Abstract
Background
Positive-pressure exhaust suits cost more than standard surgical gowns, and recent evidence suggests that they do not decrease infection risk. As a result, some hospitals and surgeons have abandoned positive-pressure exhaust suits in favor of less expensive alternatives. We propose that in addition to their original purpose of decreasing infection rates, positive-pressure exhaust suits may also improve personal protection for the surgeon and assistants, perhaps justifying their added costs.
Questions/purposes
(1) Do positive-pressure exhaust suits decrease exposure to particulate matter during TKA? (2) What areas covered by gowning systems are at risk of exposure to particulate matter?
Methods
Three surgical gowning systems were tested: (1) surgical gown, face mask, surgical skull cap, protective eyewear; (2) surgical gown, face mask, surgical protective hood, protective eyewear; and (3) positive-pressure exhaust suit. For each procedure, a cadaver knee was injected intraarticularly and intraosseously with a 5-µm fluorescent powder mixed with water (1 g/10 mL). After gowning in the standard sterile fashion, the primary surgeon and two assistants performed two TKAs with each gowning system for a total of six TKAs. After each procedure, three independent observers graded skin exposure of each surgical participant under ultraviolet light using a standardized scale from 0 (no exposure) to 4 (gross exposure). Statistical analysis was performed using Friedman’s and Nemenyi tests. The interrater reliability for the independent observers was also calculated.
Results
The positive-pressure exhaust suits had less surgeon and assistant exposure compared with other systems (p < 0.001). The median overall exposure grade for each gowning system was 4 for System 1 (range, 3–4), 2.5 for System 2 (range, 2–3), and 0 for System 3 (range, 0–0). In pairwise comparisons between gowning systems, the positive-pressure exhaust suits had less exposure than gowning System 1 (difference of medians: 4, p < 0.001) and gowning System 2 (difference of medians: 2.5, p = 0.038). There was no difference found in exposure between Systems 1 and 2 (difference of medians: 1.5, p = 0.330). When gowning Systems 1 and 2 were removed, particulate matter was found in places that were covered such as the surgeon’s beard, lips, inside the nostrils, behind the protective eyewear around the surgeon’s eye, and in both eyebrows and eyelashes.
Conclusions
The positive-pressure exhaust suits provided greater personal protection with each procedure than the other two gowning systems.
Clinical Relevance
With conventional gowns, particulate matter was found in the surgeon’s eyelashes, under the face mask around the mouth, and inside the nostrils. Despite recent evidence that certain types of positive-pressure exhaust suits may not decrease infection, there is a clear benefit of surgeon protection from potentially infectious and harmful patient substances. Despite their added costs, hospitals and surgeons should weigh this protective benefit when considering the use of positive-pressure exhaust suits.
Introduction
Orthopaedic procedures put surgeons and their assistants at risk for exposure to bloodborne pathogens such as HIV or hepatitis B/C through splash or dissemination of particulate matter mist from the patient created by orthopaedic instruments. These exposures are often unrecognized by the surgeon and can occur beyond the areas protected by traditional protection methods.
Negative-pressure body exhaust suits were pioneered by Dr John Charnley in the 1970s and their use was determined to decrease deep joint infections even further when compared with conventional pattern clothing [9]. This led to the widespread integration of these suits into practice with the eventual evolution into the modern positive-pressure exhaust suits used today. In contrast to early negative-pressure suits, with both air intake and exhaust tubing, positive-pressure systems have air that is drawn in through a helmet fan and flows out the bottom of the gown or any other gown opening [20]. Several recent studies have suggested that body exhaust suits may not provide more patient protection against microbial contamination when compared with conventional clothing [2, 11, 15]; one study found that positive-pressure suits may, in fact, increase the risk of infection [7]. Thus, their use and role in infection prevention remain controversial.
