619 | 277/64 | Patients undergoing PCI | 5 years and 3 months | Cardiovascular death, cerebrovascular death, ACS including non-fatal AMI and unstable angina, non-fatal stroke and hospitalization due to worsening CHF |
| Bansal et al., 201615 | 2410/40,2 ± 3,6 | Patients at risk for cardiovascular events who underwent echocardiography | 10 years | Left ventricular hypertrophy |
| Abid et al., 20167 | 127/58 ± 11,65 | Patients with STEMI and NSTEMI | 1 year | Cardiovascular death, myocardial reinfarction, NSTEMI, HF |
| Woitas et al., 201318 | 2356/64 ± 10 | Patients with CAD and healthy individuals | 10 years | Cardiovascular death and death from any cause |
| Dupont et al., 20128 | 615/65 ± 11 | Patients with CHF who underwent coronary angiography | 3 years | Death from any cause, non-fatal AMI and non-fatal stroke |
| Gao et al., 201121 | 13 8/65,4 ± 11,0 | Patients with chronic or new onset systolic CHF | 3 years | Cardiovascular death, development or progression of HF requiring hospitalization, intravenous treatment of HF within the first 3 days after admission, cardiac transplantation |
| Keller et al., 200917 | 1827/62 | Patients with stable CAD or ACS | 4 years | Cardiovascular death |
| Gao et al., 200922 | 160/60 | Patients with stable, unstable angina and AMI and healthy individuals | 6 months | AMI, cardiovascular death, refractory angina, PCI and angiography |
| Alehagen et al., 200920 | 464/65 to 87 | Patients with CHF | 10 years | Cardiovascular death |
| Acuna et al., 200916 | 203/66,6 ± 13,16 | Patients with STEMI and NSTEMI | 1 years and 3 months | Cardiovascular death and HF |
| Koenig et al., 200724 | 466 3/≥ 65 | Elderly subjects (≥ 65 years) | 9,3 years | Death from any cause, cardiovascular death, incident HF, stroke and AMI |
| Ix et al., 200723 | 990/67 | Patients with a history of AMI, angiographic evidence of stenosis greater than 50% in 1 or more coronary vessels, evidence of treadmill-induced ischemia or nuclear testing, or history of coronary artery bypass grafting | 3 years and 1 month | Cardiovascular death, non-fatal AMI, stroke, death from all causes and HF |
AMI: Acute Myocardial Infarction; HF: Heart failure; CHF: congestive heart failure; NSTEMI: Non-ST-segment elevation myocardial infarction; PCI: Percutaneous coronary intervention; ACS: acute coronary syndrome; STEMI: ST-segment elevation myocardial infarction; CAD: Coronary artery disease.
Population
The population of the studies analyzed consisted of patients at risk for cardiovascular events,15 with ST-elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI),7,16 and stable coronary artery disease (CAD),17,18 ACS,17 patients undergoing percutaneous coronary intervention,19 with congestive heart failure (CHF),20,21 with CHF who underwent coronary angiography,9 with stable angina and AMI,22 with a history of AMI that had angiographic evidence of stenosis greater than 50%,23 or healthy elderly individuals (older than 65 years).24
Sample size, age group and follow-up time
The sample size varied from 127 to 4,663 individuals, and the sample number of 25% (n = 3)8,20,23 of the studies ranged from 400 to 1000 individuals, 41.67% (n = 5)7,16,19,21,22 of the studies had a sample number of less than 300 patients, and 33.33% (n = 4)15,17,18,24 had a sample size greater than 1000. The mean age ranged from 37 to 87 years, with 41.66% (n =5)7,8,16,18,21 of the studies evaluating both adult and elderly population (over 60 years), 50% (n = 6)17,19,20,22-24 evaluating only the elderly population, and one study [8,33% (n = 1)]15 analyzing only the adult population (below 60 years). The study follow-up time ranged from 6 months to 10 years, with 25% (n = 3)7,16,22 accompanying patients for less than 15 months, 41.67% (n = 5)8,17,19,21,23 following for 3 to 6 years, and 33.33% (n = 4)15,18,20,24 following for a period of more than 9 years.
Outcome
The main outcomes evaluated by the studies were cardiovascular death (n = 10; 83.33%),7,16-24 heart failure (n = 6; 50%),7,16,19,21,23,24 and acute myocardial infarction (n = 6; 50%),7,8,19,22-24 followed by stroke (n = 4; 33,33%),8,19,23,24 death from any cause (n = 3; 35%,)8,23,24 and unstable angina (n = 2; 16,67%).19,22 Only one study (8.33%) evaluated each of the following outcomes: cerebrovascular death,19 left ventricular hypertrophy,15 myocardial reinfarction,7 need for percutaneous coronary intervention,22 and angiography.22
Method for dosing cystatin C and criteria for the definition of normal renal function
The cystatin C dosing method and the criteria used to define normal renal function in the selected studies are shown in Table 2. The methods used for cystatin C dosing were immunonephelometry [41.67% (n = 5)],15-18,23 immunoturbimetry [33.33% (n = 4)],7,8,19,20 and immunoenzymatic assay [8.33% (n = 1)].22 Two studies (16.66%)21,24 did not report the method used for cystatin C dosing. The criteria used to define normal renal function were the GFR, estimated by the MDRD equation, above 60 mL/min/1.73 m2 [66.67% (n = 8)],7,8,16-19,23,24 the GFR, estimated by the CKD-EPI equation based on cystatin C, above 60 mL/min/1.73 m2, and normal albuminuria [8,33% (n = 1)]15 and serum creatinine levels below 115 µmol/L [8,33% (n = 1)].20 Two studies (16.67%)21,22 did not mention the method of evaluation of renal function.
Table 2.
Method of dosing cystatin C and criteria for the definition of normal renal function in the selected studies
| Author/Year | Method of dosing cystatin C | Criteria used to define normal renal function |
|---|---|---|
| Sai et al., 201619 | Immunoturbimetry | GFR calculated using the MDRD equation > 60 mL/min/1.73m2 |
| Bansal et al., 201615 | Immunonephelometry | GFR based on cystatin C using the equation CKD-EPI > 60 mL/min/1.73 m2 and normal albuminuria |
| Abid et al., 20167 | Immunoturbimetry | GFR calculated using the MDRD equation > 60 mL/min/1.73 m2 |
| Woitas et al., 201318 | Immunonephelometry | GFR calculated using the MDRD equation > 60 mL/min/1.73 m2 |
| Dupont et al., 20128 | Immunoturbimetry | GFR calculated using the MDRD equation > 60 mL/min/1.73 m2 |
| Gao et al., 201121 | NI | NI |
| Keller et al., 200917 | Immunonephelometry | GFR calculated using the MDRD equation > 60 mL/min/1.73 m2 |
| Gao et al., 200922 | Enzyme immunoassay | NI |
| Alehagen et al., 200920 | Immunoturbimetry | Creatinine < 115 µmol/L |
| Acuna et al., 200916 | Immunonephelometry | GFR calculated using the MDRD equation > 60 mL/min/1.73 m2 |
| Koenig et al., 200724 | NI | GFR calculated using the MDRD equation > 60 mL/min/1.73 m2 |
| Ix et al., 200723 | Immunonephelometry | GFR calculated using the MDRD equation > 60 mL/min/1.73 m2 |
MDRD: Modification of diet in renal disease; NI: Not informed; GFR: Glomerular filtration rate.
Classification of patients and variables included in the multivariate regression analysis
The way patients were classified in each of the selected studies, and the variables included in the multivariate Cox proportional hazards regression analysis are presented in Table 3, while the results of the studies are presented in Table 4. Among the studies included in this systematic review, five (41.66%)8,17,18,20,23 classified patients according to cystatin C quartiles; three (25%)8,21 classified patients according to whether or not there were fatal or non-fatal cardiovascular events; two (16.66%)19,21 divided the patients according to the median of cystatin C; one study (8.33%)17 classified patients according to whether or not they developed cardiovascular death; another study (8.33%)18 compared patients with coronary disease in relation to the healthy control group; a study (8.33%)22 classified the patients into four groups: stable angina, unstable angina, AMI and healthy control group; another study (8.33%)15 classified patients according to the GFR estimated by the CKD-EPI equation based on cystatin C: between 60 and 75 mL/min/1.73 m2; between 76 and 90 mL/min/1.73 m2; and above 90 mL/min/1.73 m2; two other studies (16.66%)7,16 further divided patients into two groups according to cystatin C levels above or below 0.95 mg/L and above and below 1.2 mg/L; and one study24 divided them according to high or low levels of cystatin C without mentioning the cutoff point.
Table 3.
Classification of patients and variables included in multivariate regression analysis of Cox proportional hazards in selected studies
| Author/Year | Classification of patients | Variables included in the multivariate regression analysis |
|---|---|---|
| Sai et al., 201619 | Patients with cystatin C levels above (n = 138) and below (n = 139) median. (Median = 0.637) | BMI, hypertension, HbA1c, HDL, BNP, cystatin C. |
| Bansal et al., 201615 | GFR between 60 and 75 mL/min/1.73 m2 (n = 29).GFR between 76 and 90 mL/min/1.73m2 (n = 153).GFR > 90 mL/min/1.73 m2 (n = 2228). | Age, gender, race, smoking, DM, LDL, HDL, albuminuria, BMI, systolic blood pressure. |
| Abid et al., 20167 | Patients who developed fatal (n = 6) or non-fatal (n = 26) cardiovascular events and patients who did not develop these events.Patients with cystatin C levels> 1.2 mg/L and <1.2 mg/L | NA |
| Woitas et al., 201318 | Patients with coronary disease (n = 2,346) and control group (n = 652).First quartile < 0.8 mg/L (n = 731).Second quartile 0.81 to 0.91 mg/L (n=769).Third quartile 0.91 to 1.06 mg/L (n=752).Fourth quartile > 1.07 mg/L (n=746) | Hypertension, HDL, LDL, triglycerides, statin use, smoking, DM, usPCR, GFR CKD-EPI based on creatinine, age, gender, BMI |
| Dupont et al., 20128 | Cystatin C quartiles. | NA |
| Gao et al., 201121 | Patients who developed fatal or non-fatal (n = 21) cardiovascular events and patients who did not develop these events (n = 117).Patients with cystatin C levels above the median and below the median (0.9 mg/L). | Male gender, history of hypertension, high creatinine, reduced triglycerides, high homocysteine, high usPCR, high cystatin C. |
| Keller et al., 200917 | Patients with cardiovascular death (n = 66) and patients without cardiovascular death (n = 1761).Cystatin C quartiles. | Age, gender, BMI, smoking, DM, hypertension, LDL/HDL ratio, PCR, GNP. |
| Gao et al., 200922 | Patients with stable angina (n = 34), patients with unstable angina (n = 56), patients with AMI (n = 36) and control group (n = 34).Patients who developed fatal or non-fatal (n = 26) cardiovascular events and patients who did not develop these events (n = 22). | NA |
| Alehagen et al., 200920 | First quartile: < 1.22 mg/L (n = 109).Second quartile: 1.22 to 1.42 mg/L (n = 120).Third quartile: 1.43 to 1.66 mg/L (n = 117).Fourth quartile: > < 1.66 mg/L (n = 118). | NA |
| Acuna et al., 200916 | Patients with cystatin C levels> 0.95 mg/L (n = 63) and ≤ 0.95 mg/L (n = 76) | NA |
| Koenig et al., 200724 | Patients with high (n = 1261) and reduced levels of cystatin C (n = 1347) | NA |
| Ix et al., 200723 | First quartile: ≤ <0.91 mg/L (n = 239).Second quartile: 0.92 to 1.05 mg/L (n = 248).Third quartile: 1.06 to 1.29 mg/L (n = 262).Fourth quartile:> ≥ <1.30 mg/L (n = 241). | Age, gender, race, smoking, DM, hypertension, previous AMI, smoking, HDL, BMI, CRP. |
DM: Diabetes mellitus; HDL-high density lipoprotein; AMI: Acute Myocardial Infarction; BMI: Body mass index; LDL: low density lipoprotein; NA: Not applicable; CRP: C-reactive protein; GFR: Glomerular filtration rate; usPCR: Ultra-sensitive C-reactive protein.