Positive-pressure suits cost more than conventional gowns, which typically incur negligible costs because they are often included in surgical draping bundles. Considering their controversial role in infection prevention and their increased cost, some hospitals and surgeons are abandoning these suits in favor of less expensive alternatives. It has been estimated that surgeons have a 42.7%, 34.8%, and 0.54% lifetime risk of acquiring hepatitis B virus, hepatitis C virus, and HIV, respectively [12]. As a result of this risk, surgeons are aware of the importance of protecting themselves from bloodborne pathogens, but much of this has focused on preventing sharps and needlestick injuries. Increased attention to the importance of protection by preventing mucous membrane exposure is warranted. Studies have shown that a single viral HIV particle can transmit the virus, and hepatitis C transmission through mucous membrane exposure has been estimated to be 0.24% [1, 14]. These less expensive alternatives may not provide the necessary protection of mucous membranes from bloodborne pathogens. We propose that in addition to their original purpose of decreasing patient infection rates, positive-pressure suits also provide improved personal protection for the orthopaedic surgeon and their surgical assistants. This alternative benefit may justify the use of these suits despite their higher cost.
Therefore, we asked: (1) Do positive-pressure exhaust suits decrease exposure to particulate matter during TKA? (2) What areas covered by gowning systems are at risk of exposure to particulate matter?
Materials and Methods
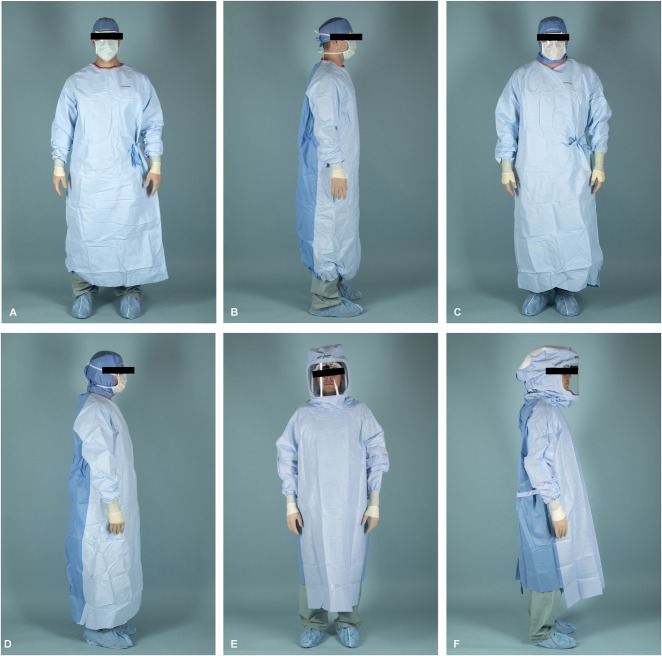
We tested three surgical gowning systems: (1) a conventional Halyard ULTRA Surgical gown (Halyard Health, Alpharetta, GA, USA), surgical skull cap, surgical face mask, and protective eyewear; (2) a conventional Halyard ULTRA Surgical gown (Halyard Health), surgical protective hood, surgical face mask, and protective eyewear; and (3) a Stryker one-piece toga (Stryker, Kalamazoo, MI, USA) positive-pressure exhaust suit (Fig. 1A-F).
Fig. 1A-F.
Front and side views of each tested gowning system are shown, including the conventional Halyard ULTRA Surgical gown (Halyard Health, Alpharetta, GA, USA), which includes a surgical skull cap, surgical face mask, and protective eyewear (A-B); a Halyard ULTRA surgical gown with a surgical protective hood, surgical face mask, and protective eyewear (Halyard Health; C-D); and a Stryker one-piece toga (Stryker, Kalamazoo, MI, USA; E-F).