Table 4.
Results of selected studies
| Author/Year | Result |
|---|---|
| Sai et al., 201619 | Proportion of patients with cystatin C levels> 0.637 mg/L who developed fatal or non-fatal cardiovascular events was higher than in patients with cystatin C < 0.637 mg/L [22 (15.9%) x 7 (5, 0%), p = 0.0025].Risk of fatal or non - fatal cardiovascular events in patients with cystatin C levels > 0.637 mg/L was greater than in patients with cystatin levels < 0.637 mg/L [(univariate) HR = 1.37 (1.10 - 1.66), p = 0.004; HR (multivariate) = 1.30 (1.01 - 1.63), p = 0.0038]. |
| Bansal et al., 201615 | Risk of left ventricle hypertrophy was higher in patients with GFR between 60 and 75 ml/min/1.73 m2 than in those with GFR > 90 ml/min/1.73 m2 [(univariate) HR = 10.12 (5.22 - 15.02), p < 0.001; HR (multivariate analysis) = 5.63 (0.90 - 10.36), p = 0.02]Risk of left ventricular hypertrophy was higher in patients with GFR between 76 and 90 mL/min/1.73m2 than in those with GFR> 90 mL/min/1.73 m2 [HR (univariate analysis) = 3.48 (1, 29 - 5.68), p = 0.002]. |
| Abid et al., 20167 | Patients who developed non-fatal cardiovascular events showed higher levels of cystatin C compared to patients who did not develop these events (1.19 ± 0.4 mg/L x 1.01 ± 0.35 mg/L, p = 0.01)Patients who developed fatal cardiovascular events showed higher levels of cystatin C compared to patients who did not develop these events (1.21 ± 0.36 mg/L x 0.96 ± 0.27 mg/L, p = 0.03)Survival of patients with cystatin C levels < 1.2 mg/L was higher than in patients with cystatin levels > 1.2 mg/L (p < 0.01). |
| Woitas et al., 201318 | Patients with CAD showed higher levels of cystatin C than the control group (1.02 ± 0.44 mg/L x 0.92 ± 0.26 mg/L, p = 0.065Risk of cardiovascular death and death from any cause of fourth quartile patients was higher than that of first quartile patients [HR (univariate) = 4.82 (3.69 - 6.29), p < 0.001; HR (multivariate) = 2.05 (1.48 - 2.84), p < 0.001].Risk of cardiovascular death and death from any cause of third quartile patients was higher than that of first quartile patients [HR (univariate) = 2.11 (1.58 - 2.81), p < 0.001; HR (multivariate) = 1.20 (0.88 - 1.65), p < 0.243]. |
| Dupont et al., 20128 | Risk of death from any cause and non-fatal cardiovascular event of patients in the fourth quartile was higher than in patients in the first quartile (p = 0.002). |
| Gao et al., 201121 | Patients who developed fatal or non-fatal cardiovascular events showed higher levels of cystatin C compared to patients who did not develop these events (1.63 ± 0.81 mg/L x 0.91 ± 0.27 mg/L, p = 0.001)Risk of fatal or non-fatal cardiovascular events in patients with cystatin C levels> 0,9 mg/L was higher than in patients with cystatin levels < 0.9 mg/L [(univariate) HR = 3.58 (2.61 - 4.82), p = 0.033; HR (multivariate) = 7.10 (3.36 - 23.75), p = 0,006]. |
| Keller et al., 200917 | Patients with cardiovascular death had higher levels of cystatin C than patients without cardiovascular death [0.94 (0.79 - 1.08 x 0.79 (0.70 - 0.90), p < 0.001].Risk of cardiovascular death of patients in the fourth quartile was higher than in patients in the other quartiles [OD (univariate) = 3.87 (2.33-6.42), p < 0.001; OD (multivariate) = 1.86 (0.90-3.81), p = 0.09]. |
| Gao et al., 200922 | Patients with AMI and unstable angina had higher levels of cystatin C than the control group (2873.55 ± 1148.48 ng/mL x 1509.99 ± 408.65 ng/mL, p < 0.01 and 2013.83 ± 633.85 ng/mL x 1509.99 ± 408.65 ng/mL, p < 0.05, respectively).Patients with AMI and unstable angina had higher levels of cystatin C than the patients with stable angina (2873.55 ± 1148.48 ng/mL x 1348.41 ± 369.62 ng/mL, p < 0.01 and 2013.83 ± 633.85 ng/mL x 1348.41 ± 369.62 ng/mL, p < 0.01, respectively).Patients with AMI had higher levels of cystatin C than the patients with stable angina (2873.55 ± 1148.48 ng/mL x 2013.83 ± 633.85 ng/mL, p < 0.05).Patients who developed fatal or non-fatal cardiovascular events showed higher levels of cystatin C compared to patients who did not develop these events (2356,73 ± 897,64 ng/L x 1469.51 ± 574.83 ng/L, p = 0.006) |
| Alehagen et al., 200920 | Risk of cardiovascular death of fourth quartile patients was higher than that of first quartile patients [HR (univariate analysis) = 3.61 (1.81 - 7.14)]. |
| Acuna et al., 200916 | The proportion of patients with cystatin C levels > 0.95 mg/L who had cardiovascular death was higher than that of patients with cystatin C levels ≤ 0.95 mg/L [16 (27.1%) x 6 (7.8%), p = 0.01].The proportion of patients with cystatin C levels> 0.95 mg/L who develop HF was higher than that of patients with cystatin C levels ≤ 0.95 mg/L [22 (40.7%) x 6 (7.5%), p = 0.01]. |
| Koenig et al., 200724 | Each increase of 0.18 mg/L cystatin C was associated with an increased risk of cardiovascular death [OD = 1.42 (1.30 -1.54)], death from any cause [OD = 1.33 1.25-1.40)], HF [OD = 1.28 (1.17-1.40)], stroke [OD = 1.22 (1.08-1.38)] and AMI [OD = 1.20 (1.06-1.36)].Patients with high levels of cystatin C had more adverse events than those with reduced levels of cystatin C (p < 0.001). |
| Ix et al., 200723 | Risk of death from any cause of fourth quartile patients was higher than that of first quartile patients [HR (univariate) = 5,7 (3,1 - 10,5), p < 0.001; HR (multivariate) = 3,6 (1,8 - 7,0), p < 0.001].Risk of cardiovascular events of fourth quartile patients was higher than that of first quartile patients [HR (univariate) = 3.8 (2.1 - 6.9), p < 0.001; HR (multivariate) = 2.0 (1.0 - 3.8), p < 0.04].Risk of CHF in patients in the fourth quartile was higher than in patients in the first quartile [HR (univariate) = 6.1 (2.5 - 14.5), p = 0.001; HR (multivariate) = 2.6 (1.0 - 6.9), p = 0.05]. |
CAD: Coronary artery disease; AMI: Acute Myocardial Infarction; GFR: Glomerular filtration rate; HR: Hazard Ratio.
Studies results
Among the included studies, two (16.66%)16,19 analyzed the difference between the proportion of patients with high levels of cystatin C who developed fatal or non-fatal cardiovascular events,19 cardiovascular death,16 and CHF16 compared with the proportion of patients with reduced levels of Cystatin C that developed these events, and all of them found a significant difference. A study (8.33%)24 further observed that patients with high levels of cystatin C had more adverse cardiovascular events than those with reduced levels of cystatin C. The difference between cystatin C levels in patients who developed fatal or non-fatal cardiovascular events, and those who did not develop these events was evaluated by four studies (33.33%),7,17,19,21 and all found significantly higher levels of cystatin C in the group of patients who developed the events. A study (8.33%)18 also found that cystatin C levels in patients with CAD were higher than in the control group and another study (8.33%)22 observed that cystatin C levels in patients with AMI were higher than in patients with unstable angina, stable angina, and control group, and that cystatin C levels in patients with unstable angina were higher than in those with stable angina and control group. Another study (8.33%)7 found a higher survival rate in patients with lower levels of cystatin C.
The risk of developing adverse outcomes was assessed by eight studies (66.66%)15,17-21,23,24 calculating the hazard ratio. Among these, two studies (22,22%)19,21 found an increased risk of fatal or non-fatal cardiovascular events in patients with higher levels of cystatin C; one study (11.11%)18 observed a higher risk of death from any cause and non-fatal cardiovascular events; another study found an increased risk of cardiovascular death and death from any cause; two studies (22.22%)17,20 found an increased risk of cardiovascular death; one study (11.11%)23 found an increased risk of death from any cause, cardiovascular events and CHF; and one study (11.11%)15 still observed a higher risk of left ventricular hypertrophy. Finally, one study24 found that each increase of 0.18 mg/L of cystatin C was associated with an increased risk of cardiovascular death, death from any cause, HF, stroke and AMI. The multivariate regression analysis was performed by six (50%)15,17-19,21,23 of these studies, with the risk of developing evaluated adverse outcomes remaining significant after the performance of this analysis in four of these studies.18,19,21,23
Methodological quality
The results of the evaluation of the methodological quality of the studies included in this review are shown in Table 5, and the detailed description of the criteria used for the distribution of the stars is presented in the legend. After the quality analysis, a study (8.33%)22 was found to have good methodological quality and 11 studies (91.66%) had excellent methodological quality.
Table 5.