A comparative study was conducted in a procedural laboratory without laminar flow and without pulse lavage irrigation on fresh-frozen disarticulated lower extremity cadaver specimens. Previous studies have determined that the size of organism-carrying particles range from 5 μm to 15 μm [3]. To simulate these particles, a 5-μm fluorescent powder (Glo Germ; Hygienic Solutions, Lincoln, UK) was used. Before each procedure, the 5-μm fluorescent powder was mixed in a syringe with water (1 g/10 mL) to achieve the consistency of patient particulate matter encountered during TKA. In all, 30 mL of this mixture was injected intraarticularly into the knee through a superolateral approach and 10 mL each intraosseously into the proximal tibia and distal femur. The mixture was allowed to passively diffuse for 10 minutes before beginning the procedure (Fig. 2A-B). Using one of the three tested surgical gowning systems, the primary surgeon, along with a first and second assistant, was gowned and gloved in the standard sterile fashion. At this point, an ultraviolet light was used to ensure that the surgeon’s and assistants’ skin surfaces were free of fluorescent exposure before beginning the procedure. If skin exposure was detected, the surgical gowning system was removed, the skin surface washed, and gowning and gloving performed again until the skin surface was free of fluorescent exposure before the procedure was started. The surgeon and assistants then performed a standardized, simulated TKA through a medial parapatellar approach following a primary TKA protocol. The procedure was concluded once implants were trialed and deemed acceptable. Two TKAs with three participants per procedure (surgeon and two assistants) were performed for each gowning system for a total of six TKAs (Fig. 3). The number of trials for each gowning system was determined by a previously published power analysis detecting a difference between contamination in a conventional gown versus a body exhaust suit [19].
Fig. 2A-B.
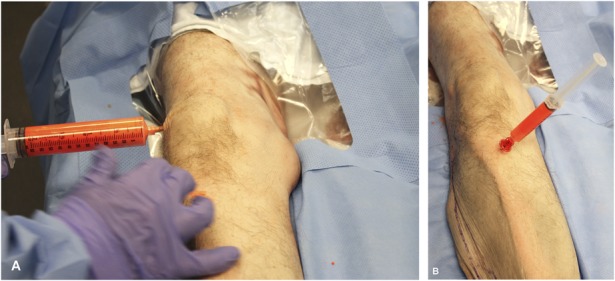
To simulate patient particulate matter, a 5-μm fluorescent powder was mixed with water and was injected intraarticularly into the knee (A) and intraosseously into the proximal tibia (B).
Fig. 3.
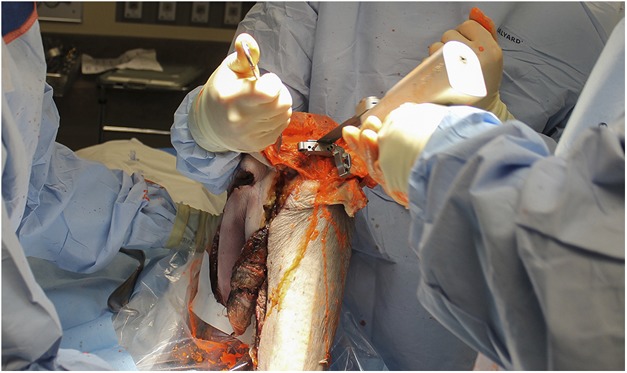
Two standardized, simulated TKAs were performed for each gowning system for a total of six procedures.
Once each procedure was completed, three independent observers used ultraviolet light to grade the exposure of each surgical participant from 0 to 4 using a previously published grading scale [5, 19]. Exposure was graded 0 (no exposure), 1 (1–5 specks), 2 (5–10 specks), 3 (10–100 specks), and 4 (> 100 specks). After grading, gowns were removed, and test subjects were reexamined for additional contamination potentially hidden in areas assumed to be protected by the gowning systems; however, scores were not changed. Additional areas of contamination were considered unexpected findings and reported descriptively. Each observer graded each observation independently with interrater reliability determined. The median grade of the three observers for each surgical participant was used for statistical analysis.
Statistical analysis was performed using an R package for Friedman’s test and post hoc tests [13]. Probability values were considered statistically significant when < 0.05. Friedman’s test was used to compare the levels of exposure for each surgical gowning system across the trials using the median grade for each surgical participant in each trial. Post hoc, the Nemenyi test was used to perform a pairwise comparison of each surgical gowning system based on the median grade of each participant in each trial.