Evaluation of study quality according to Newcastle-Ottawa Scale
| Author/Year | Selection 1 2 3 4 | Comparability 5 | Outcomes 6 7 8 | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sai et al., 201619 | * | * | * | - | ** | * | * | * | 8 |
| Bansal et al., 201615 | * | * | * | - | ** | * | * | * | 8 |
| Abid et al., 20167 | * | * | * | - | * | * | * | * | 7 |
| Woitas et al., 201318 | * | * | * | - | ** | * | * | * | 8 |
| Dupont et al., 20128 | * | * | * | - | * | * | * | * | 7 |
| Gao et al., 201121 | * | * | - | - | ** | * | * | * | 7 |
| Keller et al., 200917 | * | * | * | - | ** | * | * | * | 8 |
| Gao et al., 200922 | * | * | - | - | * | * | - | * | 5 |
| Alehagen et al., 200920 | * | * | * | - | * | * | * | * | 7 |
| Acuna et al., 200916 | * | * | * | - | * | * | * | * | 7 |
| Koenig et al., 200724 | * | * | - | * | * | * | * | * | 7 |
| Ix et al., 200723 | * | * | * | - | ** | * | * | * | 9 |
1 - Representativeness of the exposed cohort: all the studies received one star, because the exposed cohort was a little representative of the average in the community; 2 - Selection of the unexposed cohort: all studies received one star, because the unexposed cohort was obtained in the same community of the exposed cohort; 3- Determination of exposure: only studies that dosed cystatin C using the immunonephelometry or immunoturbidimetry methods received a star; 4 - Demonstration that the outcome of interest was not present at the beginning of the study: studies in which patients did not present any cardiovascular disease at the beginning of the study received one star; 5 - Cohort comparability based on design and analysis: studies that performed multivariate regression analysis of Cox proportional hazards and defined normal renal function as GFR > 60 mL/min/1.73 m2 received 2 stars. Studies that only defined normal renal function as GFR > 60 mL/min/1.73 m2 but did not perform multivariate regression analysis of Cox proportional hazards received 1 star. 6 - Determination of outcome: all studies received one star, because the evaluation of the outcome was performed by the physicians independently; 7 - Adequate follow-up period for the occurrence of outcome (s): studies in which patients were followed for at least six months received one star, and studies in which patients were followed for less than six months did not receive a star; 8 - Adequacy of the follow-up period of the cohort: studies in which at least 90% of the patients were followed to the end or who did not comment if there were significant loss of patients during follow-up received one star.
Meta-analysis
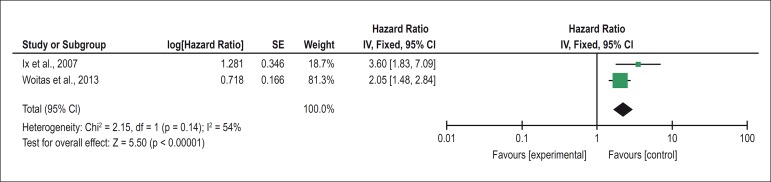
Only two studies evaluated the outcome of all-cause mortality, compared the fourth quartile of cystatin C with the first quartile, and performed a multivariate regression analysis of Cox proportional hazards and were therefore included in the meta-analysis, the result of which is shown in Figure 2. Homogeneity was observed among the studies (I2 = 53,423 and p = 0,14); therefore, the fixed-effect model was used to calculate the hazard ratio. The result of the meta-analysis [HR = 2.28 (1.70 - 3.05), p < 0.001] indicates that there is a significant association between high levels of cystatin C and the risk of all-cause mortality in individuals with normal renal function. A symmetric distribution of the articles included in the meta-analysis was observed in the funnel plot, indicating that there is no publication bias.
Figure 2.
Metanalysis of studies evaluating the association between high levels of cystatin C and the risk of mortality from any cause through the comparison between the fourth and first quartiles of cystatin C.
Discussion
The present study aimed to evaluate the association between high levels of cystatin C and the risk of cardiovascular events or mortality in subjects with normal renal function through a systematic review of the scientific literature and meta-analysis.
The difference between the proportion of patients with high levels of cystatin C who developed cardiovascular events or mortality, compared with the proportion of patients with reduced levels of Cystatin C that developed these events was evaluated by two studies and both of them found a significant difference. The difference between cystatin C levels in patients who developed fatal or non-fatal cardiovascular events and those who did not develop these events was assessed by four studies (33.3%) and all found significantly higher levels of cystatin C in the group of patients who had the events. The risk of developing adverse outcomes was assessed by eight studies (66.66%) calculating the hazard ratio. Among these, six studies found an increased risk of cardiovascular events or mortality. The multivariate regression analysis was performed by six (50%) of these studies, with the risk of developing the adverse outcomes remaining significant after the performance of this analysis in four of these studies.
The meta-analysis also demonstrated that there is a significant association between high levels of cystatin C and the risk of all-cause mortality. Thus, the results presented by the studies included in this systematic review and meta-analysis indicate that there is a significant association between high levels of cystatin C and the development of cardiovascular events or mortality in subjects with normal renal function assessed by serum creatinine-based GFR.
A possible mechanism for the association between high levels of cystatin C and the development of cardiovascular events is related to the atherogenic process. The development of lesions in the arteries endothelium results in the accumulation of cholesterol in the artery wall, and in the development of the atherosclerotic plaque.25 It has been suggested that lysosomal cathepsins, whose production is stimulated by inflammatory cytokines, may contribute to the degradation of the atherosclerotic plaque. As cystatin C is able to inhibit lysosomal cathepsins, it is possible to suggest that elevated levels of cystatin C may contribute to non-degradation of atherosclerotic plaque, resulting in increased risk of cardiovascular events.26,27
Another possible mechanism is related to the fact that cystatin C presents a greater sensitivity for the detection of the initial stages of renal dysfunction than serum creatinine or creatinine-based GFR.28,29 Several authors have already demonstrated that renal dysfunction is associated with an increased risk of cardiovascular events.30,31 Thus, it is possible to suggest that patients who have normal renal function assessed by GFR based on creatinine or serum creatinine but who have high levels of cystatin C may present with renal dysfunction at an earlier stage, which could be associated with an increased risk of cardiovascular events.
Although cystatin C is a more sensitive marker for detecting the early stages of CKD than creatinine, especially in groups at risk for CKD, such as patients with diabetes mellitus and renal transplant recipients, it has some limitations.32,33 High doses of glucocorticoids and hyperthyroidism may result in increased serum levels of cystatin C, whereas hypothyroidism may result in a decrease.34 Some factors, such as age, male gender, body weight, smoking, C-reactive protein, cancer, inflammatory processes and steroid therapy may also influence serum levels of cystatin C, limiting its assessment in clinical practice.35
Renal weight and volume decrease gradually between the ages of 30 and 90 years, resulting in a natural decline of renal function with increasing age.36 Thus, elderly patients have a lower GFR, which may be associated with higher levels of cystatin C and an increased risk of cardiovascular events.28 As most of the studies that performed the multivariate regression analysis [66.66% (n = 4)]15,17,18,23 included age in this analysis, and nonetheless found a significant association between high levels of cystatin C and the development of adverse outcomes, it is possible to conclude that this association is age-independent. It should be noted that the two studies20,25 that were included in the meta-analysis are among these studies that included age in the multivariate regression analysis, indicating that the association between high levels of cystatin C and any cause-related mortality observed in meta-analysis is age-independent.
All selected studies have described the renal function of patients as being normal. The estimated GFR calculated by the MDRD formula, greater than 60 mL/min/1.73 m2, was used as a criterion for normal renal function in 66.67% of the studies, and 8.33% used serum creatinine levels below 115 µmol/L. The estimated GFR is a better marker for renal function evaluation than serum creatinine, because it undergoes interference of muscle mass, gender, age, physical activity and diet. Moreover, unlike GFR, serum creatinine is not able to detect the presence of chronic renal disease early because its levels increase only when renal disease is already at an advanced stage.31 The inclusion of individuals with estimated GFR greater than 60 mL/min/1.73 m2 by most studies, including studies of the meta-analysis, supports the information that the association between high levels of cystatin C and the risk of cardiovascular events or mortality is not dependent on the renal function of the patient evaluated by creatinine-based estimated GFR, which is a marker that has good sensitivity for the detection of renal dysfunction in the early stages.
Immunonephelometry and immunoturbidimetry were the most commonly used methods [75% (n = 9)] for the laboratory dosage of cystatin C and were even used by the studies included in the meta-analysis. These methods have good precision, specificity, adequate time to result, and minimum amount of sample required, being the methods of choice for cystatin C37,38 dosage. Therefore, the use of these methods by most of the studies included in the systematic review brings greater reliability to the results.
The sample size of the studies ranged from 127 to 4,663 individuals, with most of them having more than 400 individuals [58.33% (n = 7)].8,15,17,18,20,23,24 The study7 that obtained the smallest sample size still included more than 100 individuals, which can be considered a significant number if the follow-up is performed for an adequate time.39 It should be noted that this study found a significant difference between patients who developed fatal or non-fatal cardiovascular events and those who did not develop these events.
This systematic review had some limitations, such as the population studied, which varied widely among the studies. Only one study24 included healthy elderly subjects, while the population of the other studies consisted of patients at risk for cardiovascular events,15 with STEMI and NSTEMI,7,16 with stable CAD,17,18 SCA,17 patients undergoing percutaneous coronary intervention,19 with CHF,20,21 with CHF who underwent coronary angiography,8 with stable angina and AMI,22 and with a history of AMI that had angiographic evidence of stenosis greater than 50%.23 This variation may lead to bias in the results, because cardiovascular impairment varied among the populations at the beginning of the studies, which may influence cystatin C levels, since patients with CHF or AMI could present higher levels of cystatin C at the beginning of the study if compared to patients who only present risk of cardiovascular events.23 Since most studies evaluated a population at risk of cardiovascular events or who already have some degree of cardiovascular impairment, it is possible to suggest that cystatin C is an interesting marker for assessing the risk of cardiovascular events or mortality in these population groups and may complement the currently available markers.
In addition to the variation of the study population, follow-up time, patient classification, and outcomes also varied widely across studies. The follow-up time ranged from six months to ten years, and three studies (25%)7,16,22 followed the patients for less than 15 months and four studies (33.33%)15,18,20,24 have followed for more than nine years. The prevalent time of follow-up of the studies was three to six years [41.67% (n = 5)].8,17,19,21,23 The follow-up time should be adequate for the outcome to be observed, and should be greater for the detection of mortality than for cardiovascular events. The study22 with shorter follow-up (6 months) found higher levels of cystatin C among patients who developed fatal and non-fatal cardiovascular events compared to patients who did not develop these outcomes, indicating that even shorter follow-up time was sufficient for the detection of both outcomes and for the observation of a significant association with Cystatin C levels. Both studies included in the meta-analysis assessed the outcome for all-cause mortality. One of them followed the patients for three years and the other for ten years, with these times being adequate for the evaluation of the outcome.