Results
Of the three gowning systems tested, the positive-pressure exhaust suit had less surgeon and assistant exposure compared with the other systems (p < 0.001). The Stryker one-piece toga provided complete surgical participant protection with each procedure, whereas gowning Systems 1 and 2 had Grade 2 or higher particulate exposure with each TKA (Fig. 4). The median overall exposure grade for each gowning system was 4 for System 1 (range, 3–4), 2.5 for System 2 (range, 2–3), and 0 for System 3 (range, 0–0). In pairwise comparisons between gowning systems, the positive-pressure exhaust suit had less exposure than gowning System 1 (difference of medians: 4, p < 0.001) and gowning System 2 (difference of medians: 2.5, p = 0.038). There was no difference found in exposure between Systems 1 and 2 (difference of medians: 1.5, p = 0.330). The interrater reliability of the independent observers was 1.
Fig. 4A-D.
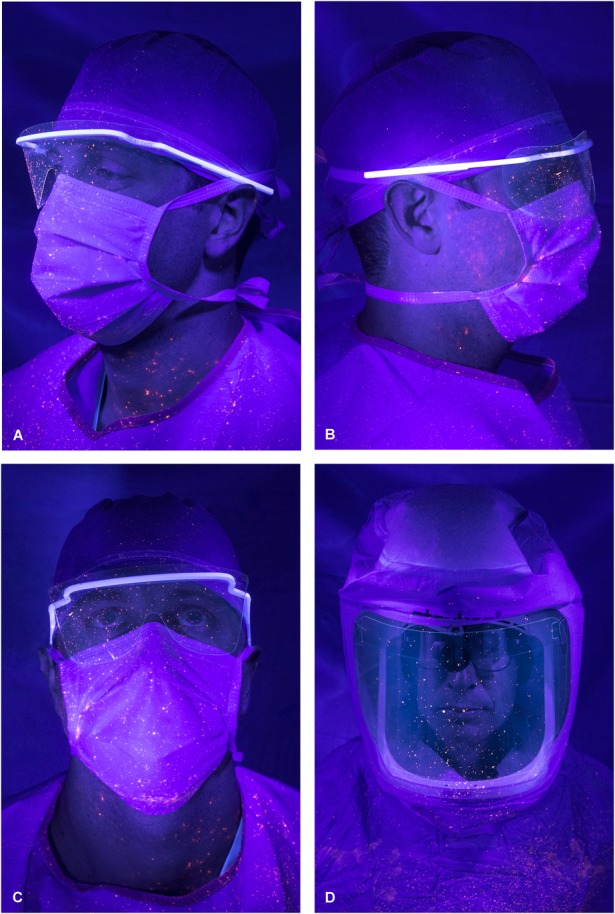
Particulate matter exposure encountered by surgical participants while wearing each gowning system shown here, including side views (A-B) and front view (C) of conventional gowning systems, and front view of the positive-pressure exhaust suit providing complete surgical participant protection (D).
Also of note, when gowning Systems 1 and 2 were removed, particulate matter was found in places that were supposedly covered by the gowning system. Exposure was found under the areas ostensibly protected by the face mask on the surgeon’s beard, lips, and even inside the nostrils. Matter was also found behind the protective eyewear around the surgeon’s eye and in both eyebrows and eyelashes (Fig. 5A-B).
Fig. 5A-B.
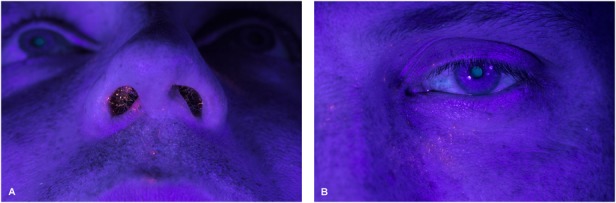
Views of particulate matter exposure encountered by surgical participants that was seen after the gowning system was removed are shown, including inside the surgeon’s nostrils (A) and on both the eyebrow and eyelashes (B).