Patients classification to carry out the statistical analysis also varied considerably among the studies. Only five studies (41.66%),8,17,18,20,23 including the studies of the meta-analysis, classified patients according to quartiles of cystatin C, which is the best classification to establish a cutoff point above which the risk of developing cardiovascular events or mortality would be higher.
Despite these study limitations, of the articles selected in this systematic review, 11 have excellent methodological quality and only one has good quality.
Conclusion
The systematic review has shown that there is a significant association between high levels of cystatin C and the risk of cardiovascular events or mortality in subjects with normal renal function. The meta-analysis also demonstrated that there is a significant association between high levels of cystatin C and the risk of all-cause mortality. As individuals included in the studies had normal renal function, it is possible to conclude that the association between high levels of cystatin C and the risk of cardiovascular events or mortality does not depend on the presence of renal dysfunction assessed by serum creatinine-based GFR. Therefore, cystatin C is a very interesting marker to assess the risk of cardiovascular events or mortality, especially in populations at risk of cardiovascular events or that already have some degree of cardiovascular impairment, and can complement the currently available markers.
Footnotes
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Author contributions
Conception and design of the research, Acquisition of data, Analysis and interpretation of the data, Statistical analysis e Writing of the manuscript: Einwoegerer CF, Domingueti CP; Critical revision of the manuscript for intellectual content: Domingueti CP.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Organização Pan Americana de Saúde [Internet] [2018 abr 10]. Disponível em: https://www.paho.org/bra/index.php?option=com_content&view=article&id=5253:doencas-cardiovasculares&Itemid=839.
- 2.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European Society of Cardiology Committee for Practice Guidelines European guidelines on cardiovascular disease prevention in clinical practice: third joint task force of European and other societies on cardiovascular disease prevention in clinical practive (constituted by representatives of eight societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2003;10(4):S1–10. doi: 10.1097/01.hjr.0000087913.96265.e2. [DOI] [PubMed] [Google Scholar]
- 3.Bi M, Huang Z, Li P, Cheng C, Huang Y, Chen W. The association between elevated cystatin C levels with myocardial infarction: a meta-analysis. Int J Clin Exp Med. 2015;8(11):20540–20547. [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Zhang L, Yue R, You G, Zeng R. Significance of cystatin C for early diagnosis of contrast-induced nephropathy in patients undergoing coronary angiography. Med Sci Monit. 2016;22:2956–2961. doi: 10.12659/MSM.897241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lameire N, Vanholder R, Biesen WV, Benoit D. Acute kidney injury in critically ill cancer patients: an update. Crit Care. 2016;20(1):209–209. doi: 10.1186/s13054-016-1382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bongartz LG, Cramer MJ, Braam B. The cardiorenal connection. Hypertension. 2004;43(4):e14. doi: 10.1161/01.HYP.0000118521.06245.b8. [DOI] [PubMed] [Google Scholar]
- 7.Abid L, Charfeddine S, Kammoun S, Turki M, Ayedi F. Cystatin C: a prognostic marker after myocardial infarction in patients without chronic kidney disease. J Saudi Heart Assoc. 2016;28(3):144–151. doi: 10.1016/j.jsha.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupont M, Wu Y, Hazen SL, Tang WH. Cystatin C identifies patients with stable chronic heart failure at increased risk for adverse cardiovascular events. Circ. Heart failure. 2012;5(5):602–609. doi: 10.1161/CIRCHEARTFAILURE.112.966960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R, Hao P, Chen Y, Zhang Y. Association of cystatin C level and cardiovascular prognosis for patients with preexisting coronary heart disease: a meta-analysis. Chin Sci Bulletin. 2014;59(5-6):539–545. [Google Scholar]
- 10.Bi M, Huang Z, Li P, Cheng C, Huang Y, Chen W. The association between elevated cystatin C levels with myocardial infarction: a meta-analysis. Int J Clin Exp Med. 2015;8(11):20540–20547. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee M, Saver JL, Huang WH, Chow J, Chang KH, Ovbiagele B. Impact of elevated cystatin C level on cardiovascular disease risk in predominantly high cardiovascular risk populations: a meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3(6):675–683. doi: 10.1161/CIRCOUTCOMES.110.957696. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Wang LP, Hu HF, Zhang L, Li YL, Ai LM, et al. Cystatin C and cardiovascular or all-cause mortality risk in the general population: a meta-analysis. Clin Chim Acta. 2015 Oct 23;450:39–45. doi: 10.1016/j.cca.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analysis [Internet] [2017 Sep 1]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15.Bansal N, Lin F, Vittinghoff E, Peralta C, Lima J, Kramer H, et al. Estimated GFR and subsequent higher left ventricular mass in young and middle-aged adults with normal kidney function: the coronary artery risk development in young adults (CARDIA) study. Am J Kidney Dis. 2016;67(2):227–234. doi: 10.1053/j.ajkd.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García Acuña JM, González-Babarro E, Grigorian Shamagian L, Peña-Gil C, Vidal Pérez R, López-Lago AM, et al. Cystatin C provides more information than other renal function parameters for stratifying risk in patients with acute coronary syndrome. Rev Esp Cardiol. 2009;62(5):510–519. doi: 10.1016/s1885-5857(09)71833-x. [DOI] [PubMed] [Google Scholar]