Discussion
Prosthetic joint infection after total joint arthroplasty is a devastating complication, and positive-pressure exhaust suits were originally pioneered to decrease the risk of infection. With the additional cost of these suits and recent studies suggesting that they may not decrease the risk of infection [2, 7, 11, 15, 17, 20], some hospitals and surgeons have abandoned them in favor of less expensive alternatives. Orthopaedic procedures have an inherent risk for surgeon exposure to patient particulate matter and splash. This exposure occurs in nearly every procedure and it can occur outside the areas protected by conventional protective gowning systems [6, 16]. We proposed that in addition to their original purpose of reducing infection, body exhaust suits also provide personal protection for both the surgeon and assistants, perhaps justifying the added cost of these suits. Therefore, we designed a study to quantitatively assess surgeon exposure during TKA with a variety of gowning systems.
This study has several limitations. The most important is determining the clinical relevance of complete protection from exposure to patient particulate matter, mist, and splash during procedures. As a result of the relatively low incidence of reported mucocutaneous occupational splash injuries from bloodborne pathogen sources, the link between the amount of exposure and the development of clinical manifestation (bloodborne pathogen transmission) is hard to determine. One recent review determined that mucocutaneous exposures were second only to percutaneous injury for occupational exposure to HIV-positive fluid, but still only found 115 healthcare workers with these injuries out of approximately 18,000 determined occupational exposures [10]. Although disease transmission is higher after percutaneous exposure, previous studies have determined the risk of HIV and hepatitis C transmission after mucous membrane exposure to be 0.09% and 0.24%, respectively [1, 8]. These findings, combined with the increased prevalence of HIV in the patient population as a result of widespread antiretroviral use and the lack of surgeon exposure awareness, should lead surgeons to protect themselves as much as possible [4, 10]. Until the direct link between the amount of exposure during procedures and transmission is determined, emphasis should be on gowning systems that provide the greatest amount of personal protection.
Despite the small sample size, the six surgical participants per gowning system were sufficient to detect a strong statistical difference. Including a larger sample size may have increased the generalizability of the study; however, obtaining more cadaveric specimens was not necessary to support our conclusions. We chose to follow a standardized primary TKA protocol, which concluded once the implants were trialed. This simulated procedure corresponded with the length of previously published protocols looking at contamination in total joint arthroplasty [5, 19]. If the length of the simulated procedures was increased, it is likely that exposure grades would have increased as well because there is a positive correlation between duration of surgery and splash occurrence [16]. As a result of the lack of availability in our procedural laboratory, the TKAs did not include the use of pulsed lavage or laminar flow. Like with increasing procedure length, one can assume the use of pulsed lavage would have increased the amount of exposure encountered. It is unlikely that the use of laminar flow would have an impact on the volume and velocity of the splash and thus not change the exposure of the participants. Laminar flow is used at our institution and face shields are routinely covered with aerosolized particles from the saw during the procedure. It is very unlikely that the addition of laminar flow would have altered our results. An additional limitation was that patient particulate matter was simulated by fluorescent powder mixed with water. It is unclear how closely this corresponds to the actual exposure of bodily fluid mist- and splash-carrying pathogens encountered during knee arthroplasty. However, organism-carrying particles have been shown to range in size from 5 µm to 15 µm, and the 5-µm fluorescent powder used in this study has been used in previous studies to simulate airborne particulate matter [3, 5, 19]. Lastly, there was a lack of blinding to the surgical gowning system being tested from both the surgeons performing the procedure and the observers grading the exposure. Blinding was not possible because each gowning system had obvious characteristics and components specific to that gowning setup. Instead, direct visualization of each gowning system by observers allowed for a more accurate determination of the degree of exposure in each gowning setup. For example, a two-dimensional picture would not have allowed the observer to see the exposure that occurred under folds or exposure found around the surgeon’s neck or under the edges of the mask. Additionally, not all fluorescent exposure seen under direct visualization with the ultraviolet light could be portrayed through photographs. Thus, by not blinding, the sensitivity of the observers to detect exposure was increased and potentially increased the strength of the study.
Wearing positive-pressure exhaust suits can decrease or eliminate particulate matter exposure to skin and mucous membranes. Most previous studies have identified surgeon exposure risk through splash evaluation. These studies prospectively analyzed the amount of splash on the visors or goggles worn during the procedures. A splash occurs in up to 100% of elective primary knee and hip arthroplasties and 86% of orthopaedic trauma procedures [4, 16]. These studies concluded that conventional gowning systems, including goggles and visors, fail to protect the surgeon because 13% to 51% of splash occurs outside the area protected by these systems [6, 16]. A sawbones study determined that contamination risk is 30% while wearing these conventional gowning systems and protection can be superior with the use of a surgical helmet system [18]. Also, most surgeons are unaware of their exposure; one study showed that only 15% of surgeons who were exposed during a procedure recognized the exposure [4]. In this study, each procedure produced patient particulate matter mist and splash, providing the potential for exposure through contact with skin or mucous membranes. We determined that this potential can be eliminated with the use of positive-pressure exhaust suits because they did not allow any exposure to skin or mucous membranes.
With conventional gowning systems, covered areas should not be considered protected. Splash has previously been found to occur outside of areas protected by goggles and visors in conventional gowning systems [6, 16]. In addition, as evidenced by this study, splash can also be found in the areas thought to be protected by these conventional systems. The exposure occurring under the face mask and protective eyewear was found to involve the surgeon’s mucous membranes. We determined that the most reliable way to eliminate mucous membrane exposure is by the surgeon wearing a positive-pressure exhaust suit.
During total joint arthroplasties, splash is likely and thus these procedures provide an inherent risk for surgeon exposure to patient particulate matter and the potential transmission of bloodborne pathogens. The potential for transmission from contact of open skin or mucous membranes warrants the use of adequate personal protection by the surgeon. Conventional gowning systems present an exposure risk for the surgeon, whereas positive-pressure exhaust suits provide complete protection during each procedure. Despite recent concerns that positive-pressure exhaust suits may not decrease infection rates, the clear added benefit to the surgeon and assistants in protection from potentially infectious and harmful patient substances is overwhelming. Despite the added costs of these suits, hospitals and surgeons should heavily weigh these protective benefits when considering whether to use a positive-pressure exhaust suit or a conventional gowning system during total joint arthroplasties.
Acknowledgments
We thank Nathan Pallace for his assistance with the photography during this project.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
This work was performed at the Mayo Clinic Arizona, Phoenix, AZ, USA.
References
- 1.Baffoy-Fayard N, Maugat S, Sapoval M, Cluzel P, Denys A, Sellier N, Desruennes E, Legmann P, Thibault V, Brücker G, Astagneau P; Study Group on Hygiene Practices in Interventional Radiology. Potential exposure to hepatitis C virus through accidental blood contact in interventional radiology. J Vasc Interv Radiol. 2003;14:173–179. [DOI] [PubMed] [Google Scholar]
- 2.Bohn WW, McKinsey DS, Dykstra M, Koppe S. The effect of a portable HEPA-filtered body exhaust system on airborne microbial contamination in a conventional operating room. Infect Control Hosp Epidemiol. 1996;17:419–422. [DOI] [PubMed] [Google Scholar]