- 17.Keller T, Martina CM, Lubos E, Nicaud V, Wild SP, Rupprecht HJ, et al. Cystatin C and cardiovascular mortality in patients with coronary artery disease and normal or mildly reduced kidney function: results from the AtheroGene study. Eur Heart J. 2009;30(3):314–320. doi: 10.1093/eurheartj/ehn598. [DOI] [PubMed] [Google Scholar]
- 18.Woitas RP, Kleber ME, Meinitzer A, Grammer TB, Silbernagel G, Stefan Pilz, et al. Cystatin C is independently associated with total and cardiovascular mortality in individuals undergoing coronary angiography. The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Atherosclerosis. 2013;229(2):541–548. doi: 10.1016/j.atherosclerosis.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Sai E, Shimada K, Miyauchi K, Masaki Y, Kojima T, Miyazaki T, et al. Increased cystatin C levels as a risk factor of cardiovascular events in patients with preserved estimated glomerular filtration rate after elective percutaneous coronary intervention with drug-eluting stents. Heart Vessels. 2016;31(5):694–701. doi: 10.1007/s00380-015-0674-0. [DOI] [PubMed] [Google Scholar]
- 20.Alehagen U, Dahlström U, Lindahl TL. Cystatin C and NT-proBNP, a powerful combination of biomarkers for predicting cardiovascular mortality in elderly patients with heart failure: results from a 10-year study in primary care. Eur J Heart Fail. 2009;11(4):354–360. doi: 10.1093/eurjhf/hfp024. [DOI] [PubMed] [Google Scholar]
- 21.Gao C, Zhong L, Gao Y, Li X, Zhang M, Wei S. Cystatin C levels are associated with the prognosis of systolic heart failure patients. Arch Cardiovasc Dis. 2011;104(11):565–571. doi: 10.1016/j.acvd.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Ge C, Ren F, Lu S, Ji F, Chen X, Wu X. Clinical prognostic significance of plasma cystatin C levels among patients with acute coronary syndrome. Clin Cardiol. 2009;32(11):644–648. doi: 10.1002/clc.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of Cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease data from the Heart and Soul Study. Circulation. 2007;115(2):173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koening W. Is elevated cystatin C a predictor of cardiovascular risk in elderly people without chronic kidney disease? Nat Clin Pract Cardiovasc Med. 2007;4(2):76–77. doi: 10.1038/ncpcardio0769. [DOI] [PubMed] [Google Scholar]
- 25.Guyton AC, Hall JE. Tratado de Fisiologia Médica. 12ª. ed. Rio de Janeiro: Elsevier; 2011. pp. 870–871. [Google Scholar]
- 26.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(8):1359–1366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson P, Jones KG, Brown LC, Greenhalgh RM, Hamsten A, Powell JT. Genetic approach to the role of cysteine proteases in the expansion of abdominal aortic aneurysms. Br J Surg. 2004;91(1):86–89. doi: 10.1002/bjs.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prates AB, Amaral FB, Vacaro MZ, Gross JL, Camargo JL, Silveiro SP. Glomerular filtration evaluation employing serum cystatin C measurement. J Bras Nefrol. 2013;35(1):48–56. [Google Scholar]
- 29.Gabriel IC, Nishida SK, Kirsztajn GM. [Serum cystatin C: a practical alternative for renal function evaluation?] J Bras Nefrol. 2011;33(2):261–267. doi: 10.1590/s0101-28002011000200023. [DOI] [PubMed] [Google Scholar]
- 30.Moura RS. Cistatina C em pacientes com hipertensão arterial essencial: Avaliação da função renal e correlação com fatores de risco cardiovascular. Brasília: Universidade de Brasília; 2010. [Dissertação] [Google Scholar]
- 31.Porto JR, Gomes KB, Fernandes AP, Domingueti CP. Avaliação da função renal na doença renal crônica. RBAC. 2017;49(1):26–35. [Google Scholar]
- 32.Pucci L, Triscorna S, Lucchesi D, Fotino C, Pellegrini G, Pardini E, et al. Cystatin C and estimatives of renal function: searching a better measure of kidney function in diabetic patients. Clin Chem. 2007;53(3):480–488. doi: 10.1373/clinchem.2006.076042. [DOI] [PubMed] [Google Scholar]
- 33.Le Bricon T, Thervet E, Froissart M, Benlakehal M, Bousquet B, Legendre C, et al. Plasma cystatin C is superior to 24 h creatinine clearance and plasma creatinine for estimation of glomerular filtration rate 3 months after kidney transplantation. Pt 1Clin Chem. 2000;46(8):1206–1207. [PubMed] [Google Scholar]
- 34.Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A. Cystatin C as a marker of GFR-history, indications, and future research. Clin Biochem. 2005;38(1):1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Macissac RJ, Premaratne E, Jerums G. Estimating glomerular filtration rate in diabetes using serum cystatin C. Clin Biochem Rev. 2011;32(2):61–67. [PMC free article] [PubMed] [Google Scholar]
- 36.Abreu PF, Sesso RC, Ramos LR. Aspectos renais no idoso. J Bras Nefrol. 1998;20(2):158–165. [Google Scholar]
- 37.Neri LA, Mendes ME, Neto ED, Sumita NM, Medeiros FS. Determinação de cistatina C como marcador de função renal. J Bras Patol Med Lab. 2010;46(6):443–453. [Google Scholar]
- 38.Soares JL, Rosa DD, Leite VR, Pasqualotto AC. Métodos diagnósticos: consulta rápida. 2ª. ed. Porto Alegre: Artmed; 2012. [Google Scholar]
- 39.Marotti J, Galhardo AP, Furuyama RJ, Pigozzo MN, Campos TN, Laganá DC. Amostragem em pesquisa clínica: tamanho da amostra. Rev Odont Univ Cid São Paulo. 2008;20(2):186–194. [Google Scholar]