- 3.Clark RP, Reed PJ, Seal DV, Stephenson ML. Ventilation conditions and air-borne bacteria and particles in operating theatres: proposed safe economies. J Hyg (Lond). 1985;95:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins D, Rice J, Nicholson P, Barry K. Quantification of facial contamination with blood during orthopaedic procedures. J Hosp Infect. 2000;45:73–75. [DOI] [PubMed] [Google Scholar]
- 5.Fraser JF, Young SW, Valentine KA, Probst NE, Spangehl MJ. The gown-glove interface is a source of contamination: a comparative study. Clin Orthop Relat Res. 2015;473:2291–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirpara KM, O’Halloran E, O’Sullivan M. A quantitative assessment of facial protection systems in elective hip arthroplasty. Acta Orthop Belg. 2011;77:375–380. [PubMed] [Google Scholar]
- 7.Hooper GJ, Rothwell AG, Frampton C, Wyatt MC. Does the use of laminar flow and space suits reduce early deep infection after total hip and knee replacement? The ten-year results of the New Zealand Joint Registry. J Bone Joint Surg Br. 2011;93:85–90. [DOI] [PubMed] [Google Scholar]
- 8.Kuhar DT, Henderson DK, Struble KA, Heneine W, Thomas V, Cheever LW, Gomaa A, Panlilio AL; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875–892. [DOI] [PubMed] [Google Scholar]
- 9.Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Stanley SJ, Lowe D. Effect of ultraclean air in operating rooms on deep sepsis in the joint after total hip or knee replacement: a randomised study. Br Med J (Clin Res Ed). 1982;285:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nwaiwu CA, Egro FM, Smith S, Harper JD, Spiess AM. Seroconversion rate among health care workers exposed to HIV-contaminated body fluids: the University of Pittsburgh 13-year experience. Am J Infect Control. 2017;45:896–900. [DOI] [PubMed] [Google Scholar]
- 11.Pasquarella C, Pitzurra O, Herren T, Poletti L, Savino A. Lack of influence of body exhaust gowns on aerobic bacterial surface counts in a mixed-ventilation operating theatre. A study of 62 hip arthroplasties. J Hosp Infect. 2003;54:2–9. [DOI] [PubMed] [Google Scholar]
- 12.Pietrabissa A, Merigliano S, Montorsi M, Poggioli G, Stella M, Borzomati D, Ciferri E, Rossi G, Doglietto G. Reducing the occupational risk of infections for the surgeon: multicentric national survey on more than 15,000 surgical procedures. World J Surg. 1997;21:573–578. [DOI] [PubMed] [Google Scholar]
- 13.Pohlert T. PMCMR: Calculate Pairwise Multiple Comparisons of Mean Rank Sums. 2018. Available at: https://cran.r-project.org/web/packages/PMCMR/index.html. Accessed January 26, 2018.
- 14.Seale J. AIDS virus infection: prognosis and transmission. J R Soc Med. 1985;78:613–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw JA, Bordner MA, Hamory BH. Efficacy of the Steri-Shield filtered exhaust helmet in limiting bacterial counts in the operating room during total joint arthroplasty. J Arthroplasty. 1996;11:469–473. [DOI] [PubMed] [Google Scholar]
- 16.Singh VK, Kalairajah Y. Splash in elective primary knee and hip replacement: are we adequately protected? J Bone Joint Surg Br. 2009;91:1074–1077. [DOI] [PubMed] [Google Scholar]
- 17.Tayton ER, Frampton C, Hooper GJ, Young SW. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. Bone Joint J. 2016;98:334–340. [DOI] [PubMed] [Google Scholar]
- 18.Wendlandt R, Thomas M, Kienast B, Schulz AP. In-vitro evaluation of surgical helmet systems for protecting surgeons from droplets generated during orthopaedic procedures. J Hosp Infect. 2016;94:75–79. [DOI] [PubMed] [Google Scholar]
- 19.Young SW, Chisholm C, Zhu M. Intraoperative contamination and space suits: a potential mechanism. Eur J Orthop Surg Traumatol. 2014;24:409–413. [DOI] [PubMed] [Google Scholar]
- 20.Young SW, Zhu M, Shirley OC, Wu Q, Spangehl MJ. Do 'surgical helmet systems' or 'body exhaust suits' affect contamination and deep infection rates in arthroplasty? A systematic review. J Arthroplasty. 2016;31:225–233. [DOI] [PubMed] [Google Scholar]